Brain Macro-Structural Alterations in Aging Rats: A Longitudinal Lifetime Approach
Abstract
1. Introduction
2. Material and Methods
2.1. Animals and Experimental Design
2.2. Magnetic Resonance Imaging (MRI)
2.3. Deformation-Based Morphometry (DBM)
2.4. Statistical Analyses
3. Results
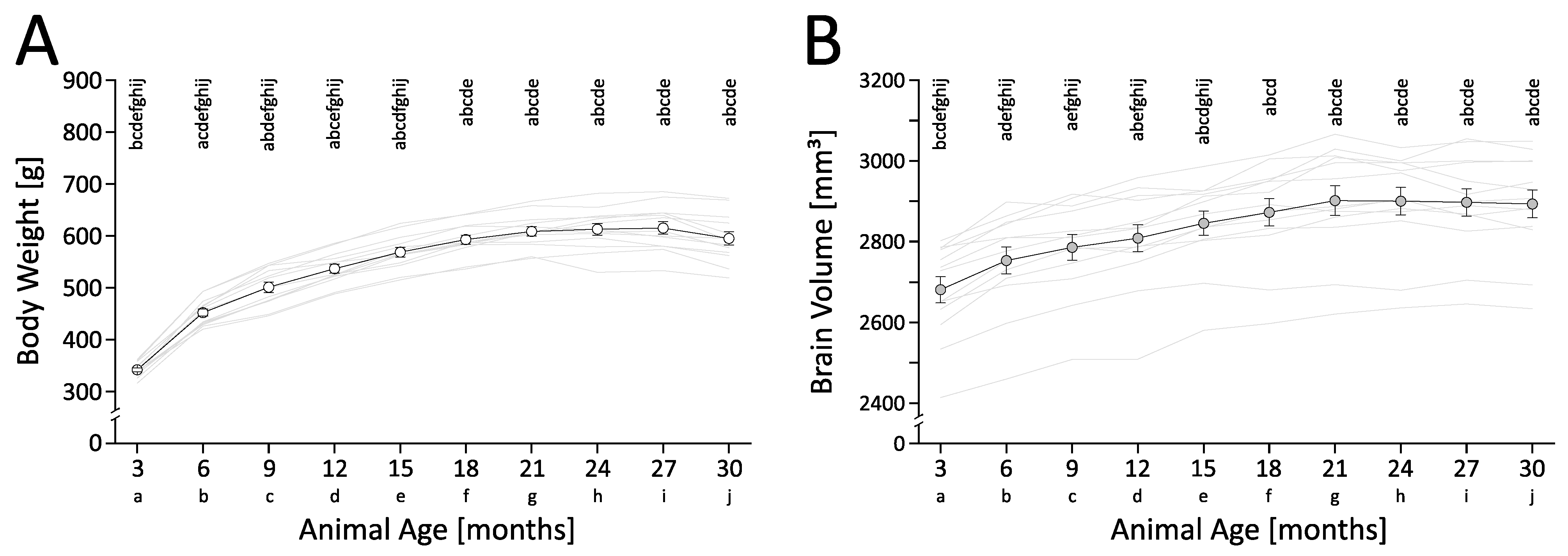
3.1. Body Weight
3.2. Total Intracranial Brain Volume
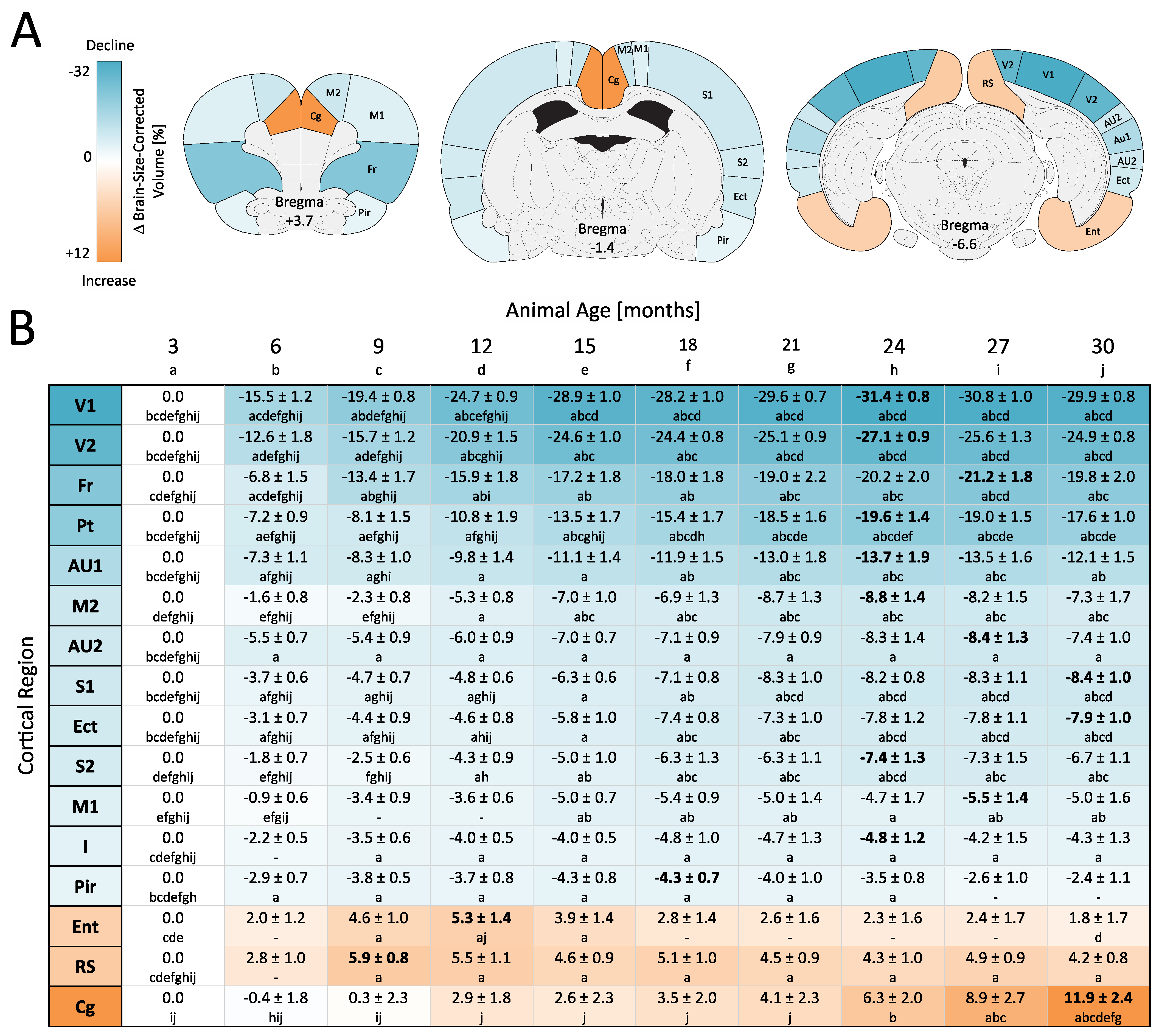
3.3. ROI-Based Analyses: Cortex
3.4. Voxel-Wise Analyses: Whole Brain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klenk, J.; Keil, U.; Jaensch, A.; Christiansen, M.C.; Nagel, G. Changes in life expectancy 1950–2010: Contributions from age- and disease-specific mortality in selected countries. Popul. Health Metr. 2016, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Kontis, V.; Bennett, J.E.; Mathers, C.D.; Li, G.; Foreman, K.; Ezzati, M. Future life expectancy in 35 industrialised countries: Projections with a Bayesian model ensemble. Lancet 2017, 389, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Trajectories of normal cognitive aging. Psychol. Aging 2019, 34, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Grady, C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 2012, 13, 491–505. [Google Scholar] [CrossRef]
- Ferguson, H.J.; Brunsdon, V.E.A.; Bradford, E.E.F. The developmental trajectories of executive function from adolescence to old age. Sci. Rep. 2021, 11, 1382. [Google Scholar] [CrossRef]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 701–710. [Google Scholar] [CrossRef]
- Park, D.C.; Polk, T.A.; Mikels, J.A.; Taylor, S.F.; Marshuetz, C. Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues Clin. Neurosci. 2001, 3, 151–165. [Google Scholar] [CrossRef]
- Malagurski, B.; Liem, F.; Oschwald, J.; Merillat, S.; Jancke, L. Longitudinal functional brain network reconfiguration in healthy aging. Hum. Brain Mapp. 2020, 41, 4829–4845. [Google Scholar] [CrossRef] [PubMed]
- Dolan, R.J. Neuroimaging of cognition: Past, present, and future. Neuron 2008, 60, 496–502. [Google Scholar] [CrossRef]
- Lovden, M.; Wenger, E.; Martensson, J.; Lindenberger, U.; Backman, L. Structural brain plasticity in adult learning and development. Neurosci. Biobehav. Rev. 2013, 37, 2296–2310. [Google Scholar] [CrossRef]
- Lockhart, S.N.; DeCarli, C. Structural imaging measures of brain aging. Neuropsychol. Rev. 2014, 24, 271–289. [Google Scholar] [CrossRef]
- Fan, Y.T.; Fang, Y.W.; Chen, Y.P.; Leshikar, E.D.; Lin, C.P.; Tzeng, O.J.L.; Huang, H.W.; Huang, C.M. Aging, cognition, and the brain: Effects of age-related variation in white matter integrity on neuropsychological function. Aging Ment. Health 2019, 23, 831–839. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Gaser, C. Magnetic resonance-based morphometry: A window into structural plasticity of the brain. Curr. Opin. Neurol. 2006, 19, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Villringer, A.; Ragert, P. Learning-related gray and white matter changes in humans: An update. Neuroscientist 2012, 18, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; May, A. Training-induced structural changes in the adult human brain. Behav. Brain Res. 2008, 192, 137–142. [Google Scholar] [CrossRef]
- Ashburner, J.; Csernansky, J.G.; Davatzikos, C.; Fox, N.C.; Frisoni, G.B.; Thompson, P.M. Computer-assisted imaging to assess brain structure in healthy and diseased brains. Lancet Neurol. 2003, 2, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Allemang-Grand, R.; Dazai, J.; Lerch, J.P. Environmental enrichment is associated with rapid volumetric brain changes in adult mice. Neuroimage 2015, 109, 190–198. [Google Scholar] [CrossRef]
