The Emerging Roles of γ-Glutamyl Peptides Produced by γ-Glutamyltransferase and the Glutathione Synthesis System
Abstract
1. Introduction
2. Overview of Glutathione Function
2.1. Glutathione Conjugation in Xenobiotic Metabolism and in the Production of Intrinsic Bioactive Compounds
2.2. The Role of GSH in Maintaining Redox Homeostasis
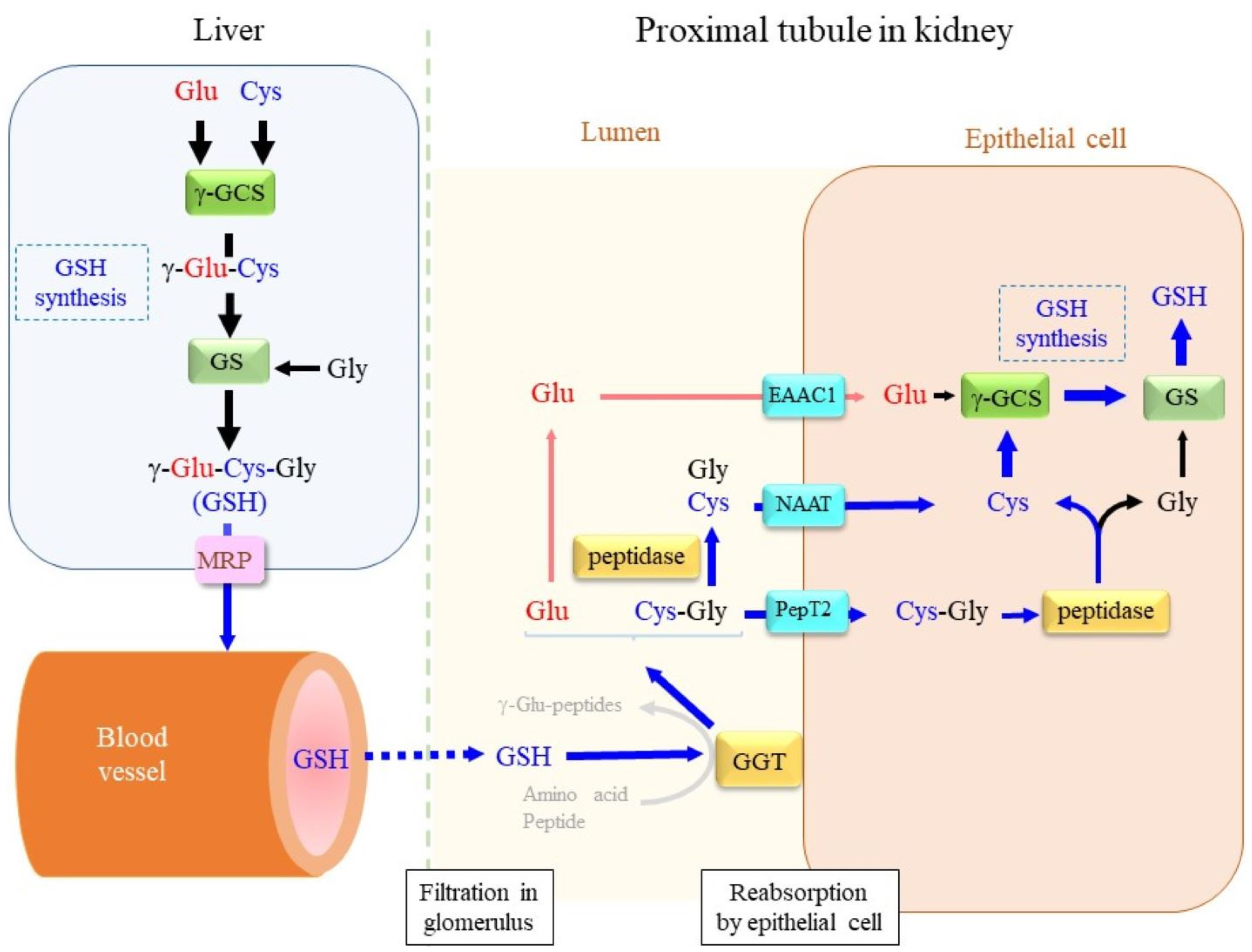
3. Maintenance of Intracellular Glutathione
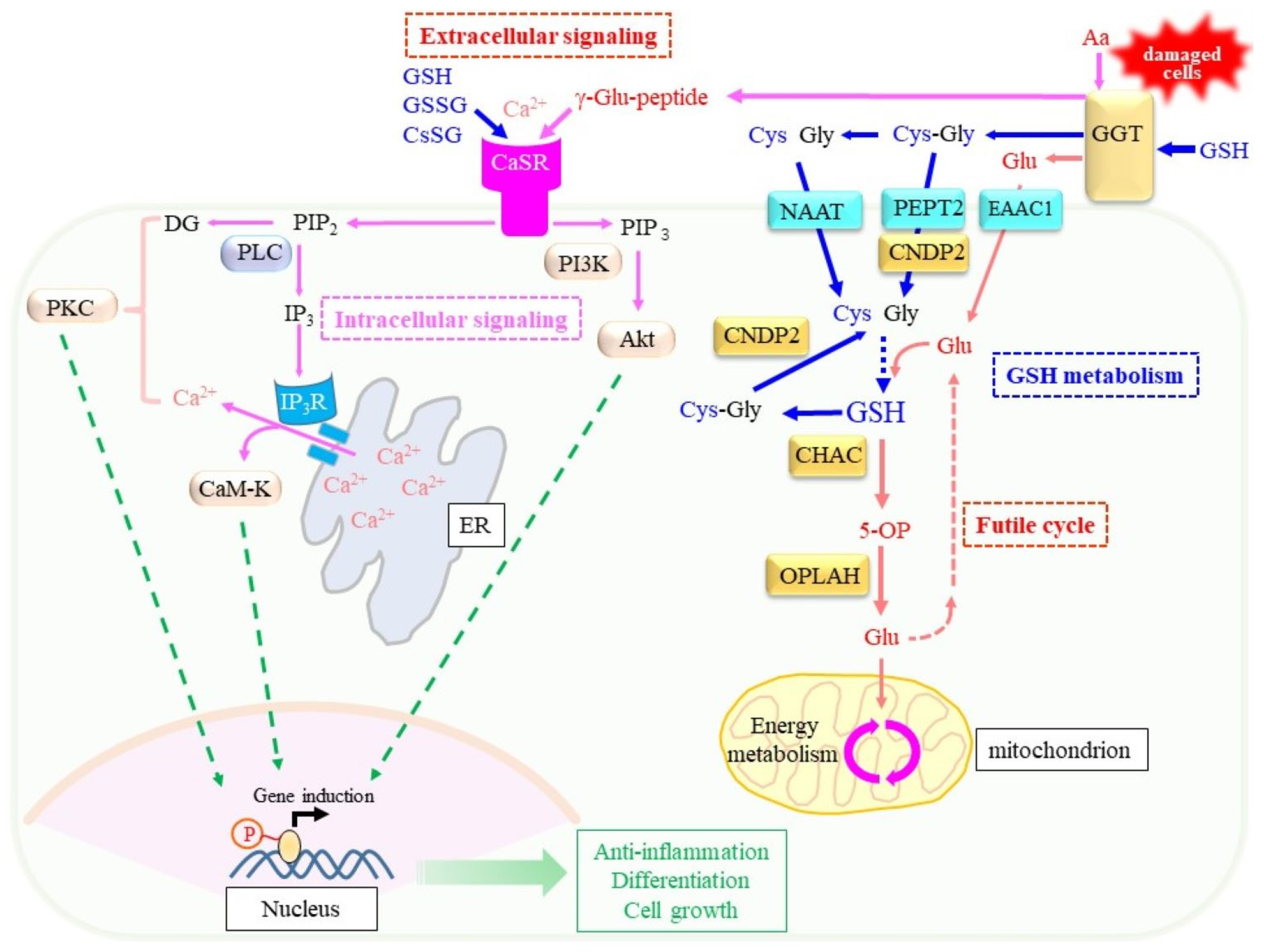
3.1. Extracellular and Intracellular Cys Sources
3.2. De Novo Glutathione Synthesis
3.3. Reductive Recycling of GSSG
4. GGT, a Key Enzyme in Extracellular Glutathione Metabolism
4.1. γ-Glutamyltransferase
4.2. Substrate Specificities and Enzyme Assay of GGT
4.3. Enzyme Reactions of GGT: Transpeptidation, Hydrolysis, and Autotranspeptidation of γ-Glutamyl Compounds
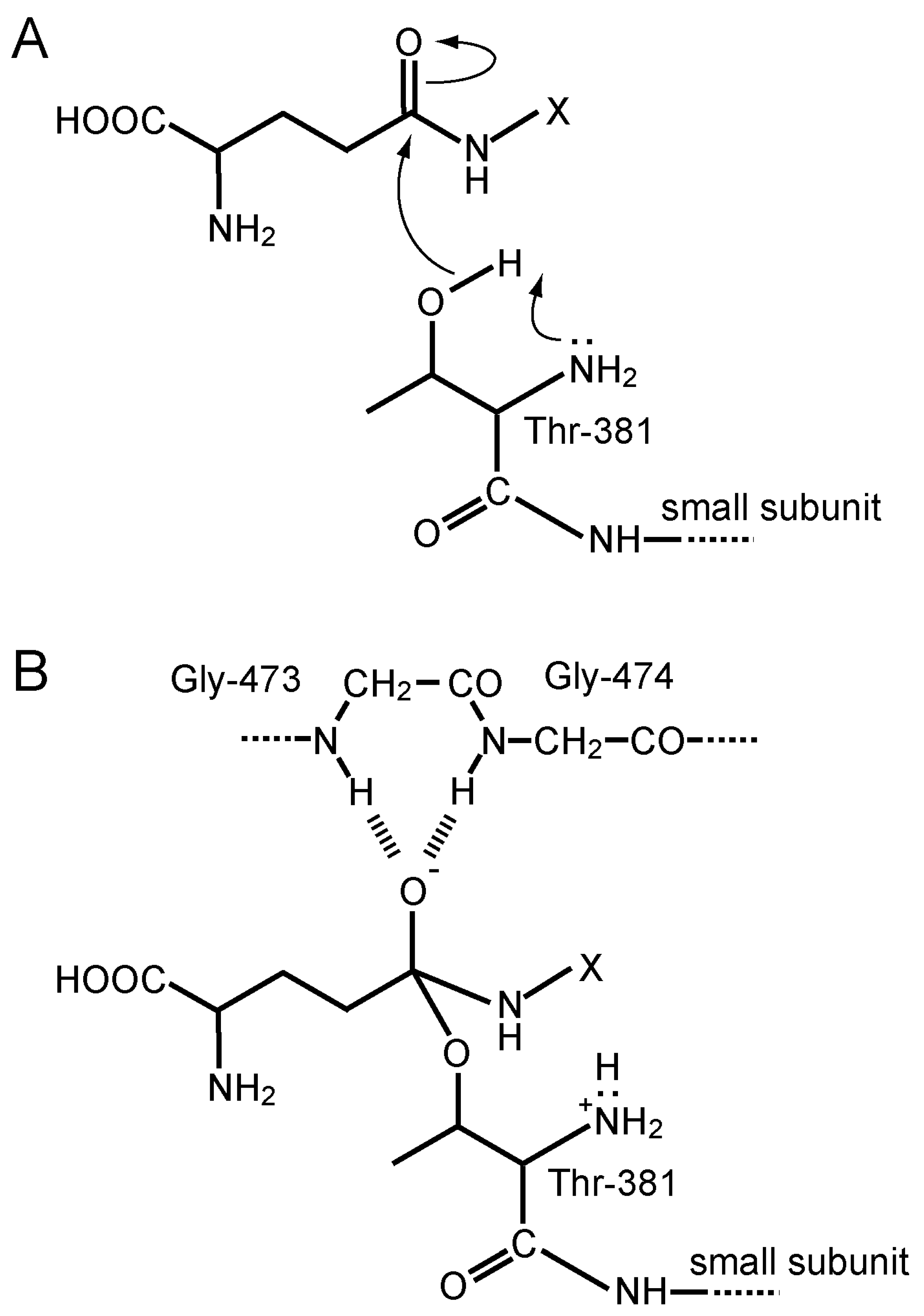
4.4. Protein Structure, Autoprocessing, and the Active-Site Chemistry of GGT
4.5. Gene Family of γ-Glutamyltransferase
4.6. Structure, Expression, and Deficiency of the GGT1 Gene
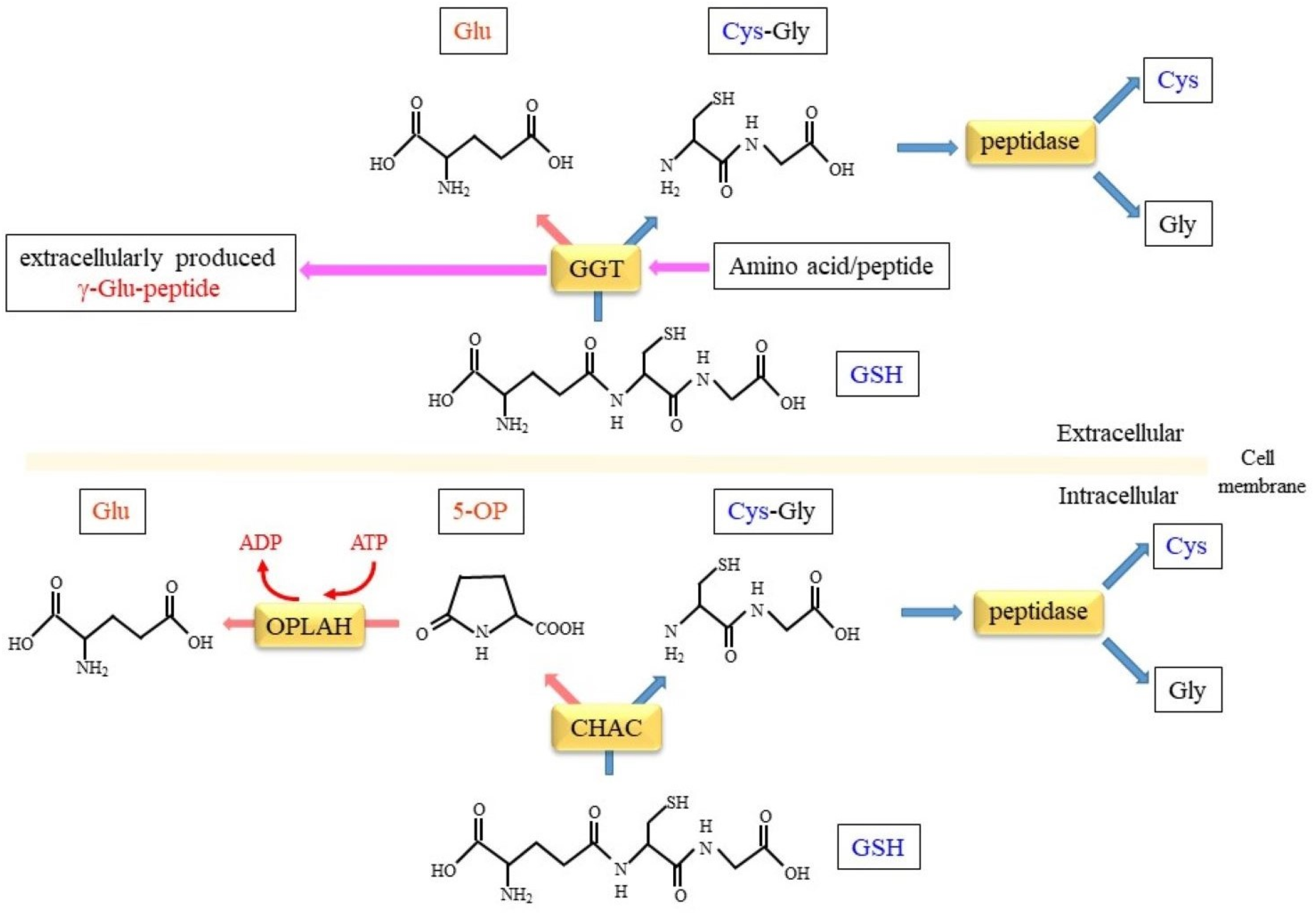
5. Enzymatic Reactions Involved in Intracellular Glutathione Metabolism
5.1. GSH-Specific γ-Glutamylcyclotransferase Activity Inside Cells
5.2. Conversion of 5-OP to Glu by 5-Oxoprolinase
5.3. Hydrolysis of Cys-Gly Dipeptide
6. Physiological Significance of γ-Glutamyl Peptide Production
6.1. γ-GCS/GS Are Intracellular Producers of γ-Glutamyl Peptides
6.2. Benefits of the Production of γ-Glutamyl Peptide by the γ-GCS-Involved Reaction Inside Cells
6.3. Extracellular Signaling Mediated by γ-Glutamyl Peptides Produced by GGT
7. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-Related Enzymes and Proteins: A Review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Asp. Med. 2009, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Kaur, A. Glutathione Degradation. Antioxid. Redox Signal. 2017, 27, 1200–1216. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Taniguchi, N. Gene expression of γ-glutamyltranspeptidase. Methods Enzymol. 2005, 401, 408–425. [Google Scholar] [CrossRef]
- Brennan, S.C.; Davies, T.S.; Schepelmann, M.; Riccardi, D. Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: Control of taste modulation and intestinal hormone secretion. Br. J. Nutr. 2014, 111 (Suppl. S1), S16–S22. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Comprehensive Review of γ-Glutamyl Peptides (γ-GPs) and Their Effect on Inflammation Concerning Cardiovascular Health. J. Agric. Food Chem. 2022, 70, 7851–7870. [Google Scholar] [CrossRef]
- Leach, K.; Hannan, F.M.; Josephs, T.M.; Keller, A.N.; Møller, T.C.; Ward, D.T.; Kallay, E.; Mason, R.S.; Thakker, R.V.; Riccardi, D.; et al. International Union of Basic and Clinical Pharmacology. CVIII. Calcium-Sensing Receptor Nomenclature, Pharmacology, and Function. Pharmacol. Rev. 2020, 72, 558–604. [Google Scholar] [CrossRef]
- Sau, A.; Pellizzari Tregno, F.; Valentino, F.; Federici, G.; Caccuri, A.M. Glutathione transferases and development of new principles to overcome drug resistance. Arch. Biochem. Biophys. 2010, 500, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Hayes, J.D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011, 43, 92–137. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.C.; Mieyal, J.J. Glutathione and Glutaredoxin-Key Players in Cellular Redox Homeostasis and Signaling. Antioxidants 2023, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Chen, Y.; Schneider, S.N.; Nebert, D.W.; Shertzer, H.G. Genetically altered mice to evaluate glutathione homeostasis in health and disease. Free Radic. Biol. Med. 2004, 37, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Ristoff, E.; Larsson, A. Inborn errors in the metabolism of glutathione. Orphanet J. Rare Dis. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ito, J.I.; Zhang, X.; Kurahashi, T. Unveiling the roles of the glutathione redox system in vivo by analyzing genetically modified mice. J. Clin. Biochem. Nutr. 2011, 49, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mazzetti, A.P.; Fiorile, M.C.; Primavera, A.; Lo Bello, M. Glutathione transferases and neurodegenerative diseases. Neurochem. Int. 2015, 82, 10–18. [Google Scholar] [CrossRef]
- Stern, S.T.; Bruno, M.K.; Hennig, G.E.; Horton, R.A.; Roberts, J.C.; Cohen, S.D. Contribution of acetaminophen-cysteine to acetaminophen nephrotoxicity in CD-1 mice: I. Enhancement of acetaminophen nephrotoxicity by acetaminophen-cysteine. Toxicol. Appl. Pharmacol. 2005, 202, 151–159. [Google Scholar] [CrossRef]
- Arodin, L.; Miranda-Vizuete, A.; Swoboda, P.; Fernandes, A.P. Protective effects of the thioredoxin and glutaredoxin systems in dopamine-induced cell death. Free Radic. Biol. Med. 2014, 3, 328–336. [Google Scholar] [CrossRef]
- Gelosa, P.; Colazzo, F.; Tremoli, E.; Sironi, L.; Castiglioni, L. Cysteinyl Leukotrienes as Potential Pharmacological Targets for Cerebral Diseases. Mediat. Inflamm. 2017, 2017, 3454212. [Google Scholar] [CrossRef] [PubMed]
