Cannabinoid Mixture Affects the Fate and Functions of B Cells through the Modulation of the Caspase and MAP Kinase Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Storage of Peripheral Blood Mononuclear Cells
2.2. Cell Culture and PBMC Exposure to a Cannabinoid Mixture
2.3. Cytotoxicity Assay
2.4. Apoptosis and CD45+/CD19+ Cell Count
2.5. Assessment of CB2R Expression
2.6. Oxidative and Anti-Oxidative Stress Responses
2.7. Mitochondrial Membrane Potential
2.8. Apoptosis PCR Array
2.9. Assessment of Caspase Activation
2.10. Assessment of DNA Damage
2.11. Analysis of Cell Signaling Pathways
2.12. Detection and Enumeration of IgG-/IgM-Secreting B Cells
2.13. Cytokine Expression Profiles of B Cells
2.14. Statistical Analysis
3. Results
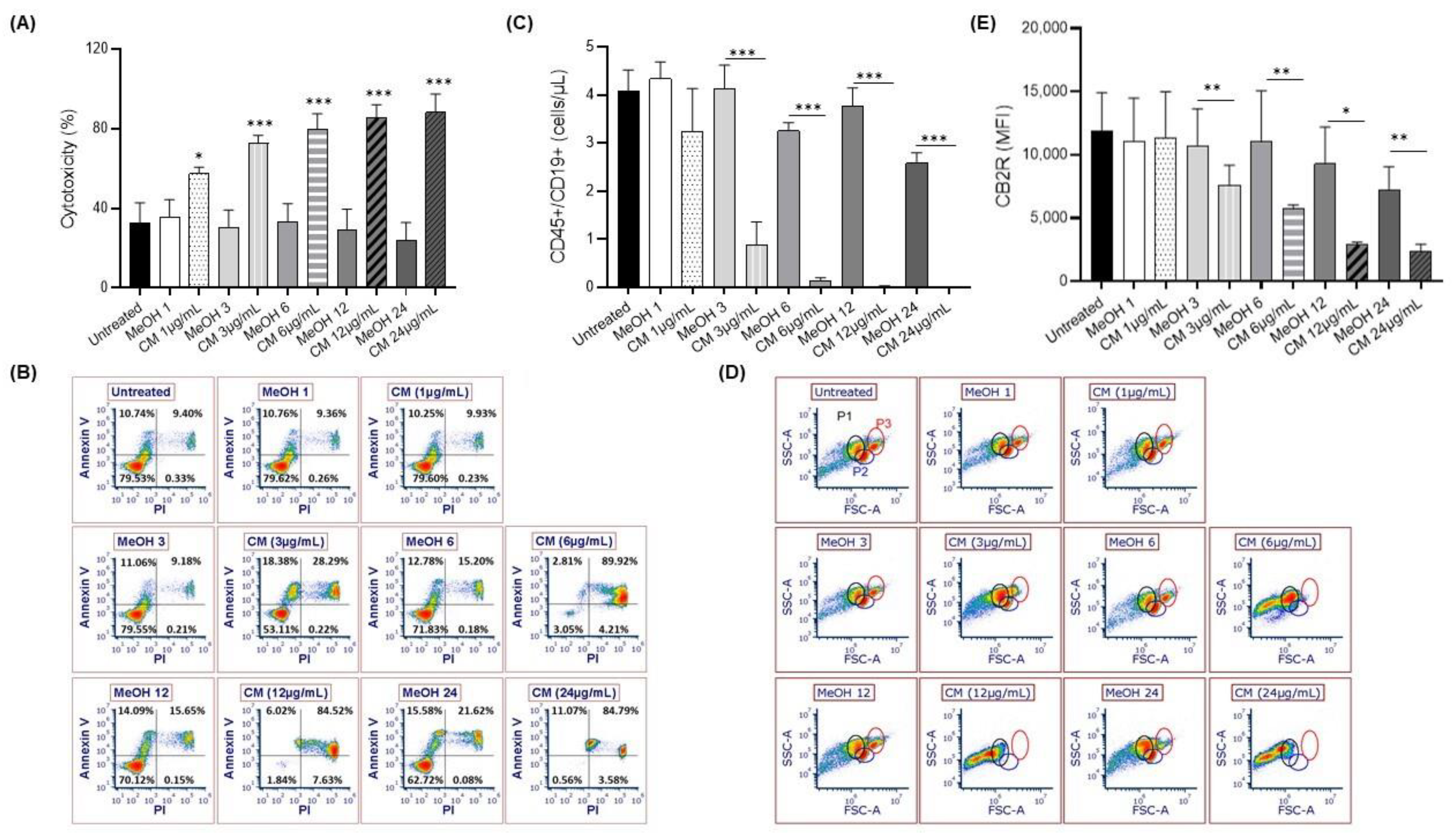
3.1. Exposure to the Cannabinoid Mixture Favors B-Cell Apoptosis
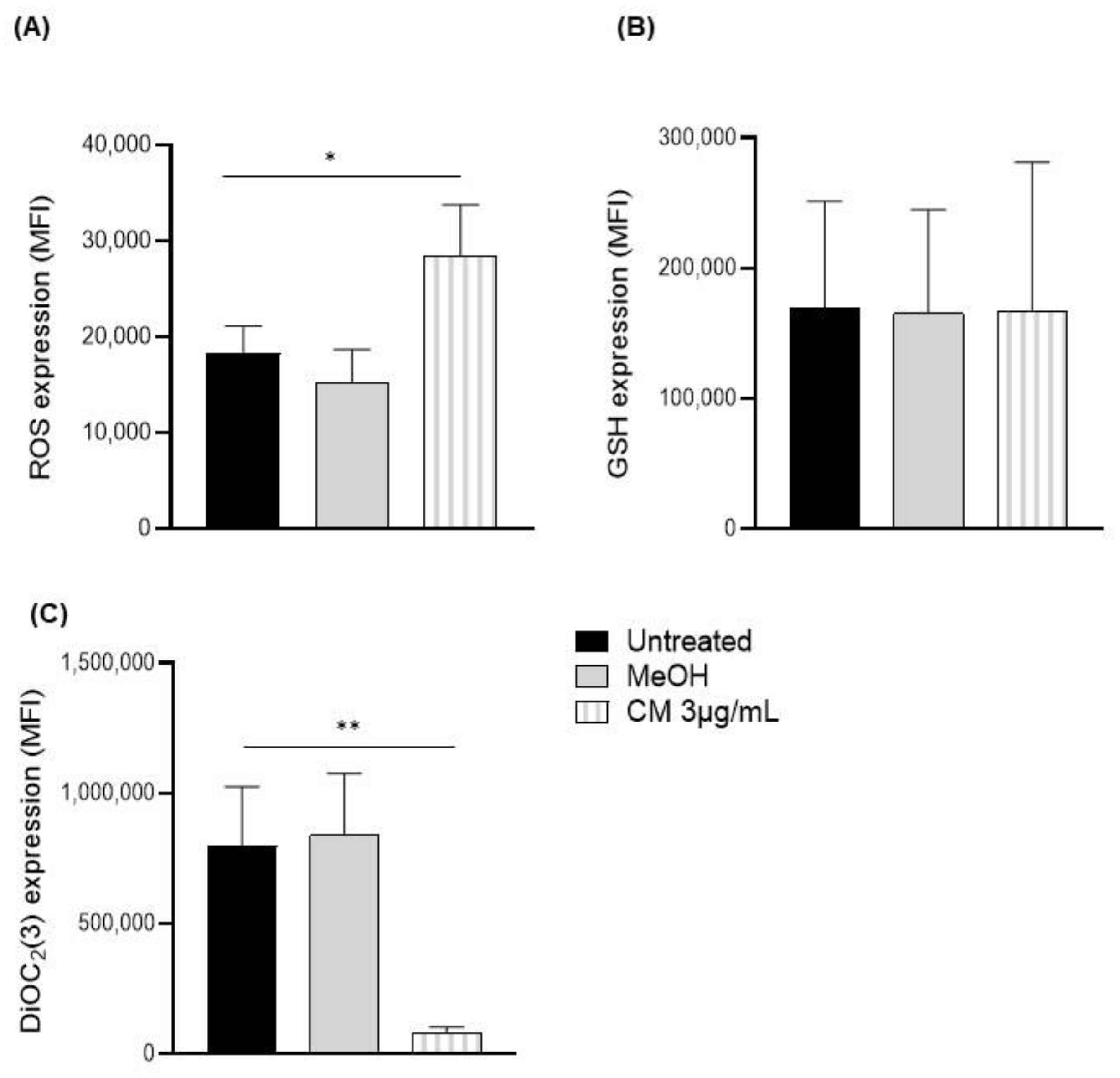
3.2. Exposure to the Cannabinoid Mixture Significantly Reduces the Mitochondrial Membrane Potential of B Cells
