Abstract
Protein citrullination is accomplished by a broad enzyme family named Peptidyl Arginine Deiminases (PADs), which makes this post-translational modification in many proteins that perform physiological and pathologic mechanisms in the body. Due to these modifications, citrullination has become a significant topic in the study of pathological processes. It has been related to some chronic and autoimmune diseases, including rheumatoid arthritis (RA), interstitial lung diseases (ILD), multiple sclerosis (MS), and certain types of cancer, among others. Antibody production against different targets, including filaggrin, vimentin, and collagen, results in an immune response if they are citrullinated, which triggers a continuous inflammatory process characteristic of autoimmune and certain chronic diseases. PAD coding genes (PADI1 to PADI4 and PADI6) harbor variations that can be important in these enzymes’ folding, activity, function, and half-life. However, few studies have considered these genetic factors in the context of chronic diseases. Exploring PAD pathways and their role in autoimmune and chronic diseases is a major topic in developing new pharmacological targets and valuable biomarkers to improve diagnosis and prevention. The present review addresses and highlights genetic, molecular, biochemical, and physiopathological factors where PAD enzymes perform a major role in autoimmune and chronic diseases.
1. Introduction
Protein citrullination is accomplished by a large enzyme family named Peptidyl Arginine Deiminases (PAD), which makes this post-translational modification in a large number of proteins that perform ordinary and pathogenic mechanisms in the body, from keratinocyte differentiation to the involvement of signaling pathway and/or estrogen regulation, to name a few examples.
The role of PAD in the pathophysiological mechanism of diseases such as autoimmune or chronic diseases is complex, including involvement in the production of antibody target molecules (autoantibodies in autoimmune diseases), which could be a consequence of abnormal modification by PAD or alteration of activity in joints as well as in other organs and systems. A change in the activity of these enzyme-functional proteins is mainly associated with an inflammatory process that triggers an exaggerated response.
This review aims to investigate PAD enzymes, which significantly affect autoimmune and chronic diseases. This modification has been implicated in various physiological and pathological processes, including chronic inflammatory diseases such as rheumatoid arthritis and lung diseases related to autoimmune and chronic diseases. We will focus on the PAD enzymes involved in rheumatoid arthritis (RA) and pulmonary diseases.
2. PAD Enzyme
2.1. Peptidyl Arginine Deiminases “PADs”
Post-translational modifications (PTM) include genetic expression, enzymatic activity, and protein stability, critical processes in cell biology. These PTMs can be made by phosphorylation, acetylation, methylation, and citrullination. This last one is catalyzed by a calcium (Ca2+)-dependent enzyme family called peptidyl arginine deiminases (PADs; EC 3.5.3.15) [1,2].
2.2. Biological Function
PADs regulate many processes in fundamental cellular mechanisms; there are five isoenzymes in mammals, PAD1 to PAD4 and PAD6, which have 70–95% of homology in the amino-acid sequence [3] and different substrates and specific distribution/expression in the body [1,4], establishing an addressed biological function. Some biological functions and distribution in the body of PADs are shown in Table 1.

Table 1.
Characteristics of peptidyl arginine deiminase (PAD) enzymes.
The most described function is keratin citrullination, which is a necessary process for the cornification of the epidermis performed by PAD1 [13]; PAD2 has a major role in astrocyte signaling on the central nervous system (CNS) [13]; PAD3 has been associated with the loss of regenerative ability in neural cells [14]; PAD4 is mainly located in cell nucleus, and among its functions is to regulate chromatin decondensation by histone citrullination, gene regulation, and neutrophil extracellular trap formation [15]; finally, PAD6 is involved in oocyte formation and growth, and microtubule regulation and movement [13].
2.3. Enzyme Mechanism
Protein citrullination mediated by PAD is conducted by hydrolysis in the guanidinium bond of peptidyl arginine producing peptidyl citrulline and ammonium, which contains a large number of guanidinium modifications; these enzymes are named aminotransferases [16]. PADs have specific inhibitors determined by chemical reactions. In PAD2, guanidinium neutralization positive charge is replaced by urea, decreasing cysteine nucleophilic pKa, compared with the reverse protonation mechanism in PAD1, 3, and 4 [1].
