Novel Zebrafish Patient-Derived Tumor Xenograft Methodology for Evaluating Efficacy of Immune-Stimulating BCG Therapy in Urinary Bladder Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Zebrafish Breeding and Maintenance
2.3. Cell Culture and Fluorescent Labelling
2.4. Clinical Tumor Tissue Samples
2.5. BCG Reconstitution, Toxicity Testing and Treatment in the Xenograft Model
2.6. Microinjections into 48 h Old Zebrafish Larvae
2.7. Analysis of Primary Tumor Size and Cell Dissemination
2.8. Statistical Analysis
3. Results
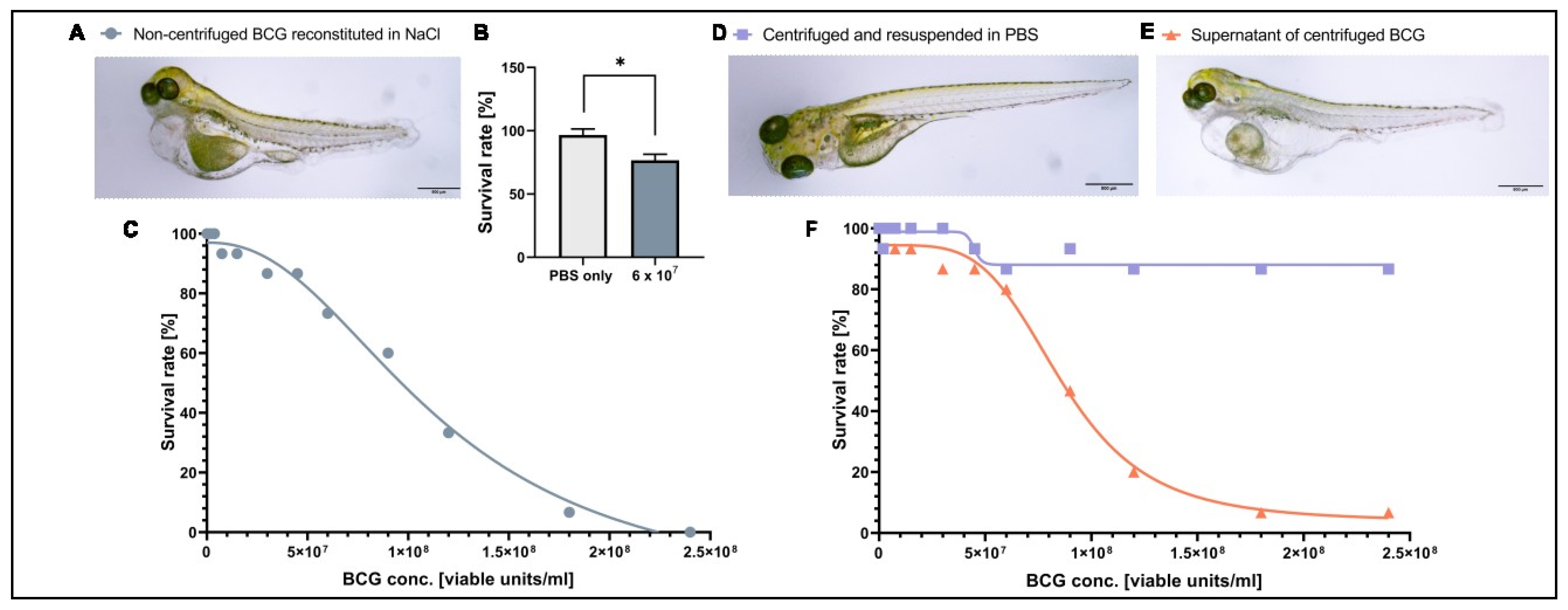
3.1. A Component of the BCG-Medac Drug Formulation Is Toxic to Zebrafish Larvae but Can Be Removed by Centrifugation
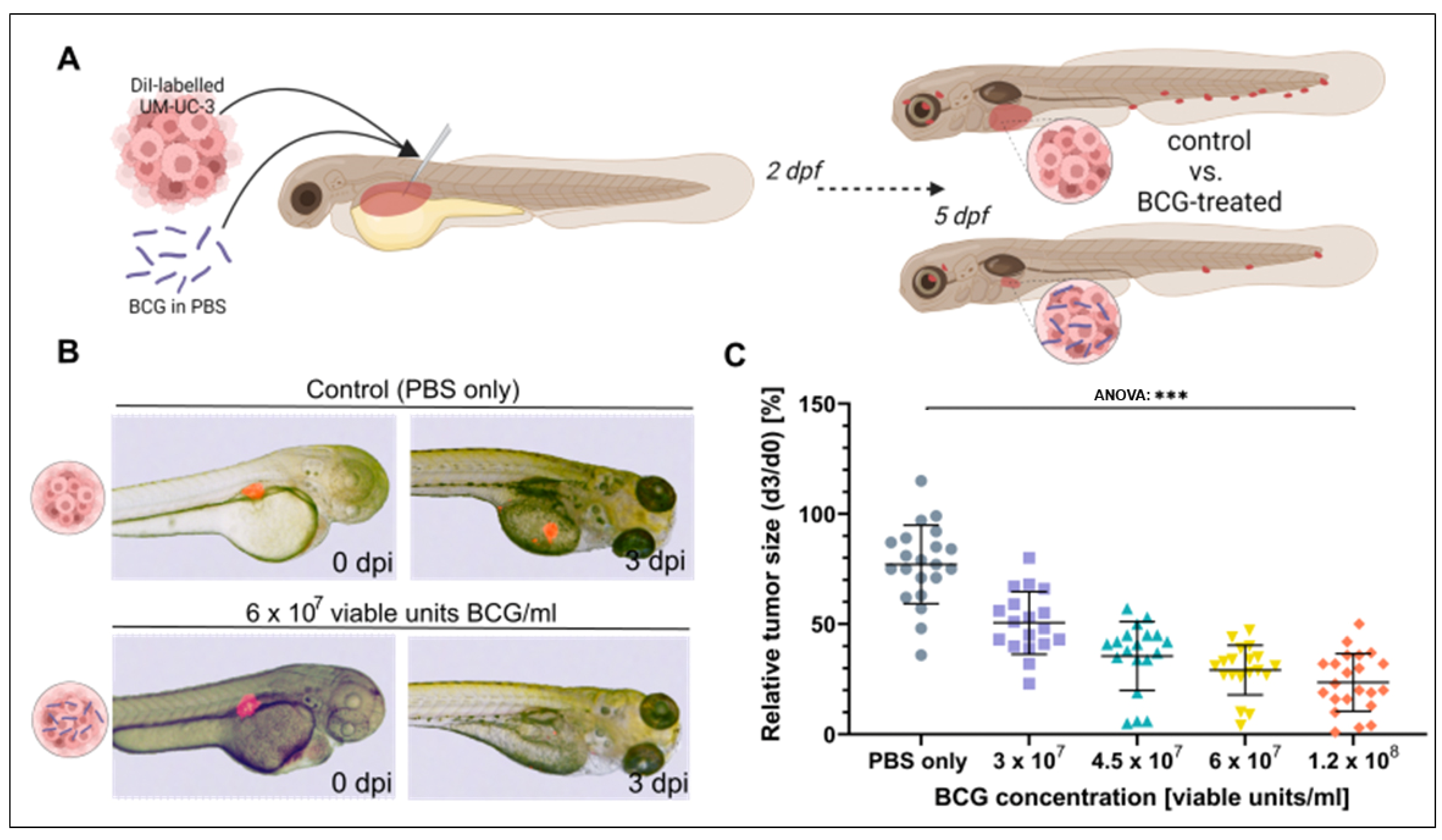
3.2. BCG Leads to Significant Regression of UM-UC-3 Xenografts
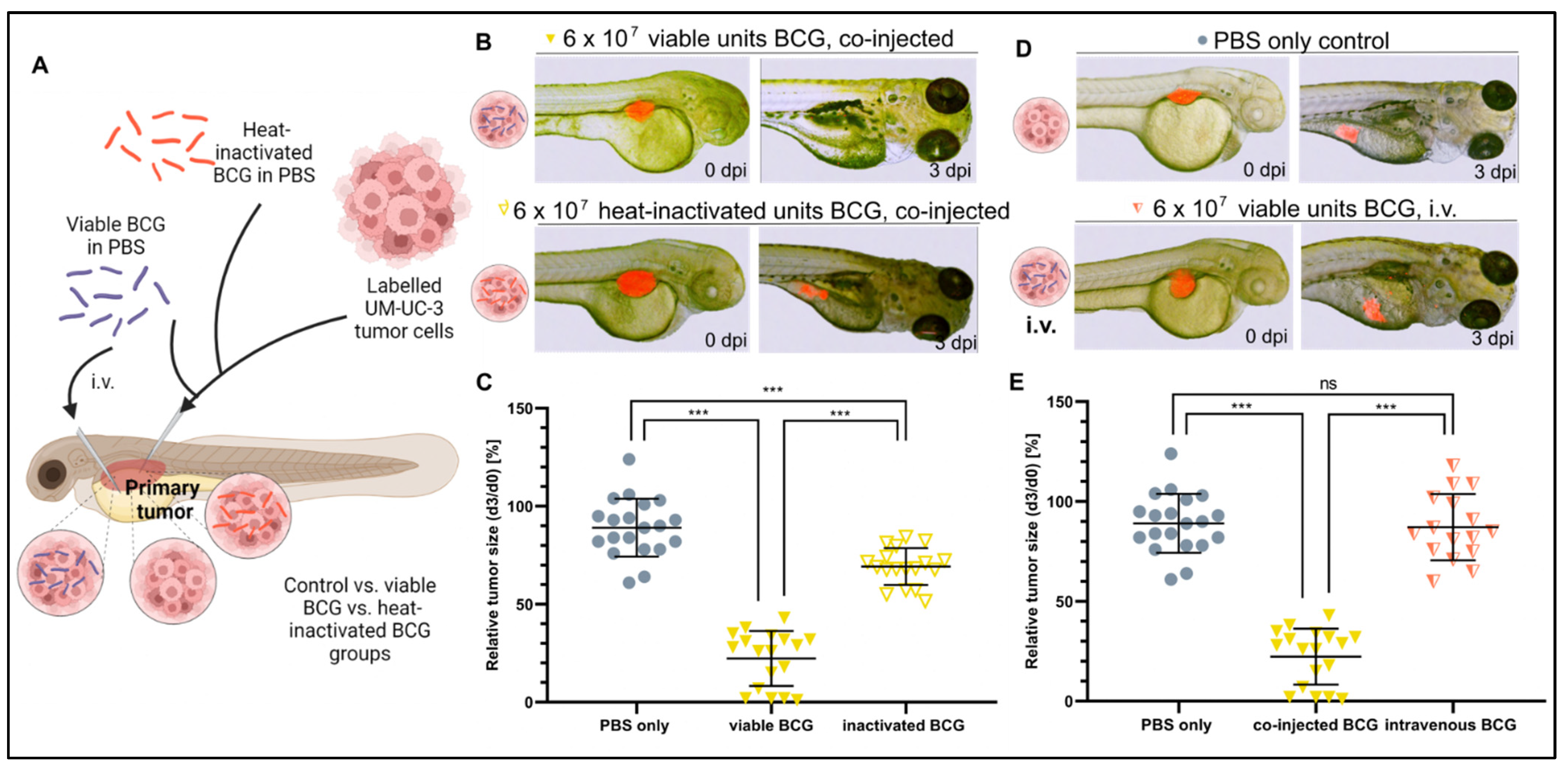
3.3. Bacterial Viability and Physical Contact with the Tumor Cells Are Both Required for Efficacy
3.4. BCG Therapy Does Not Impact on Bladder Cancer Invasiveness and Dissemination
3.5. ZTX Models Predicted Responses to BCG Therapy in NMIBC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Functional precision cancer medicine—Moving beyond pure genomics. Nat. Med. 2017, 23, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, J.; Tang, S.; Sun, G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int. Immunopharmacol. 2020, 85, 106677. [Google Scholar] [CrossRef] [PubMed]
- Letai, A. Functional Precision Medicine: Putting Drugs on Patient Cancer Cells and Seeing What Happens. Cancer Discov. 2022, 12, 290–292. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-Derived Xenograft Models: An Emerging Platform for Translational Cancer Research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Willyard, C. The mice with human tumours: Growing pains for a popular cancer model. Nature 2018, 560, 156–157. [Google Scholar] [CrossRef]
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A.L.M. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef]