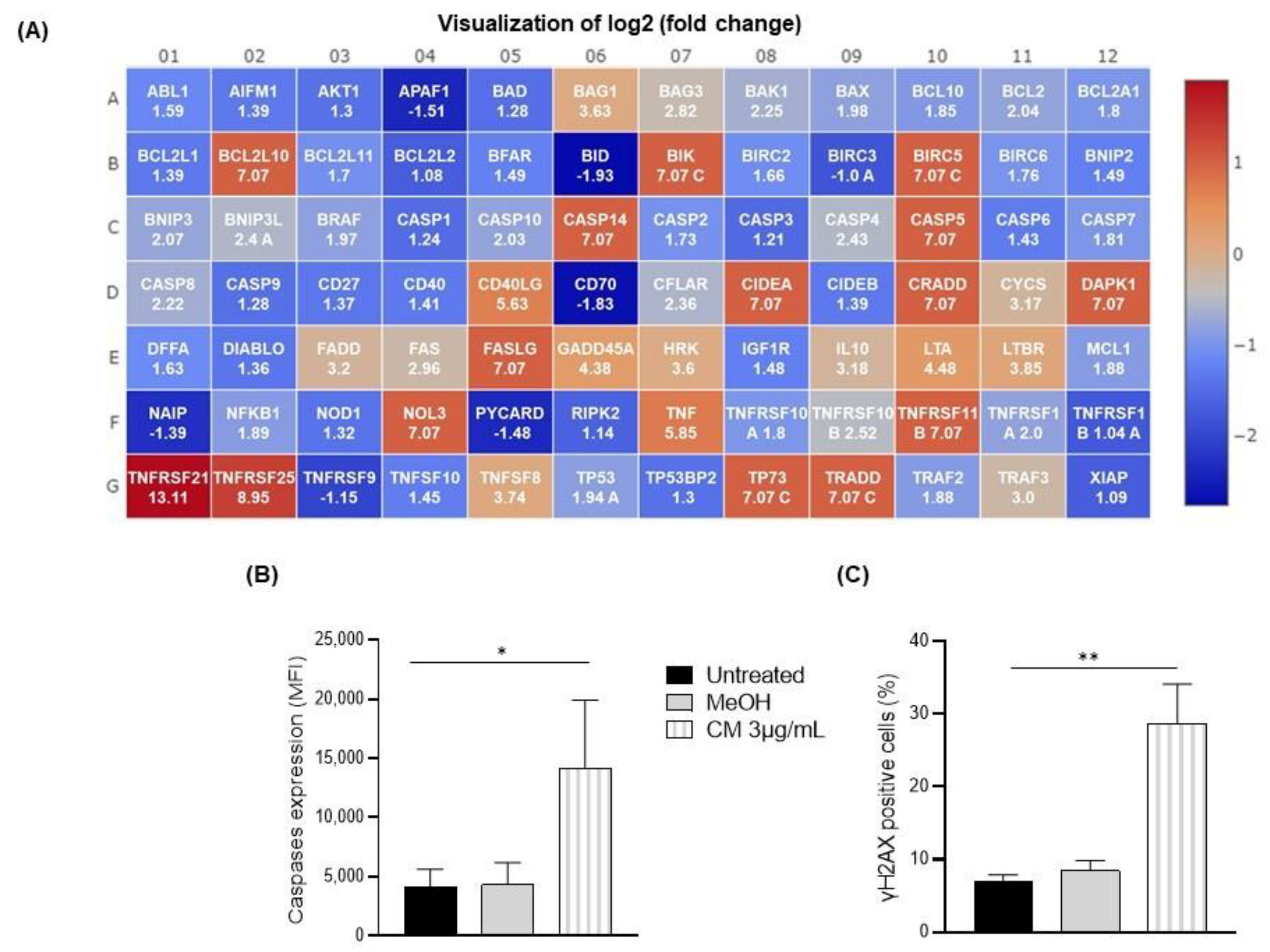
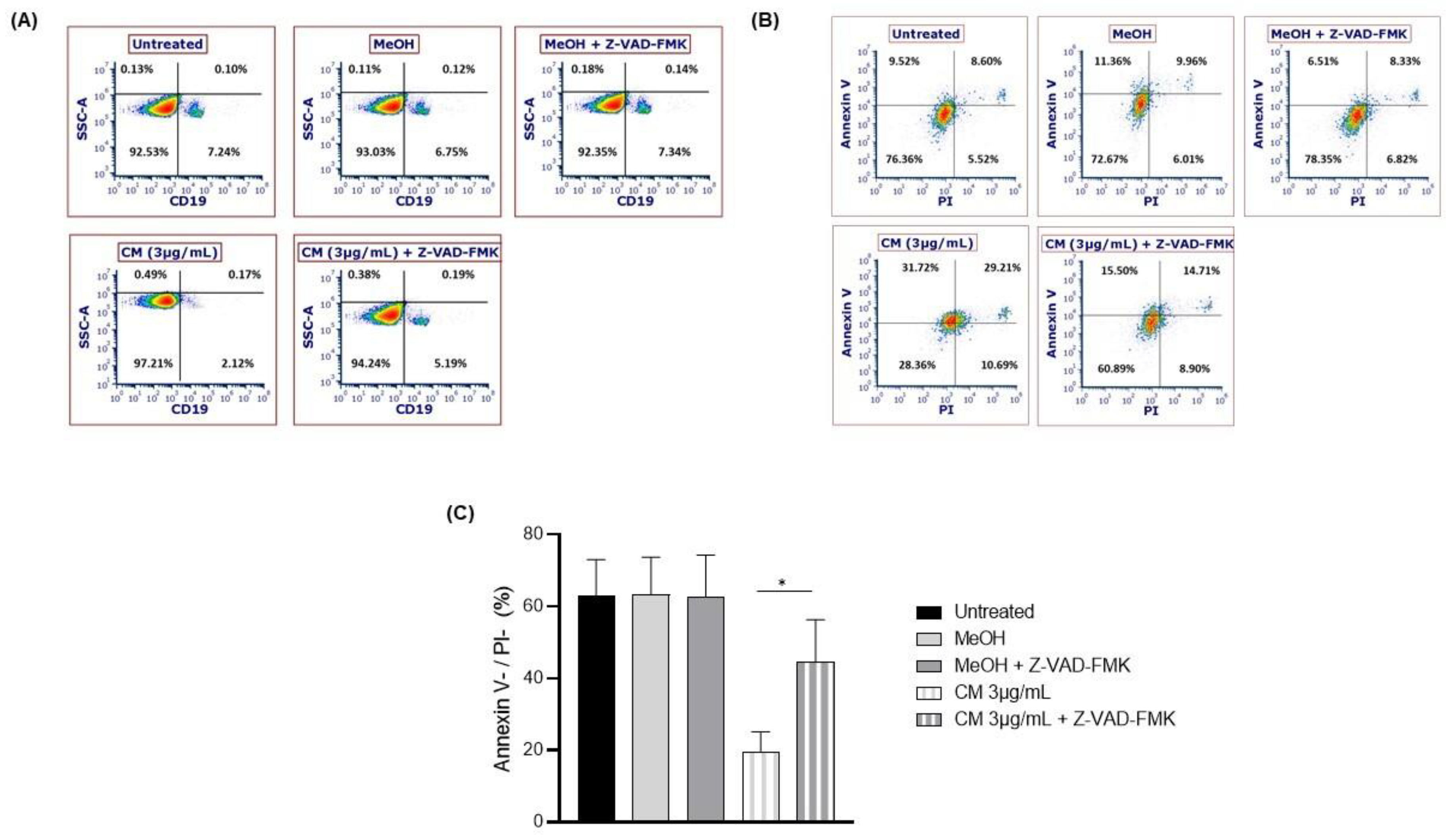
3.3. The Cannabinoid Mixture Induces B-Cell Apoptosis through the Caspase Pathway
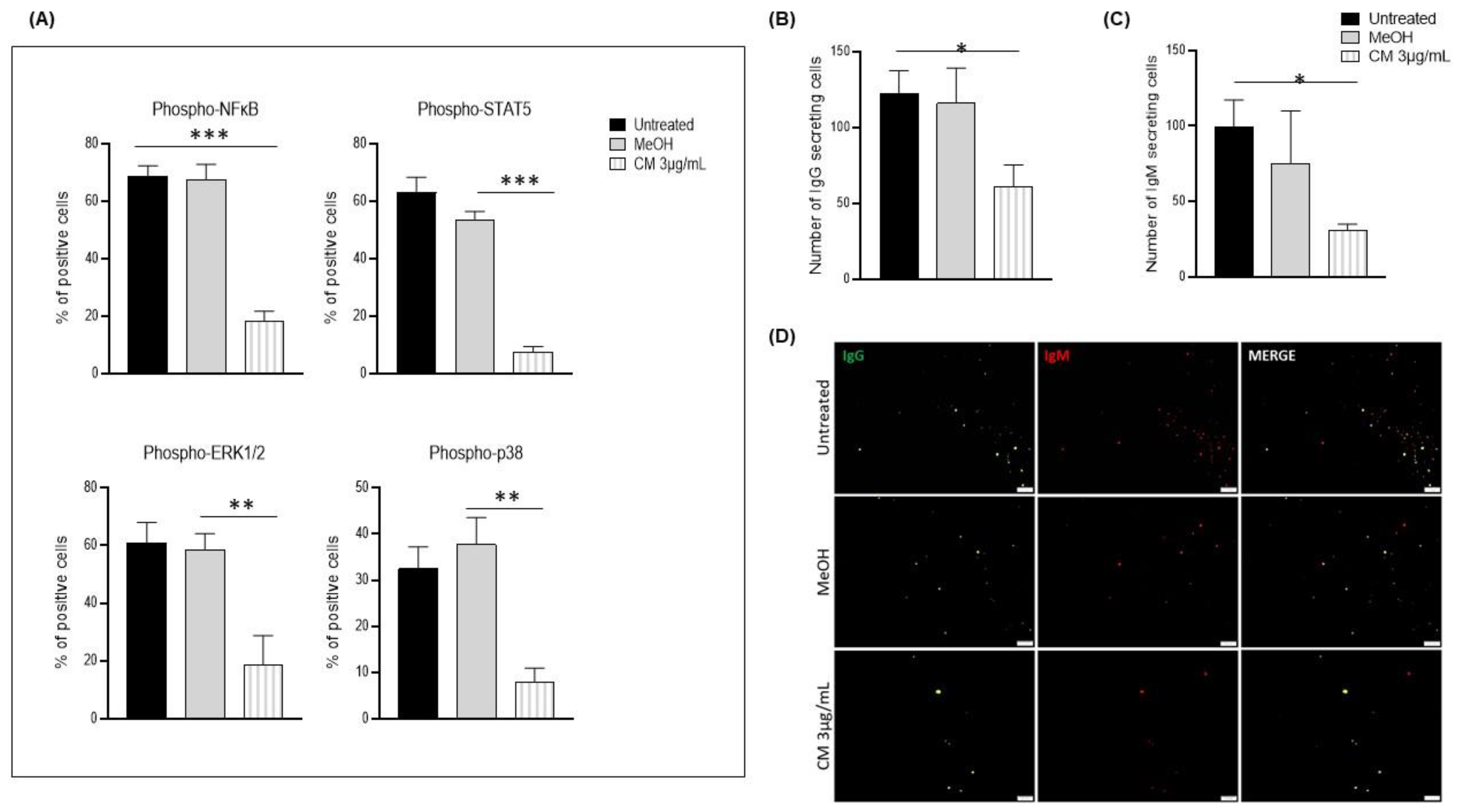
3.4. The Cannabinoid Mixture Affects B-Cell Signaling Pathways and Function
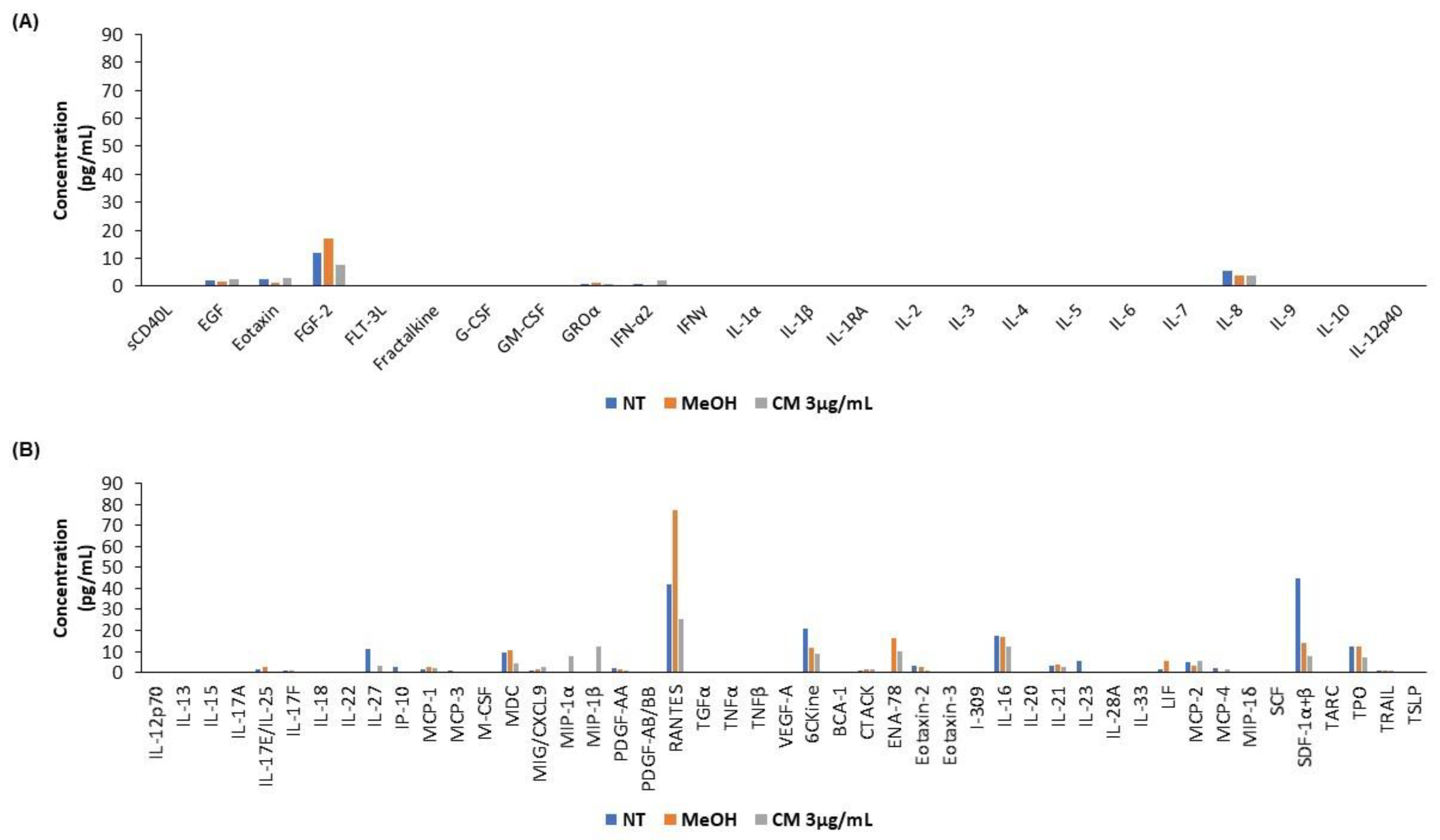
3.5. The Cannabinoid Mixture Affects the Cytokine Secretion Profile of B Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Alcohol and Drugs Survey (CADS): Summary of Results for 2019. Available online: https://www.canada.ca/en/health-canada/services/canadian-alcohol-drugs-survey/2019summary.html (accessed on 25 November 2022).
