Infrapatellar Fat Pad Modulates Osteoarthritis-Associated Cytokine and MMP Expression in Human Articular Chondrocytes
Abstract
1. Introduction
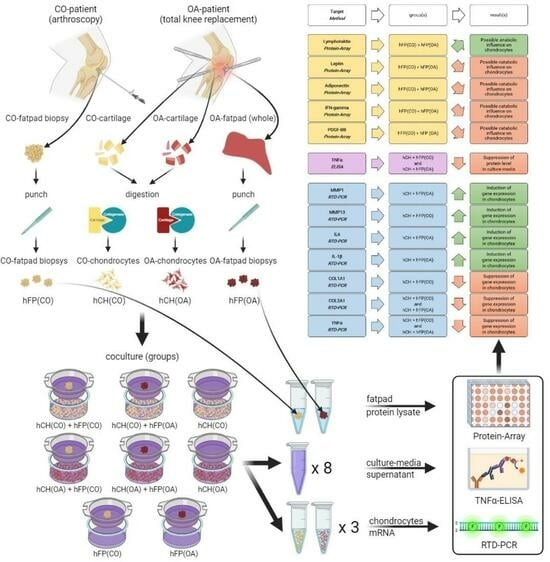
2. Materials and Methods
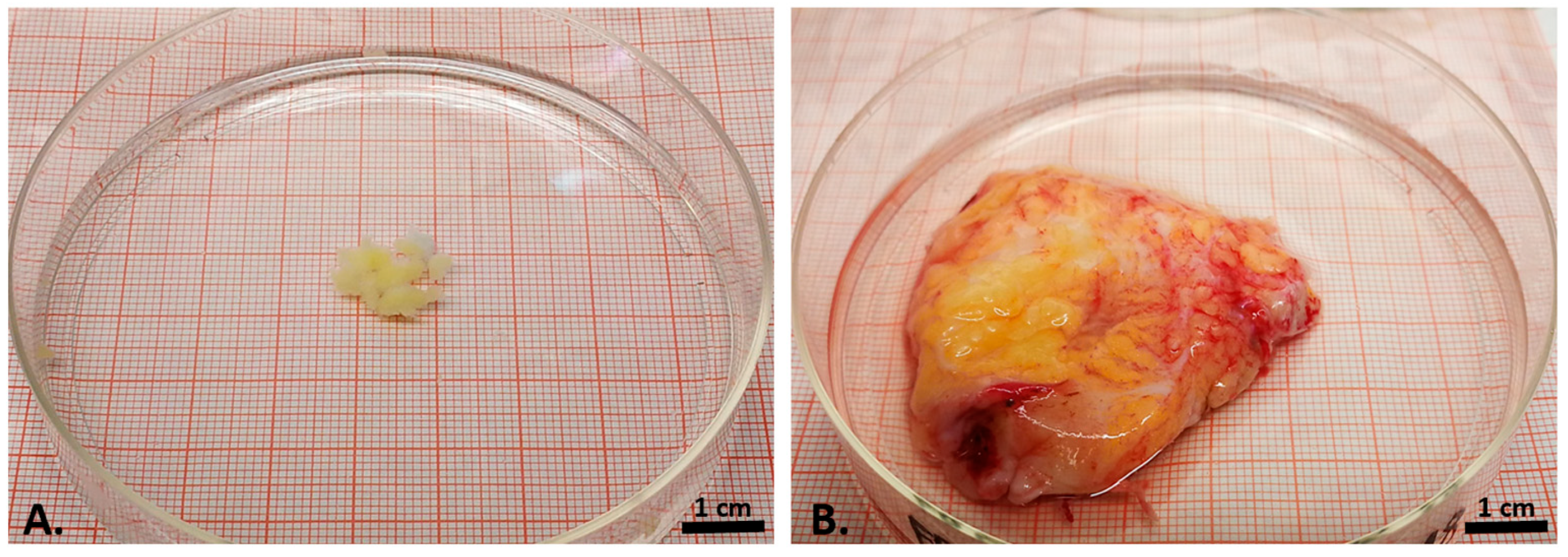
2.1. Sample Acquisition
2.2. Isolation of Human Articular Chondrocytes
2.3. Co-Culture Experiments
2.4. RNA Extraction and RTD-PCR (Real-Time Detection Polymerase Chain Reaction)
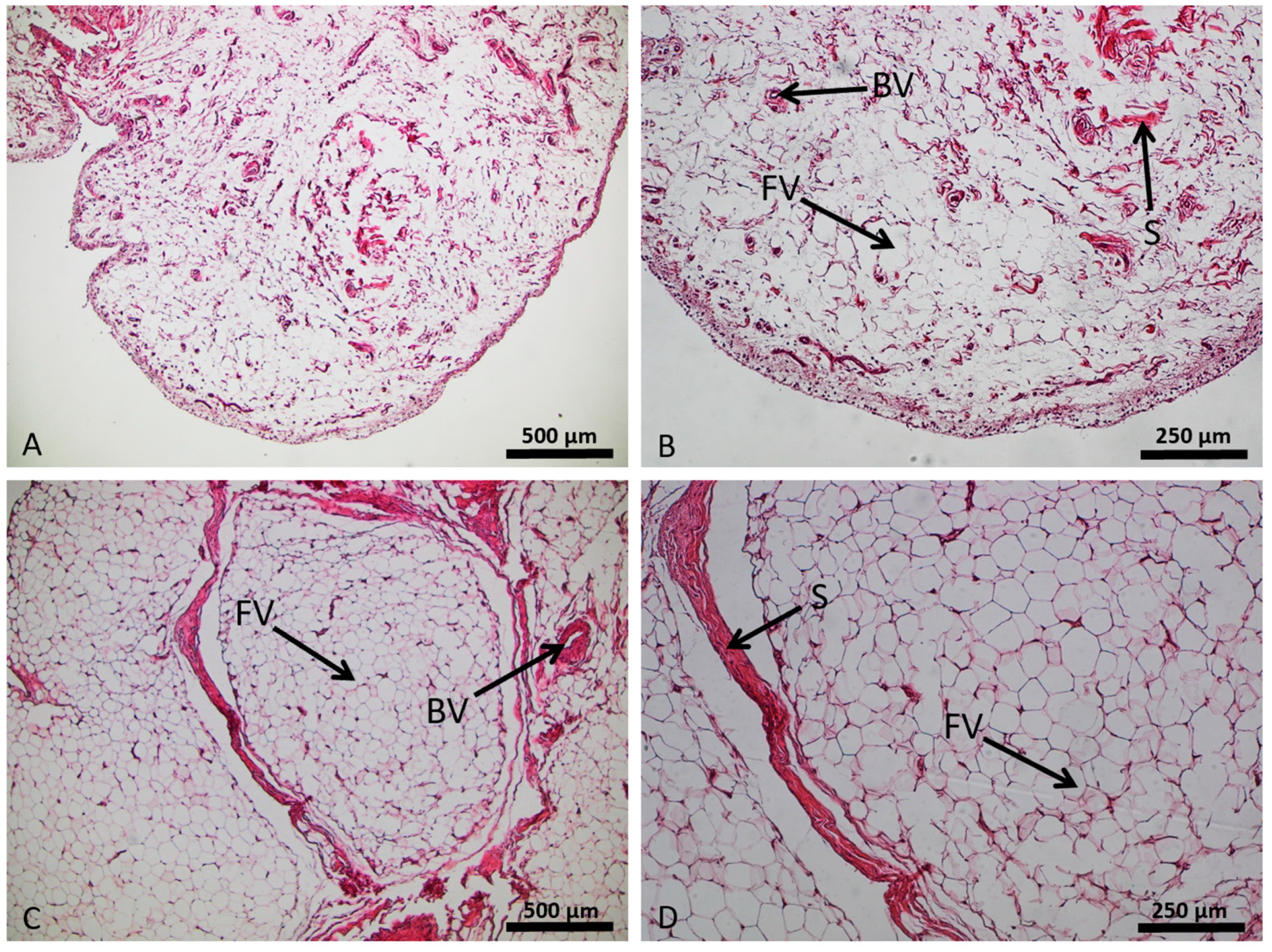
2.5. Histological Analysis by Hematoxylin and Eosin Staining (HE)
2.6. ELISA
2.7. Protein Array
2.8. Statistical Analysis
3. Results
3.1. Histological Characteristics of the Control and OA Infrapatellar Fat Pads
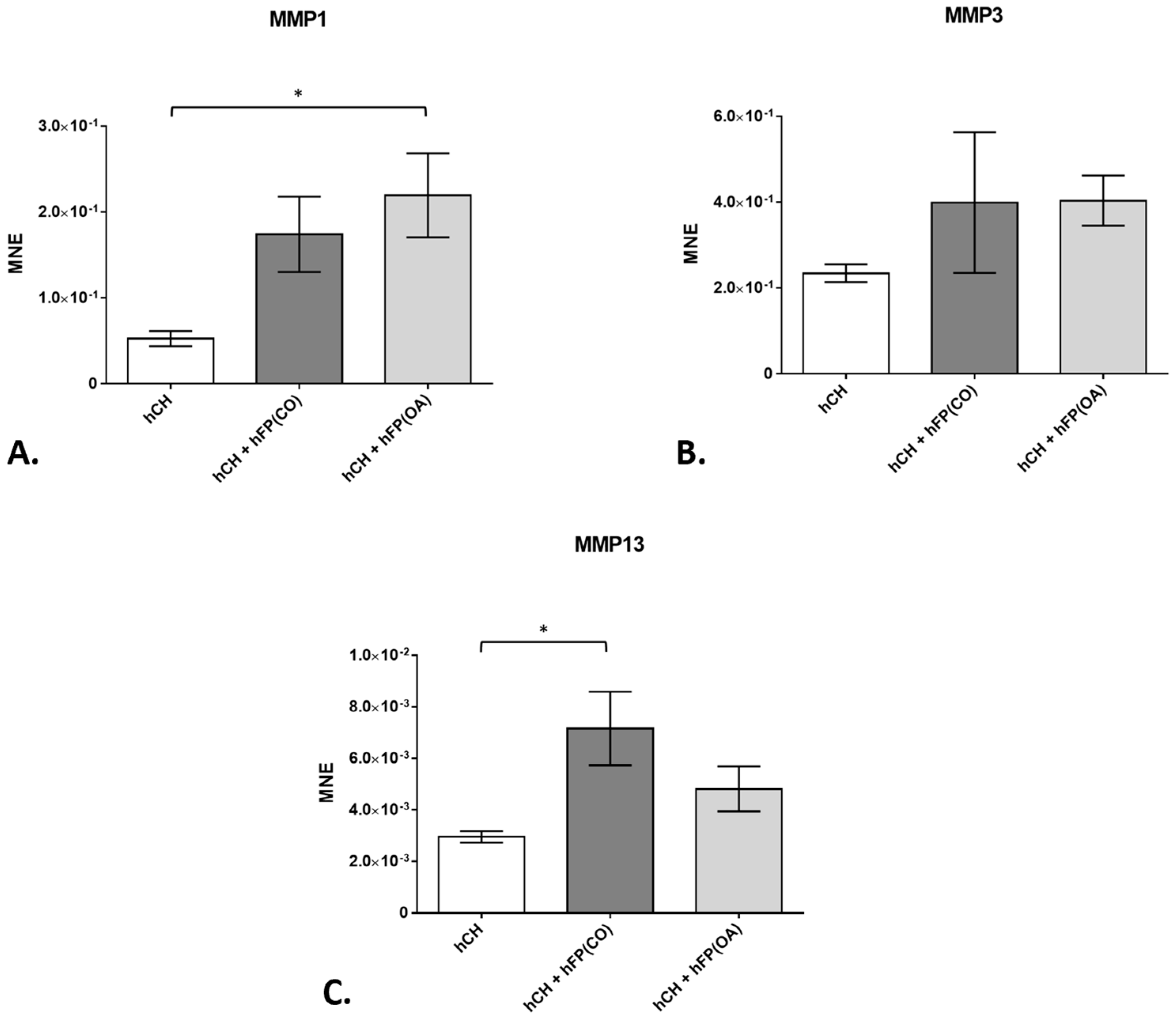
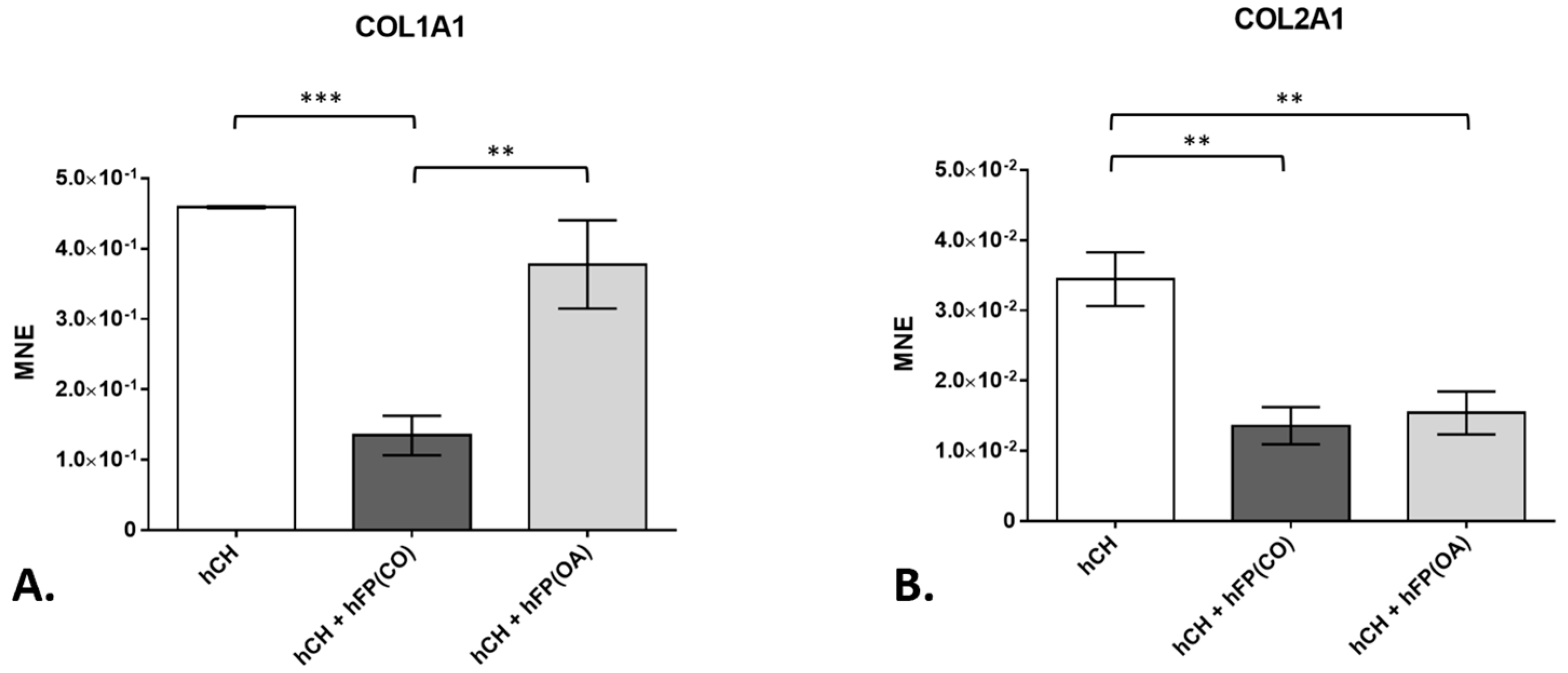
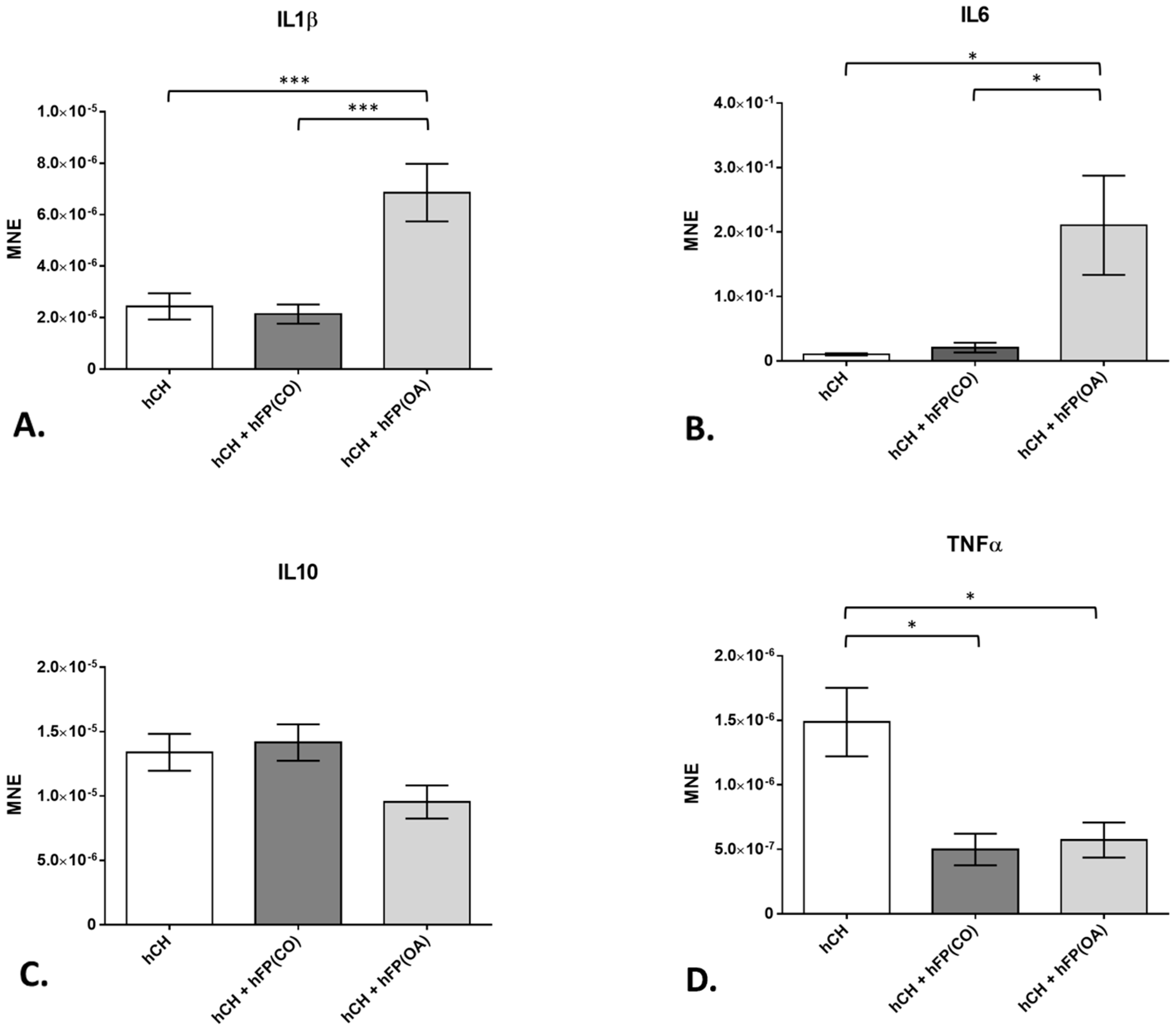
3.2. Chondrocyte Gene Expression Profiles by Real-Time Detection Polymerase Chain Reaction (RTD-PCR)
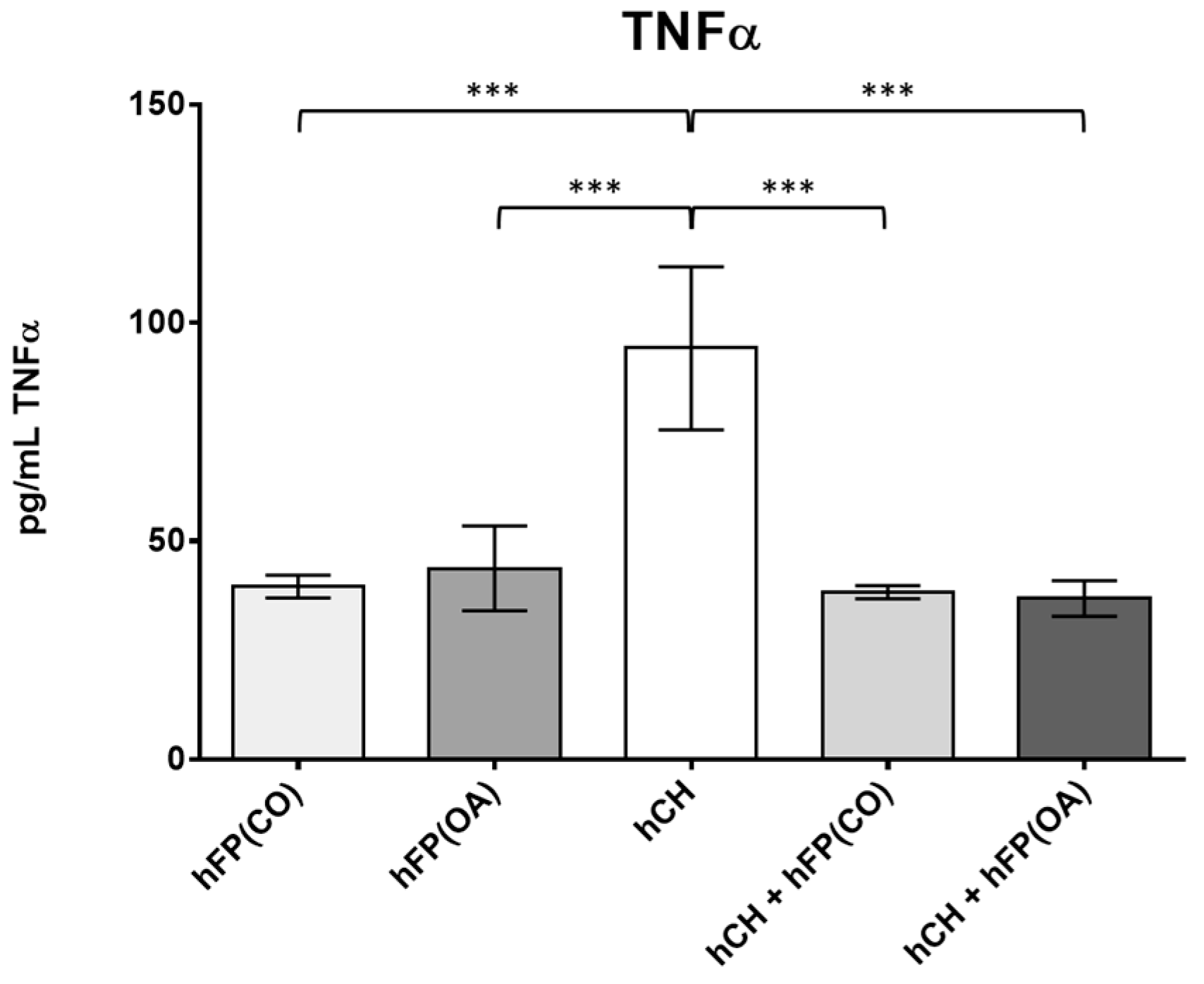
3.3. TNFα Secretion
3.4. Protein Expression in IPFP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CO | Control samples from healthy patients |
| COL1A1 | Collagen type I: alpha I |
| COL2A1 | Collagen type II, alpha I |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DMSO | Dimethylsulfoxid |
| cDNA | Complementary deoxyribonucleic acid |
| ECM | Extracellular matrix |
| FCS | Fetal calf serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IL6 | Interleukin 6 |
| IPFP | Infrapatellar fat pad |
| MMP1 | Matrix metalloproteinase 1 |
| MNE | Mean normalized expression value |
| MSC | Mesenchymal stem cells |
| NCS | Normalized chemiluminescence signal |
| NM | Accession number for the reference sequences |
| OA | Osteoarthritis |
| PCR | Polymerase chain reaction |
| PFA | Paraformaldehyde |
| RNA | Ribonucleic acid |
| RTD | Real-time detection |
| SEM | Standard error of the mean |
| TNFα | Tumor necrosis factor α |
