Effects of Fatty Acid Metabolites on Adipocytes Britening: Role of Thromboxane A2
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Adiponectin Levels
2.4. Isolation and Analysis of RNA
2.5. Western Blot Analysis
2.6. Statistical Analyses
3. Results
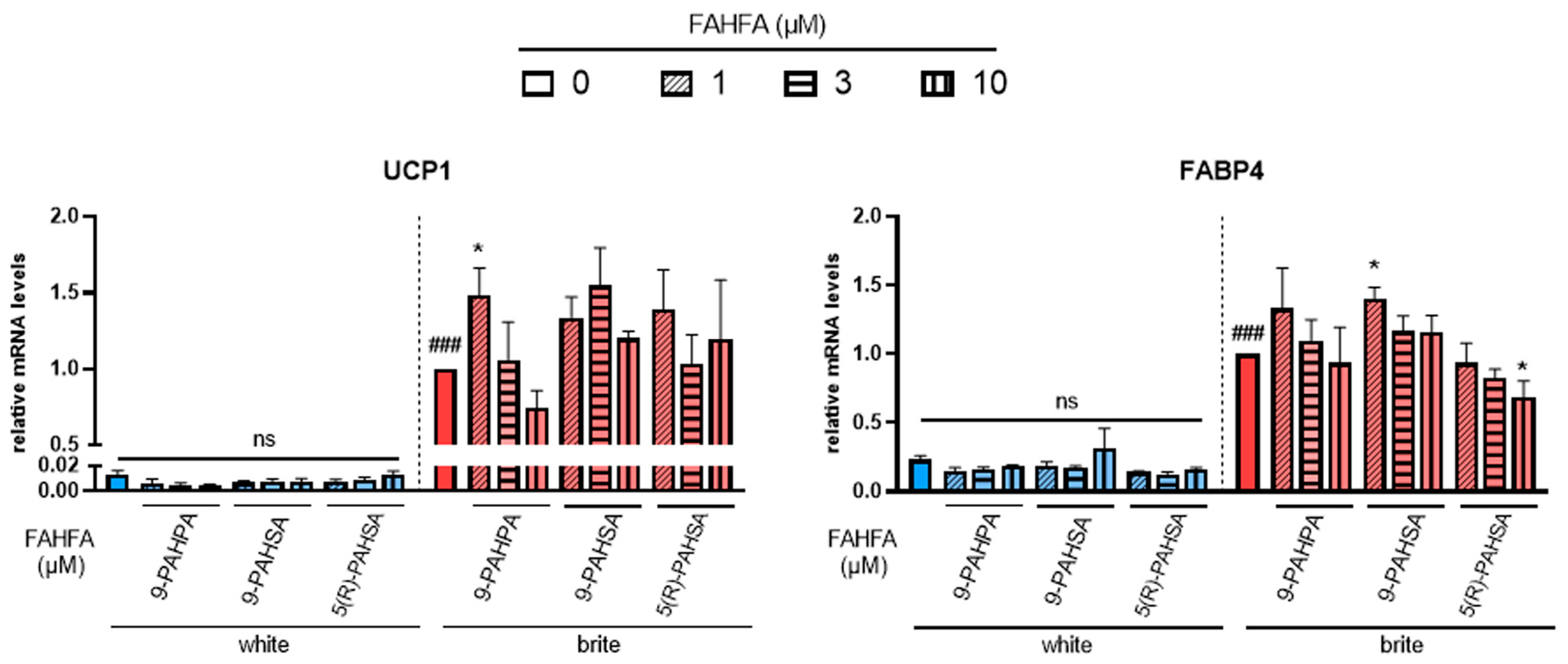
3.1. 9-PAHSA and 9-PAHPA Effects on Browning of White hMADS Adipocytes
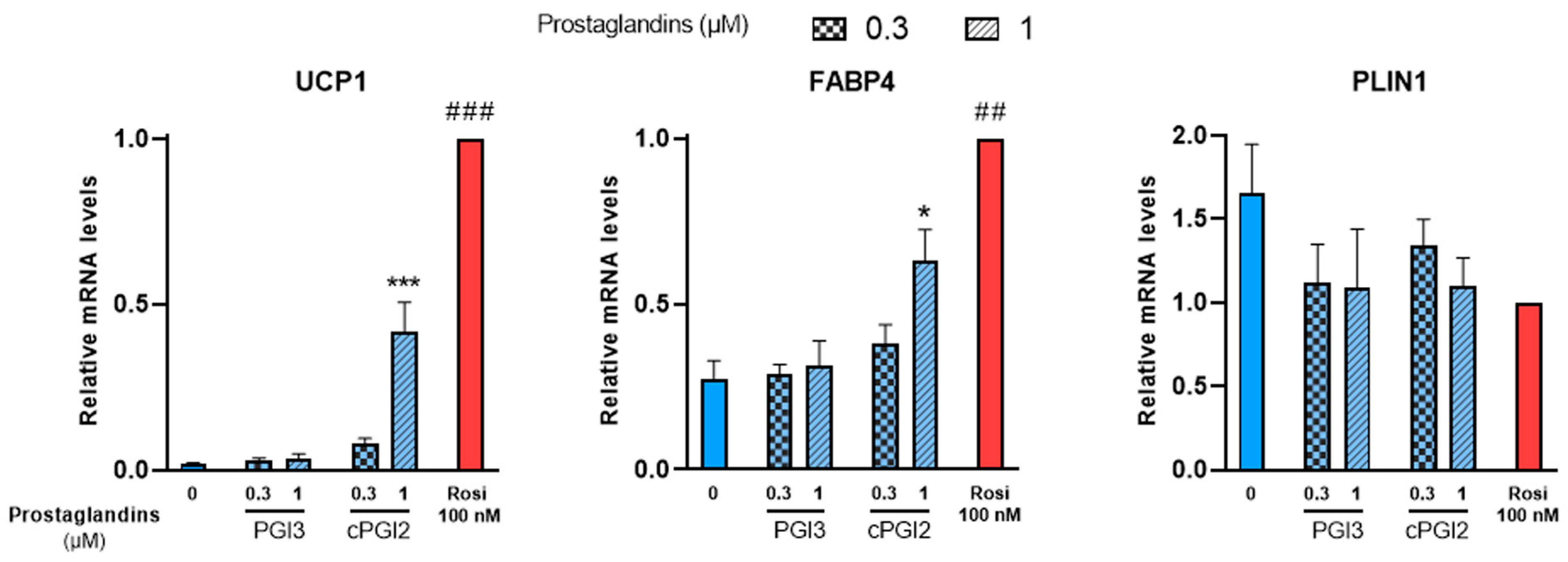
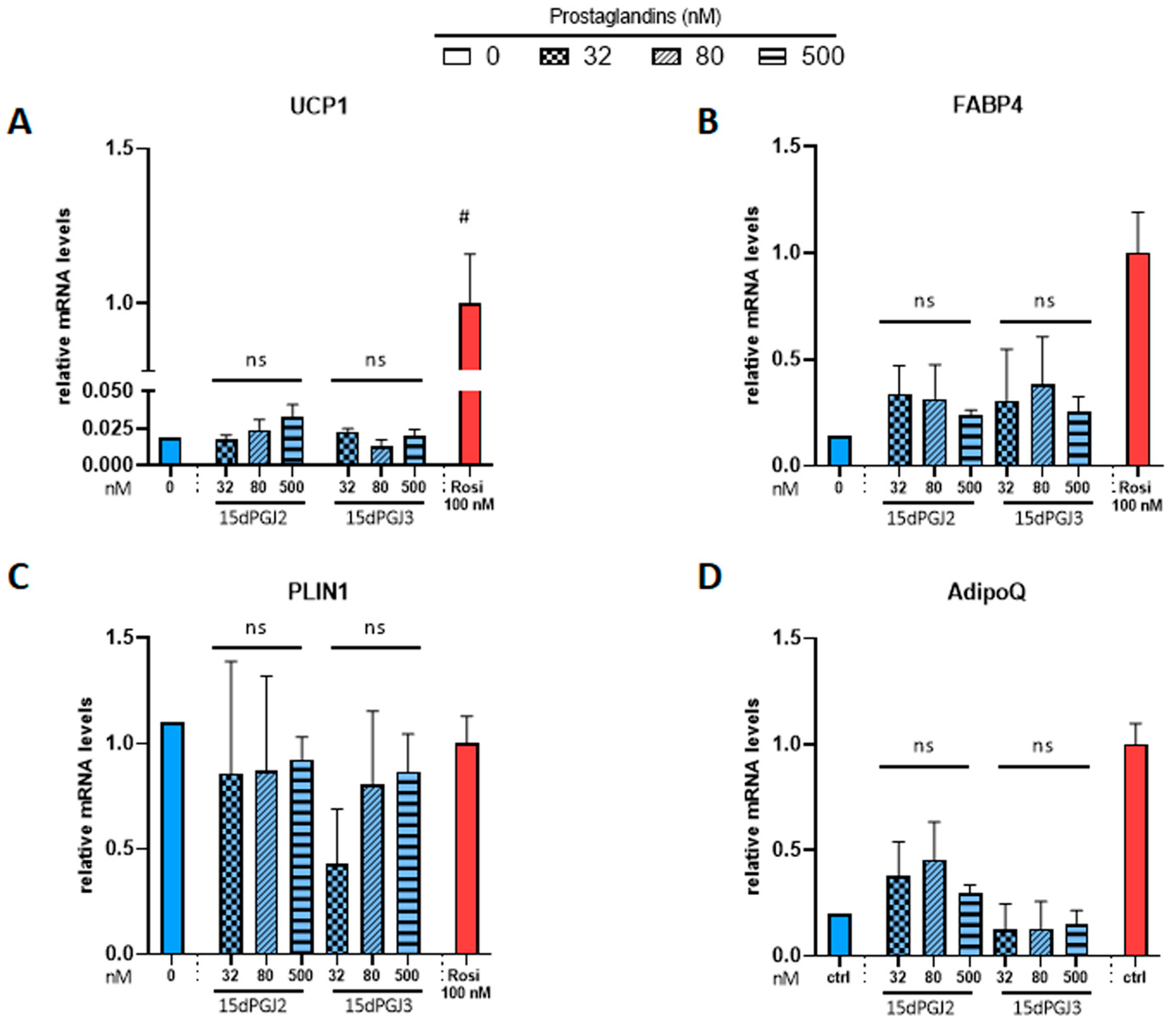
3.2. Effects of Prostaglandins and Their Derivatives on Browning of White Adipocytes
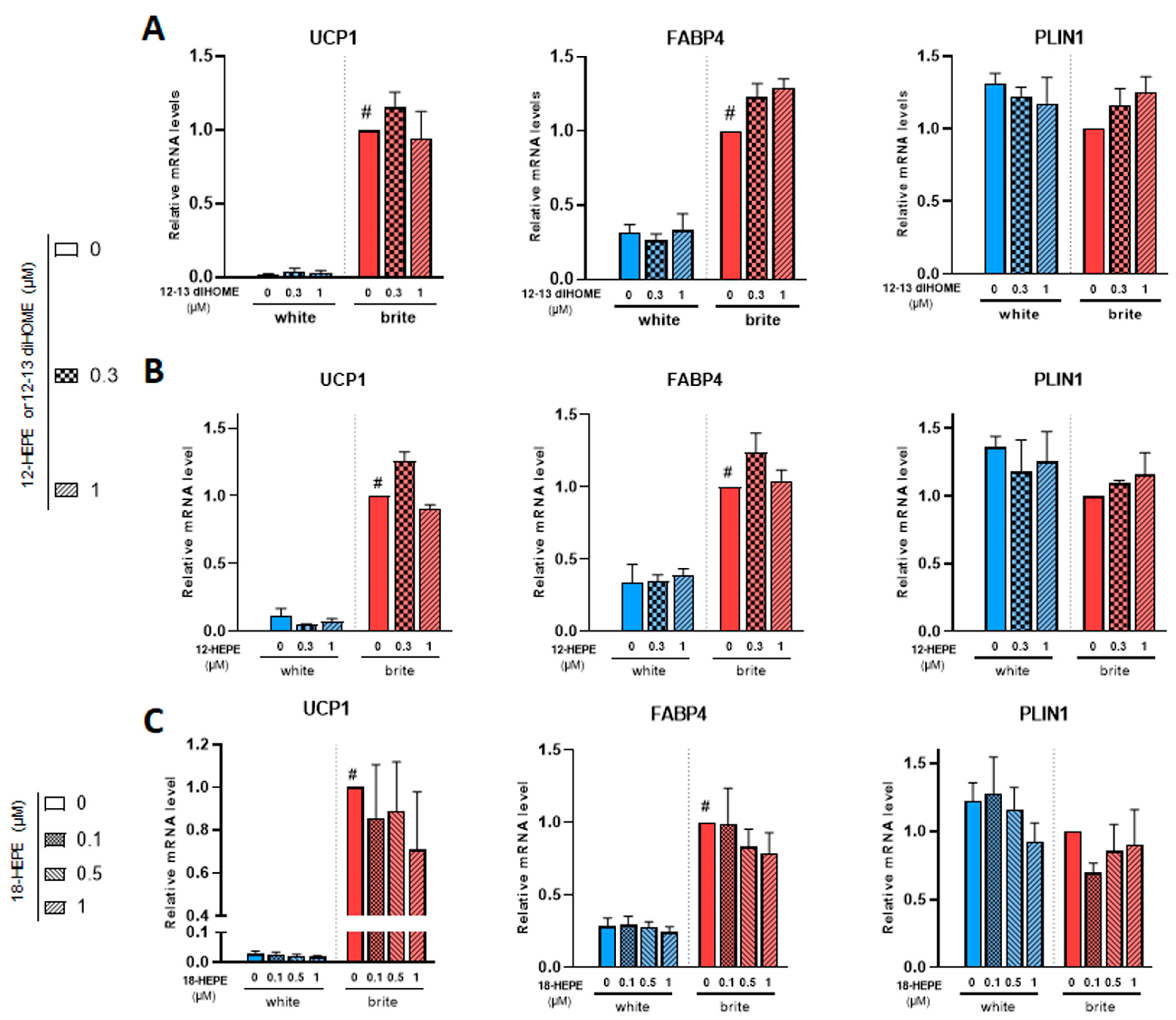
3.3. Effects of 12,13 di-HOME, 12-HEPE and 18-HEPE on Browning of White Adipocytes
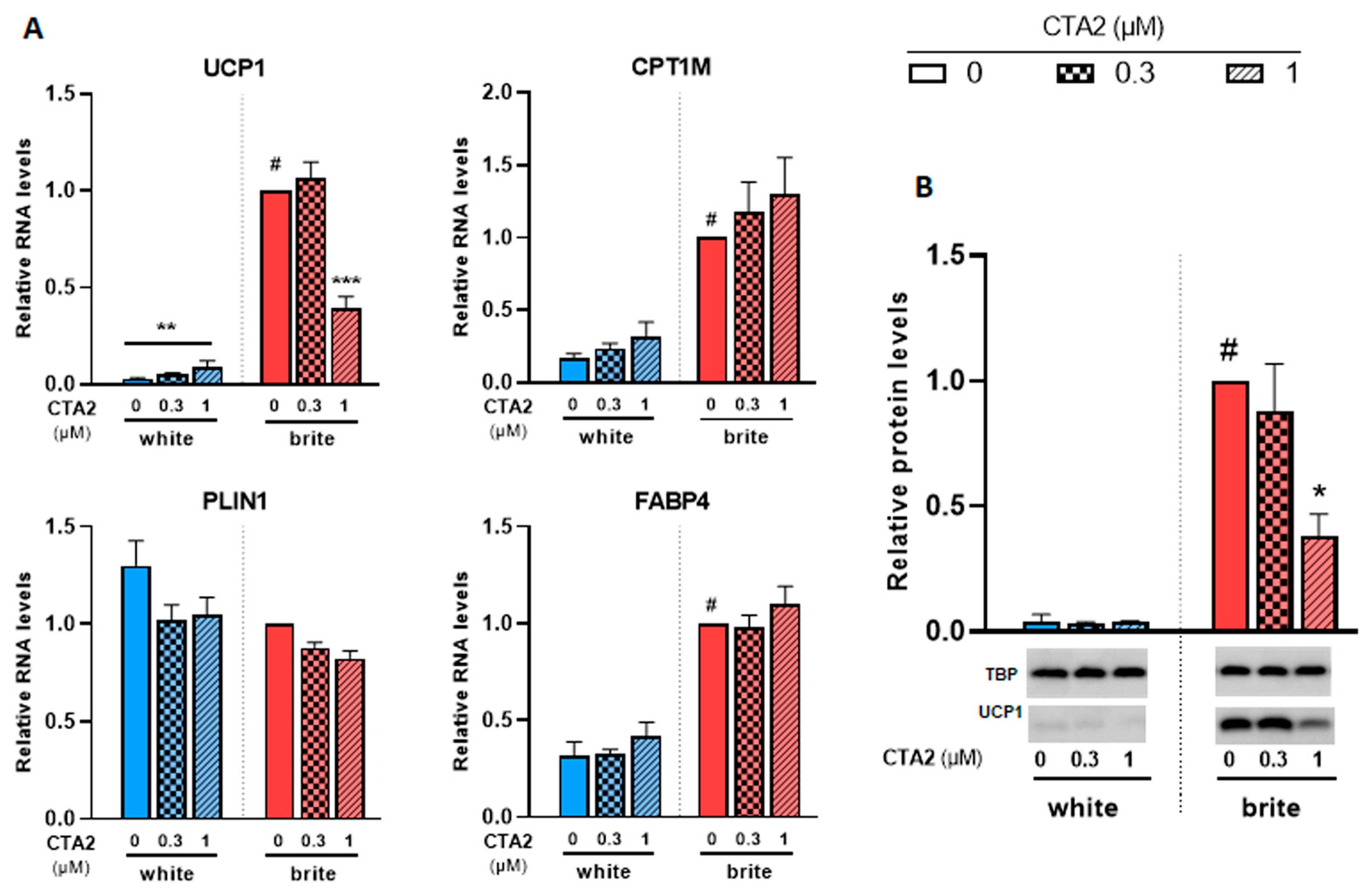
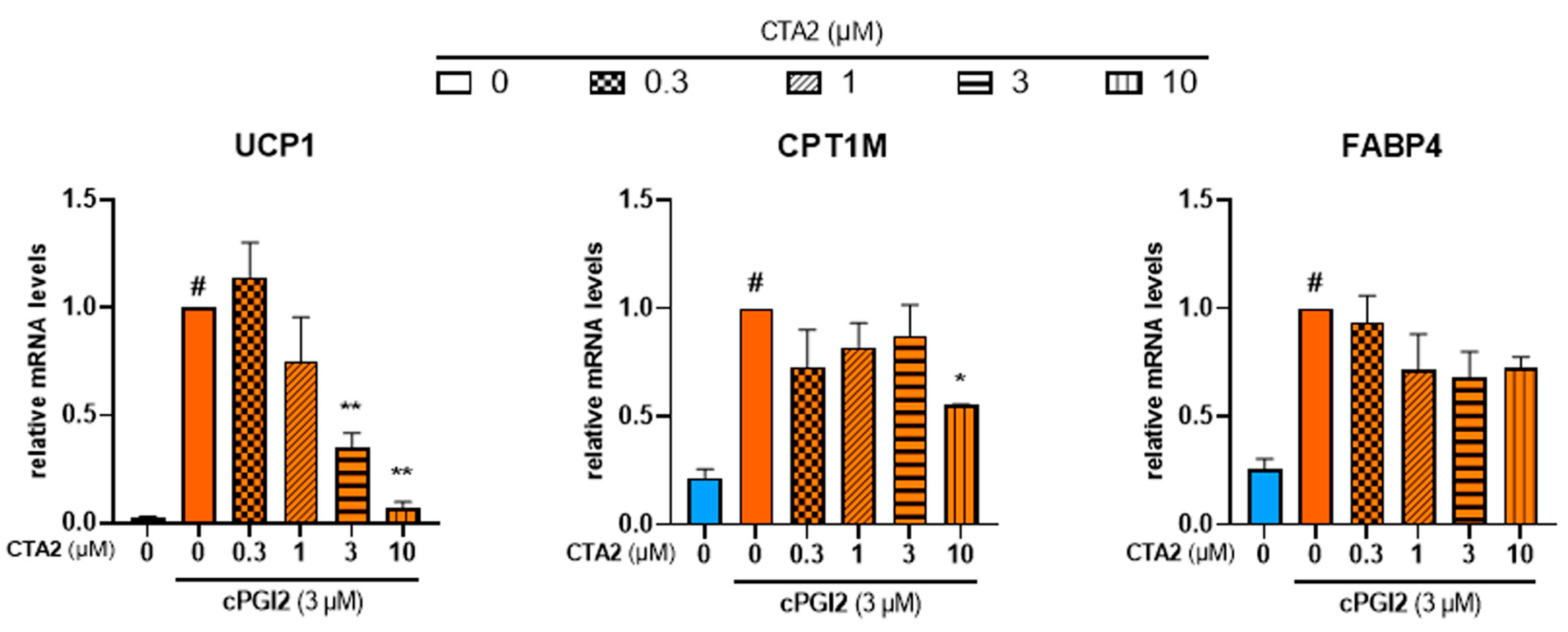
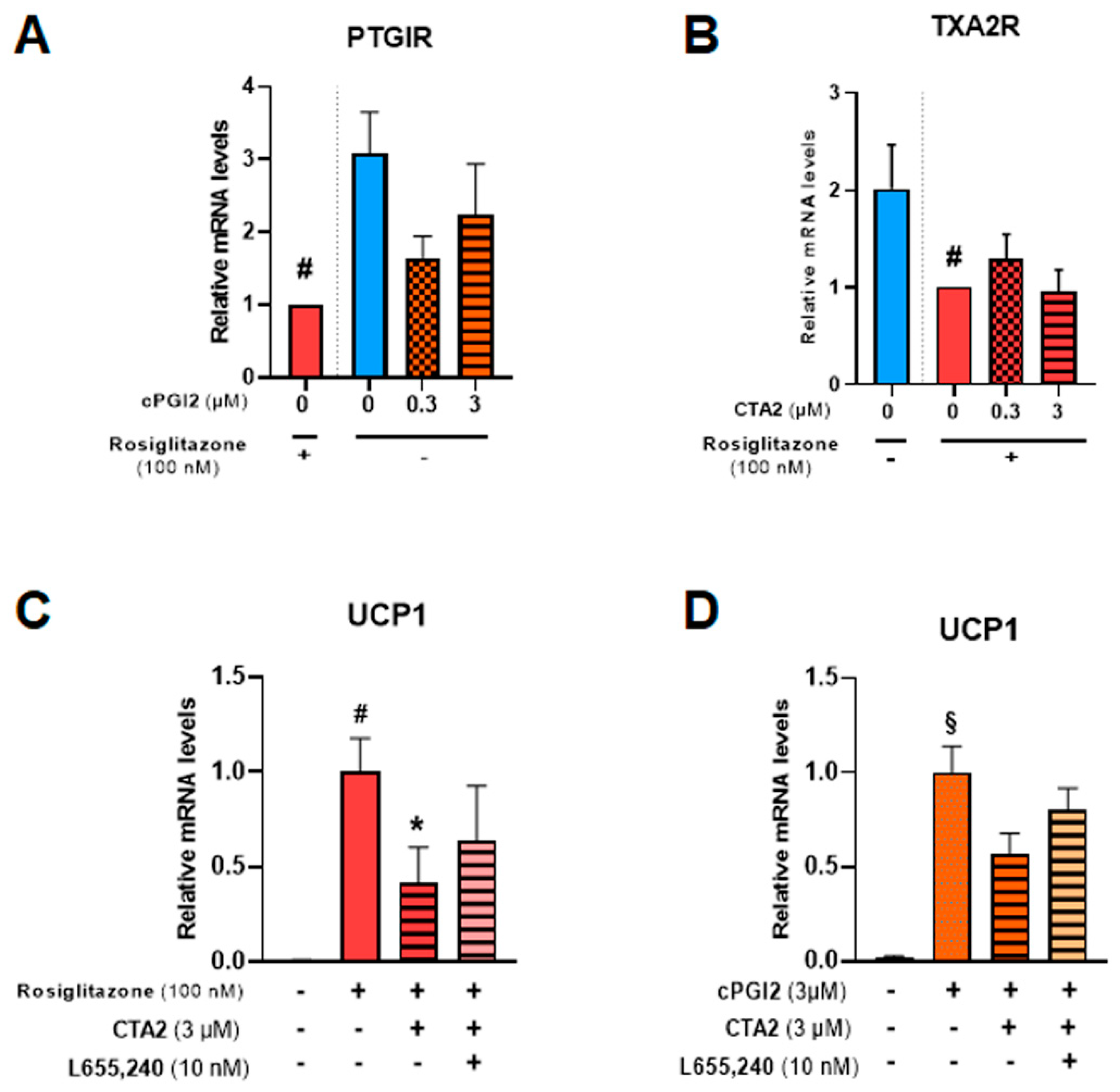
3.4. Effects of Thromboxane A2 on Browning of White Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARA | arachidonic acid |
| CTA2 | carbocyclic thromboxane A2 |
| BMI | body mass index |
| FAHFA | fatty acid esters of hydroxy fatty acids |
| TXA2 | thromboxane A2 |
| TXB2 | thromboxane B2 |
| 5(R)-PAHSA | palmitic-acid-5-hydroxy-stearic acid |
| 9-PAHPA | palmitic-acid-9-hydroxy-palmitic acid |
| 9-PAHSA | palmitic-acid-9-hydroxy-stearic acid |
| 15dPGJ2 | 15-deoxy-Delta(12,14)-prostaglandin J2 |
| 15dPGJ3 | 15-deoxy-Delta(12,14)-prostaglandin J3 |
References
- WHO. Obesity and Overweight. Fact Sheet N°311. 2020. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef]
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E350–E357. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2020, 41, 53–65. [Google Scholar] [CrossRef]
- Nedergaard, J.; Bengtsson, T.; Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E444–E452. [Google Scholar] [CrossRef]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef]