- Kanaoka, Y.; Austen, K.F. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv. Immunol. 2019, 142, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.K. Leukotriene C(4) synthase. Prostaglandins Leukot. Essent. Fat. Acids. 2003, 69, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Zhang, S.; Li, C.; Zhang, L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: Implications for cerebral ischemia and neurodegenerative diseases. Neurobiol. Aging 2020, 87, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory Phenomena in the Glutathione Peroxidase Superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Sato, K. Glutathione transferases and cancer. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 337–384. [Google Scholar] [CrossRef]
- Fisher, A.B.; Martínez, R.; de Villavicencio-Díaz, T.N.; Sánchez, A.; Ramos, Y.; Ferro, J.N.; Gil González, L.; Méndez, M.; Rodríguez, E.; Marcos, E.; et al. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A₂ activities. Antioxid. Redox Signal. 2011, 15, 831–844. [Google Scholar] [CrossRef]
- Ye, Z.W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2015, 1850, 1607–1621. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2019, 133, 144–152. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294.e5. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Mishima, E.; Conrad, M. Nutritional and Metabolic Control of Ferroptosis. Annu. Rev. Nutr. 2022, 42, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S. Mitochondria and ferroptosis. Curr. Opin. Physiol. 2022, 25, 100483. [Google Scholar] [CrossRef]
- Fujii, J.; Yamada, K.I. Defense systems to avoid ferroptosis caused by lipid peroxidation-mediated membrane damage. Free Radic. Res. 2023, 57, 353–372. [Google Scholar] [CrossRef]
- Lillig, C.H.; Berndt, C.; Holmgren, A. Glutaredoxin systems. Biochim. Biophys. Acta 2008, 1780, 1304–1317. [Google Scholar] [CrossRef]
- Janssen-Heininger, Y.M.; Mossman, B.T.; Heintz, N.H.; Forman, H.J.; Kalyanaraman, B.; Finkel, T.; Stamler, J.S.; Rhee, S.G.; van der Vliet, A. Redox-based regulation of signal transduction: Principles, pitfalls, and promises. Free Radic. Biol. Med. 2008, 45, 1–17. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, G.; Giustarini, D.; Milzani, A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009, 34, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Ferran, B.; Oh, A.; Croteau, D.; Shao, D.; Han, J.; Pimentel, D.R.; Bachschmid, M.M. Redox Regulation via Glutaredoxin-1 and Protein S-Glutathionylation. Antioxid. Redox Signal. 2020, 32, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Ogata, F.T.; Branco, V.; Vale, F.F.; Coppo, L. Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 2021, 43, 101975. [Google Scholar] [CrossRef] [PubMed]
- McBean, G.J. The transsulfuration pathway: A source of cysteine for glutathione in astrocytes. Amino Acids 2012, 42, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Massie, A.; Boillée, S.; Hewett, S.; Knackstedt, L.; Lewerenz, J. Main path and byways: Non-vesicular glutamate release by system xc(-) as an important modifier of glutamatergic neurotransmission. J. Neurochem. 2015, 135, 1062–1079. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Hediger, M.A. The glutamate and neutral amino acid transporter family: Physiological and pharmacological implications. Eur. J. Pharmacol. 2003, 479, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, K.; Nakaki, T. Glutathione in Cellular Redox Homeostasis: Association with the Excitatory Amino Acid Carrier 1 (EAAC1). Molecules 2015, 20, 8742–8758. [Google Scholar] [CrossRef]
- Conrad, M.; Sato, H. The oxidative stress-inducible cystine/glutamate antiporter, system xc-: Cystine supplier and beyond. Amino Acids 2012, 42, 231–246. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Pader, I.; Sengupta, R.; Cebula, M.; Xu, J.; Lundberg, J.O.; Holmgren, A.; Johansson, K.; Arnér, E.S. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc. Natl. Acad. Sci. USA 2014, 111, 6964–6969. [Google Scholar] [CrossRef]
- Sato, H.; Nomura, S.; Maebara, K.; Sato, K.; Tamba, M.; Bannai, S. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 2004, 325, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mann, G.E. Redox status in mammalian cells and stem cells during culture in vitro: Critical roles of Nrf2 and cystine transporter activity in the maintenance of redox balance. Redox Biol. 2014, 2, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sato, M.; Kasakoshi, T.; Tsutsui, T.; Sugimoto, M.; Osaki, M.; Okada, F.; Igarashi, K.; Hiratake, J.; Homma, T.; et al. Cystathionine is a novel substrate of cystine/glutamate transporter: Implications for immune function. J. Biol. Chem. 2015, 290, 8778–8788. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W.; Anderson, M.E.; Meister, A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J. Biol. Chem. 1979, 254, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M.; Meister, A. Partial reactions catalyzed by γ-glutamylcysteine synthetase and evidence for an activated glutamate intermediate. J. Biol. Chem. 1971, 246, 7095–7105. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Osei-Frimpong, J.; Kala, G.; Kala, S.V.; Barrios, R.J.; Habib, G.M.; Lukin, D.J.; Danney, C.M.; Matzuk, M.M.; Lieberman, M.W. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc. Natl. Acad. Sci. USA 2000, 97, 5101–5106. [Google Scholar] [CrossRef] [PubMed]