References
- Leardini, G.; Vaccaro, E. Osteoarthritis: Socioeconomic problems. Semin. Arthritis Rheum. 2005, 34 (Suppl. S2), 35–37. [Google Scholar] [CrossRef]
- Arden, N.; Nevitt, M.C. Osteoarthritis: Epidemiology. Best. Pr. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.W.; Schluter-Brust, K.U.; Eysel, P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef]
- Sarmanova, A.; Fernandes, G.S.; Richardson, H.; Valdes, A.M.; Walsh, D.A.; Zhang, W.; Doherty, M. Contribution of central and peripheral risk factors to prevalence, incidence and progression of knee pain: A community-based cohort study. Osteoarthr. Cartil. 2018, 26, 1461–1473. [Google Scholar] [CrossRef]
- van der Weegen, W.; Wullems, J.A.; Bos, E.; Noten, H.; van Drumpt, R.A. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: A randomized, controlled, double-blind trial. J. Arthroplast. 2015, 30, 754–757. [Google Scholar] [CrossRef]
- Haenle, M.; Skripitz, C.; Mittelmeier, W.; Skripitz, R. Economic impact of infected total hip arthroplasty in the German diagnosis-related groups system. Orthopade 2012, 41, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Schroer, W.C.; Berend, K.R.; Lombardi, A.V.; Barnes, C.L.; Bolognesi, M.P.; Berend, M.E.; Ritter, M.A.; Nunley, R.M. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J. Arthroplast. 2013, 28, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Silawal, S.; Triebel, J.; Bertsch, T.; Schulze-Tanzil, G. Osteoarthritis and the Complement Cascade. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751430. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Pengas, I.; Eldridge, S.; Assiotis, A.; McNicholas, M.; Mendes, J.E.; Laver, L. MMP-3 in the peripheral serum as a biomarker of knee osteoarthritis, 40 years after open total knee meniscectomy. J. Exp. Orthop. 2018, 5, 21. [Google Scholar] [CrossRef]