- Potvin, O.; Mouiha, A.; Dieumegarde, L.; Duchesne, S.; Alzheimer’s Disease Neuroimaging, I. Normative data for subcortical regional volumes over the lifetime of the adult human brain. Neuroimage 2016, 137, 9–20. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–221. [Google Scholar] [CrossRef]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef]
- Raz, N.; Gunning-Dixon, F.; Head, D.; Rodrigue, K.M.; Williamson, A.; Acker, J.D. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences in volume. Neurobiol. Aging 2004, 25, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Hedman, A.M.; van Haren, N.E.; Schnack, H.G.; Kahn, R.S.; Hulshoff Pol, H.E. Human brain changes across the life span: A review of 56 longitudinal magnetic resonance imaging studies. Hum. Brain Mapp. 2012, 33, 1987–2002. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R.M.; Klein, M.; Grasby, K.L.; Schnack, H.G.; Jahanshad, N.; Teeuw, J.; Thomopoulos, S.I.; Sprooten, E.; Franz, C.E.; Gogtay, N.; et al. Genetic variants associated with longitudinal changes in brain structure across the lifespan. Nat. Neurosci. 2022, 25, 421–432. [Google Scholar] [CrossRef]
- Bethlehem, R.A.I.; Seidlitz, J.; White, S.R.; Vogel, J.W.; Anderson, K.M.; Adamson, C.; Adler, S.; Alexopoulos, G.S.; Anagnostou, E.; Areces-Gonzalez, A.; et al. Brain charts for the human lifespan. Nature 2022, 604, 525–533. [Google Scholar] [CrossRef]
- Barre-Sinoussi, F.; Montagutelli, X. Animal models are essential to biological research: Issues and perspectives. Future Sci. OA 2015, 1, FSO63. [Google Scholar] [CrossRef] [PubMed]
- Colon, E.; Bittner, E.A.; Kussman, B.; McCann, M.E.; Soriano, S.; Borsook, D. Anesthesia, brain changes, and behavior: Insights from neural systems biology. Prog. Neurobiol. 2017, 153, 121–160. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.H.; Schmidt, S.; Kretz, A.; Haenold, R.; Krumbein, I.; Metzler, M.; Gaser, C.; Witte, O.W.; Reichenbach, J.R. Possibilities and limitations for high resolution small animal MRI on a clinical whole-body 3T scanner. MAGMA 2012, 25, 233–244. [Google Scholar] [CrossRef]
- Gaser, C.; Schmidt, S.; Metzler, M.; Herrmann, K.H.; Krumbein, I.; Reichenbach, J.R.; Witte, O.W. Deformation-based brain morphometry in rats. Neuroimage 2012, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Andersson, J.L.; Friston, K.J. High-dimensional image registration using symmetric priors. Neuroimage 1999, 9, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Academic Press: Sydney, Australia, 2005. [Google Scholar]
- Peelle, J.E.; Cusack, R.; Henson, R.N. Adjusting for global effects in voxel-based morphometry: Gray matter decline in normal aging. Neuroimage 2012, 60, 1503–1516. [Google Scholar] [CrossRef]
- Hamezah, H.S.; Durani, L.W.; Ibrahim, N.F.; Yanagisawa, D.; Kato, T.; Shiino, A.; Tanaka, S.; Damanhuri, H.A.; Ngah, W.Z.W.; Tooyama, I. Volumetric changes in the aging rat brain and its impact on cognitive and locomotor functions. Exp. Gerontol. 2017, 99, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Adalsteinsson, E.; Sood, R.; Mayer, D.; Bell, R.; McBride, W.; Li, T.K.; Pfefferbaum, A. Longitudinal brain magnetic resonance imaging study of the alcohol-preferring rat. Part I: Adult brain growth. Alcohol. Clin. Exp. Res. 2006, 30, 1234–1247. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Muthusamy, S.; Wakim, P.G.; Sinharay, S.; Lentz, M.R.; Reid, W.C.; Hammoud, D.A. MR brain volumetric measurements are predictive of neurobehavioral impairment in the HIV-1 transgenic rat. Neuroimage Clin. 2018, 17, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, I.; Howard, S.R.; Stone, J.C.; Monfils, M.H.; Tomanek, B.; Brooks, W.M.; Sutherland, R.J. The aging hippocampus: A multi-level analysis in the rat. Neuroscience 2006, 139, 1173–1185. [Google Scholar] [CrossRef] [PubMed]