- Min, S.Y.; Kady, J.; Nam, M.; Rojas-Rodriguez, R.; Berkenwald, A.; Kim, J.H.; Noh, H.L.; Kim, J.K.; Cooper, M.P.; Fitzgibbons, T.; et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med. 2016, 22, 312–318. [Google Scholar] [CrossRef]
- Srivastava, S.; Veech, R.L. Brown and Brite: The Fat Soldiers in the Anti-obesity Fight. Front. Physiol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerback, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Horvath, C.; Wolfrum, C. Feeding brown fat: Dietary phytochemicals targeting non-shivering thermogenesis to control body weight. Proc. Nutr. Soc. 2020, 79, 338–356. [Google Scholar] [CrossRef] [PubMed]
- Leiria, L.O.; Tseng, Y.H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158788. [Google Scholar] [CrossRef]
- Park, H.; He, A.; Lodhi, I.J. Lipid Regulators of Thermogenic Fat Activation. Trends Endocrinol. Metab. 2019, 30, 710–723. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Kodani, S.D.; Tseng, Y.H. Lipokines and Thermogenesis. Endocrinology 2019, 160, 2314–2325. [Google Scholar] [CrossRef]
- Lynes, M.D.; Leiria, L.O.; Lundh, M.; Bartelt, A.; Shamsi, F.; Huang, T.L.; Takahashi, H.; Hirshman, M.F.; Schlein, C.; Lee, A.; et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med. 2017, 23, 631–637. [Google Scholar] [CrossRef]
- Kulterer, O.C.; Niederstaetter, L.; Herz, C.T.; Haug, A.R.; Bileck, A.; Pils, D.; Kautzky-Willer, A.; Gerner, C.; Kiefer, F.W. The Presence of Active Brown Adipose Tissue Determines Cold-Induced Energy Expenditure and Oxylipin Profiles in Humans. J. Clin. Endocrinol. Metab. 2020, 105, 2203–2216. [Google Scholar] [CrossRef]
- Leiria, L.O.; Wang, C.H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, 768–783.e7. [Google Scholar] [CrossRef]