At the protein level, citrullination induces molecular mass reduction to 1.0 Da by each modified arginine, losing its positive charge [3,17]. The isoelectric point provided by the hydrogen bond allows interaction with other proteins [2,10,18]. The negative net charge is believed to recognize the substrate of arginine residues with a positive charge and the union of essential calcium ions [19].
Citrullinated neoepitope production in extracellular space makes them reachable to the immune system and then initiates an immune response, which modifies citrullinated protein function and its half-life [20].
2.4. Enzyme Regulation
Specific biochemical reactions mediate the PAD regulation process. Ca2+ ion is the most described regulator. Low intracellular Ca2+ concentrations make PAD inactive [3]. However, when there is an alteration (triggered by apoptosis, necrosis, or oxidative stress), Ca2+ levels increase and activate the enzyme [3,7,21,22], which makes the citrullination unspecific, reducing the positive charges and stability of the nucleosome as illustrated in Figure 1A [23].
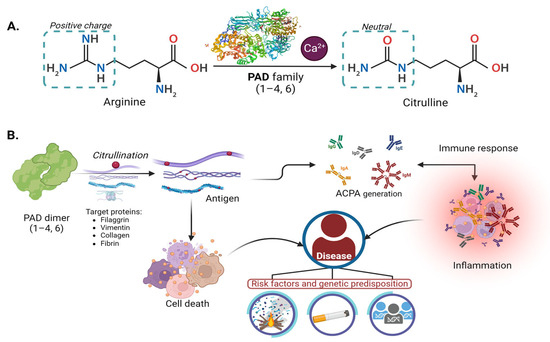
Figure 1.
Citrullination molecular mechanism of PAD enzyme physiology and pathology: (A) Mechanism of deimination (citrullination) mediated by PAD enzymes dependent on calcium. (B) Participation of PAD enzymes in physiopathological mechanisms. The PADs play a role in the citrullination of arginine in proteins such as filaggrin, vimentin, keratin, collagen, and others.
PAD enzymatic activity is also regulated by pH, exhibiting a maximum peak between 7.0 and 8.0 on PAD2 and PAD4 in the ionization process [24]. Although PAD has the same covalent catalysis mechanism by a cysteine residue in its active site, they show differences in kinetic properties [25,26,27].
PAD substrates affect enzymatic regulation. The most reported are estradiol, filaggrin, vimentin, myelin basic protein (MBP), fibrinogen, some chemokines, and histones [17,28,29]. Estrogens regulate PAD4 expression by improving promoter binding sites of PADI4 [2], and nuclear substrate for PAD4 are histones H2A, H3, and H4 [25].
Figure 1B illustrates the physiological mechanism that generates antigens, potentially initiating an autoimmune, inflammatory response leading to relevant diseases, as described in Section 4. Modifications within the gene structure of each isoform were observed to alter the expression of PAD proteins, as detailed below.
3. Genes
3.1. Localization and Structure of the Gene
PAD coding genes are located in chromosomal region 1p35-36, with a ~355Kb length; they are named PADI genes [25,30]. However, as shown in Figure 2, each gene has a different length and structure.
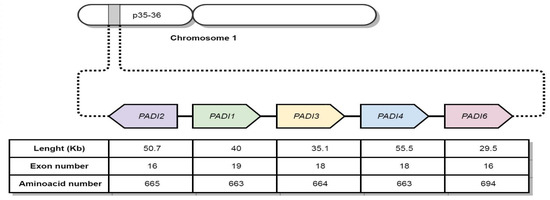
Figure 2.
Localization of PADI genes encoding PAD enzymes.
3.2. Genetic Variants
Many genetic variants have been described in coding regions (exons), non-coding regions (introns), and nearby regions. These genetic variants are classified as single-nucleotide polymorphisms (SNPs), insertions, deletions, etc., [31,32,33], and details are shown in Table 2.

Table 2.
Location of PADI genes and variants present.