- Almusafri, F.; Elamin, H.E.; Khalaf, T.E.; Ali, A.; Ben-Omran, T.; El-Hattab, A.W. Clinical and molecular characterization of 6 children with glutamate-cysteine ligase deficiency causing hemolytic anemia. Blood Cells Mol. Dis. 2017, 65, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Ye, J.; Wang, L.; Zhu, J.; He, Z. A case of severe glutathione synthetase deficiency with novel GSS mutations. Braz. J. Med. Biol. Res. 2018, 51, e6853. [Google Scholar] [CrossRef]
- Guney Varal, I.; Dogan, P.; Gorukmez, O.; Dorum, S.; Akdag, A. Glutathione synthetase deficiency: A novel mutation with femur agenesis. Fetal Pediatr. Pathol. 2020, 39, 38–44. [Google Scholar] [CrossRef]
- Ristoff, E.; Hebert, C.; Njålsson, R.; Norgren, S.; Rooyackers, O.; Larsson, A. Glutathione synthetase deficiency: Is γ-glutamylcysteine accumulation a way to cope with oxidative stress in cells with insufficient levels of glutathione? J. Inherit. Metab. Dis. 2002, 25, 577–584. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The pentose phosphate pathway in health and disease. Nat. Metab. 2023, 5, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Grayson, C.; Mailloux, R.J. Coenzyme Q10 and nicotinamide nucleotide transhydrogenase: Sentinels for mitochondrial hydrogen peroxide signaling. Free Radic. Biol. Med. 2023, 208, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Nkhoma, E.T.; Poole, C.; Vannappagari, V.; Hall, S.A.; Beutler, E. The global prevalence of glucose-6-phosphate dehydrogenase deficiency: A systematic review and meta-analysis. Blood Cells Mol. Dis. 2009, 42, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Zakany, F.; Mándity, I.M.; Varga, Z.; Panyi, G.; Nagy, P.; Kovacs, T. Effect of the Lipid Landscape on the Efficacy of Cell-Penetrating Peptides. Cells 2023, 12, 1700. [Google Scholar] [CrossRef] [PubMed]
- Bourbouloux, A.; Shahi, P.; Chakladar, A.; Delrot, S.; Bachhawat, A.K. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 13259–13265. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Wang, Y.; Yen, F.S.; Zhu, X.G.; Timson, R.C.; Weber, R.; Xing, C.; Liu, Y.; Allwein, B.; Luo, H.; Yeh, H.W.; et al. SLC25A39 is necessary for mitochondrial glutathione import in mammalian cells. Nature 2021, 599, 136–140. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione and glutathione delivery compounds. Adv. Pharmacol. 1997, 38, 65–78. [Google Scholar] [CrossRef]
- Heisterkamp, N.; Groffen, J.; Warburton, D.; Sneddon, T.P. The human γ-glutamyltransferase gene family. Hum. Genet. 2008, 123, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Hanes, C.S.; Hird, F.J.R.; Isherwood, F.A. Synthesis of peptides in enzymatic reactions involving glutathione. Nature 1950, 166, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Hanes, C.S.; Hird, F.J.R.; Isherwood, F.A. Enzymic transpeptidation reactions involving γ-glutamyl peptides and γ-amino-acy peptides. Biochem. J. 1952, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.S.; Meister, A. Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of γ-glutamyl transpeptidase by maleate; Identification of a rat kidney maleate-stimulated glutaminase and γ-glutamyl transpeptidase. Proc. Natl. Acad. Sci. USA 1974, 71, 3320–3333. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.S.; Meister, A. Identity of maleate-stimulated glutaminase with γ-glutamyl transpeptidase in rat kidney. J. Biol. Chem. 1975, 250, 4619–4627. [Google Scholar] [CrossRef] [PubMed]
- West, M.B.; Wickham, S.; Quinalty, L.M.; Pavlovicz, R.E.; Li, C.; Hanigan, M.H. Autocatalytic cleavage of human γ-glutamyl transpeptidase is highly dependent on N-glycosylation at asparagine 95. J. Biol. Chem. 2011, 286, 28876–28888. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. Clinical significance of appearance of serum γ-glutamyl transpeptidase isozyme. Gastroenterol. Jpn. 1981, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, C.; Shimojo, N.; Naka, K.; Okuda, K.; Ohkawa, J. Separation of hepatoma-associated γ-glutamyltransferase isoenzyme on cellulose acetate media with Triton X-100 and concanavalin A. Clin. Chim. Acta 1989, 185, 317–323. [Google Scholar] [CrossRef]
- Franzini, M.; Bramanti, E.; Ottaviano, V.; Ghiri, E.; Scatena, F.; Barsacchi, R.; Pompella, A.; Donato, L.; Emdin, M.; Paolicchi, A. A high performance gel filtration chromatography method for γ-glutamyltransferase fraction analysis. Anal. Biochem. 2008, 374, 1–6. [Google Scholar] [CrossRef]