- Brejchova, K.; Balas, L.; Paluchova, V.; Brezinova, M.; Durand, T.; Kuda, O. Understanding FAHFAs: From structure to metabolic regulation. Prog. Lipid Res. 2020, 79, 101053. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Goonapienuwala, B.L.; Moustaid-Moussa, N. Omega-3 Fatty Acids and Adipose Tissue: Inflammation and Browning. Annu. Rev. Nutr. 2020, 40, 25–49. [Google Scholar] [CrossRef]
- Fujimori, K. Prostaglandin D(2) and F(2alpha) as Regulators of Adipogenesis and Obesity. Biol. Pharm. Bull. 2022, 45, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Yun, D.H.; Song, H.Y.; Lee, M.J.; Kim, M.R.; Kim, M.Y.; Lee, J.S.; Kim, J.H. Thromboxane A(2) modulates migration, proliferation, and differentiation of adipose tissue-derived mesenchymal stem cells. Exp. Mol. Med. 2009, 41, 17–24. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Elabd, C.; Delteil, F.; Astier, J.; Vernochet, C.; Saint-Marc, P.; Guesnet, J.; Guezennec, A.; Amri, E.Z.; Dani, C.; et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 2004, 315, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Chiellini, C.; Carmona, M.; Galitzky, J.; Cochet, O.; Petersen, R.; Penicaud, L.; Kristiansen, K.; Bouloumie, A.; Casteilla, L.; et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells 2009, 27, 2753–2760. [Google Scholar] [CrossRef]
- Barquissau, V.; Beuzelin, D.; Pisani, D.F.; Beranger, G.E.; Mairal, A.; Montagner, A.; Roussel, B.; Tavernier, G.; Marques, M.A.; Moro, C.; et al. White-to-brite conversion in human adipocytes promotes metabolic reprogramming towards fatty acid anabolic and catabolic pathways. Mol. Metab. 2016, 5, 352–365. [Google Scholar] [CrossRef]
- Pisani, D.F.; Barquissau, V.; Chambard, J.C.; Beuzelin, D.; Ghandour, R.A.; Giroud, M.; Mairal, A.; Pagnotta, S.; Cinti, S.; Langin, D.; et al. Mitochondrial fission is associated with UCP1 activity in human brite/beige adipocytes. Mol. Metab. 2018, 7, 35–44. [Google Scholar] [CrossRef]
