Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatment
2.2. Measurement of Chlorophyll Fluorescence
2.3. Protein Extraction
2.4. Trypsin Digestion
2.5. LC-MS/MS Analysis and Database Search
2.6. Proteomics and Bioinformatics Analysis
2.7. Protein Functional Enrichment
2.7.1. GO Enrichment Analysis
2.7.2. KEGG Pathway Enrichment Analysis
2.7.3. Protein Domain Enrichment Analysis
2.8. Statistical Analysis
2.9. Acetylated Proteins Homology Modeling
3. Results
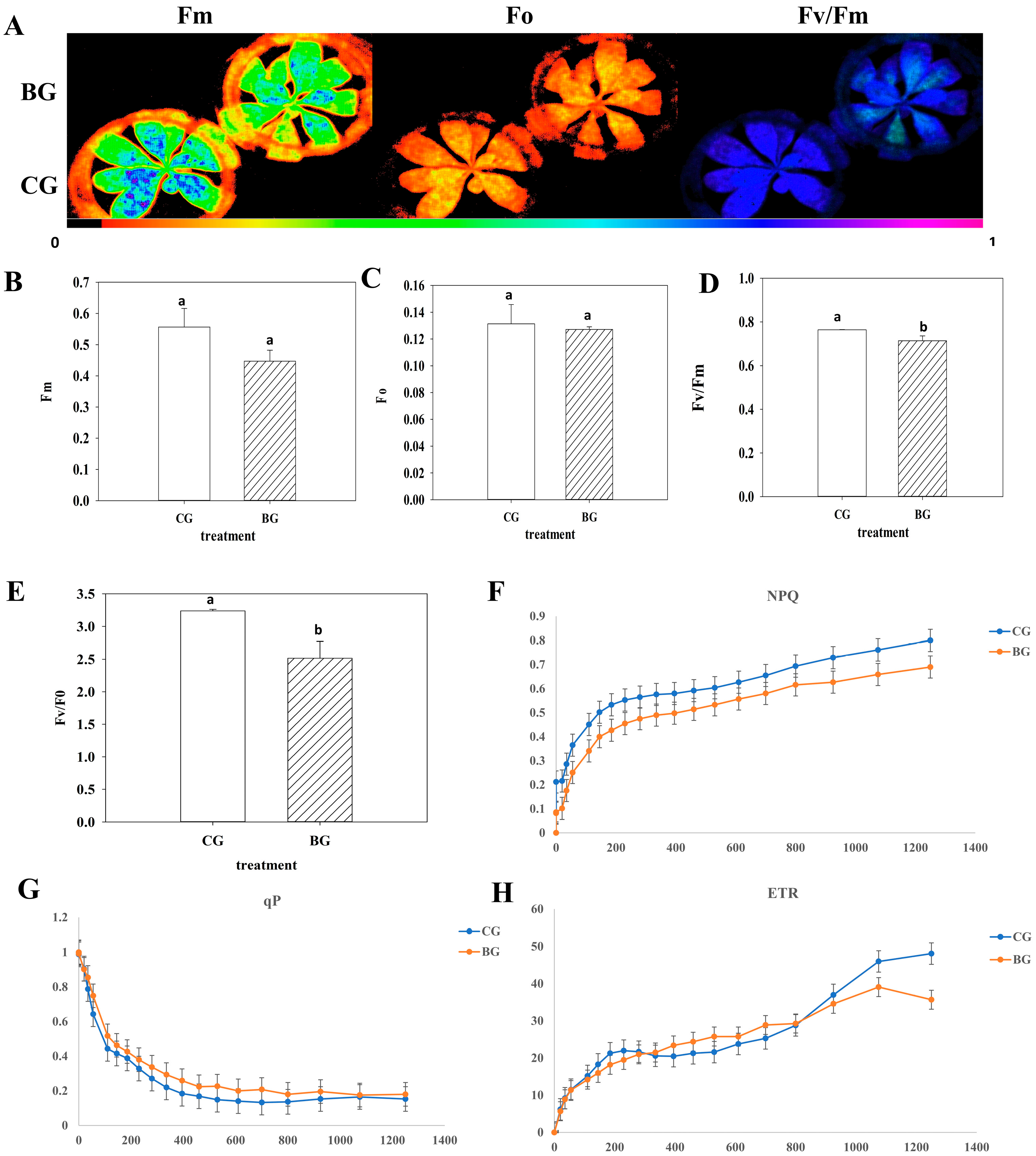
3.1. Rhododendron chrysanthum Photosynthesis Decreased in the Presence of UV-B Stress
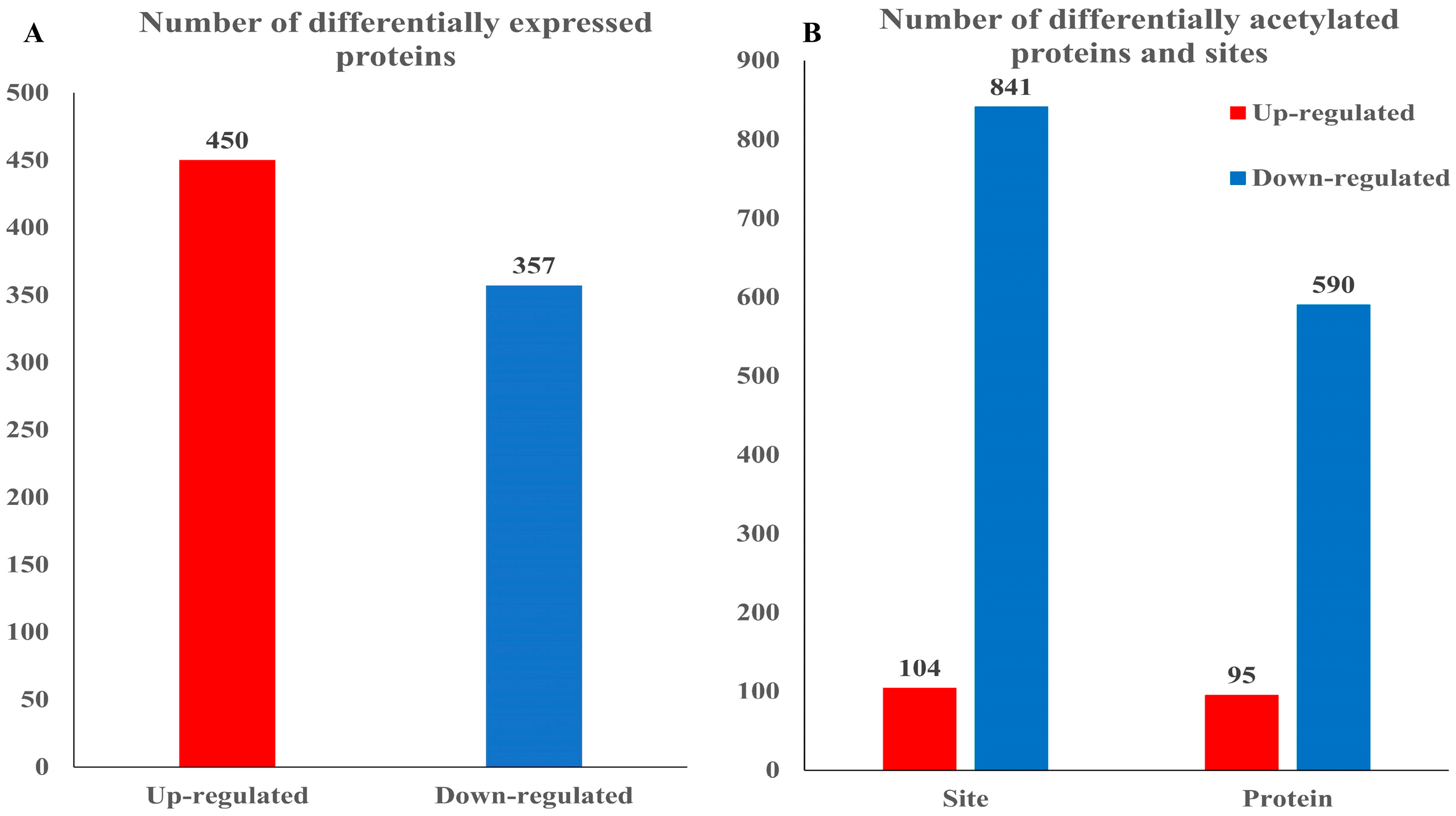
3.2. Acetylated Proteome in Rhododendron chrysanthum Leaves Responds to UV-B Stress
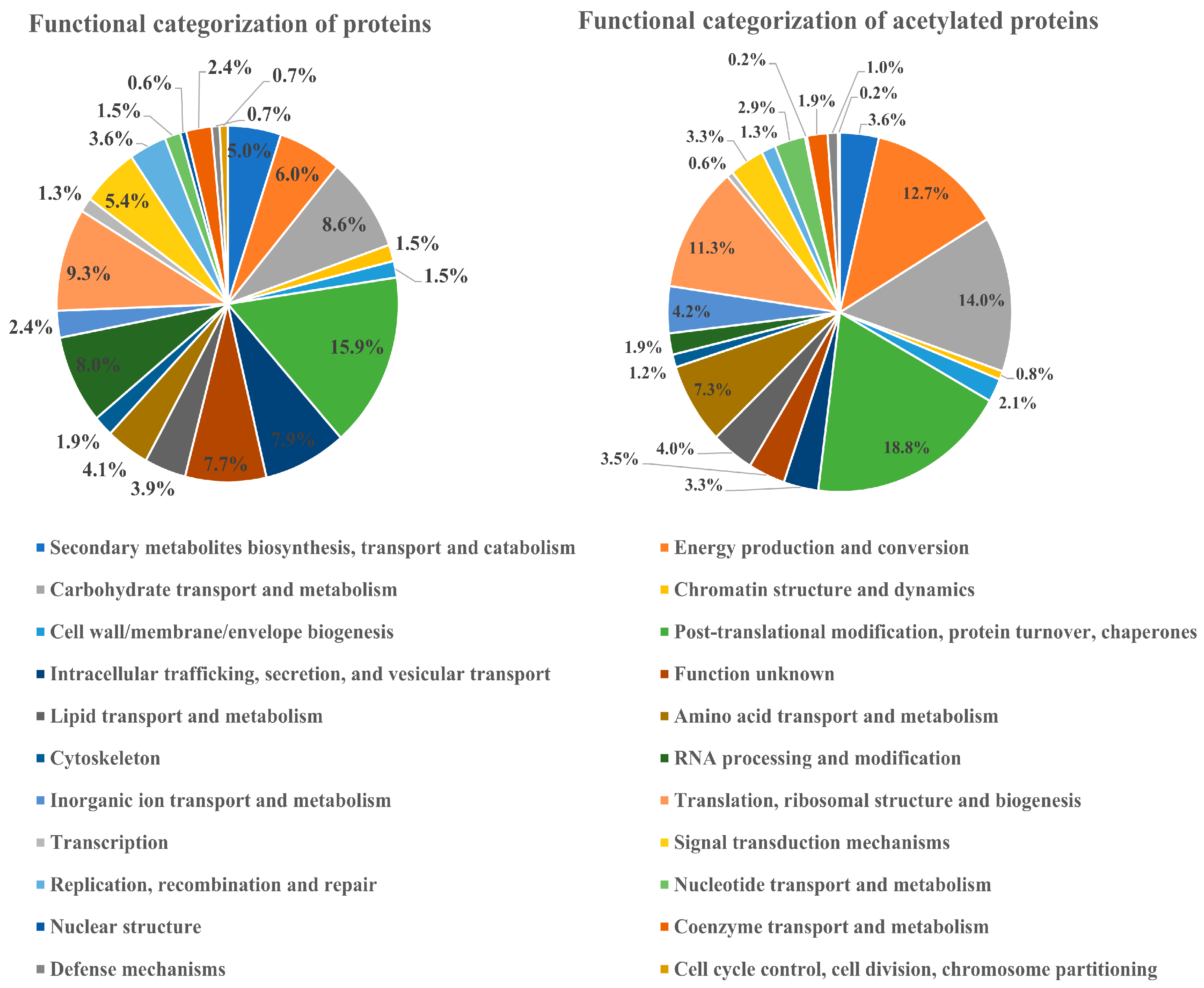
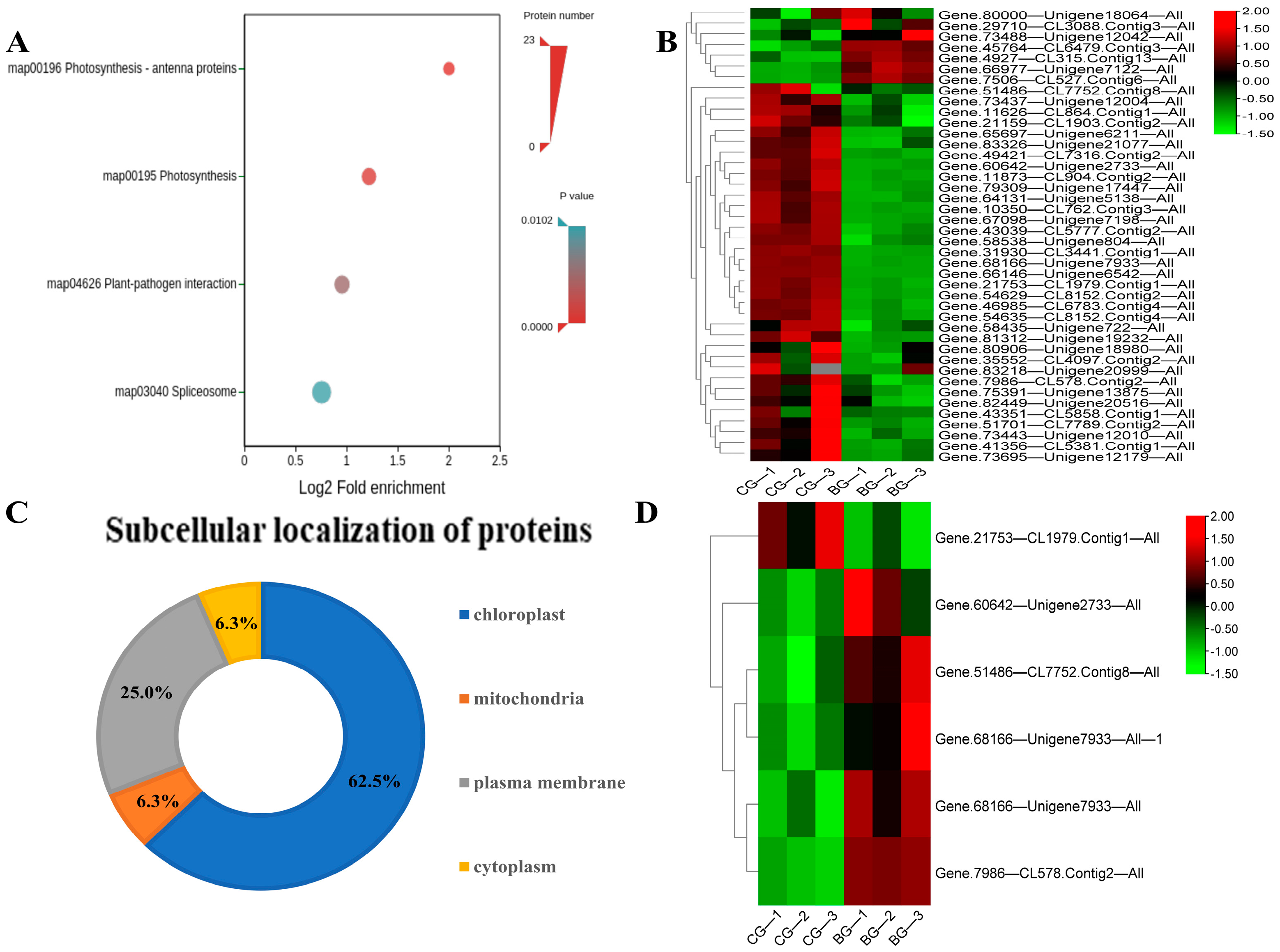
3.3. COG Classification of the Differential Acetylated Proteins in Rhododendron chrysanthum Leaves under UV-B Stress Conditions
3.4. GO Functional Annotation Analysis of Differential Acetylated Proteins in Rhododendron chrysanthum Leaves under UV-B Stress
3.5. KEGG Enrichment Analysis of Differential Proteins in Rhododendron chrysanthum Leaves under UV-B Stress
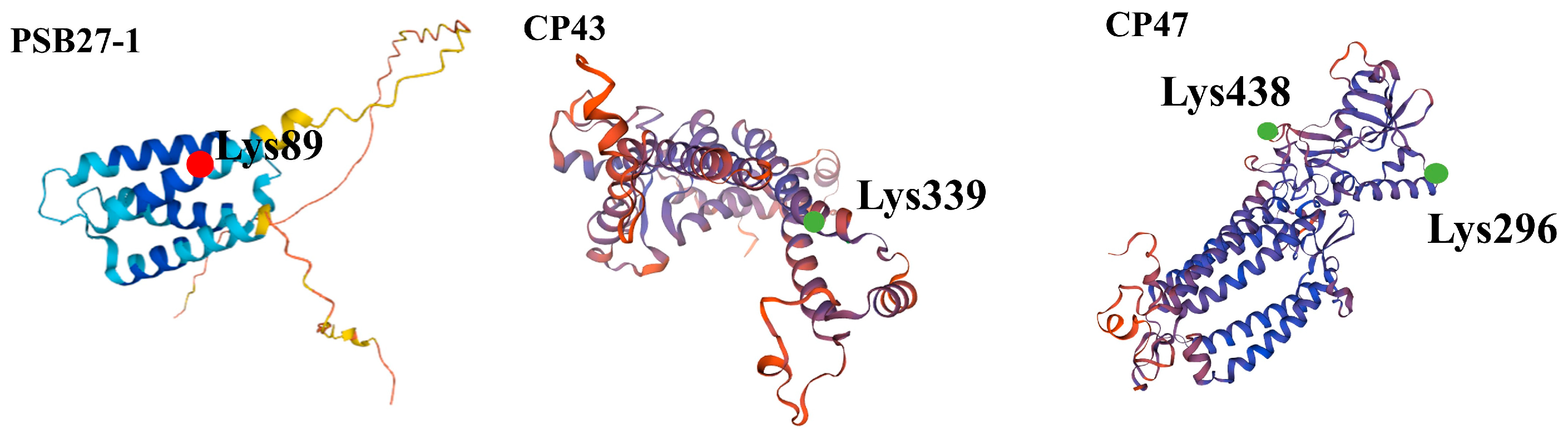
3.6. Three-Dimensional Structure Modeling of UV-B Stress-Responsive Acetylated Proteins
