Lipid ROS- and Iron-Dependent Ferroptotic Cell Death in Unicellular Algae Chlamydomonas reinhardtii
Abstract
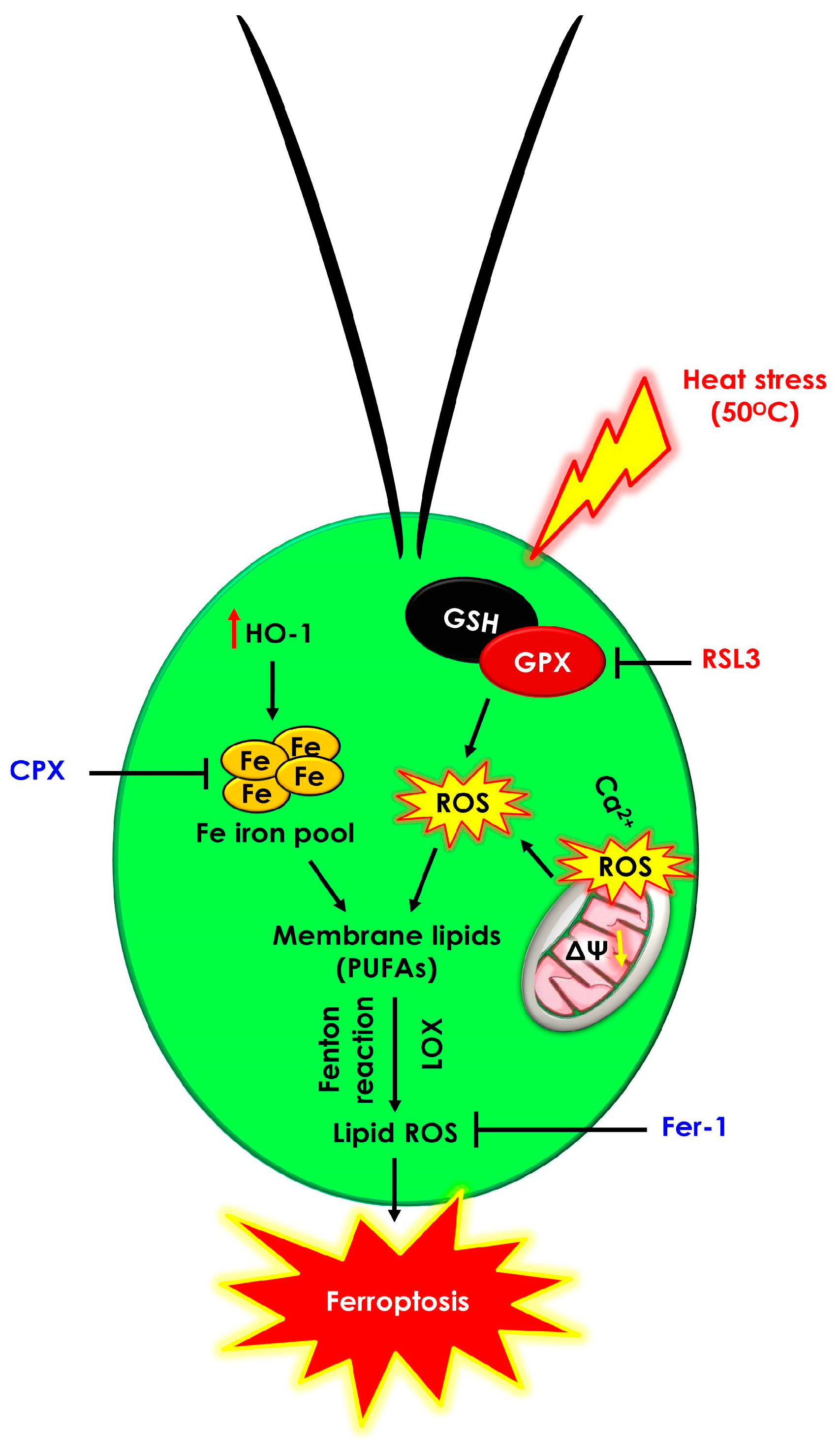
1. Introduction
2. Materials and Methods
2.1. Chalmydomonas and Culture Treatment Conditions
2.2. Fluorescence Microscopy and Measurements
2.3. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR) Analysis
2.4. Lipid Peroxidation Assay and Lipoxygenase (LOX) Activity
2.5. Iron Content Measurement and HO-1 Activity
2.6. Determination of Ascorbate (AsA) and Glutathione (GSH) Contents
2.7. DNA Fragmentation Assay
2.8. Statistical Analysis
3. Results and Discussion
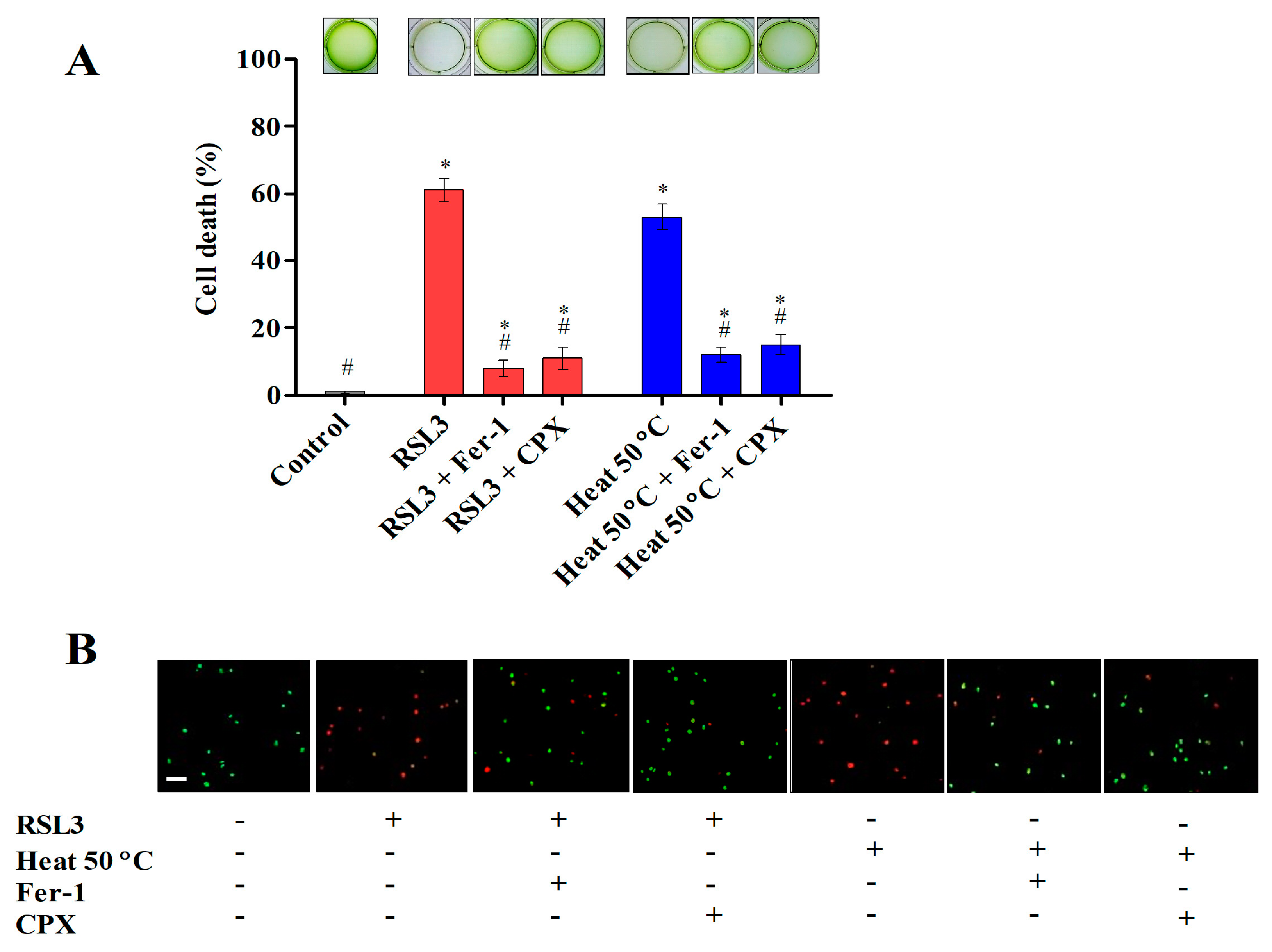
3.1. Ferroptosis Inhibitors Prevent Cell Death Induced by Heat Shock
3.2. Hallmarks of Ferroptosis-like Cell Death in Chlamydomonas during Heat Shock
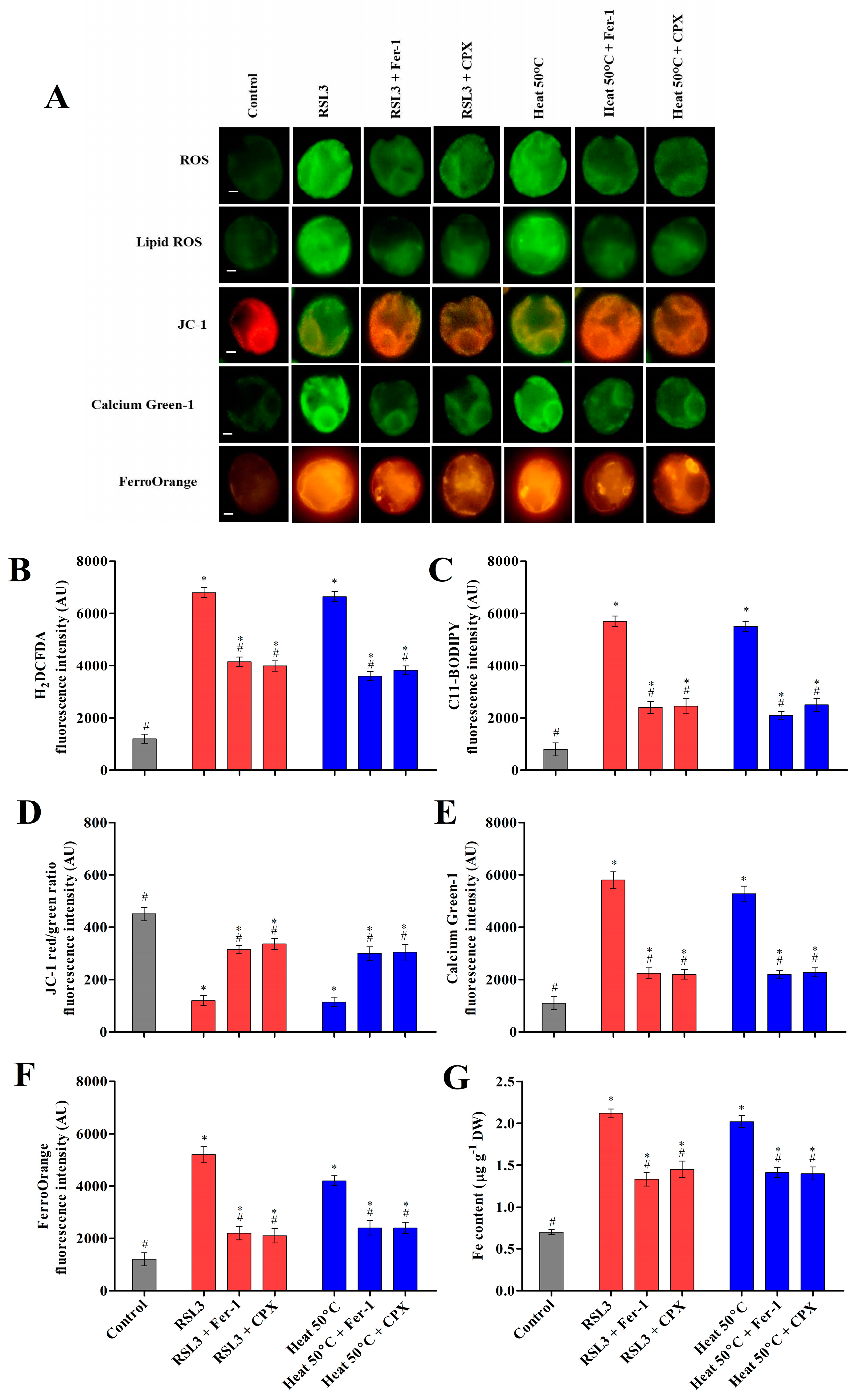
3.2.1. Heat Shock Induced Cytosolic ROS and Lipid Peroxides
3.2.2. Heat Shock Induced Mitochondrial Dysfunction and Cytosolic Calcium Levels
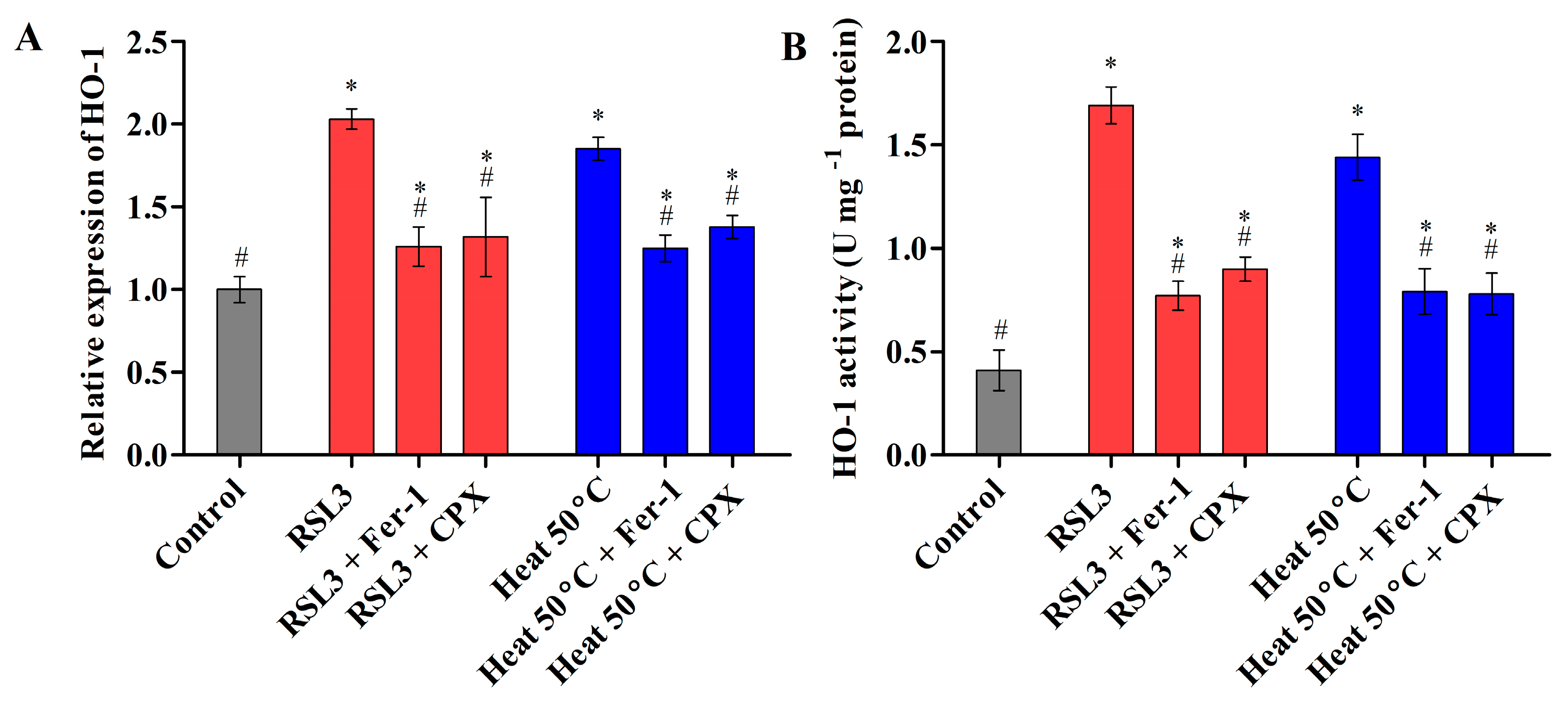
3.2.3. Heat Shock Triggered Fe2+ Labile Ferrous Iron Accumulation via Modulating HO-1 Activity
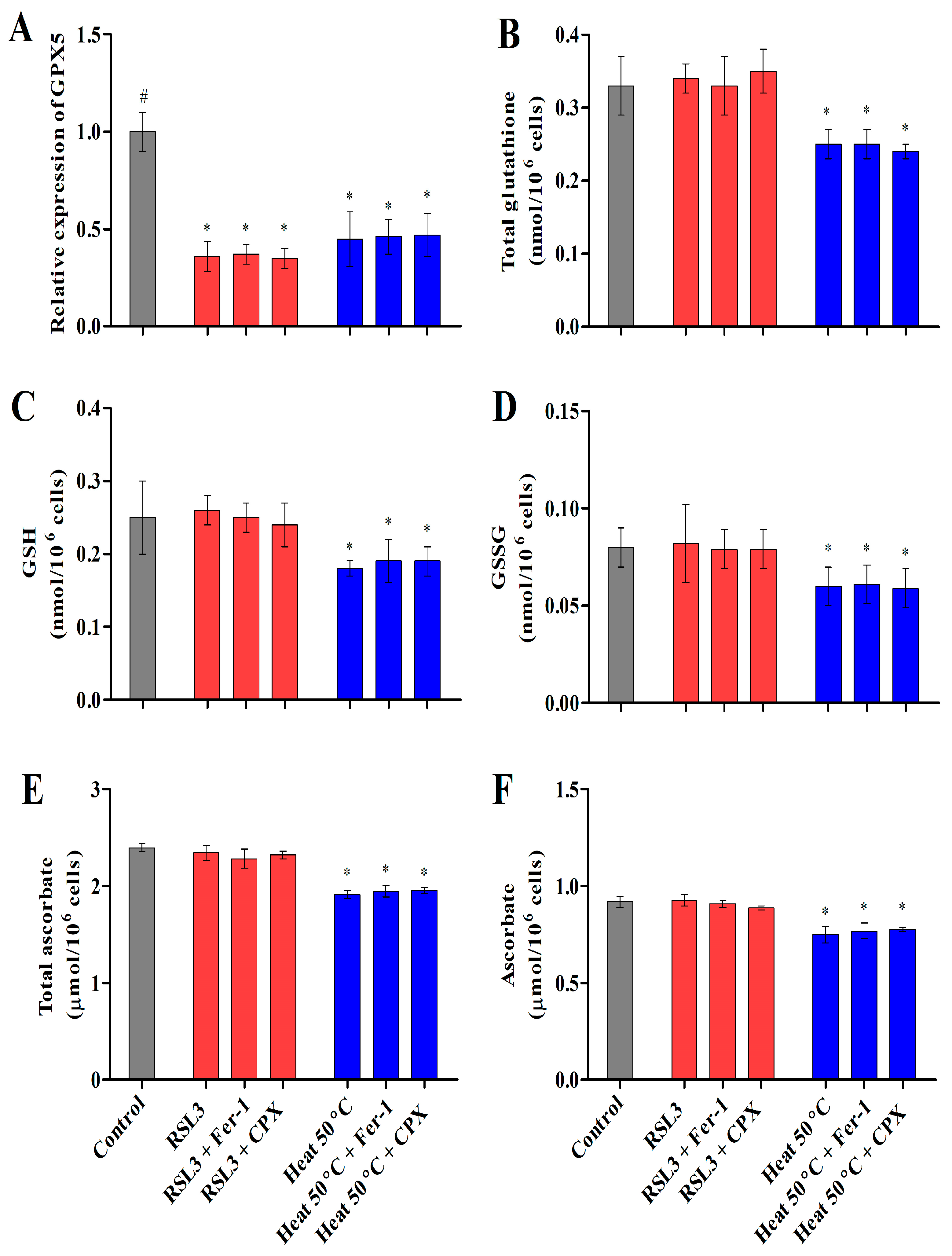
3.2.4. Heat Shock Inactivates Glutathione Peroxidase (GPX) via Glutathione and Ascorbate Depletion