- Ghandour, R.A.; Colson, C.; Giroud, M.; Maurer, S.; Rekima, S.; Ailhaud, G.; Klingenspor, M.; Amri, E.Z.; Pisani, D.F. Impact of dietary omega3 polyunsaturated fatty acid supplementation on brown and brite adipocyte function. J. Lipid Res. 2018, 59, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Lefils-Lacourtablaise, J.; Socorro, M.; Geloen, A.; Daira, P.; Debard, C.; Loizon, E.; Guichardant, M.; Dominguez, Z.; Vidal, H.; Lagarde, M.; et al. The eicosapentaenoic acid metabolite 15-deoxy-delta(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARgamma-dependent mechanism. PLoS ONE 2013, 8, e63997. [Google Scholar] [CrossRef]
- Balas, L.; Bertrand-Michel, J.; Viars, F.; Faugere, J.; Lefort, C.; Caspar-Bauguil, S.; Langin, D.; Durand, T. Regiocontrolled syntheses of FAHFAs and LC-MS/MS differentiation of regioisomers. Org. Biomol. Chem. 2016, 14, 9012–9020. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Pisani, D.; Dechesne, C.A.; Turc-Carel, C.; Kurzenne, J.Y.; Wdziekonski, B.; Villageois, A.; Bagnis, C.; Breittmayer, J.P.; Groux, H.; et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005, 201, 1397–1405. [Google Scholar] [CrossRef]
- Pisani, D.F.; Djedaini, M.; Beranger, G.E.; Elabd, C.; Scheideler, M.; Ailhaud, G.; Amri, E.Z. Differentiation of Human Adipose-Derived Stem Cells into “Brite” (Brown-in-White) Adipocytes. Front. Endocrinol. 2011, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Pisani, D.F.; Ghandour, R.A.; Beranger, G.E.; Le Faouder, P.; Chambard, J.C.; Giroud, M.; Vegiopoulos, A.; Djedaini, M.; Bertrand-Michel, J.; Tauc, M.; et al. The omega6-fatty acid, arachidonic acid, regulates the conversion of white to brite adipocyte through a prostaglandin/calcium mediated pathway. Mol. Metab. 2014, 3, 834–847. [Google Scholar] [CrossRef]