- von Kienlin, M.; Kunnecke, B.; Metzger, F.; Steiner, G.; Richards, J.G.; Ozmen, L.; Jacobsen, H.; Loetscher, H. Altered metabolic profile in the frontal cortex of PS2APP transgenic mice, monitored throughout their life span. Neurobiol. Dis. 2005, 18, 32–39. [Google Scholar] [CrossRef]
- Oberg, J.; Spenger, C.; Wang, F.H.; Andersson, A.; Westman, E.; Skoglund, P.; Sunnemark, D.; Norinder, U.; Klason, T.; Wahlund, L.O.; et al. Age related changes in brain metabolites observed by 1H MRS in APP/PS1 mice. Neurobiol. Aging 2008, 29, 1423–1433. [Google Scholar] [CrossRef]
- Reichel, J.M.; Bedenk, B.T.; Czisch, M.; Wotjak, C.T. Age-related cognitive decline coincides with accelerated volume loss of the dorsal but not ventral hippocampus in mice. Hippocampus 2017, 27, 28–35. [Google Scholar] [CrossRef]
- Maheswaran, S.; Barjat, H.; Rueckert, D.; Bate, S.T.; Howlett, D.R.; Tilling, L.; Smart, S.C.; Pohlmann, A.; Richardson, J.C.; Hartkens, T.; et al. Longitudinal regional brain volume changes quantified in normal aging and Alzheimer’s APP x PS1 mice using MRI. Brain Res. 2009, 1270, 19–32. [Google Scholar] [CrossRef]
- Fowler, C.; Goerzen, D.; Madularu, D.; Devenyi, G.A.; Chakravarty, M.M.; Near, J. Longitudinal characterization of neuroanatomical changes in the Fischer 344 rat brain during normal aging and between sexes. Neurobiol. Aging 2022, 109, 216–228. [Google Scholar] [CrossRef]
- Fotenos, A.F.; Snyder, A.Z.; Girton, L.E.; Morris, J.C.; Buckner, R.L. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 2005, 64, 1032–1039. [Google Scholar] [CrossRef]
- Grajauskas, L.A.; Siu, W.; Medvedev, G.; Guo, H.; D’Arcy, R.C.N.; Song, X. MRI-based evaluation of structural degeneration in the ageing brain: Pathophysiology and assessment. Ageing Res. Rev. 2019, 49, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Wrigglesworth, J.; Ward, P.; Harding, I.H.; Nilaweera, D.; Wu, Z.; Woods, R.L.; Ryan, J. Factors associated with brain ageing—A systematic review. BMC Neurol. 2021, 21, 312. [Google Scholar] [CrossRef]
- Mos, J.; Hollander, C.F. Analysis of survival data on aging rat cohorts: Pitfalls and some practical considerations. Mech. Ageing Dev. 1987, 38, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Deerberg, F. Age-associated versus husbandry-related pathology of aging rats. Neurobiol. Aging 1991, 12, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Taubert, M.; Roggenhofer, E.; Melie-Garcia, L.; Muller, S.; Lehmann, N.; Preisig, M.; Vollenweider, P.; Marques-Vidal, P.; Lutti, A.; Kherif, F.; et al. Converging patterns of aging-associated brain volume loss and tissue microstructure differences. Neurobiol. Aging 2020, 88, 108–118. [Google Scholar] [CrossRef]
- Kiyosawa, I. Age-related changes in visual function and visual organs of rats. Exp. Anim. 1996, 45, 103–114. [Google Scholar] [CrossRef]
- Keithley, E.M.; Feldman, M.L. Hair cell counts in an age-graded series of rat cochleas. Hear Res. 1982, 8, 249–262. [Google Scholar] [CrossRef]