3.2.5. Heat-Shock-Induced Cell Death Involves Caspase-like Activity but Does Not Cause DNA Fragmentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozik, C.; Young, E.B.; Sandgren, C.D.; Berges, J.A. Cell death in individual freshwater phytoplankton species: Relationships with population dynamics and environmental factors. Eur. J. Phycol. 2019, 54, 369–379. [Google Scholar] [CrossRef]
- Durand, P.M.; Barreto Filho, M.M.; Michod, R.E. Cell death in evolutionary transitions in individuality. Yale J. Biol. Med. 2019, 92, 651–662. [Google Scholar] [PubMed]
- Ashkenazi, A.; Salvesen, G. Regulated cell death: Signaling and mechanisms. Annu. Rev. Cell Dev. Biol. 2014, 30, 337–356. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Martin, M.V.; Córdoba, J.P.; Bellido, A.M.; D’Ippólito, S.; Colman, S.L.; Soto, D.; Roldán, J.A.; Bartoli, C.G.; Zabaleta, E.J.; et al. Heat stress induces ferroptosis-like cell death in plants. J. Cell Biol. 2017, 216, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Distéfano, A.M.; López, G.A.; Setzes, N.; Marchetti, F.; Cainzos, M.; Cascallares, M.; Zabaleta, E.; Pagnussat, G.C. Ferroptosis in plants: Triggers, proposed mechanisms, and the role of iron in modulating cell death. J. Exp. Bot. 2021, 72, 2125–2135. [Google Scholar] [CrossRef]
- Aguilera, A.; Berdun, F.; Bartoli, C.; Steelheart, C.; Alegre, M.; Bayir, H.; Tyurina, Y.Y.; Kagan, V.E.; Salerno, G.; Pagnussat, G.; et al. C-ferroptosis is an iron-dependent form of regulated cell death in cyanobacteria. J. Cell Biol. 2022, 221, e201911005. [Google Scholar] [CrossRef]