- Yore, M.M.; Syed, I.; Moraes-Vieira, P.M.; Zhang, T.; Herman, M.A.; Homan, E.A.; Patel, R.T.; Lee, J.; Chen, S.; Peroni, O.D.; et al. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 2014, 159, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.M.; Liu, H.X.; Fang, N.Y. 9-PAHSA promotes browning of white fat via activating G-protein-coupled receptor 120 and inhibiting lipopolysaccharide/NF-kappa B pathway. Biochem. Biophys. Res. Commun. 2018, 506, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Benlebna, M.; Balas, L.; Bonafos, B.; Pessemesse, L.; Fouret, G.; Vigor, C.; Gaillet, S.; Grober, J.; Bernex, F.; Landrier, J.F.; et al. Long-term intake of 9-PAHPA or 9-OAHPA modulates favorably the basal metabolism and exerts an insulin sensitizing effect in obesogenic diet-fed mice. Eur. J. Nutr. 2021, 60, 2013–2027. [Google Scholar] [CrossRef] [PubMed]
- Ghandour, R.A.; Giroud, M.; Vegiopoulos, A.; Herzig, S.; Ailhaud, G.; Amri, E.Z.; Pisani, D.F. IP-receptor and PPARs trigger the conversion of human white to brite adipocyte induced by carbaprostacyclin. Biochim. Biophys. Acta 2016, 1861, 285–293. [Google Scholar] [CrossRef]
- Babaei, R.; Schuster, M.; Meln, I.; Lerch, S.; Ghandour, R.A.; Pisani, D.F.; Bayindir-Buchhalter, I.; Marx, J.; Wu, S.; Schoiswohl, G.; et al. Jak-TGFbeta cross-talk links transient adipose tissue inflammation to beige adipogenesis. Sci. Signal. 2018, 11, eaai7838. [Google Scholar] [CrossRef]
- Babaei, R.; Bayindir-Buchhalter, I.; Meln, I.; Vegiopoulos, A. Immuno-Magnetic Isolation and Thermogenic Differentiation of White Adipose Tissue Progenitor Cells. Methods Mol. Biol. 2017, 1566, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Forman, B.M.; Tontonoz, P.; Chen, J.; Brun, R.P.; Spiegelman, B.M.; Evans, R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell 1995, 83, 803–812. [Google Scholar] [CrossRef]