- Hoffa, A. The influence of the adipose tissue with regard to the pathology of the knee joint. J. Am. Med. Assoc. 1904, XLIII, 795–796. [Google Scholar] [CrossRef]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Runhaar, J.; Van Osch, G.J.; Van Offel, J.F.; Verhaar, J.A.; De Clerck, L.S.; Somville, J. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: A narrative review. Osteoarthr. Cartil. 2010, 18, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Oba, H.; Miura, R.; Morimoto, M.; Gamada, K. Movement and volume of infrapatellar fat pad and knee kinematics during quasi-static knee extension at 30 and 0 degrees flexion in young healthy individuals. Knee 2020, 27, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Jagielski, M.; Wolf, J.; Marzahn, U.; Volker, A.; Lemke, M.; Meier, C.; Ertel, W.; Godkin, O.; Arens, S.; Schulze-Tanzil, G. The influence of IL-10 and TNFalpha on chondrogenesis of human mesenchymal stromal cells in three-dimensional cultures. Int. J. Mol. Sci. 2014, 15, 15821–15844. [Google Scholar] [CrossRef] [PubMed]
- Distel, E.; Cadoudal, T.; Durant, S.; Poignard, A.; Chevalier, X.; Benelli, C. The infrapatellar fat pad in knee osteoarthritis: An important source of interleukin-6 and its soluble receptor. Arthritis Rheum. 2009, 60, 3374–3377. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Vuolteenaho, K.; Koskinen, A.; Kukkonen, M.; Nieminen, R.; Paivarinta, U.; Moilanen, T.; Moilanen, E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage--mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediat. Inflamm. 2009, 2009, 345838. [Google Scholar] [CrossRef]
- Koskinen, A.; Juslin, S.; Nieminen, R.; Moilanen, T.; Vuolteenaho, K.; Moilanen, E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res. Ther. 2011, 13, R184. [Google Scholar] [CrossRef]