- Tran, L.; Xiao, J.-F.; Agarwal, N.; Duex, J.E.; Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 2020, 21, 104–121. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M.D. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Han, J.; Gu, X.; Li, Y.; Wu, Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed. Pharmacother. 2020, 129, 110393. [Google Scholar] [CrossRef] [PubMed]
- Vasekar, M.; Degraff, D.; Joshi, M. Immunotherapy in Bladder Cancer. Curr. Mol. Pharmacol. 2016, 9, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Old, L.J.; Clarke, D.A.; Benacerraf, B. Effect of Bacillus Calmette-Guérin Infection on Transplanted Tumours in the Mouse. Nature 1959, 184, 291–292. [Google Scholar] [CrossRef]
- Coe, J.E.; Feldman, J.D. Extracutaneous delayed hypersensitivity, particularly in the guinea-pig bladder. Immunology 1966, 10, 127–136. [Google Scholar] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-guerin in the Treatment of Superficial Bladder Tumors. J. Urol. 1976, 116, 180–182. [Google Scholar] [CrossRef]
- Lamm, D.L. BCG immunotherapy for transitional-cell carcinoma in situ of the bladder. Oncology 1995, 9, 947–952. [Google Scholar]
- Larsen, E.S.; Joensen, U.N.; Poulsen, A.M.; Goletti, D.; Johansen, I.S. Bacillus Calmette–Guérin immunotherapy for bladder cancer: A review of immunological aspects, clinical effects and BCG infections. APMIS 2020, 128, 92–103. [Google Scholar] [CrossRef]
- Kamat, A.M.; Li, R.; O’Donnell, M.A.; Black, P.C.; Roupret, M.; Catto, J.W.; Comperat, E.; Ingersoll, M.A.; Witjes, W.P.; McConkey, D.J.; et al. Predicting Response to Intravesical Bacillus Calmette-Guérin Immunotherapy: Are We There Yet? A Systematic Review. Eur. Urol. 2018, 73, 738–748. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Salome, B.; Daza, J.; Farkas, A.; Bhardwaj, N.; Horowitz, A. Bacillus Calmette-Guerin (BCG): Its fight against pathogens and cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 39, 121–129. [Google Scholar] [CrossRef]
- Tully, K.H.; Cole, A.P.; Krimphove, M.J.; Friedlander, D.F.; Mossanen, M.; Herzog, P.; Noldus, J.; Sonpavde, G.P.; Trinh, Q.D. Contemporary Treatment Patterns for Non-Muscle-Invasive Bladder Cancer: Has the Use of Radical Cystectomy Changed in the BCG Shortage Era? Urology 2021, 147, 199–204. [Google Scholar] [CrossRef]