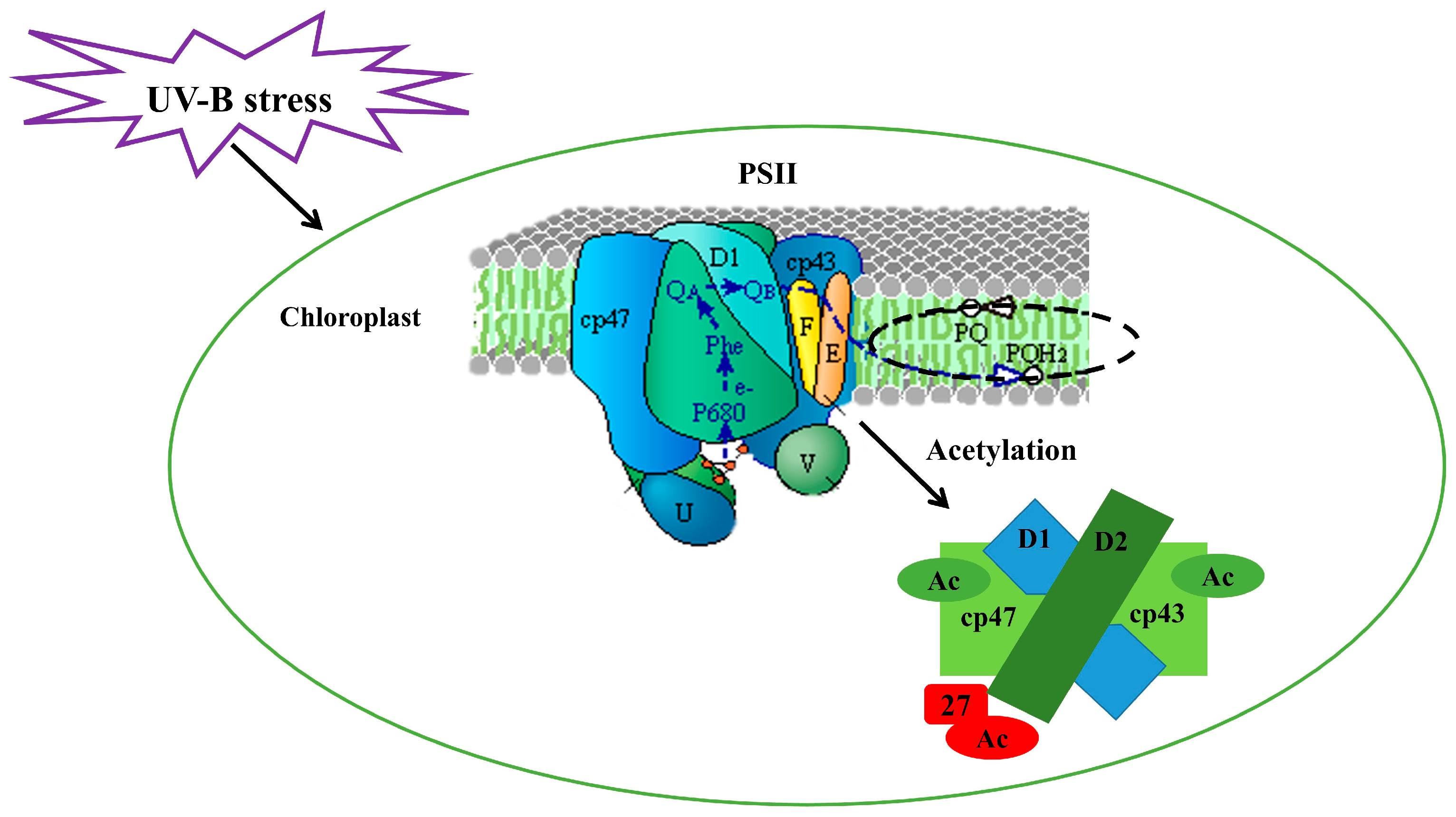
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rastogi, R.P.; Kumar, A.; Tyagi, M.B.; Sinha, R.P. Molecular Mechanisms of Ultraviolet Radiation-Induced DNA Damage and Repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.R.; Mikkelsen, T.N.; Ro-Poulsen, H.; Arndal, M.F.; Michelsen, A. Ambient UV-B radiation reduces PSII performance and net photosynthesis in high Arctic Salix arctica. Environ. Exp. Bot. 2011, 73, 10–18. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Widel, M.; Krzywon, A.; Gajda, K.; Skonieczna, M.; Rzeszowska-Wolny, J. Induction of bystander effects by UVA, UVB, and UVC radiation in human fibroblasts and the implication of reactive oxygen species. Free Radic. Biol. Med. 2014, 68, 278–287. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, Z.; Qiang, W.; An, L.; Xu, S.; Wang, X. Effects of enhanced UV-B radiation on the hormonal content of vegetative and reproductive tissues of two tomato cultivars and their relationships with reproductive characteristics. Plant Growth Regul. 2004, 43, 251–258. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, Y.; Xu, G.; Li, C. Growth and physiological responses to drought and elevated ultraviolet-B in two contrasting populations of Hippophae rhamnoides. Physiol. Plant. 2010, 124, 431–440. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, S.; Agrawal, S.B. Response of ultraviolet-B induced antioxidant defense system in a medicinal plant, Acorus calamus. J. Environ. Biol. 2010, 31, 907–911. [Google Scholar]
- Tripathi, R.; Sarkar, A.; Pandey Rai, S.; Agrawal, S.B. Supplemental ultraviolet-B and ozone: Impact on antioxidants, proteome and genome of linseed (Linum usitatissimum L. cv. Padmini). Plant Biol. 2011, 13, 93–104. [Google Scholar] [CrossRef]
- Zhou, X.; Lyu, J.; Sun, L.; Dong, J.; Xu, H.; Allakhverdiev, S. Metabolic programming of Rhododendron chrysanthum leaves following exposure to UVB irradiation. Funct. Plant Biol. 2021, 48, 1175–1185. [Google Scholar] [CrossRef]
- Yamasaki, T.; Yamakawa, T.; Yamane, Y.; Koike, H.; Satoh, K.; Katoh, S. Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 2002, 128, 1087–1097. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Tyystjaervi, E. Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord. Chem. Rev. 2008, 252, 361–376. [Google Scholar] [CrossRef]
- Vass, I.; Szilárd, A.; Sicora, C. 43 Adverse Effects of UV-B Light on the Structure and Function of the Photosynthetic Apparatus. In Handbook of Photosynthesis; Francis and Taylor Publisher: London, UK, 2005. [Google Scholar]