- Bohnsack, M.; Meier, F.; Walter, G.F.; Hurschler, C.; Schmolke, S.; Wirth, C.J.; Ruhmann, O. Distribution of substance-P nerves inside the infrapatellar fat pad and the adjacent synovial tissue: A neurohistological approach to anterior knee pain syndrome. Arch. Orthop. Trauma. Surg. 2005, 125, 592–597. [Google Scholar] [CrossRef]
- Emmi, A.; Stocco, E.; Boscolo-Berto, R.; Contran, M.; Belluzzi, E.; Favero, M.; Ramonda, R.; Porzionato, A.; Ruggieri, P.; De Caro, R.; et al. Infrapatellar Fat Pad-Synovial Membrane Anatomo-Fuctional Unit: Microscopic Basis for Piezo1/2 Mechanosensors Involvement in Osteoarthritis Pain. Front. Cell Dev. Biol. 2022, 10, 886604. [Google Scholar] [CrossRef] [PubMed]
- Dowsey, M.M.; Nikpour, M.; Dieppe, P.; Choong, P.F. Associations between pre-operative radiographic changes and outcomes after total knee joint replacement for osteoarthritis. Osteoarthr. Cartil. 2012, 20, 1095–1102. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Sriram, M.; Kothari, A.; Katti, D.S. Co-culture of infrapatellar fat pad-derived mesenchymal stromal cells and articular chondrocytes in plasma clot for cartilage tissue engineering. Cytotherapy 2017, 19, 881–894. [Google Scholar] [CrossRef]
- Lopez-Ruiz, E.; Peran, M.; Cobo-Molinos, J.; Jimenez, G.; Picon, M.; Bustamante, M.; Arrebola, F.; Hernandez-Lamas, M.C.; Delgado-Martinez, A.D.; Montanez, E.; et al. Chondrocytes extract from patients with osteoarthritis induces chondrogenesis in infrapatellar fat pad-derived stem cells. Osteoarthr. Cartil. 2013, 21, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; Kloppenburg, M. An emerging player in knee osteoarthritis: The infrapatellar fat pad. Arthritis Res. Ther. 2013, 15, 225. [Google Scholar] [CrossRef]
- Hui, W.; Litherland, G.J.; Elias, M.S.; Kitson, G.I.; Cawston, T.E.; Rowan, A.D.; Young, D.A. Leptin produced by joint white adipose tissue induces cartilage degradation via upregulation and activation of matrix metalloproteinases. Ann. Rheum. Dis. 2012, 71, 455–462. [Google Scholar] [CrossRef]