- Henshaw, F.R.; Dewsbury, L.S.; Lim, C.K.; Steiner, G.Z. The Effects of Cannabinoids on Pro- and Anti-Inflammatory Cytokines: A Systematic Review of In Vivo Studies. Cannabis Cannabinoid Res. 2021, 6, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis Sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Argenziano, M.; Tortora, C.; Bellini, G.; Di Paola, A.; Punzo, F.; Rossi, F. The Endocannabinoid System in Pediatric Inflammatory and Immune Diseases. Int. J. Mol. Sci. 2019, 20, 5875, Erratum in IJMS 2020, 21, 2757. [Google Scholar] [CrossRef]
- Begg, M.; Pacher, P.; Batkai, S.; Oseihyiaman, D.; Offertaler, L.; Mo, F.; Liu, J.; Kunos, G. Evidence for Novel Cannabinoid Receptors. Pharmacol. Ther. 2005, 106, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Cencioni, M.T.; Chiurchiù, V.; Catanzaro, G.; Borsellino, G.; Bernardi, G.; Battistini, L.; Maccarrone, M. Anandamide Suppresses Proliferation and Cytokine Release from Primary Human T-Lymphocytes Mainly via CB2 Receptors. PLoS ONE 2010, 5, e8688. [Google Scholar] [CrossRef]
- Coopman, K.; Smith, L.D.; Wright, K.L.; Ward, S.G. Temporal Variation in CB2R Levels Following T Lymphocyte Activation: Evidence That Cannabinoids Modulate CXCL12-Induced Chemotaxis. Int. Immunopharmacol. 2007, 7, 360–371. [Google Scholar] [CrossRef] [PubMed]
- McHugh, D.; Tanner, C.; Mechoulam, R.; Pertwee, R.G.; Ross, R.A. Inhibition of Human Neutrophil Chemotaxis by Endogenous Cannabinoids and Phytocannabinoids: Evidence for a Site Distinct from CB1 and CB2. Mol. Pharm. 2008, 73, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutiérrez, S.; Molina-Holgado, E.; Guaza, C. Effect of Anandamide Uptake Inhibition in the Production of Nitric Oxide and in the Release of Cytokines in Astrocyte Cultures. Glia 2005, 52, 163–168. [Google Scholar] [CrossRef]
- Carayon, P.; Marchand, J.; Dussossoy, D.; Derocq, J.M.; Jbilo, O.; Bord, A.; Bouaboula, M.; Galiègue, S.; Mondière, P.; Pénarier, G.; et al. Modulation and Functional Involvement of CB2 Peripheral Cannabinoid Receptors during B-Cell Differentiation. Blood 1998, 92, 3605–3615. [Google Scholar] [CrossRef]
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid Receptors CB1 and CB2: A Characterization of Expression and Adenylate Cyclase Modulation within the Immune System. Toxicol. Appl. Pharm. 1997, 142, 278–287. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lee, S.T.; Lin, W.W. Effects of Cannabinoids on LPS-Stimulated Inflammatory Mediator Release from Macrophages: Involvement of Eicosanoids. J. Cell Biochem. 2001, 81, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Nagarkatti, P.; Pandey, R.; Rieder, S.A.; Hegde, V.L.; Nagarkatti, M. Cannabinoids as Novel Anti-Inflammatory Drugs. Future Med. Chem. 2009, 1, 1333–1349. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Lombard, C.; Martin, B.R.; Nagarkatti, M.; Nagarkatti, P.S. Δ9-Tetrahydrocannabinol-Induced Apoptosis in the Thymus and Spleen as a Mechanism of Immunosuppression In Vitro and In Vivo. J. Pharm. Exp. 2002, 302, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, G.; Chatterjee, N. Endocannabinoids Alleviate Proinflammatory Conditions by Modulating Innate Immune Response in Muller Glia during Inflammation. Glia 2012, 60, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Berdyshev, E.V.; Boichot, E.; Germain, N.; Allain, N.; Anger, J.-P.; Lagente, V. Influence of Fatty Acid Ethanolamides and Δ9-Tetrahydrocannabinol on Cytokine and Arachidonate Release by Mononuclear Cells. Eur. J. Pharmacol. 1997, 330, 231–240. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Pan, H.; Rajesh, M.; Bátkai, S.; Patel, V.; Harvey-White, J.; Mukhopadhyay, B.; Haskó, G.; Gao, B.; Mackie, K.; et al. CB 1 Cannabinoid Receptors Promote Oxidative/Nitrosative Stress, Inflammation and Cell Death in a Murine Nephropathy Model: CB 1 Antagonists for Nephropathy. Br. J. Pharmacol. 2010, 160, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Cohen, I.; Bar-Sela, G. “The Two Sides of the Same Coin”—Medical Cannabis, Cannabinoids and Immunity: Pros and Cons Explained. Pharmaceutics 2022, 14, 389. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. ATVB 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of Inflammation by Cannabinoids, the Endocannabinoids 2-Arachidonoyl-Glycerol and Arachidonoyl-Ethanolamide, and Their Metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- El-Gohary, M.; Eid, M.A. Effect of Cannabinoid Ingestion (in the Form of Bhang) on the Immune System of High School and University Students. Hum. Exp. Toxicol. 2004, 23, 149–156. [Google Scholar] [CrossRef]