- Fornaciari, I.; Fierabracci, V.; Corti, A.; Elawadi, H.A.; Lorenzini, E.; Emdin, M.; Paolicchi, A.; Franzini, M. γ-Glutamyltransferase fractions in human plasma and bile: Characteristics and biogenesis. PLoS ONE 2014, 9, e88532. [Google Scholar] [CrossRef]
- Corti, A.; Belcastro, E.; Dominici, S.; Maellaro, E.; Pompella, A. The dark side of γ-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radic. Biol. Med. 2020, 160, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Hitoi, A.; Taniguchi, N.; Yokosawa, N.; Tsukada, Y.; Kobata, A. Comparative study of the sugar chains of γ-glutamyltranspeptidases purified from rat liver and rat AH-66 hepatoma cells. Cancer Res. 1983, 43, 5059–5063. [Google Scholar] [PubMed]
- Wickham, S.; West, M.B.; Cook, P.F.; Hanigan, M.H. γ-Glutamyl compounds: Substrate specificity of γ-glutamyl transpeptidase enzymes. Anal. Biochem. 2011, 414, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Taniguchi, N. γ-Glutamyl transpeptidase (GGT) activity assay. In Current Protocols in Toxicology; Maines, M.D., Costa, L.G., Reed, D.J., Sassa, S., Sipes, I.G., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2000; Volume 5, pp. 6.6.1–6.6.8. [Google Scholar]
- Szewczuk, A.; Kuropatwa, N.; Lang, D. Colorimetric method for assay of serum γ-glutamyltransferase activity with some l-γ-glutamyl-carboxyanilides. Clin. Chim. Acta 1988, 178, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.A.; Meister, A. Hydrolysis and transfer reactions catalyzed by γ-glutamyl transpeptidase; evidence for separate substrate sites and for high affinity of L-cystine. Biochem. Biophys. Res. Commun. 1976, 71, 32–36. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M.; Curthoys, N.P. Comparison of the hydrolytic and transfer activities of rat renal γ-glutamyltranspeptidase. J. Biol. Chem. 1979, 254, 6499–6504. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.A.; Griffith, O.W.; Meister, A. γ-Glutamyl-glutathione: Natural occurrence and enzymology. J. Biol. Chem. 1986, 261, 13657–13661. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, M.; Meister, A. Isolation of γ-glutamyl transpeptidase from hog kidney. J. Biol. Chem. 1965, 240, 338–347. [Google Scholar] [CrossRef]
- Fodor, P.J.; Miller, A.; Waelsch, H. Quantitative aspects of enzymatic cleavage of glutathione. J. Biol. Chem. 1953, 202, 551–565. [Google Scholar] [CrossRef]
- Ikeda, Y.; Fujii, J.; Taniguchi, N. Effects of substitutions of the conserved histidine residues in human γ-glutamyl transpeptidase. J. Biochem. 1996, 119, 1166–1170. [Google Scholar] [CrossRef]
- West, M.B.; Chen, Y.; Wickham, S.; Heroux, A.; Cahill, K.; Hanigan, M.H.; Mooers, B.H. Novel insights into eukaryotic γ-glutamyltranspeptidase 1 from the crystal structure of the glutamate-bound human enzyme. J. Biol. Chem. 2013, 288, 31902–31913. [Google Scholar] [CrossRef] [PubMed]
- Terzyan, S.S.; Burgett, A.W.; Heroux, A.; Smith, C.A.; Mooers, B.H.; Hanigan, M.H. Human γ-Glutamyl Transpeptidase 1: Structures Of The Free Enzyme, Inhibitor-Bound Tetrahedral Transition States, and Glutamate-Bound Enzyme Reveal Novel Movement Within The Active Site During Catalysis. J. Biol. Chem. 2015, 290, 17576–17586. [Google Scholar] [CrossRef] [PubMed]
- Terzyan, S.S.; Cook, P.F.; Heroux, A.; Hanigan, M.H. Structure of 6-diazo-5-oxo-norleucine-bound human γ-glutamyl transpeptidase 1, a novel mechanism of inactivation. Protein Sci. 2017, 26, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Terzyan, S.S.; Nguyen, L.T.; Burgett, A.W.G.; Heroux, A.; Smith, C.A.; You, Y.; Hanigan, M.H. Crystal structures of glutathione- and inhibitor-bound human GGT1: Critical interactions within the cysteinylglycine binding site. J. Biol. Chem. 2021, 296, 100066. [Google Scholar] [CrossRef] [PubMed]
- Heisterkamp, N.; Rajpert-De Meyts, E.; Uribe, L.; Forman, H.J.; Groffen, J. Identification of a human γ-glutamyl cleaving enzyme related to, but distinct from, γ-glutamyl transpeptidase. Proc. Natl. Acad. Sci. USA 1991, 88, 6303–6307. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.Z.; Shi, Z.Z.; Barrios, R.; Lieberman, M.W. γ-Glutamyl leukotrienase, a γ-glutamyl transpeptidase gene family member, is expressed primarily in spleen. J. Biol. Chem. 1998, 273, 28277–28285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Han, B.; Habib, G.M.; Matzuk, M.M.; Lieberman, M.W. Disruption of γ-glutamyl leukotrienase results in disruption of leukotriene D(4) synthesis in vivo and attenuation of the acute inflammatory response. Mol. Cell. Biol. 2001, 21, 5389–5395. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Luo, G.; Shi, Z.Z.; Barrios, R.; Atwood, D.; Liu, W.; Habib, G.M.; Sifers, R.N.; Corry, D.B.; Lieberman, M.W. γ-Glutamyl leukotrienase, a novel endothelial membrane protein, is specifically responsible for leukotriene D(4) formation in vivo. Am. J. Pathol. 2002, 161, 481–490. [Google Scholar] [CrossRef]
- Carter, B.Z.; Wiseman, A.L.; Orkiszewski, R.; Ballard, K.D.; Ou, C.N.; Lieberman, M.W. Metabolism of leukotriene C4 in γ-glutamyl transpeptidase-deficient mice. J. Biol. Chem. 1997, 272, 12305–12310. [Google Scholar] [CrossRef]