- Bastiaansen-Jenniskens, Y.M.; Clockaerts, S.; Feijt, C.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Bridts, C.; de Clerck, L.; De Groot, J.; Verhaar, J.A.; Kloppenburg, M.; et al. Infrapatellar fat pad of patients with end-stage osteoarthritis inhibits catabolic mediators in cartilage. Ann. Rheum. Dis. 2012, 71, 288–294. [Google Scholar] [CrossRef]
- Housmans, B.A.C.; Neefjes, M.; Surtel, D.A.M.; Vitik, M.; Cremers, A.; van Rhijn, L.W.; van der Kraan, P.M.; van den Akker, G.G.H.; Welting, T.J.M. Synovial fluid from end-stage osteoarthritis induces proliferation and fibrosis of articular chondrocytes via MAPK and RhoGTPase signaling. Osteoarthr. Cartil. 2022, 30, 862–874. [Google Scholar] [CrossRef]
- Clockaerts, S.; Bastiaansen-Jenniskens, Y.M.; Feijt, C.; De Clerck, L.; Verhaar, J.A.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Somville, J.; Kloppenburg, M.; van Osch, G.J. Cytokine production by infrapatellar fat pad can be stimulated by interleukin 1beta and inhibited by peroxisome proliferator activated receptor alpha agonist. Ann. Rheum. Dis. 2012, 71, 1012–1018. [Google Scholar] [CrossRef]
- Labusca, L.; Zugun-Eloae, F. The Unexplored Role of Intra-articular Adipose Tissue in the Homeostasis and Pathology of Articular Joints. Front. Vet. Sci. 2018, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Toussirot, E.; Streit, G.; Wendling, D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr. Med. Chem. 2007, 14, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.M.; Du, S.H.; Huang, L.G.; Li, J.H.; Xiao, L.; Tong, P. Leptin promotes apoptosis and inhibits autophagy of chondrocytes through upregulating lysyl oxidase-like 3 during osteoarthritis pathogenesis. Osteoarthr. Cartil. 