- Kates, M.; Matoso, A.; Choi, W.; Baras, A.S.; Daniels, M.J.; Lombardo, K.; Brant, A.; Mikkilineni, N.; McConkey, D.J.; Kamat, A.M.; et al. Adaptive Immune Resistance to Intravesical BCG in Non–Muscle Invasive Bladder Cancer: Implications for Prospective BCG-Unresponsive Trials. Clin. Cancer Res. 2020, 26, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Mostafid, A.H.; Redorta, J.P.; Sylvester, R.; Witjes, J.A. Therapeutic Options in High-risk Non–muscle-invasive Bladder Cancer During the Current Worldwide Shortage of Bacille Calmette-Guérin. Eur. Urol. 2015, 67, 359–360. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, P.; Jensen, L.; Cao, Z.; Hosaka, K.; Länne, T.; Wahlberg, E.; Steffensen, J.F.; Cao, Y. Hypoxia-induced metastasis model in embryonic zebrafish. Nat. Protoc. 2010, 5, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Vildevall, M.; Rodriguez, G.V.; Tandiono, D.; Vamvakaris, I.; Evangelou, G.; Lolas, G.; Syrigos, K.N.; Villanueva, A.; Wick, M.; et al. Zebrafish patient-derived xenograft models predict lymph node involvement and treatment outcome in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2022, 41, 1–18. [Google Scholar] [CrossRef]
- Lee, S.L.C.; Rouhi, P.; Jensen, L.D.; Zhang, D.; Ji, H.; Hauptmann, G.; Ingham, P.; Cao, Y. Hypoxia-induced pathological angiogenesis mediates tumor cell dissemination, invasion, and metastasis in a zebrafish tumor model. Proc. Natl. Acad. Sci. USA 2009, 106, 19485–19490. [Google Scholar] [CrossRef]
- Fior, R.; Póvoa, V.; Mendes, R.V.; Carvalho, T.; Gomes, A.; Figueiredo, N.; Ferreira, M.G. Single-cell functional and chemosensitive profiling of combinatorial colorectal therapy in zebrafish xenografts. Proc. Natl. Acad. Sci. USA 2017, 114, E8234–E8243. [Google Scholar] [CrossRef]
- Wu, J.-Q.; Zhai, J.; Li, C.-Y.; Tan, A.-M.; Wei, P.; Shen, L.-Z.; He, M.-F. Patient-derived xenograft in zebrafish embryos: A new platform for translational research in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 160. [Google Scholar] [CrossRef]