Among the genetic variants reported in PADI genes, SNPs represent ~90% of the total variation, of which ~50% are located in structural/regulatory regions (srSNP: 3′UTR, 5′UTR, and intronic), and only 3–8% are in coding regions (cSNP) that can be either synonymous or non-synonymous [34,35]. These variants can affect the proteins by modifying their structure, function, electric charge, or substrate affinity. Due to the many SNPs located in different regions in PADI genes and the effect of their biological consequence, they have been evaluated in genetic association studies with complex traits.
4. Citrullination, PAD, and Physiopathology
Citrullination causes structural and functional changes in their biological targets (irreversible modification), such as a loss of positive charge, conformational modification, alterations in protease, or interaction protein affinity (Figure 1B). This physiological process may generate new antigens that induce a systemic response [13].
Due to these modifications, citrullination has become a major topic in the study of pathological processes. It has been related to some chronic and autoimmune diseases, including RA, chronic lung diseases, multiple sclerosis (MS), Alzheimer’s disease, and certain types of cancer [1,4,18,36].
Multiple sclerosis (MS) is a severe autoimmune and complex disease affecting electrical neural conduction in the CNS by gradually losing myelin in oligodendroglial cells. It has been proposed that MBP hypercitrullination plays a significant role in this disease [3]. MBP is a component in myelin sheaths that naturally protects neural axons and is synthesized by oligodendrocytes; MBP deimination can alter proteolysis sensibility by unsettling CNS cells due to an altered interaction of myelin sheaths phospholipids [37,38]. PAD4 has increased levels and activity in MS patients caused by either a demyelination process or a dysfunction in myelin repairment; either way, oligodendrocyte survival is affected [39].
An increase in PAD2 and citrullinated proteins like vimentin have been detected in some neurodegenerative diseases like Alzheimer’s [13,40]. Additionally, PAD3 has been related to decreased regenerative ability in neural cells [41,42]. It has been addressed in other neurodegenerative diseases like schizophrenia; however, it has not presented meaningful results associated with PADI2 [43].
Some types of cancer have been linked to altered roles of PAD and their coding genes. It has been found that tumors, including squamous cell esophageal carcinoma, have an abnormally increased expression in PADI4. Furthermore, this increase has been associated with carcinogenesis, progress, and metastasis in other tumors [44]. However, expression decreases when the tumor is extirpated [45]. It has been argued that tumors can induce autoantibodies for assembling after new epitope recognition [46].
In breast cancer, PAD2 has a major role in cell proliferation [47], and PADI2 is over-expressed in luminal subtype cells [48]. PADI4 has been related to regulating gene expression, transcriptional activation, and interaction with other genes and proteins in mammary glands [49].
PADI2 has been proposed in multiple myeloma as a therapeutic target due to its capacity to remove plasmatic cells’ signaling portion to their abnormal proliferation [50].
The higher PAD4 levels in patients with lung cancer secondary to tobacco smoking suggest that citrullination increase in lung tissue is not necessarily associated with ACPA production; however, it can enhance or be a product of tumor development [46].
Thanks to in vitro studies performed in cancer cells, it has been possible to identify citrullinated proteins like ENO1, HSP60, KRT8, and TUBB, which are citrullinated and expressed in tumors. These proteins affect signaling pathways and cytoskeleton components, but their physiological role changes to an abnormal pathological grade when they are citrullinated [51].
In the following section, we will explore the involvement of PAD proteins in rheumatoid arthritis and lung disease, from protein to genetic alterations, and their impact on these diseases.
4.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by a symmetric inflammation of peripheral synovial joints [52]. There has been reported an increase in autoantibodies against different biological targets like collagen type II, vimentin, filaggrin, and heat shock proteins, besides highly specific antibodies like anti-citrullinated peptide antibodies (ACPAs), in synovial fluid and serum samples of RA patients [4,53,54,55].
4.1.1. Molecular Mechanisms and Role of PADs in RA
ACPAs are one of the biochemical markers used in the diagnosis of RA, with a sensitivity of >80% and a specificity of 98% in RA patients. However, one of the methods these autoantibodies are produced may be due to the catalytic activity in arginine to citrulline modification (carried out by PAD proteins), which will lead to changes in peptides or proteins that will be found to be citrullinated. These will be the proteins identified by ACPAs [56,57], which can promote the generation of more specific epitopes to ACPAs [58], and PAD2 activity in synovial fluid in this ACPA generation has been described [59]. These processes suggest that RA can be detected in the early stages because of the recognition of peptidyl citrulline epitopes, thus facilitating RA diagnosis [60,61].