- Rachelefsky, G.S.; Opelz, G.; Mickey, M.R.; Lessin, P.; Kiuchi, M.; Silverstein, M.J.; Stiehm, E.R. Intact Humoral and Cell-Mediated Immunity in Chronic Marijuana Smoking. J. Allergy Clin. Immunol. 1976, 58, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Newton, C.A.; Klein, T.W. Cannabinoid 2 (CB2) Receptor Involvement in the down-Regulation but Not up-Regulation of Serum IgE Levels in Immunized Mice. J. Neuroimmune Pharm. 2012, 7, 591–598. [Google Scholar] [CrossRef]
- Nahas, G.G.; Osserman, E.F. Altered Serum Immunoglobulin Concentration in Chronic Marijuana Smokers. In Drugs of Abuse, Immunity, and Immunodeficiency; Friedman, H., Specter, S., Klein, T.W., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1991; Volume 288, pp. 25–32. ISBN 978-1-4684-5927-2. [Google Scholar]
- Ngaotepprutaram, T.; Kaplan, B.L.F.; Carney, S.; Crawford, R.; Kaminski, N.E. Suppression by Δ9-Tetrahydrocannabinol of the Primary Immunoglobulin M Response by Human Peripheral Blood B Cells Is Associated with Impaired STAT3 Activation. Toxicology 2013, 310, 84–91. [Google Scholar] [CrossRef]
- Derocq, J.M.; Ségui, M.; Marchand, J.; Le Fur, G.; Casellas, P. Cannabinoids Enhance Human B-Cell Growth at Low Nanomolar Concentrations. FEBS Lett. 1995, 369, 177–182. [Google Scholar] [CrossRef]
- Klein, T.W.; Newton, C.A.; Widen, R.; Friedman, H. The Effect of Delta-9-Tetrahydrocannabinol and 11-Hydroxy-Delta-9-Tetrahydrocannabinol on T-Lymphocyte and B-Lymphocyte Mitogen Responses. J. Immunopharmacol. 1985, 7, 451–466. [Google Scholar] [CrossRef]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the Immune System: Potential for the Treatment of Inflammatory Diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Tanasescu, R.; Constantinescu, C.S. Cannabinoids and the Immune System: An Overview. Immunobiology 2010, 215, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Lampron, M.-C.; Desbiens-Tremblay, C.; Loubaki, L. In Vitro Exposure of Whole Blood to a Cannabinoid Mixture Impairs the Quality of Red Blood Cells and Platelets. Blood Transfus. 2022. [Google Scholar] [CrossRef]
- Nahas, G.G. The Pharmacokinetics of THC in Fat and Brain: Resulting Functional Responses to Marihuana Smoking. Hum. Psychopharmacol. Clin. Exp. 2001, 16, 247–255. [Google Scholar] [CrossRef]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef]
- Loubaki, L.; Rouabhia, M.; Zahrani, M.A.; Amri, A.A.; Semlali, A. Oxidative Stress and Autophagy Mediate Anti-Cancer Properties of Cannabis Derivatives in Human Oral Cancer Cells. Cancers 2022, 14, 4924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting Mitochondrial Reactive Oxygen Species as Novel Therapy for Inflammatory Diseases and Cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Lopez, K.E.; Bouchier-Hayes, L. Lethal and Non-Lethal Functions of Caspases in the DNA Damage Response. Cells 2022, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, H.; Hikida, M.; Aiba, Y.; Mori, Y.; Hatano, N.; Ogata, M.; Kurosaki, T. Extracellular Signal-Regulated Protein Kinase 2 Is Required for Efficient Generation of B Cells Bearing Antigen-Specific Immunoglobulin G. Mol. Cell Biol. 2007, 27, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Heltemes-Harris, L.M.; Farrar, M.A. The Role of STAT5 in Lymphocyte Development and Transformation. Curr. Opin. Immunol. 2012, 24, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Khiem, D.; Cyster, J.G.; Schwarz, J.J.; Black, B.L. A P38 MAPK-MEF2C Pathway Regulates B-Cell Proliferation. Proc. Natl. Acad. Sci. USA 2008, 105, 17067–17072. [Google Scholar] [CrossRef]
- Sasaki, Y.; Iwai, K. Roles of the NF-ΚB Pathway in B-Lymphocyte Biology. In B Cell Receptor Signaling; Kurosaki, T., Wienands, J., Eds.; Current Topics in Microbiology and Immunology; Springer International Publishing: Cham, Germany, 2015; Volume 393, pp. 177–209. ISBN 978-3-319-26131-7. [Google Scholar]
- Owens, S.M.; McBay, A.J.; Reisner, H.M.; Perez-Reyes, M. 125I Radioimmunoassay of Delta-9-Tetrahydrocannabinol in Blood and Plasma with a Solid-Phase Second-Antibody Separation Method. Clin. Chem. 1981, 27, 619–624. [Google Scholar] [CrossRef]
- Vandevenne, M.; Vandenbussche, H.; Verstraete, A. Detection Time of Drugs of Abuse in Urine. Acta Clin. Belg. 2000, 55, 323–333. [Google Scholar] [CrossRef]
- Vandrey, R.; Herrmann, E.S.; Mitchell, J.M.; Bigelow, G.E.; Flegel, R.; LoDico, C.; Cone, E.J. Pharmacokinetic Profile of Oral Cannabis in Humans: Blood and Oral Fluid Disposition and Relation to Pharmacodynamic Outcomes. J. Anal. Toxicol. 2017, 41, 83–99. [Google Scholar] [CrossRef]
- Agurell, S.; Halldin, M.; Lindgren, J.E.; Ohlsson, A.; Widman, M.; Gillespie, H.; Hollister, L. Pharmacokinetics and Metabolism of Delta 1-Tetrahydrocannabinol and Other Cannabinoids with Emphasis on Man. Pharm. Rev. 1986, 38, 21–43. [Google Scholar]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Recalchi, S.; Manganelli, V.; Riitano, G.; Garofalo, T.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2021, 27, 64. [Google Scholar] [CrossRef]