2016, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J.; Blain, E.J.; Mason, D.J. Interferon-gamma modulates articular chondrocyte and osteoblast metabolism through protein kinase R-independent and dependent mechanisms. Biochem. Biophys. Rep. 2022, 32, 101323. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight 2020, 5, e135446. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Chiu, Y.C.; Tan, T.W.; Yang, R.S.; Fu, W.M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J. Immunol. 2007, 179, 5483–5492. [Google Scholar] [CrossRef] [PubMed]
- Lago, R.; Gomez, R.; Otero, M.; Lago, F.; Gallego, R.; Dieguez, C.; Gomez-Reino, J.J.; Gualillo, O. A new player in cartilage homeostasis: Adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthr. Cartil. 2008, 16, 1101–1109. [Google Scholar] [CrossRef]
- Francin, P.J.; Abot, A.; Guillaume, C.; Moulin, D.; Bianchi, A.; Gegout-Pottie, P.; Jouzeau, J.Y.; Mainard, D.; Presle, N. Association between adiponectin and cartilage degradation in human osteoarthritis. Osteoarthr. Cartil. 2014, 22, 519–526. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U.; Tarkowski, A. Resistin, an adipokine with potent proinflammatory properties. J. Immunol. 2005, 174, 5789–5795. [Google Scholar] [CrossRef]
- Endres, M.; Andreas, K.; Kalwitz, G.; Freymann, U.; Neumann, K.; Ringe, J.; Sittinger, M.; Haupl, T.; Kaps, C. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthr. Cartil. 2010, 18, 1458–1466. [Google Scholar] [CrossRef]
- Chaganti, R.K.; Lane, N.E. Risk factors for incident osteoarthritis of the hip and knee. Curr. Rev. Musculoskelet. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Afifi, A.E.A.; Shaat, R.M.; Gharbia, O.M.; Boghdadi, Y.E.; Eshmawy, M.M.E.; El-Emam, O.A. Osteoarthritis of knee joint in metabolic syndrome. Clin. Rheumatol. 2018, 37, 2855–2861. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; Kwekkeboom, J.C.; Kloppenburg, M.; Toes, R.E. Adipocytes from the infrapatellar fat pad of OA patients modulate CD4+ T cell cytokine production. J. Transl. Med. 2011, 9 (Suppl. S2), P45. [Google Scholar] [CrossRef][Green Version]