- Glaesener, A.G.; Merchant, S.S.; Blaby-Haas, C.E. Iron economy in Chlamydomonas reinhardtii. Front. Plant Sci. 2013, 4, 337. [Google Scholar] [CrossRef]
- Kasuba, K.C.; Vavilala, S.L.; D’Souza, J.S. Apoptosis-like cell death in unicellular photosynthetic organisms—A review. Algal Res. 2015, 12, 126–133. [Google Scholar] [CrossRef]
- Mühlhaus, T.; Weiss, J.; Hemme, D.; Sommer, F.; Schroda, M. Quantitative shotgun proteomics using a uniform 15N-labeled standard to monitor proteome dynamics in time course experiments reveals new insights into the heat stress response of Chlamydomonas reinhardtii. Mol. Cell. Proteom. 2011, 10, M110.004739. [Google Scholar] [CrossRef]
- Prasad, A.; Ferretti, U.; Sedlaová, M.; Pospíšil, P. Singlet oxygen production in Chlamydomonas reinhardtii under heat stress. Sci. Rep. 2016, 6, 20094. [Google Scholar] [CrossRef]
- Durand, P.M.; Rashidi, A.; Michod, R.E. How an organism dies affects the fitness of its neighbors. Am. Nat. 2011, 177, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Reimann, R.; Zeng, B.; Jakopec, M.; Burdukiewicz, M.; Petrick, I.; Schierack, P.; Rödiger, S. Classification of dead and living microalgae Chlorella vulgaris by bioimage informatics and machine learning. Algal Res. 2020, 48, 101908. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar]