- Dieckmann, S.; Maurer, S.; Fromme, T.; Colson, C.; Virtanen, K.A.; Amri, E.Z.; Klingenspor, M. Fatty Acid Metabolite Profiling Reveals Oxylipins as Markers of Brown but Not Brite Adipose Tissue. Front. Endocrinol. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Dhamoon, A.S. Physiology, Thromboxane A2. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nakahata, N. Thromboxane A2: Physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol. Ther. 2008, 118, 18–35. [Google Scholar] [CrossRef]
- Rahman, M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell. Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Claiborn, K.C.; Hotamisligil, G.S. De Novo Lipogenesis Products and Endogenous Lipokines. Diabetes 2016, 65, 1800–1807. [Google Scholar] [CrossRef]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.; Amri, E.Z.; Pisani, D.F. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 2017, 136, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lynes, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1357. [Google Scholar] [CrossRef]
- Paluchova, V.; Oseeva, M.; Brezinova, M.; Cajka, T.; Bardova, K.; Adamcova, K.; Zacek, P.; Brejchova, K.; Balas, L.; Chodounska, H.; et al. Lipokine 5-PAHSA Is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for De Novo Lipogenesis in Mice. Diabetes 2020, 69, 300–312. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Paluchova, V.; Vik, A.; Cajka, T.; Brezinova, M.; Brejchova, K.; Bugajev, V.; Draberova, L.; Draber, P.; Buresova, J.; Kroupova, P.; et al. Triacylglycerol-Rich Oils of Marine Origin are Optimal Nutrients for Induction of Polyunsaturated Docosahexaenoic Acid Ester of Hydroxy Linoleic Acid (13-DHAHLA) with Anti-Inflammatory Properties in Mice. Mol. Nutr. Food Res. 2020, 64, e1901238. [Google Scholar] [CrossRef]
- Benlebna, M.; Balas, L.; Bonafos, B.; Pessemesse, L.; Vigor, C.; Grober, J.; Bernex, F.; Fouret, G.; Paluchova, V.; Gaillet, S.; et al. Long-term high intake of 9-PAHPA or 9-OAHPA increases basal metabolism and insulin sensitivity but disrupts liver homeostasis in healthy mice. J. Nutr. Biochem. 2020, 79, 108361. [Google Scholar] [CrossRef]