- Cowan, S.M.; Hart, H.F.; Warden, S.J.; Crossley, K.M. Infrapatellar fat pad volume is greater in individuals with patellofemoral joint osteoarthritis and associated with pain. Rheumatol. Int. 2015, 35, 1439–1442. [Google Scholar] [CrossRef]
- Silawal, S.; Kohl, B.; Girke, G.; Schneider, T.; Schulze-Tanzil, G. Complement regulation in tenocytes under the influence of leukocytes in an indirect co-culture model. Inflamm. Res. 2021, 70, 495–507. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Antoniades, L.; Matson, M.; Andrew, T.; Spector, T.D. The genetic contribution to radiographic hip osteoarthritis in women: Results of a classic twin study. Arthritis Rheum. 2000, 43, 2410–2416. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Cicuttini, F.; Baker, J.; Loughlin, J.; Hart, D. Genetic influences on osteoarthritis in women: A twin study. BMJ 1996, 312, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Macchi, V.; Stocco, E.; Stecco, C.; Belluzzi, E.; Favero, M.; Porzionato, A.; De Caro, R. The infrapatellar fat pad and the synovial membrane: An anatomo-functional unit. J. Anat. 2018, 233, 146–154. [Google Scholar] [CrossRef]
- Balistreri, C.R.; Caruso, C.; Candore, G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediat. Inflamm. 2010, 2010, 802078. [Google Scholar] [CrossRef]

| Gene | NM-Number | Forward/Reverse Primer Sequence | PCR Product Size (bp) |
|---|---|---|---|
| MMP1 | NM_002421 | 5′ GGGAATAAGTACTGGGCTGTTCA 3′ 3′ TCCAGGAAAGTCATGTGCTATCA 5′ | 231 |
| MMP3 | NM_002422 | 5′ CTATCAGAGGAAATGAGGTACGAGC 3′ 3′ GCCTGGCTCCATGGAATTTC 5′ | 179 |
| MMP13 | NM_002427.3 | 5′ CCCCAGGCATCACCATTCAA 3′ 3′ CAGGTAGCGCTCTGCAAACT 5′ | 150 |
| IL1β | NM_000576 | 5′ CCCTAAACAGATGAAGTGCTCC 3′ 3′ AGAAGGTGCTCAGGTCATTCTC 5′ | 197 |