- Awad, N.; Vega-Estévez, S.; Griffiths, G. Salicylic acid and aspirin stimulate growth of Chlamydomonas and inhibit lipoxygenase and chloroplast desaturase pathways. Plant Physiol. Biochem. 2020, 149, 256–265. [Google Scholar] [CrossRef]
- Dhanushkodi, R.; Matthew, C.; McManus, M.T.; Dijkwel, P.P. Drought-induced senescence of Medicago truncatula nodules involves serpin and ferritin to control proteolytic activity and iron levels. New Phytol. 2018, 220, 196–208. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Noriega, G.O.; Batlle, A.; Tomaro, M.L. Involvement of heme oxygenase as antioxidant defense in soybean nodules. Free Radic. Res. 2005, 39, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Millhollon, E.P.; Lucas, M.C. Antioxidant response to NaCl stress in salt-tolerant and salt-sensitive cultivars of cotton. Crop Sci. 1994, 34, 706–714. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Schroda, M.; Hemme, D.; Mühlhaus, T. The Chlamydomonas heat stress response. Plant J. 2015, 82, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Légeret, B.; Schulz-Raffelt, M.; Nguyen, H.M.; Auroy, P.; Beisson, F.; Peltier, G.; Blanc, G.; Li-Beisson, Y. Lipidomic and transcriptomic analyses of Chlamydomonas reinhardtii under heat stress unveil a direct route for the conversion of membrane lipids into storage lipids. Plant Cell Environ. 2016, 39, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, C.; Zhao, Y.; Gao, G. Mitochondria regulation in ferroptosis. Eur. J. Cell Biol. 2020, 99, 151058. [Google Scholar] [CrossRef]