- Leh, H.; Chikhi, N.; Ichino, K.; Guellaën, G.; Wellman, M.; Siest, G.; Visvikis, A. An intronic promoter controls the expression of truncated human γ-glutamyltransferase mRNAs. FEBS Lett. 1998, 434, 51–56. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Habib, G.M.; Lebovitz, R.M.; Lieberman, M.W. Cloning of cDNA and genomic structure of the mouse γ-glutamyl transpeptidase-encoding gene. Gene 1995, 167, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Darin, N.; Leckström, K.; Sikora, P.; Lindgren, J.; Almén, G.; Asin-Cayuela, J. γ-Glutamyl transpeptidase deficiency caused by a large homozygous intragenic deletion in GGT1. Eur. J. Hum. Genet. 2018, 26, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Tattoli, I.; Sorbara, M.T.; Vuckovic, D.; Ling, A.; Soares, F.; Carneiro, L.A.; Yang, C.; Emili, A.; Philpott, D.J.; Girardin, S.E. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 2012, 11, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Mungrue, I.N.; Pagnon, J.; Kohannim, O.; Gargalovic, P.S.; Lusis, A.J. CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. J. Immunol. 2009, 182, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Gautam, R.; Srivastava, R.; Chandel, A.; Kumar, A.; Karthikeyan, S.; Bachhawat, A.K. ChaC2, an Enzyme for Slow Turnover of Cytosolic Glutathione. J. Biol. Chem. 2017, 92, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S.; Jainarayanan, A.K.; Dubey, P. Heart failure and the glutathione cycle: An integrated view. Biochem. J. 2020, 477, 3123–3130. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Patel, D.N.; Welsch, M.; Skouta, R.; Lee, E.D.; Hayano, M.; Thomas, A.G.; Gleason, C.E.; Tatonetti, N.P.; Slusher, B.S.; et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 2014, 3, e02523. [Google Scholar] [CrossRef]
- Chen, M.S.; Wang, S.F.; Hsu, C.Y.; Yin, P.H.; Yeh, T.S.; Lee, H.C.; Tseng, L.M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2α-ATF4 pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef]
- Crawford, R.R.; Prescott, E.T.; Sylvester, C.F.; Higdon, A.N.; Shan, J.; Kilberg, M.S.; Mungrue, I.N. Human CHAC1 Protein Degrades Glutathione, and mRNA Induction Is Regulated by the Transcription Factors ATF4 and ATF3 and a Bipartite ATF/CRE Regulatory Element. J. Biol. Chem. 2015, 290, 15878–15891. [Google Scholar] [CrossRef]
- Oyabu, M.; Takigawa, K.; Mizutani, S.; Hatazawa, Y.; Fujita, M.; Ohira, Y.; Sugimoto, T.; Suzuki, O.; Tsuchiya, K.; Suganami, T.; et al. FOXO1 cooperates with C/EBPδ and ATF4 to regulate skeletal muscle atrophy transcriptional program during fasting. FASEB J. 2022, 36, e22152. [Google Scholar] [CrossRef]
- Carraro, V.; Combaret, L.; Coudy-Gandilhon, C.; Parry, L.; Averous, J.; Maurin, A.C.; Jousse, C.; Voyard, G.; Fafournoux, P.; Papet, I.; et al. Activation of the eIF2α-ATF4 Pathway by Chronic Paracetamol Treatment Is Prevented by Dietary Supplementation with Cysteine. Int. J. Mol. Sci. 2022, 23, 7196. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Zhang, J.; Tokunaga, A.; Harraz, M.M.; Byrne, S.T.; Dolinko, A.; Xu, J.; Blackshaw, S.; Gaiano, N.; Dawson, T.M.; et al. Botch promotes neurogenesis by antagonizing Notch. Dev. Cell 2012, 22, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zheng, X.; Mao, S.; Zhang, Q.; Hu, G.; Wei, Y. DJ-1 inhibits glutathione degradation by downregulating CHAC1 expression in astrocytes. Neurosci. Res. 2022, 184, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, M.; Ahn, Y.; Cao, K.; Pinkus, C.A.; Stansfield, J.C.; Wu, Z.; Zhang, B.B. CHAC1 inactivation is effective to preserve muscle glutathione but is insufficient to protect against muscle wasting in cachexia. PLoS ONE 2023, 18, e0283806. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.K.; Yang, S.C.; Hsu, S.C.; Chang, F.P.; Lin, Y.T.; Chen, S.F.; Cheng, C.L.; Hsiao, M.; Lu, F.L.; Lu, J. CHAC2 is essential for self-renewal and glutathione maintenance in human embryonic stem cells. Free Radic. Biol. Med. 2017, 113, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fei, W.; Shi, Q.; Li, Q.; Kuang, Y.; Wang, C.; He, C.; Hu, X. CHAC2, downregulated in gastric and colorectal cancers, acted as a tumor suppressor inducing apoptosis and autophagy through unfolded protein response. Cell Death Dis. 2017, 8, e3009. [Google Scholar] [CrossRef] [PubMed]
- Chand, S.; Mehta, V.; Sharma, R.K.; Anvikar, A.R.; Chander, H. Cancer informatics analysis indicates high CHAC2 associated with unfavorable prognosis in breast cancer. Front. Oncol. 2022, 12, 1058931. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, J.; Qiao, Y. A metabolism-associated gene signature for prognosis prediction of hepatocellular carcinoma. Front. Mol. Biosci. 2022, 9, 988323. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Van der Werf, P.; Orlowski, M.; Meister, A. Enzymatic conversion of 5-oxo-L-proline (L-pyrrolidone carboxylate) to L-glutamate coupled with cleavage of adenosine triphosphate to adenosine diphosphate, a reaction in the γ-glutamyl cycle. Proc. Natl. Acad. Sci. USA 1971, 68, 2982–2985. [Google Scholar] [CrossRef] [PubMed]