- Kobzar, G.; Mardla, V.; Jarving, I.; Samel, N. Comparison of anti-aggregatory effects of PGI2, PGI3 and iloprost on human and rabbit platelets. Cell. Physiol. Biochem. 2001, 11, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Lenhard, J.M.; Willson, T.M.; Patel, I.; Morris, D.C.; Lehmann, J.M. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell 1995, 83, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Rocca, B. Measurement of Thromboxane Biosynthesis in Health and Disease. Front. Pharmacol. 2019, 10, 1244. [Google Scholar] [CrossRef]
- Lei, X.; Li, Q.; Rodriguez, S.; Tan, S.Y.; Seldin, M.M.; McLenithan, J.C.; Jia, W.; Wong, G.W. Thromboxane synthase deficiency improves insulin action and attenuates adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E792–E804. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Biasucci, L.M.; Cialdella, P.; Liuzzo, G.; Giubilato, S.; Della Bona, R.; Pulcinelli, F.M.; Iaconelli, A.; Mingrone, G.; Crea, F. Thromboxane production in morbidly obese subjects. Am. J. Cardiol. 2011, 107, 1656–1661. [Google Scholar] [CrossRef]
- Bunting, S.; Moncada, S.; Vane, J.R. The prostacyclin-thromboxane A2 balance: Pathophysiological and therapeutic implications. Br. Med. Bull. 1983, 39, 271–276. [Google Scholar] [CrossRef]
- Hayashi, Y.; Shimizu, I.; Yoshida, Y.; Ikegami, R.; Suda, M.; Katsuumi, G.; Fujiki, S.; Ozaki, K.; Abe, M.; Sakimura, K.; et al. Coagulation factors promote brown adipose tissue dysfunction and abnormal systemic metabolism in obesity. iScience 2022, 25, 104547. [Google Scholar] [CrossRef]
- Rodbell, M. Metabolism of Isolated Fat Cells: I. Effects of Hormones on Glucose Metabolism and Lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar] [CrossRef]