- Atwood, B.K.; Wager-Miller, J.; Haskins, C.; Straiker, A.; Mackie, K. Functional Selectivity in CB 2 Cannabinoid Receptor Signaling and Regulation: Implications for the Therapeutic Potential of CB 2 Ligands. Mol. Pharm. 2012, 81, 250–263. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Huang, C.-H.; Lin, Y.-H.; Wang, C.-C.; Jan, T.-R. Cannabidiol Induced Apoptosis in Human Monocytes through Mitochondrial Permeability Transition Pore-Mediated ROS Production. Free Radic. Biol. Med. 2018, 124, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.; Bektay, M.; Akyildiz, A.; Sisman, B.; Izzettin, F.; Kocyigit, A. Investigation of DNA Damage, Oxidative Stress, and Inflammation in Synthetic Cannabinoid Users. Hum. Exp. Toxicol. 2020, 39, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P. CB2 Cannabinoid Receptor Agonist, JWH-015, Triggers Apoptosis in Immune Cells: Potential Role for CB2-Selective Ligands as Immunosuppressive Agents. Clin. Immunol. 2007, 122, 259–270. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Peng, Y.; Xiao, Y.; Zhu, H.; Ding, Z.-M.; Hua, H. Phosphorylation of Extracellular Signal-Regulated Kinase as a Biomarker for Cannabinoid Receptor 2 Activation. Heliyon 2018, 4, e00909. [Google Scholar] [CrossRef] [PubMed]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-ΚB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 716469. [Google Scholar] [CrossRef] [PubMed]
- Kimata, H.; Yoshida, A.; Ishioka, C.; Fujimoto, M.; Lindley, I.; Furusho, K. RANTES and Macrophage Inflammatory Protein 1 Alpha Selectively Enhance Immunoglobulin (IgE) and IgG4 Production by Human B Cells. J. Exp. Med. 1996, 183, 2397–2402. [Google Scholar] [CrossRef]
- Morita, Y.; Masters, E.A.; Schwarz, E.M.; Muthukrishnan, G. Interleukin-27 and Its Diverse Effects on Bacterial Infections. Front. Immunol. 2021, 12, 678515. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Caramalho, I.; Demengeot, J. IFN-α/β Enhances BCR-dependent B Cell Responses. Int. Immunol. 2002, 14, 411–419. [Google Scholar] [CrossRef]
- Muehlinghaus, G.; Cigliano, L.; Huehn, S.; Peddinghaus, A.; Leyendeckers, H.; Hauser, A.E.; Hiepe, F.; Radbruch, A.; Arce, S.; Manz, R.A. Regulation of CXCR3 and CXCR4 Expression during Terminal Differentiation of Memory B Cells into Plasma Cells. Blood 2005, 105, 3965–3971. [Google Scholar] [CrossRef] [PubMed]
- Kagen, S.; Kurup, V.; Sohnle, P.; Fink, J. Marijuana Smoking and Fungal Sensitization. J. Allergy Clin. Immunol. 1983, 71, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.L.; Wyatt, T.A.; Katafiasz, D.M.; Taylor, K.W.; Heires, A.J.; Sisson, J.H.; Romberger, D.J.; Burnham, E.L. Alcohol and Cannabis Use Alter Pulmonary Innate Immunity. Alcohol 2019, 80, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Caiaffa, W.T.; Vlahov, D.; Graham, N.M.; Astemborski, J.; Solomon, L.; Nelson, K.E.; Muñoz, A. Drug Smoking, Pneumocystis Carinii Pneumonia, and Immunosuppression Increase Risk of Bacterial Pneumonia in Human Immunodeficiency Virus-Seropositive Injection Drug Users. Am. J. Respir. Crit. Care Med. 1994, 150, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Melén, C.M.; Merrien, M.; Wasik, A.M.; Panagiotidis, G.; Beck, O.; Sonnevi, K.; Junlén, H.-R.; Christensson, B.; Sander, B.; Wahlin, B.E. Clinical Effects of a Single Dose of Cannabinoids to Patients with Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2022, 63, 1387–1397. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampron, M.-C.; Paré, I.; Al-Zharani, M.; Semlali, A.; Loubaki, L. Cannabinoid Mixture Affects the Fate and Functions of B Cells through the Modulation of the Caspase and MAP Kinase Pathways. Cells 2023, 12, 588. https://doi.org/10.3390/cells12040588
Lampron M-C, Paré I, Al-Zharani M, Semlali A, Loubaki L. Cannabinoid Mixture Affects the Fate and Functions of B Cells through the Modulation of the Caspase and MAP Kinase Pathways. Cells. 2023; 12(4):588. https://doi.org/10.3390/cells12040588
Chicago/Turabian StyleLampron, Marie-Claude, Isabelle Paré, Mohammed Al-Zharani, Abdelhabib Semlali, and Lionel Loubaki. 2023. "Cannabinoid Mixture Affects the Fate and Functions of B Cells through the Modulation of the Caspase and MAP Kinase Pathways" Cells 12, no. 4: 588. https://doi.org/10.3390/cells12040588
APA StyleLampron, M.-C., Paré, I., Al-Zharani, M., Semlali, A., & Loubaki, L. (2023). Cannabinoid Mixture Affects the Fate and Functions of B Cells through the Modulation of the Caspase and MAP Kinase Pathways. Cells, 12(4), 588. https://doi.org/10.3390/cells12040588