- Almaghlouth, I.A.; Mohamed, J.Y.; Al-Amoudi, M.; Al-Ahaidib, L.; Al-Odaib, A.; Alkuraya, F.S. 5-Oxoprolinase deficiency: Report of the first human OPLAH mutation. Clin. Genet. 2012, 82, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Sass, J.O.; Gemperle-Britschgi, C.; Tarailo-Graovac, M.; Patel, N.; Walter, M.; Jordanova, A.; Alfadhel, M.; Barić, I.; Çoker, M.; Damli-Huber, A.; et al. Unravelling 5-oxoprolinuria (pyroglutamic aciduria) due to bi-allelic OPLAH mutations: 20 new mutations in 14 families. Mol. Genet. Metab. 2016, 119, 44–49. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, A.; Gil, A.; Tromp, J.; Silljé, H.H.W.; van Veldhuisen, D.J.; Voors, A.A.; Hoendermis, E.S.; Grote Beverborg, N.; Schouten, E.M.; de Boer, R.A.; et al. OPLAH ablation leads to accumulation of 5-oxoproline, oxidative stress, fibrosis, and elevated fillings pressures: A murine model for heart failure with a preserved ejection fraction. Cardiovasc. Res. 2018, 114, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Liss, D.B.; Paden, M.S.; Schwarz, E.S.; Mullins, M.E. What is the clinical significance of 5-oxoproline (pyroglutamic acid) in high anion gap metabolic acidosis following paracetamol (acetaminophen) exposure? Clin. Toxicol. 2013, 51, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Dieck, S.T.; Heuer, H.; Ehrchen, J.; Otto, C.; Bauer, K. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala-Lys-Nepsilon-AMCA in astrocytes. Glia 1999, 25, 10–20. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Döring, F.; Nickolaus, M.; Daniel, H.; Fischer, A. Expression of PEPT2 peptide transporter mRNA and protein in glial cells of rat dorsal root ganglia. Neurosci. Lett. 2001, 304, 181–184. [Google Scholar] [CrossRef]
- Kaur, H.; Kumar, C.; Junot, C.; Toledano, M.B.; Bachhawat, A.K. Dug1p Is a Cys-Gly peptidase of the γ-glutamyl cycle of Saccharomyces cerevisiae and represents a novel family of Cys-Gly peptidases. J. Biol. Chem. 2009, 284, 14493–14502. [Google Scholar] [CrossRef]
- Bellia, F.; Vecchio, G.; Rizzarelli, E. Carnosinases, their substrates and diseases. Molecules 2014, 19, 2299–2329. [Google Scholar] [CrossRef]
- Kobayashi, S.; Homma, T.; Okumura, N.; Han, J.; Nagaoka, K.; Sato, H.; Konno, H.; Yamada, S.; Takao, T.; Fujii, J. Carnosine dipeptidase II (CNDP2) protects cells under cysteine insufficiency by hydrolyzing glutathione-related peptides. Free Radic. Biol. Med. 2021, 174, 12–27. [Google Scholar] [CrossRef]
- Ahluwalia, T.S.; Lindholm, E.; Groop, L.C. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia 2011, 54, 2295–2302. [Google Scholar] [CrossRef]
- Zhang, P.; Chan, D.W.; Zhu, Y.; Li, J.J.; Ng, I.O.; Wan, D.; Gu, J. Identification of carboxypeptidase of glutamate like-B as a candidate suppressor in cell growth and metastasis in human hepatocellular carcinoma. Clin. Cancer Res. 2006, 12, 6617–6625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Z.; Miao, L.; Xin, X.; Zhang, J.; Yang, S.; Miao, M.; Kong, X.; Jiao, B. Underexpressed CNDP2 participates in gastric cancer growth inhibition through activating the MAPK signaling pathway. Mol. Med. 2014, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Zhang, Z.; Yu, H.; Yu, M.; Yuan, K.; Yang, T.; Miao, M.; Shi, H. Up-regulation of CNDP2 facilitates the proliferation of colon cancer. BMC Gastroenterol. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Yang, H.Q.; Yang, S.Q.; Wang, Y.; Chen, X.J.; Lu, H.S.; Zhao, L.P. CNDP2 Acts as an Activator for Human Ovarian Cancer Growth and Metastasis via the PI3K/AKT Pathway. Technol. Cancer Res. Treat. 2019, 18, 1533033819874773. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Liu, S.; Zhao, Q.; Liang, X.; Ma, Z.; Gupta, P.K.; Zhao, M.; Wang, A. Autophagy flux inhibition, G2/M cell cycle arrest and apoptosis induction by ubenimex in glioma cell lines. Oncotarget 2017, 8, 107730–107743. [Google Scholar] [CrossRef]
- Jansen, R.S.; Addie, R.; Merkx, R.; Fish, A.; Mahakena, S.; Bleijerveld, O.B.; Altelaar, M.; IJlst, L.; Wanders, R.J.; Borst, P.; et al. N-lactoyl-amino acids are ubiquitous metabolites that originate from CNDP2-mediated reverse proteolysis of lactate and amino acids. Proc. Natl. Acad. Sci. USA 2015, 112, 6601–6606. [Google Scholar] [CrossRef] [PubMed]