- Schoedl, K.; Schuhmacher, R.; Forneck, A. Correlating physiological parameters with biomarkers for UV-B stress indicators in leaves of grapevine cultivars Pinot noir and Riesling. J. Agric. Sci. 2013, 151, 189–200. [Google Scholar] [CrossRef]
- Yoon, H.I.; Kim, D.; Son, J.E. Spatial and Temporal Bioactive Compound Contents and Chlorophyll Fluorescence of Kale (Brassica oleracea L.) Under UV-B Exposure Near Harvest Time in Controlled Environments. Photochem. Photobiol. 2020, 96, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Skorska, E. The effect of UV-B radiation on the chlorophyll fluorescence parameters of the husked and the naked oat. Acta Agrobot. 1999, 52, 149–152. [Google Scholar] [CrossRef]
- Yang, S.H.; Wang, L.J.; Li, S.H.; Duan, W.; Loescher, W.; Liang, Z.C. The effects of UV-B radiation on photosynthesis in relation to Photosystem II photochemistry, thermal dissipation and antioxidant defenses in winter wheat (Triticum aestivum L.) seedlings at different growth temperatures. Funct. Plant Biol. 2007, 34, 907–917. [Google Scholar] [CrossRef]
- Dehariya, P.; Kataria, S.; Guruprasad, K.N.; Pandey, G.P. Photosynthesis and yield in cotton (Gossypium hirsutum L.) Var. Vikram after exclusion of ambient solar UV-B/A. Acta Physiol. Plant. 2012, 34, 1133–1144. [Google Scholar] [CrossRef]