- Shaffer, S.W.; Harrison, A.L. Aging of the somatosensory system: A translational perspective. Phys. Ther. 2007, 87, 193–207. [Google Scholar] [CrossRef]
- Reinke, H.; Dinse, H.R. Functional characterization of cutaneous mechanoreceptor properties in aged rats. Neurosci. Lett. 1996, 216, 171–174. [Google Scholar] [CrossRef]
- Lee, A.C.; Tian, H.; Grosmaitre, X.; Ma, M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem. Senses 2009, 34, 695–703. [Google Scholar] [CrossRef]
- Khan, M.; Vaes, E.; Mombaerts, P. Temporal patterns of odorant receptor gene expression in adult and aged mice. Mol. Cell Neurosci. 2013, 57, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Enwere, E.; Shingo, T.; Gregg, C.; Fujikawa, H.; Ohta, S.; Weiss, S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 8354–8365. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Hegg, C.C. Effect of IP3R3 and NPY on age-related declines in olfactory stem cell proliferation. Neurobiol. Aging 2015, 36, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Curcio, C.A.; McNelly, N.A.; Hinds, J.W. Aging in the rat olfactory system: Relative stability of piriform cortex contrasts with changes in olfactory bulb and olfactory epithelium. J. Comp. Neurol. 1985, 235, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Godde, B.; Berkefeld, T.; David-Jurgens, M.; Dinse, H.R. Age-related changes in primary somatosensory cortex of rats: Evidence for parallel degenerative and plastic-adaptive processes. Neurosci. Biobehav. Rev. 2002, 26, 743–752. [Google Scholar] [CrossRef]
- Spengler, F.; Godde, B.; Dinse, H.R. Effects of ageing on topographic organization of somatosensory cortex. Neuroreport 1995, 6, 469–473. [Google Scholar] [CrossRef]
- Suta, D.; Rybalko, N.; Pelanova, J.; Popelar, J.; Syka, J. Age-related changes in auditory temporal processing in the rat. Exp. Gerontol. 2011, 46, 739–746. [Google Scholar] [CrossRef]
- Chen, B.; Zhong, Y.; Peng, W.; Sun, Y.; Kong, W.J. Age-related changes in the central auditory system: Comparison of D-galactose-induced aging rats and naturally aging rats. Brain Res. 2010, 1344, 43–53. [Google Scholar] [CrossRef]
- Lehmann, K.; Schmidt, K.F.; Lowel, S. Vision and visual plasticity in ageing mice. Restor. Neurol. Neurosci. 2012, 30, 161–178. [Google Scholar] [CrossRef]
- Lehmann, K.; Lowel, S. Age-dependent ocular dominance plasticity in adult mice. PLoS ONE 2008, 3, e3120. [Google Scholar] [CrossRef]
- Mutlu, U.; Ikram, M.K.; Roshchupkin, G.V.; Bonnemaijer, P.W.M.; Colijn, J.M.; Vingerling, J.R.; Niessen, W.J.; Ikram, M.A.; Klaver, C.C.W.; Vernooij, M.W. Thinner retinal layers are associated with changes in the visual pathway: A population-based study. Hum. Brain Mapp. 2018, 39, 4290–4301. [Google Scholar] [CrossRef]
- Ong, Y.T.; Hilal, S.; Cheung, C.Y.; Venketasubramanian, N.; Niessen, W.J.; Vrooman, H.; Anuar, A.R.; Chew, M.; Chen, C.; Wong, T.Y.; et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci. Lett. 2015, 584, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Cute, S.L.; Vaden, K.I., Jr.; Kuchinsky, S.E.; Dubno, J.R. Auditory cortex signs of age-related hearing loss. J. Assoc. Res. Otolaryngol. 2012, 13, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.T.; Medina, R.E.; Davis, C.W.; Szymko-Bennett, Y.; Simonyan, K.; Pajor, N.M.; Horwitz, B. Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res. 2011, 1369, 74–88. [Google Scholar] [CrossRef]