- Li, V.L.; He, Y.; Contrepois, K.; Liu, H.; Kim, J.T.; Wiggenhorn, A.L.; Tanzo, J.T.; Tung, A.S.; Lyu, X.; Zushin, P.H.; et al. An exercise-inducible metabolite that suppresses feeding and obesity. Nature 2022, 606, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ikeda, Y.; Shigeno, Y.; Konno, H.; Fujii, J. γ-Glutamylcysteine synthetase and γ-glutamyl transferase as differential enzymatic sources of γ-glutamylpeptides in mice. Amino Acids 2020, 52, 555–566. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Soga, T.; Sugimoto, M.; Honma, M.; Mori, M.; Igarashi, K.; Kashikura, K.; Ikeda, S.; Hirayama, A.; Yamamoto, T.; Yoshida, H.; et al. Serum metabolomics reveals γ-glutamyl dipeptides as biomarkers for discrimination among different forms of liver disease. J. Hepatol. 2011, 55, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Homma, T.; Kobayashi, S. Ferroptosis caused by cysteine insufficiency and oxidative insult. Free Radic. Res. 2020, 54, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Baran, R.; Suematsu, M.; Ueno, Y.; Ikeda, S.; Sakurakawa, T.; Kakazu, Y.; Ishikawa, T.; Robert, M.; Nishioka, T.; et al. Differential metabolomics reveals ophthalmic acid as an oxidative stress biomarker indicating hepatic glutathione consumption. J. Biol. Chem. 2006, 281, 16768–16776. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Lee, J.; Takao, T.; Fujii, J. Increased ophthalmic acid production is supported by amino acid catabolism under fasting conditions in mice. Biochem. Biophys. Res. Commun. 2017, 491, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.; Bridge, W. Glutamate cysteine ligase and the age-related decline in cellular glutathione: The therapeutic potential of γ-glutamylcysteine. Arch. Biochem. Biophys. 2016, 593, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zarka, M.H.; Bridge, W.J. Oral administration of γ-glutamylcysteine increases intracellular glutathione levels above homeostasis in a randomised human trial pilot study. Redox Biol. 2017, 11, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Hang, Q.; Fang, Y.; Dong, X.; Cao, P.; Yin, Z.; Luo, L. γ-Glutamylcysteine exhibits anti-inflammatory effects by increasing cellular glutathione level. Redox Biol. 2019, 20, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Fernandez-Fernandez, S.; Bobo-Jimenez, V.; Escobar, J.; Sastre, J.; Almeida, A.; Bolaños, J.P. γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat. Commun. 2012, 3, 718. [Google Scholar] [CrossRef]
- Kumar, A.; Bachhawat, A.K. A futile cycle, formed between two ATP-dependant γ-glutamyl cycle enzymes, γ-glutamyl cysteine synthetase and 5-oxoprolinase: The cause of cellular ATP depletion in nephrotic cystinosis? J. Biosci. 2010, 35, 21–25. [Google Scholar] [CrossRef]
- Emmett, M. Acetaminophen toxicity and 5-oxoproline (pyroglutamic acid): A tale of two cycles, one an ATP-depleting futile cycle and the other a useful cycle. Clin. J. Am. Soc. Nephrol. 2014, 9, 191–200. [Google Scholar] [CrossRef]
- Armenian, P.; Gerona, R.R.; Blanc, P.D.; Wu, A.H.; Mookherjee, S. 5-oxoprolinemia causing elevated anion gap metabolic acidosis in the setting of acetaminophen use. J. Emerg. Med. 2012, 43, 54–57. [Google Scholar] [CrossRef]
- Kaur, G.; Leslie, E.M.; Tillman, H.; Lee, W.M.; Swanlund, D.P.; Karvellas, C.J.; US Acute Liver Failure Study Group. Detection of Ophthalmic Acid in Serum from Acetaminophen-Induced Acute Liver Failure Patients Is More Frequent in Non-Survivors. PLoS ONE 2015, 10, e0139299. [Google Scholar] [CrossRef]
- Kang, Y.P.; Mockabee-Macias, A.; Jiang, C.; Falzone, A.; Prieto-Farigua, N.; Stone, E.; Harris, I.S.; DeNicola, G.M. Non-canonical Glutamate-Cysteine Ligase Activity Protects against Ferroptosis. Cell Metab. 2021, 33, 174–189.e7. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol. Cell 2019, 73, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Homma, T.; Kobayashi, S.; Sato, H.; Fujii, J. Superoxide produced by mitochondrial complex III plays a pivotal role in the execution of ferroptosis induced by cysteine starvation. Arch. Biochem. Biophys. 2021, 700, 108775. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.E.; Tallan, H.H.; Lin, Y.Y.; Gaull, G.E. Taurine: Biological update. Annu. Rev. Biochem. 1986, 55, 427–453. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.; Win, T.; Gupta, R. γ-L-Glutamyltaurine. Amino Acids 2005, 28, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Lopes Bastos, B.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Varga, V.; Török, K.; Feuer, L.; Gulyás, J.; Somogyi, J. γ-Glutamyltransferase in the brain and its role in formation of γ-L-glutamyl-taurine. Prog. Clin. Biol. Res. 1985, 179, 115–125. [Google Scholar]
- Varga, V.; Janáky, R.; Marnela, K.M.; Saransaari, P.; Oja, S.S. Interactions of γ-L-glutamyltaurine with excitatory aminoacidergic neurotransmission. Neurochem. Res. 1994, 19, 243–248. [Google Scholar] [CrossRef]
- Uemura, S.; Ienaga, K.; Higashiura, K.; Kimura, H. γ-Glutamyltaurine has potent and long-lasting antiepileptic action as demonstrated by intra-amygdaloid injection in amygdala-kindled rats. Brain Res. 1992, 594, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate excitotoxicity: Potential therapeutic target for ischemic stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.C.; Lin, C.H.; Lane, H.Y. Cystine/Glutamate Antiporter in Schizophrenia: From Molecular Mechanism to Novel Biomarker and Treatment. Int. J. Mol. Sci. 2021, 22, 9718. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yao, Y.; Kuang, D.; Hampson, D.R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 2006, 281, 8864–8870. [Google Scholar] [CrossRef] [PubMed]