- Lyu, J.; Wang, C.; Liang, D.Y.; Liu, L.; Pandey, L.K.; Xu, H.W.; Zhou, X.F. Sensitivity of wild and domesticated Rhododendron chrysanthum to different light regime (UVA, UVB, and PAR). Photosynthetica 2020, 57, 841–849. [Google Scholar] [CrossRef]
- Majeran, W.; Friso, G.; Ponnala, L.; Connolly, B.; Huang, M.; Reidel, E.; Zhang, C.; Asakura, Y.; Bhuiyan, N.H.; Sun, Q.; et al. Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics in maize. Plant Cell 2010, 22, 3509–3542. [Google Scholar] [CrossRef]
- Olinares, P.D.; Ponnala, L.; Van Wijk, K.J. Megadalton complexes in the chloroplast stroma of Arabidopsis thaliana characterized by size exclusion chromatography, mass spectrometry, and hierarchical clustering. Mol. Cell Proteom. 2010, 9, 1594–1615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Q.; Chen, S.; Dai, S. C4 photosynthetic machinery: Insights from maize chloroplast proteomics. Front Plant Sci. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Zybailov, B.; Friso, G.; Kim, J.; Rudella, A.; Rodríguez, V.R.; Asakura, Y.; Sun, Q.; van Wijk, K.J. Large scale comparative proteomics of a chloroplast Clp protease mutant reveals folding stress, altered protein homeostasis, and feedback regulation of metabolism. Mol. Cell Proteom. 2009, 8, 1789–1810. [Google Scholar] [CrossRef] [PubMed]