- Murholm, M.; Dixen, K.; Qvortrup, K.; Hansen, L.H.; Amri, E.Z.; Madsen, L.; Barbatelli, G.; Quistorff, B.; Hansen, J.B. Dynamic regulation of genes involved in mitochondrial DNA replication and transcription during mouse brown fat cell differentiation and recruitment. PLoS ONE 2009, 4, e8458. [Google Scholar] [CrossRef]
- Colson, C.; Batrow, P.L.; Gautier, N.; Rochet, N.; Ailhaud, G.; Peiretti, F.; Amri, E.Z. The Rosmarinus Bioactive Compound Carnosic Acid Is a Novel PPAR Antagonist That Inhibits the Browning of White Adipocytes. Cells 2020, 9, 2433. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colson, C.; Batrow, P.-L.; Dieckmann, S.; Contu, L.; Roux, C.H.; Balas, L.; Vigor, C.; Fourmaux, B.; Gautier, N.; Rochet, N.; et al. Effects of Fatty Acid Metabolites on Adipocytes Britening: Role of Thromboxane A2. Cells 2023, 12, 446. https://doi.org/10.3390/cells12030446
Colson C, Batrow P-L, Dieckmann S, Contu L, Roux CH, Balas L, Vigor C, Fourmaux B, Gautier N, Rochet N, et al. Effects of Fatty Acid Metabolites on Adipocytes Britening: Role of Thromboxane A2. Cells. 2023; 12(3):446. https://doi.org/10.3390/cells12030446
Chicago/Turabian StyleColson, Cécilia, Pierre-Louis Batrow, Sebastian Dieckmann, Laura Contu, Christian H. Roux, Laurence Balas, Claire Vigor, Baptiste Fourmaux, Nadine Gautier, Nathalie Rochet, and et al. 2023. "Effects of Fatty Acid Metabolites on Adipocytes Britening: Role of Thromboxane A2" Cells 12, no. 3: 446. https://doi.org/10.3390/cells12030446
APA StyleColson, C., Batrow, P.-L., Dieckmann, S., Contu, L., Roux, C. H., Balas, L., Vigor, C., Fourmaux, B., Gautier, N., Rochet, N., Bernoud-Hubac, N., Durand, T., Langin, D., Klingenspor, M., & Amri, E.-Z. (2023). Effects of Fatty Acid Metabolites on Adipocytes Britening: Role of Thromboxane A2. Cells, 12(3), 446. https://doi.org/10.3390/cells12030446