- Goralski, T.; Ram, J.L. Extracellular Calcium Receptor as a Target for Glutathione and Its Derivatives. Int. J. Mol. Sci. 2022, 23, 717. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Tfelt-Hansen, J.; Chattopadhyay, N. Diverse roles of extracellular calcium-sensing receptor in the central nervous system. J. Neurosci. Res. 2010, 88, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Ohsu, T.; Amino, Y.; Nagasaki, H.; Yamanaka, T.; Takeshita, S.; Hatanaka, T.; Maruyama, Y.; Miyamura, N.; Eto, Y. Involvement of the calcium-sensing receptor in human taste perception. J. Biol. Chem. 2010, 285, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Brandi, M.L. The calcium-sensing receptor in inflammation: Recent updates. Front. Physiol. 2022, 13, 1059369. [Google Scholar] [CrossRef]
- Giudice, M.L.; Mihalik, B.; Dinnyés, A.; Kobolák, J. The Nervous System Relevance of the Calcium Sensing Receptor in Health and Disease. Molecules 2019, 24, 2546. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Gardenal, E.; Gui, L.; Dal Prà, I. Amyloid β-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143-Further Implications for Alzheimer’s Therapy. Front. Neurosci. 2017, 11, 217. [Google Scholar] [CrossRef]
- Dal Prà, I.; Armato, U.; Chiarini, A. Family C G-Protein-Coupled Receptors in Alzheimer’s Disease and Therapeutic Implications. Front. Pharmacol. 2019, 10, 1282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kovacs-Nolan, J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. Biochim. Biophys. Acta 2015, 1852, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Hewson, C.K.; Capraro, A.; Wong, S.L.; Pandzic, E.; Zhong, L.; Fernando, B.S.M.; Awatade, N.T.; Hart-Smith, G.; Whan, R.M.; Thomas, S.R.; et al. Novel Antioxidant Therapy with the Immediate Precursor to Glutathione, γ-Glutamylcysteine (GGC), Ameliorates LPS-Induced Cellular Stress in In Vitro 3D-Differentiated Airway Model from Primary Cystic Fibrosis Human Bronchial Cells. Antioxidants 2020, 9, 1204. [Google Scholar] [CrossRef] [PubMed]
- Bi, A.; Wang, Y.; Chen, L.; Yin, Z.; Luo, L. γ-Glutamylcysteine attenuates amyloid-β oligomers-induced neuroinflammation in microglia via blocking NF-κB signaling pathway. Chem. Biol. Interact. 2022, 363, 110019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yan, X.; Bi, X.; Lu, S.; Liu, X.; Yang, C.; Shi, Y.; Luo, L.; Yin, Z. γ-Glutamylcysteine rescues mice from TNBS-driven inflammatory bowel disease through regulating macrophages polarization. Inflamm. Res. 2023, 72, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Xia, S.N.; Xu, S.Y.; Liu, P.Y.; Gu, Y.; Bao, X.Y.; Xu, Y.; Cao, X. γ-Glutamylcysteine Alleviates Ischemic Stroke-Induced Neuronal Apoptosis by Inhibiting ROS-Mediated Endoplasmic Reticulum Stress. Oxidative Med. Cell. Longev. 2021, 2021, 2961079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Z.; Li, B.; Yao, H.; Zarka, M.; Welch, J.; Sachdev, P.; Bridge, W.; Braidy, N. Supplementation with γ-glutamylcysteine (γ-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem. Int. 2021, 144, 104931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lei, J.; Chen, L.; Wang, Y.; Yang, G.; Yin, Z.; Luo, L. γ-Glutamylcysteine Exerts Neuroprotection Effects against Cerebral Ischemia/Reperfusion Injury through Inhibiting Lipid Peroxidation and Ferroptosis. Antioxidants 2022, 11, 1653. [Google Scholar] [CrossRef]
- Fujii, J.; Osaki, T.; Soma, Y.; Matsuda, Y. Critical Roles of the Cysteine-Glutathione Axis in the Production of γ-Glutamyl Peptides in the Nervous System. Int. J. Mol. Sci. 2023, 24, 8044. [Google Scholar] [CrossRef]
- Dukić, M.; Radonjić, T.; Jovanović, I.; Zdravković, M.; Todorović, Z.; Kraišnik, N.; Aranđelović, B.; Mandić, O.; Popadić, V.; Nikolić, N.; et al. Alcohol, Inflammation, and Microbiota in Alcoholic Liver Disease. Int. J. Mol. Sci. 2023, 24, 3735. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, Y.; Fujii, J. The Emerging Roles of γ-Glutamyl Peptides Produced by γ-Glutamyltransferase and the Glutathione Synthesis System. Cells 2023, 12, 2831. https://doi.org/10.3390/cells12242831
Ikeda Y, Fujii J. The Emerging Roles of γ-Glutamyl Peptides Produced by γ-Glutamyltransferase and the Glutathione Synthesis System. Cells. 2023; 12(24):2831. https://doi.org/10.3390/cells12242831
Chicago/Turabian StyleIkeda, Yoshitaka, and Junichi Fujii. 2023. "The Emerging Roles of γ-Glutamyl Peptides Produced by γ-Glutamyltransferase and the Glutathione Synthesis System" Cells 12, no. 24: 2831. https://doi.org/10.3390/cells12242831
APA StyleIkeda, Y., & Fujii, J. (2023). The Emerging Roles of γ-Glutamyl Peptides Produced by γ-Glutamyltransferase and the Glutathione Synthesis System. Cells, 12(24), 2831. https://doi.org/10.3390/cells12242831