- Peelle, J.E.; Troiani, V.; Grossman, M.; Wingfield, A. Hearing loss in older adults affects neural systems supporting speech comprehension. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 12638–12643. [Google Scholar] [CrossRef] [PubMed]
- de Boer, T.G.; Rigters, S.C.; Croll, P.H.; Niessen, W.J.; Ikram, M.A.; van der Schroeff, M.P.; Vernooij, M.W.; Goedegebure, A. The Effect of Hearing Aid Use on the Association Between Hearing Loss and Brain Structure in Older Adults. Ear Hear. 2022, 43, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Lindenberger, U.; Baltes, P.B. Sensory functioning and intelligence in old age: A strong connection. Psychol. Aging 1994, 9, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Monge, Z.A.; Madden, D.J. Linking cognitive and visual perceptual decline in healthy aging: The information degradation hypothesis. Neurosci. Biobehav. Rev. 2016, 69, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Wayne, R.V.; Johnsrude, I.S. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res. Rev. 2015, 23, 154–166. [Google Scholar] [CrossRef]
- Rong, H.; Lai, X.; Jing, R.; Wang, X.; Fang, H.; Mahmoudi, E. Association of Sensory Impairments With Cognitive Decline and Depression Among Older Adults in China. JAMA Netw. Open 2020, 3, e2014186. [Google Scholar] [CrossRef]
- Sydnor, V.J.; Larsen, B.; Bassett, D.S.; Alexander-Bloch, A.; Fair, D.A.; Liston, C.; Mackey, A.P.; Milham, M.P.; Pines, A.; Roalf, D.R.; et al. Neurodevelopment of the association cortices: Patterns, mechanisms, and implications for psychopathology. Neuron 2021, 109, 2820–2846. [Google Scholar] [CrossRef] [PubMed]
- Henschke, J.U.; Ohl, F.W.; Budinger, E. Crossmodal Connections of Primary Sensory Cortices Largely Vanish During Normal Aging. Front. Aging Neurosci. 2018, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Braga, R.; Sharp, D.J. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Ash, J.A.; Lu, H.; Taxier, L.R.; Long, J.M.; Yang, Y.; Stein, E.A.; Rapp, P.R. Functional connectivity with the retrosplenial cortex predicts cognitive aging in rats. Proc. Natl. Acad. Sci. USA 2016, 113, 12286–12291. [Google Scholar] [CrossRef]
- Liska, A.; Galbusera, A.; Schwarz, A.J.; Gozzi, A. Functional connectivity hubs of the mouse brain. Neuroimage 2015, 115, 281–291. [Google Scholar] [CrossRef]
- Gozzi, A.; Schwarz, A.J. Large-scale functional connectivity networks in the rodent brain. Neuroimage 2016, 127, 496–509. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Storsve, A.B.; Fjell, A.M.; Tamnes, C.K.; Westlye, L.T.; Overbye, K.; Aasland, H.W.; Walhovd, K.B. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: Regions of accelerating and decelerating change. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 8488–8498. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B.; Reinvang, I.; Lundervold, A.; Salat, D.; Quinn, B.T.; Fischl, B.; Dale, A.M. Selective increase of cortical thickness in high-performing elderly--structural indices of optimal cognitive aging. Neuroimage 2006, 29, 984–994. [Google Scholar] [CrossRef]
- Gaser, C.; Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 9240–9245. [Google Scholar] [CrossRef]
- Mechelli, A.; Crinion, J.T.; Noppeney, U.; O’Doherty, J.; Ashburner, J.; Frackowiak, R.S.; Price, C.J. Neurolinguistics: Structural plasticity in the bilingual brain. Nature 2004, 431, 757. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A.; Woollett, K.; Spiers, H.J. London taxi drivers and bus drivers: A structural MRI and neuropsychological analysis. Hippocampus 2006, 16, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Aydin, K.; Ucar, A.; Oguz, K.K.; Okur, O.O.; Agayev, A.; Unal, Z.; Yilmaz, S.; Ozturk, C. Increased gray matter density in the parietal cortex of mathematicians: A voxel-based morphometry study. AJNR Am. J. Neuroradiol. 2007, 28, 1859–1864. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Toga, A.W.; Lepore, N.; Gaser, C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage 2009, 45, 672–678. [Google Scholar] [CrossRef]