- Zhai, J.; Wu, J.; Wang, Y.; Fan, R.; Xie, G.; Wu, F.; He, Y.; Qian, S.; Tan, A.; Yao, X.; et al. Prediction of Sensitivity and Efficacy of Clinical Chemotherapy Using Larval Zebrafish Patient-Derived Xenografts of Gastric Cancer. Front. Cell Dev. Biol. 2021, 9, 680491. [Google Scholar] [CrossRef]
- Gauert, A.; Olk, N.; Pimentel-Gutiérrez, H.; Astrahantseff, K.; Jensen, L.D.; Cao, Y.; Eggert, A.; Eckert, C.; Hagemann, A.I. Fast, In Vivo Model for Drug-Response Prediction in Patients with B-Cell Precursor Acute Lymphoblastic Leukemia. Cancers 2020, 12, 1883. [Google Scholar] [CrossRef]
- Sahu, D.; Huan, J.; Wang, H.; Sahoo, D.; Casteel, D.E.; Klemke, R.L.; Boss, G.R.; Hansel, D.E. Bladder Cancer Invasion Is Mediated by Mammalian Target of Rapamycin Complex 2–Driven Regulation of Nitric Oxide and Invadopodia Formation. Am. J. Pathol. 2021, 191, 2203–2218. [Google Scholar] [CrossRef]
- Lawson, N.D.; Weinstein, B.M. In Vivo Imaging of Embryonic Vascular Development Using Transgenic Zebrafish. Dev. Biol. 2002, 248, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, E.; Rochelet, M.; Gal, L.; Boschiroli, M.L.; Hartmann, A. Impact of temperature and soil type on Mycobacterium bovis survival in the environment. PLoS ONE 2017, 12, e0176315. [Google Scholar] [CrossRef]
- Diaz Acosta, C.C.; Dias, A.A.; Rosa, T.; Batista-Silva, L.R.; Rosa, P.S.; Toledo-Pinto, T.G.; Costa, F.D.M.R.; Lara, F.A.; Rodrigues, L.S.; Mattos, K.A.; et al. PGL I expression in live bacteria allows activation of a CD206/PPARgamma cross-talk that may contribute to successful Mycobacterium leprae colonization of peripheral nerves. PLoS Pathog. 2018, 14, e1007151. [Google Scholar] [CrossRef] [PubMed]
- Sabiiti, W.; Azam, K.; Esmeraldo, E.; Bhatt, N.; Rachow, A.; Gillespie, S.H. Heat Inactivation Renders Sputum Safe and Preserves Mycobacterium tuberculosis RNA for Downstream Molecular Tests. J. Clin. Microbiol. 2019, 57, e01778-18. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Van Leeuwen, L.M.; van der Sar, A.M.; Bitter, W. Animal models of tuberculosis: Zebrafish. Cold Spring Harb. Perspect. Med. 2014, 5, a018580. [Google Scholar] [CrossRef]