- Diallo, I.; Seve, M.; Cunin, V.; Minassian, F.; Poisson, J.F.; Michelland, S.; Bourgoin-Voillard, S. Current trends in protein acetylation analysis. Expert Rev. Proteom. 2019, 16, 139–159. [Google Scholar] [CrossRef]
- Seo, J.; Lee, K.J. Post-translational modifications and their biological functions: Proteomic analysis and systematic approaches. J. Biochem. Mol. Biol. 2004, 37, 35–44. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, S.; Wu, H.; Yang, Y.; Xu, H. Biochemical and proteomics analyses of antioxidant enzymes reveal the potential stress tolerance in Rhododendron chrysanthum Pall. Biol. Direct 2017, 12, 10. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, M.; Cao, K.; Xu, H.; Zhou, X. UV-B Irradiation to Amino Acids and Carbohydrate Metabolism in Rhododendron chrysanthum Leaves by Coupling Deep Transcriptome and Metabolome Analysis. Plants 2022, 11, 2730. [Google Scholar] [CrossRef]
- Akaya, M.; Takenaka, C. Effects of aluminum stress on photosynthesis of Quercus glauca Thumb. Plant Soil 2001, 237, 137–146. [Google Scholar] [CrossRef]
- Chen, Y.E.; Su, Y.Q.; Zhang, C.M.; Ma, J.; Mao, H.T.; Yang, Z.H.; Yuan, M.; Zhang, Z.W.; Yuan, S.; Zhang, H.Y. Comparison of Photosynthetic Characteristics and Antioxidant Systems in Different Wheat Strains. J. Plant Growth Regul. 2018, 37, 347–359. [Google Scholar] [CrossRef]