- Pivato, M.; Ballottari, M. Chlamydomonas reinhardtii cellular compartments and their contribution to intracellular calcium signalling. J. Exp. Bot. 2021, 72, 5312–5335. [Google Scholar] [CrossRef]
- Dai, E.; Meng, L.; Kang, R.; Wang, X.; Tang, D. ESCRT-III–dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 2020, 522, 415–421. [Google Scholar] [CrossRef]
- Pedrera, L.; Espiritu, R.A.; Ros, U.; Weber, J.; Schmitt, A.; Stroh, J.; Hailfinger, S.; von Karstedt, S.; García-Sáez, A.J. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021, 28, 1644–1657. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Consoli, V.; Sorrenti, V.; Grosso, S.; Vanella, L. Heme oxygenase-1 signaling and redox homeostasis in physiopathological conditions. Biomolecules 2021, 11, 589. [Google Scholar] [CrossRef]
- Immenschuh, S.; Ramadori, G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000, 60, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Zheng, Q.; Liu, Z.P.; Yang, Z.M. Regulation of tolerance of Chlamydomonas reinhardtii to heavy metal toxicity by heme oxygenase-1 and carbon monoxide. Plant Cell Physiol. 2011, 52, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.K.; Chen, S.E.; Chang, L.C. A dual role of heme oxygenase-1 in cancer cells. Int. J. Mol. Sci. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhatla, S.C. Heme oxygenase-nitric oxide crosstalk-mediated iron homeostasis in plants under oxidative stress. Free Radic. Biol. Med. 2022, 182, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Akagi, R.; Kubo, T.; Hatori, Y.; Miyamoto, T.; Inouye, S. Heme oxygenase-1 induction by heat shock in rat hepatoma cell line is regulated by the coordinated function of HSF1, NRF2 and BACH1. J. Biochem. 2021, 170, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Lv, W.; Su, R.; Yu, C.; Jing, X.; Bai, N.; Hasi, S. Hydrolyzed camel whey protein alleviated heat stress-induced hepatocyte damage by activated Nrf2/HO-1 signaling pathway and inhibited NF-κB/NLRP3 axis. Cell Stress Chaperones 2021, 26, 387–401. [Google Scholar] [CrossRef]
- Imai, H.; Matsuoka, M.; Kumagai, T.; Sakamoto, T.; Koumura, T. Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 2017, 403, 143–170. [Google Scholar]
- Miao, R.; Ma, X.; Deng, X.; Huang, K. High level of reactive oxygen species inhibits triacylglycerols accumulation in Chlamydomonas reinhardtii. Algal Res. 2019, 38, 101400. [Google Scholar] [CrossRef]
- Fischer, B.B.; Dayer, R.; Wiesendanger, M.; Eggen, R.I.L. Independent regulation of the GPXH gene expression by primary and secondary effects of high light stress in Chlamydomonas reinhardtii. Physiol. Plant. 2007, 130, 195–206. [Google Scholar] [CrossRef]