- Grossman, H.B.; Wedemeyer, G.; Ren, L.; Wilson, G.N.; Cox, B. Improved Growth of Human Urothelial Carcinoma Cell Cultures. J. Urol. 1986, 136, 953–959. [Google Scholar] [CrossRef]
- Pasco, S.; Anguita, J. Lessons from Bacillus Calmette-Guérin: Harnessing Trained Immunity for Vaccine Development. Cells 2020, 9, 2109. [Google Scholar] [CrossRef]
- Svensson, S.; Abrahamsson, A.; Rodriguez, G.V.; Olsson, A.-K.; Jensen, L.; Cao, Y.; Dabrosin, C. CCL2 and CCL5 Are Novel Therapeutic Targets for Estrogen-Dependent Breast Cancer. Clin. Cancer Res. 2015, 21, 3794–3805. [Google Scholar] [CrossRef]
- Rodriguez, G.V.; Abrahamsson, A.; Jensen, L.D.E.; Dabrosin, C. Estradiol Promotes Breast Cancer Cell Migration via Recruitment and Activation of Neutrophils. Cancer Immunol. Res. 2017, 5, 234–247. [Google Scholar] [CrossRef]
- Póvoa, V.; de Almeida, C.R.; Maia-Gil, M.; Sobral, D.; Domingues, M.; Martinez-Lopez, M.; Fuzeta, M.D.A.; Silva, C.; Grosso, A.R.; Fior, R. Innate immune evasion revealed in a colorectal zebrafish xenograft model. Nat. Commun. 2021, 12, 1156. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Bareham, B.; Georgakopoulos, N.; Matas-Cespedes, A.; Curran, M.; Saeb-Parsy, K. Modeling human tumor-immune environments in vivo for the preclinical assessment of immunotherapies. Cancer Immunol. Immunother. 2021, 70, 2737–2750. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, Z.; Zhang, X.-M.; Nakamura, M.; Sun, M.; Hartman, J.; Harris, R.A.; Sun, Y.; Cao, Y. Novel Mechanism of Macrophage-Mediated Metastasis Revealed in a Zebrafish Model of Tumor Development. Cancer Res. 2015, 75, 306–315. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yin, X.; Wu, J.; Wickström, S.L.; Duo, Y.; Du, Q.; Qin, S.; Yao, S.; Jing, X.; Hosaka, K.; et al. Visualization of human T lymphocyte-mediated eradication of cancer cells in vivo. Proc. Natl. Acad. Sci. USA 2020, 117, 22910–22919. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Z.; Wickstrom, S.L.; Gao, J.; He, X.; Jing, X.; Wu, J.; Du, Q.; Yang, M.; Chen, Y.; et al. Interleukin-33 is a Novel Immunosuppressor that Protects Cancer Cells from TIL Killing by a Macrophage-Mediated Shedding Mechanism. Adv. Sci. 2021, 8, e2101029. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Iyer, G.; Solit, D.B.; Glickman, M.S. Oncogenic Activation of Pak1-Dependent Pathway of Macropinocytosis Determines BCG Entry into Bladder Cancer Cells. Cancer Res. 2013, 73, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Wu, J.; Liu, Y.; Wu, N.; Ruan, J. Drug Carrier-Oriented Polygeline for Preparing Novel Polygeline-Bound Paclitaxel Nanoparticles. J. Pharm. Sci. 2019, 108, 2012–2021. [Google Scholar] [CrossRef]
- Alvarez, Y.; Chen, K.; Reynolds, A.; Waghorne, N.; O’Connor, J.; Kennedy, B. Predominant cone photoreceptor dysfunction in a hyperglycaemic model of non-proliferative diabetic retinopathy. Dis. Model. Mech. 2010, 3, 236–245. [Google Scholar] [CrossRef]
- Coors, E.A.; Seybold, H.; Merk, H.F.; Mahler, V. Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann. Allergy Asthma Immunol. 2005, 95, 593–599. [Google Scholar] [CrossRef]
- Yang, R.; Lao, Q.-C.; Yu, H.-P.; Zhang, Y.; Liu, H.-C.; Luan, L.; Sun, H.-M.; Li, C.-Q. Tween-80 and impurity induce anaphylactoid reaction in zebrafish. J. Appl. Toxicol. 2015, 35, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Mukherjee, N.; Morales, E.E.; Tomasini, M.E.; Hurez, V.; Curiel, T.J.; Abate, G.; Hoft, D.F.; Zhao, X.-R.; Gelfond, J.; et al. Percutaneous BCG enhances innate effector antitumor cytotoxicity during treatment of bladder cancer: A translational clinical trial. Oncoimmunology 2019, 8, 1614857. [Google Scholar] [CrossRef]
- Redelman-Sidi, G.; Glickman, M.S.; Bochner, B.H. The mechanism of action of BCG therapy for bladder cancer—A current perspective. Nat. Rev. Urol. 2014, 11, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Zhang, G.; Chen, F.; Cao, Y.; Kalyanaraman, B.; See, W. Loss of Bacillus Calmette-Guérin Viability Adversely Affects the Direct Response of Urothelial Carcinoma Cells to Bacillus Calmette-Guérin Exposure. J. Urol. 2014, 191, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Esteso, G.; Aguiló, N.; Julián, E.; Ashiru, O.; Ho, M.M.; Martín, C.; Valés-Gómez, M. Natural Killer Anti-Tumor Activity Can Be Achieved by In Vitro Incubation With Heat-Killed BCG. Front. Immunol. 2021, 12, 622995. [Google Scholar] [CrossRef] [PubMed]