- Barber, J. Photosystem two. Biochim. Biophys. Acta 1998, 1365, 269–277. [Google Scholar] [CrossRef]
- Nelson, N.; Yocum, C.F. Structure and function of photosystems I and II. Annu. Rev. Plant Biol. 2006, 57, 521–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassi, R.; Høyer-Hansen, G.; Barbato, R.; Giacometti, G.M.; Simpson, D.J. Chlorophyll-proteins of the photosystem II antenna system. J. Biol. Chem. 1987, 262, 13333–13341. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fu, A.; Garcia, V.J.; Buchanan, B.B.; Luan, S. PSB27: A thylakoid protein enabling Arabidopsis to adapt to changing light intensity. Proc. Natl. Acad. Sci. USA 2015, 112, 1613–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
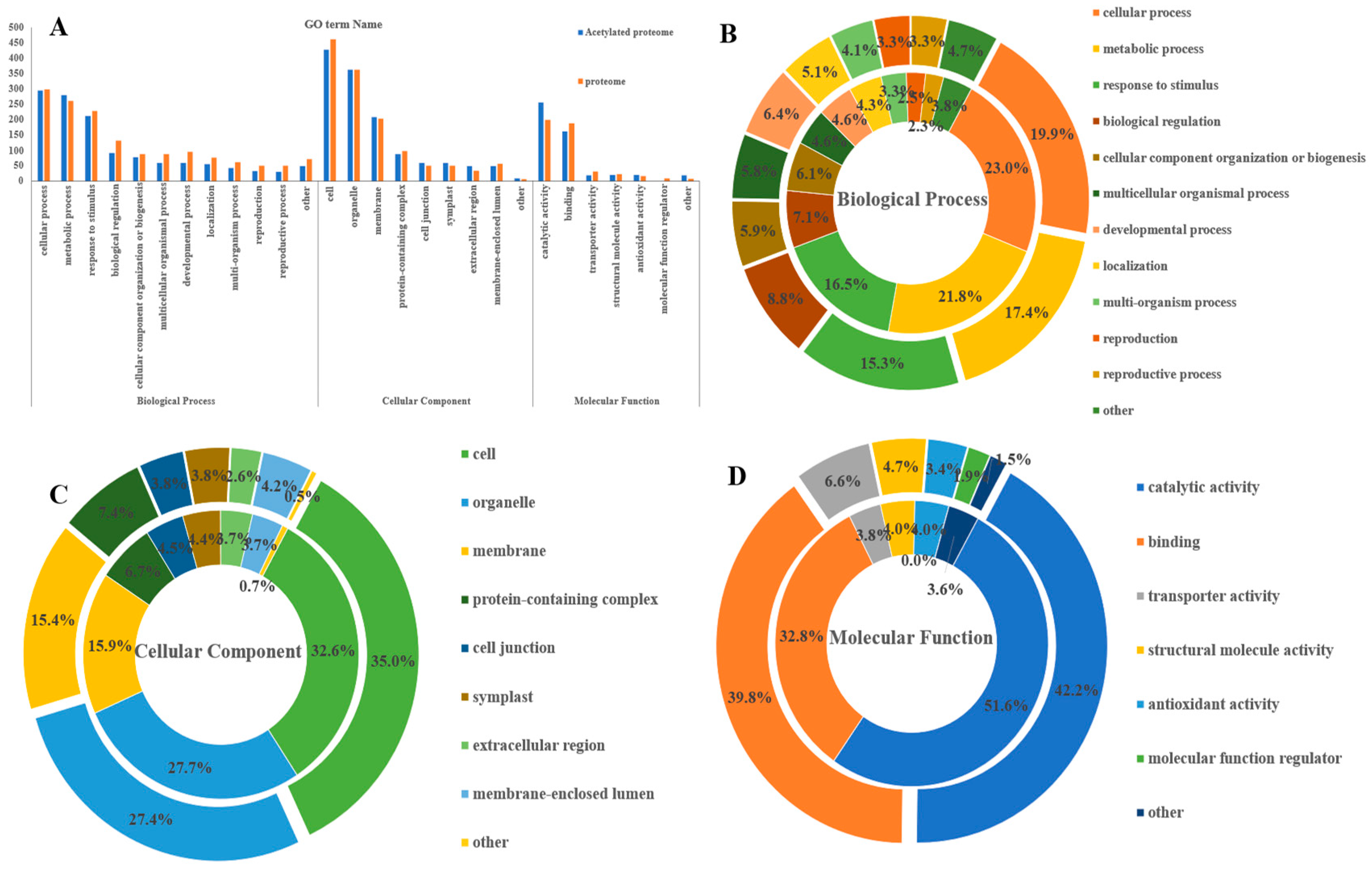

| GO Terms Level 1 | GO Terms Level 2 | Number of Proteins | Number of Acetylated Proteins |
|---|---|---|---|
| Biological Process | Cellular process | 298 | 295 |
| Metabolic process | 260 | 280 | |
| Response to stimulus | 228 | 212 | |
| Biological regulation | 131 | 91 | |
| Cellular component organization or biogenesis | 88 | 78 | |
| Multicellular organismal process | 87 | 59 | |
| Developmental process | 95 | 59 | |
| Localization | 76 | 55 | |
| Multi-organism process | 61 | 42 | |
| Reproduction | 50 | 32 | |
| Reproductive process | 50 | 30 | |