- Schmidt, S.; Gull, S.; Herrmann, K.H.; Boehme, M.; Irintchev, A.; Urbach, A.; Reichenbach, J.R.; Klingner, C.M.; Gaser, C.; Witte, O.W. Experience-dependent structural plasticity in the adult brain: How the learning brain grows. Neuroimage 2021, 225, 117502. [Google Scholar] [CrossRef]
- May, A. Experience-dependent structural plasticity in the adult human brain. Trends Cogn. Sci. 2011, 15, 475–482. [Google Scholar] [CrossRef]
- Kelly, A.M.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Lissek, S.; Wilimzig, C.; Stude, P.; Pleger, B.; Kalisch, T.; Maier, C.; Peters, S.A.; Nicolas, V.; Tegenthoff, M.; Dinse, H.R. Immobilization impairs tactile perception and shrinks somatosensory cortical maps. Curr. Biol. 2009, 19, 837–842. [Google Scholar] [CrossRef]
- Gilland, K.E.; Fox, E.A. Effect of food deprivation or short-term Western diet feeding on BDNF protein expression in the hypothalamic arcuate, paraventricular, and ventromedial nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R611–R625. [Google Scholar] [CrossRef]
- Hurst, P.; Garfield, A.S.; Marrow, C.; Heisler, L.K.; Evans, M.L. Recurrent hypoglycemia is associated with loss of activation in rat brain cingulate cortex. Endocrinology 2012, 153, 1908–1914. [Google Scholar] [CrossRef]
- Takahashi, M.; Shinoda, Y. Neural Circuits of Inputs and Outputs of the Cerebellar Cortex and Nuclei. Neuroscience 2021, 462, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H.; Pettorossi, V.E. Adaptive Balance in Posterior Cerebellum. Front. Neurol. 2021, 12, 635259. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Q.; Seemungal, B.M. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front. Neurol. 2016, 7, 231. [Google Scholar] [PubMed]
- Nickl-Jockschat, T.; Kleiman, A.; Schulz, J.B.; Schneider, F.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B.; Reetz, K. Neuroanatomic changes and their association with cognitive decline in mild cognitive impairment: A meta-analysis. Brain Struct. Funct. 2012, 217, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Lan, K.; Huang, X.; He, Y.; Yang, F.; Li, J.; Hu, Q.; Xu, J.; Yu, H. Gray Matter Atrophy in Amnestic Mild Cognitive Impairment: A Voxel-Based Meta-Analysis. Front. Aging Neurosci. 2021, 13, 627919. [Google Scholar] [CrossRef]
- Pessoa, L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?”. Neuropsychologia 2010, 48, 3416–3429. [Google Scholar] [CrossRef]
- Milczarek, M.M.; Vann, S.D.; Sengpiel, F. Spatial Memory Engram in the Mouse Retrosplenial Cortex. Curr. Biol. 2018, 28, 1975–1980.e1976. [Google Scholar] [CrossRef]
- Miller, A.M.P.; Mau, W.; Smith, D.M. Retrosplenial Cortical Representations of Space and Future Goal Locations Develop with Learning. Curr. Biol. 2019, 29, 2083–2090.e2084. [Google Scholar] [CrossRef]
- Czajkowski, R.; Jayaprakash, B.; Wiltgen, B.; Rogerson, T.; Guzman-Karlsson, M.C.; Barth, A.L.; Trachtenberg, J.T.; Silva, A.J. Encoding and storage of spatial information in the retrosplenial cortex. Proc. Natl. Acad. Sci. USA 2014, 111, 8661–8666. [Google Scholar] [CrossRef]