- Fischer, B.B.; Krieger-Liszkay, A.; Eggen, R.I.L. Photosensitizers neutral red (type I) and rose bengal (type II) cause light-dependent toxicity in Chlamydomonas reinhardtii and induce the Gpxh gene via increased singlet oxygen formation. Environ. Sci. Technol. 2004, 38, 6307–6313. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, B.; Miao, R.; Deng, X.; Duan, Y.; Cheng, Y.; Zhang, W.; Shi, M.; Huang, K.; Xia, X.Q. Transcriptomic and physiological responses to oxidative stress in a Chlamydomonas reinhardtii glutathione peroxidase mutant. Genes 2020, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Hajdinák, P.; Czobor, Á.; Szarka, A. The potential role of acrolein in plant ferroptosis-like cell death. PLoS ONE 2019, 14, e0227278. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 drives ferroptosis through GPX4 inactivation and ros production in colorectal cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef]
- Sun, Q.; Zhou, M.; Zuo, Z. Toxic mechansim of eucalyptol and β-cyclocitral on Chlamydomonas reinhartii by inducing programmed cell death. J. Hazard. Mater. 2020, 389, 121910. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Ye, B.; Zhu, K.; Yin, J.; Zheng, T.; Xu, S.; Sun, Q.; Li, Y.; Zuo, Z. Programmed cell death of Chlamydomonas reinhardtii induced by three cyanobacterial volatiles β-ionone, limonene and longifolene. Sci. Total Environ. 2021, 762, 144539. [Google Scholar] [CrossRef]