| Other | 71 | 49 | |
| Cellular Component | Cell | 461 | 427 |
| Organelle | 362 | 362 | |
| Membrane | 203 | 208 | |
| Protein-containing complex | 97 | 88 | |
| Cell junction | 50 | 59 | |
| Symplast | 50 | 58 | |
| Extracellular region | 34 | 49 | |
| Membrane-enclosed lumen | 56 | 48 | |
| Other | 0 | 9 | |
| Molecular Function | Catalytic activity | 199 | 255 |
| Binding | 188 | 162 | |
| Structural molecule activity | 22 | 20 | |
| Antioxidant activity | 16 | 20 | |
| Transporter activity | 31 | 19 | |
| Other | 0 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Sun, Q.; Cao, K.; Xu, H.; Zhou, X. Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells 2023, 12, 478. https://doi.org/10.3390/cells12030478
Liu M, Sun Q, Cao K, Xu H, Zhou X. Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells. 2023; 12(3):478. https://doi.org/10.3390/cells12030478
Chicago/Turabian StyleLiu, Meiqi, Qi Sun, Kun Cao, Hongwei Xu, and Xiaofu Zhou. 2023. "Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum" Cells 12, no. 3: 478. https://doi.org/10.3390/cells12030478
APA StyleLiu, M., Sun, Q., Cao, K., Xu, H., & Zhou, X. (2023). Acetylated Proteomics of UV-B Stress-Responsive in Photosystem II of Rhododendron chrysanthum. Cells, 12(3), 478. https://doi.org/10.3390/cells12030478