- Cowansage, K.K.; Shuman, T.; Dillingham, B.C.; Chang, A.; Golshani, P.; Mayford, M. Direct reactivation of a coherent neocortical memory of context. Neuron 2014, 84, 432–441. [Google Scholar] [CrossRef]
- Hafting, T.; Fyhn, M.; Molden, S.; Moser, M.B.; Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 2005, 436, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Tsao, A.; Sugar, J.; Lu, L.; Wang, C.; Knierim, J.J.; Moser, M.B.; Moser, E.I. Integrating time from experience in the lateral entorhinal cortex. Nature 2018, 561, 57–62. [Google Scholar] [CrossRef]
- Garcia, A.D.; Buffalo, E.A. Anatomy and Function of the Primate Entorhinal Cortex. Annu. Rev. Vis. Sci. 2020, 6, 411–432. [Google Scholar] [CrossRef]
- Maguire, E.A.; Valentine, E.R.; Wilding, J.M.; Kapur, N. Routes to remembering: The brains behind superior memory. Nat. Neurosci. 2003, 6, 90–95. [Google Scholar] [CrossRef]
- Vann, S.D.; Aggleton, J.P.; Maguire, E.A. What does the retrosplenial cortex do? Nat. Rev. Neurosci. 2009, 10, 792–802. [Google Scholar] [CrossRef]
- Fournier, D.I.; Cheng, H.Y.; Robinson, S.; Todd, T.P. Cortical Contributions to Higher-Order Conditioning: A Review of Retrosplenial Cortex Function. Front. Behav. Neurosci. 2021, 15, 682426. [Google Scholar] [CrossRef]
- Roy, D.S.; Park, Y.G.; Kim, M.E.; Zhang, Y.; Ogawa, S.K.; DiNapoli, N.; Gu, X.; Cho, J.H.; Choi, H.; Kamentsky, L.; et al. Brain-wide mapping reveals that engrams for a single memory are distributed across multiple brain regions. Nat. Commun. 2022, 13, 1799. [Google Scholar] [CrossRef]
- Murray, M.M.; Lewkowicz, D.J.; Amedi, A.; Wallace, M.T. Multisensory Processes: A Balancing Act across the Lifespan. Trends Neurosci. 2016, 39, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Vetere, G.; Restivo, L.; Cole, C.J.; Ross, P.J.; Ammassari-Teule, M.; Josselyn, S.A.; Frankland, P.W. Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. USA 2011, 108, 8456–8460. [Google Scholar] [CrossRef]
- Kitamura, T.; Ogawa, S.K.; Roy, D.S.; Okuyama, T.; Morrissey, M.D.; Smith, L.M.; Redondo, R.L.; Tonegawa, S. Engrams and circuits crucial for systems consolidation of a memory. Science 2017, 356, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Abdou, K.; Shehata, M.; Choko, K.; Nishizono, H.; Matsuo, M.; Muramatsu, S.I.; Inokuchi, K. Synapse-specific representation of the identity of overlapping memory engrams. Science 2018, 360, 1227–1231. [Google Scholar] [CrossRef]
- Langille, J.J.; Gallistel, C.R. Locating the engram: Should we look for plastic synapses or information-storing molecules? Neurobiol. Learn Mem. 2020, 169, 107164. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Guo, J.; Sigmon, H.C.; Sloan, R.P.; Brickman, A.M.; Provenzano, F.A.; Small, S.A.; Alzheimer’s Disease Neuroimaging, I. Brain regions vulnerable and resistant to aging without Alzheimer’s disease. PLoS ONE 2020, 15, e0234255. [Google Scholar] [CrossRef] [PubMed]
- Chetelat, G.; Fouquet, M.; Kalpouzos, G.; Denghien, I.; De la Sayette, V.; Viader, F.; Mezenge, F.; Landeau, B.; Baron, J.C.; Eustache, F.; et al. Three-dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel-based morphometry. Neuropsychologia 2008, 46, 1721–1731. [Google Scholar] [CrossRef] [PubMed]
- Frisoni, G.B.; Ganzola, R.; Canu, E.; Rüb, U.; Pizzini, F.B.; Alessandrini, F.; Zoccatelli, G.; Beltramello, A.; Caltagirone, C.; Thompson, P.M. Mapping local hippocampal changes in Alzheimer’s disease and normal ageing with MRI at 3 Tesla. Brain 2008, 131, 3266–3276. [Google Scholar] [CrossRef]