- Nedelcu, A.M. Evidence for p53-like-mediated stress responses in green algae. FEBS Lett. 2006, 580, 3013–3017. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, R.; Han, H.-S.; Subramanian, P.; Mageswari, A.; Kim, S.-H.; Tirumani, S.; Maurya, V.K.; Muthukaliannan, G.K.; Ramya, M. Lipid ROS- and Iron-Dependent Ferroptotic Cell Death in Unicellular Algae Chlamydomonas reinhardtii. Cells 2023, 12, 553. https://doi.org/10.3390/cells12040553
Srinivasan R, Han H-S, Subramanian P, Mageswari A, Kim S-H, Tirumani S, Maurya VK, Muthukaliannan GK, Ramya M. Lipid ROS- and Iron-Dependent Ferroptotic Cell Death in Unicellular Algae Chlamydomonas reinhardtii. Cells. 2023; 12(4):553. https://doi.org/10.3390/cells12040553
Chicago/Turabian StyleSrinivasan, Ramachandran, Hyo-Shim Han, Parthiban Subramanian, Anbazhagan Mageswari, Seong-Hoon Kim, Srikanth Tirumani, Vaibhav Kumar Maurya, Gothandam Kodiveri Muthukaliannan, and Mohandass Ramya. 2023. "Lipid ROS- and Iron-Dependent Ferroptotic Cell Death in Unicellular Algae Chlamydomonas reinhardtii" Cells 12, no. 4: 553. https://doi.org/10.3390/cells12040553
APA StyleSrinivasan, R., Han, H.-S., Subramanian, P., Mageswari, A., Kim, S.-H., Tirumani, S., Maurya, V. K., Muthukaliannan, G. K., & Ramya, M. (2023). Lipid ROS- and Iron-Dependent Ferroptotic Cell Death in Unicellular Algae Chlamydomonas reinhardtii. Cells, 12(4), 553. https://doi.org/10.3390/cells12040553