| IL6 | NM_000600.4 | 5′ GGCACTGGCAGAAAACAACC 3′ 3′ GCAAGTCTCCTCATTGAATCC 5′ | 85 |
| IL10 | NM_000572.2 | 5′ AAGACCCAGACATCAAGGCG 3′ 3′ AATCGATGACAGCGCCGTAG 5′ | 85 |
| COL1A1 | NM_000088 | 5′ GGCAACGATGGTGCTAAGG 3′ 3′ GACCAGCATCACCTCTGTCA 5′ | 139 |
| COL2A1 | NM_001844 | 5′ GATGGCTGCACGAAACATACC 3′ 3′ AAGAAGCAGACCGGCCCTAT 5′ | 155 |
| TNFα | NM_000594.3 | 5′ ATGTTGTAGCAAACCCTCAAGC 3′ 3′ CTTGGTCTGGTAGGAGACGG 5′ | 227 |
| GAPDH | NM_002046 | 5′ GAAGGTGAAGGTCGGAGTC 3′ 3′ GAAGATGGTGATGGGATTTC 5′ | 226 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wisniewska, E.; Laue, D.; Spinnen, J.; Kuhrt, L.; Kohl, B.; Bußmann, P.; Meier, C.; Schulze-Tanzil, G.; Ertel, W.; Jagielski, M. Infrapatellar Fat Pad Modulates Osteoarthritis-Associated Cytokine and MMP Expression in Human Articular Chondrocytes. Cells 2023, 12, 2850. https://doi.org/10.3390/cells12242850
Wisniewska E, Laue D, Spinnen J, Kuhrt L, Kohl B, Bußmann P, Meier C, Schulze-Tanzil G, Ertel W, Jagielski M. Infrapatellar Fat Pad Modulates Osteoarthritis-Associated Cytokine and MMP Expression in Human Articular Chondrocytes. Cells. 2023; 12(24):2850. https://doi.org/10.3390/cells12242850
Chicago/Turabian StyleWisniewska, Ewa, Dominik Laue, Jacob Spinnen, Leonard Kuhrt, Benjamin Kohl, Patricia Bußmann, Carola Meier, Gundula Schulze-Tanzil, Wolfgang Ertel, and Michal Jagielski. 2023. "Infrapatellar Fat Pad Modulates Osteoarthritis-Associated Cytokine and MMP Expression in Human Articular Chondrocytes" Cells 12, no. 24: 2850. https://doi.org/10.3390/cells12242850
APA StyleWisniewska, E., Laue, D., Spinnen, J., Kuhrt, L., Kohl, B., Bußmann, P., Meier, C., Schulze-Tanzil, G., Ertel, W., & Jagielski, M. (2023). Infrapatellar Fat Pad Modulates Osteoarthritis-Associated Cytokine and MMP Expression in Human Articular Chondrocytes. Cells, 12(24), 2850. https://doi.org/10.3390/cells12242850