- La Joie, R.; Fouquet, M.; Mezenge, F.; Landeau, B.; Villain, N.; Mevel, K.; Pelerin, A.; Eustache, F.; Desgranges, B.; Chetelat, G. Differential effect of age on hippocampal subfields assessed using a new high-resolution 3T MR sequence. Neuroimage 2010, 53, 506–514. [Google Scholar] [CrossRef]
- Wolf, D.; Fischer, F.U.; de Flores, R.; Chetelat, G.; Fellgiebel, A. Differential associations of age with volume and microstructure of hippocampal subfields in healthy older adults. Hum. Brain Mapp. 2015, 36, 3819–3831. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kitanishi, T.; Mizuseki, K. The subiculum: Unique hippocampal hub and more. Neurosci. Res. 2019, 143, 1–12. [Google Scholar] [CrossRef]
- Mizuseki, K.; Kitanishi, T. Oscillation-coordinated, noise-resistant information distribution via the subiculum. Curr. Opin. Neurobiol. 2022, 75, 102556. [Google Scholar] [CrossRef]
- Eldridge, L.L.; Engel, S.A.; Zeineh, M.M.; Bookheimer, S.Y.; Knowlton, B.J. A dissociation of encoding and retrieval processes in the human hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 3280–3286. [Google Scholar] [CrossRef]
- Gabrieli, J.D.; Brewer, J.B.; Desmond, J.E.; Glover, G.H. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science 1997, 276, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.W.; Cheong, C. Functional dissociation of hippocampal subregions corresponding to memory types and stages. J. Physiol. Anthr. 2020, 39, 15. [Google Scholar] [CrossRef] [PubMed]
- Zeineh, M.M.; Engel, S.A.; Thompson, P.M.; Bookheimer, S.Y. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science 2003, 299, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.S.; Kitamura, T.; Okuyama, T.; Ogawa, S.K.; Sun, C.; Obata, Y.; Yoshiki, A.; Tonegawa, S. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell 2017, 170, 1000–1012.e1019. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef]
- Burke, D.M.; Light, L.L. Memory and aging: The role of retrieval processes. Psychol. Bull. 1981, 90, 513–514. [Google Scholar] [CrossRef]
- Bowles, N.L.; Poon, L.W. Aging and retrieval of words in semantic memory. J. Gerontol. 1985, 40, 71–77. [Google Scholar] [CrossRef]
- St Jacques, P.L.; Rubin, D.C.; Cabeza, R. Age-related effects on the neural correlates of autobiographical memory retrieval. Neurobiol. Aging 2012, 33, 1298–1310. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gull, S.; Gaser, C.; Herrmann, K.-H.; Urbach, A.; Boehme, M.; Afzal, S.; Reichenbach, J.R.; Witte, O.W.; Schmidt, S. Brain Macro-Structural Alterations in Aging Rats: A Longitudinal Lifetime Approach. Cells 2023, 12, 432. https://doi.org/10.3390/cells12030432
Gull S, Gaser C, Herrmann K-H, Urbach A, Boehme M, Afzal S, Reichenbach JR, Witte OW, Schmidt S. Brain Macro-Structural Alterations in Aging Rats: A Longitudinal Lifetime Approach. Cells. 2023; 12(3):432. https://doi.org/10.3390/cells12030432
Chicago/Turabian StyleGull, Sidra, Christian Gaser, Karl-Heinz Herrmann, Anja Urbach, Marcus Boehme, Samia Afzal, Jürgen R. Reichenbach, Otto W. Witte, and Silvio Schmidt. 2023. "Brain Macro-Structural Alterations in Aging Rats: A Longitudinal Lifetime Approach" Cells 12, no. 3: 432. https://doi.org/10.3390/cells12030432
APA StyleGull, S., Gaser, C., Herrmann, K.-H., Urbach, A., Boehme, M., Afzal, S., Reichenbach, J. R., Witte, O. W., & Schmidt, S. (2023). Brain Macro-Structural Alterations in Aging Rats: A Longitudinal Lifetime Approach. Cells, 12(3), 432. https://doi.org/10.3390/cells12030432