- Gnosa, S.; Capodanno, A.; Murthy, R.V.; Jensen, L.D.E.; Sun, X.-F. AEG-1 knockdown in colon cancer cell lines inhibits radiation-enhanced migration and invasion in vitro and in a novel in vivo zebrafish model. Oncotarget 2016, 7, 81634–81644. [Google Scholar] [CrossRef] [Green Version]


| Parameter | Value |
|---|---|
| Number of patients | 6 |
| Gender: Male/female | 5/1 |
| Age: Median (interval) | 76 (66–84) |
| Tumor stage: T1G2/T1G3/T1G3+CIS | 1/3/2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowald, S.; Huge, Y.; Tandiono, D.; Ali, Z.; Vazquez-Rodriguez, G.; Erkstam, A.; Fahlgren, A.; Sherif, A.; Cao, Y.; Jensen, L.D. Novel Zebrafish Patient-Derived Tumor Xenograft Methodology for Evaluating Efficacy of Immune-Stimulating BCG Therapy in Urinary Bladder Cancer. Cells 2023, 12, 508. https://doi.org/10.3390/cells12030508
Kowald S, Huge Y, Tandiono D, Ali Z, Vazquez-Rodriguez G, Erkstam A, Fahlgren A, Sherif A, Cao Y, Jensen LD. Novel Zebrafish Patient-Derived Tumor Xenograft Methodology for Evaluating Efficacy of Immune-Stimulating BCG Therapy in Urinary Bladder Cancer. Cells. 2023; 12(3):508. https://doi.org/10.3390/cells12030508
Chicago/Turabian StyleKowald, Saskia, Ylva Huge, Decky Tandiono, Zaheer Ali, Gabriela Vazquez-Rodriguez, Anna Erkstam, Anna Fahlgren, Amir Sherif, Yihai Cao, and Lasse D. Jensen. 2023. "Novel Zebrafish Patient-Derived Tumor Xenograft Methodology for Evaluating Efficacy of Immune-Stimulating BCG Therapy in Urinary Bladder Cancer" Cells 12, no. 3: 508. https://doi.org/10.3390/cells12030508
APA StyleKowald, S., Huge, Y., Tandiono, D., Ali, Z., Vazquez-Rodriguez, G., Erkstam, A., Fahlgren, A., Sherif, A., Cao, Y., & Jensen, L. D. (2023). Novel Zebrafish Patient-Derived Tumor Xenograft Methodology for Evaluating Efficacy of Immune-Stimulating BCG Therapy in Urinary Bladder Cancer. Cells, 12(3), 508. https://doi.org/10.3390/cells12030508