Despite the relatively high frequency of inflammatory processes in daily life, only a tiny fraction of the population develops ACPAs, which is highly related to RA development. In synovial fluid samples, PAD enzymes’ presence and activity influence ACPA production [3,59].
The expression of PAD2 and PAD4 is unique to individuals with RA [62]. PAD2 levels are elevated in synovial fluid, along with the presence of ACPA-positive [63].
At the clinical level, antibodies generated by peptide citrullination and antibodies against these proteins (anti-PAD) have been linked to the activity and duration of RA. ACPAs derived from peptide citrullination are associated with RA disease activity, and in carrying anti-PAD (3/4) antibody patients, 53% exhibit low disease activity indices (measured by the CDAI: clinical disease activity index). On average, individuals with ACPAs have a 9.5-year longer duration of RA compared to those with ACPA-negative [64].
Nonetheless, it has been noted that during the pre-diagnostic phase, both anti-PAD4 and anti-CCP antibodies are initially detected at low levels and gradually increase as the disease progresses. These autoantibodies are also linked to erosion and damage that can be visualized through radiographic examination [65]. In early diagnosis RA, PAD levels were elevated and subsequently decreased after treatment with DMARDs (Disease-Modifying Antirheumatic Drugs) [66].
Concerning the PAD2 isoform, it has been established that the presence of anti-PAD2 antibodies is correlated with reduced severity of RA and diminished articular progression, as observed radiographically, regardless of the administration of DMARDs [67]. The presence of these antibodies in RA patients indicates an elevated risk, particularly in cases where multiple autoantibodies are concurrently present [68].
The possible mechanism by which the response to ACPAs increases involves a hapten-carrier model (hapten-citrullinated proteins/-carrier mechanism), with the participation of citrullinated proteins and genetics (HLA-DRB1 alleles). In this model, peptides are recognized by T-cells, leading to the production of ACPAs [69,70]. However, it is important to note that this mechanism is a hypothesis, and the production mechanism remains unknown.
PAD2, PAD4, and antibody expression have been associated with various proteins mediating inflammation resulting from the autoimmune response. These proteins include TNF-α, where PAD4 may contribute alongside TNF-α in amplifying ACPA production, suggesting a complex positive regulation involving PAD4, citrullinated antigens, ACPAs, and TNF-α, particularly in RA exacerbation [71].
Furthermore, the involvement of cells such as granulocytes and neutrophils has been described at the cellular response level. In the latter, the contribution of PAD enzymes plays an essential role in carrying out chromatin compaction; however, in diseases such as RA, the formation of neutrophil extracellular traps (NETs) has been linked to an inflammatory process [72] where the function of PADs, specifically PAD4, performs hypercitrullination of histone 3. This process is known as NETosis, which is a type of neutrophil cell death, leading to the generation of new antigens that ACPAs and the generation of an inflammatory response can target [73,74].
Among the risk factors described for the development of ACPAs, cultured targets, and increased antigen production are environmental factors such as smoking, occupational exposures, and genetic predisposition, which play an important role in the development and prognosis of RA [75,76,77].
4.1.2. Genetic Associations: Variations in PAD Genes and Their Potential Contribution to RA Susceptibility and Severity
Among the primary risk factors associated with developing this disease and contributing to generating ACPAs, tobacco smoking has been extensively studied. It plays a crucial role in the development of RA by inducing citrullination, a critical mechanism in the development of autoimmune and inflammatory diseases, ultimately leading to an increase in ACPA blood levels [78,79]. As previously mentioned, genetic predisposition factors play an essential role in the development of rheumatoid arthritis. One or more alleles of the shared epitope (SE) of HLA-DRB1 have been linked to ACPAs and anti-PAD4 antibodies, although other genetic factors can also influence ACPA production. Smokers with RA have shown up to 20 times greater ACPA levels when carrying the HLA-DRB1*04:01 allele [80,81].
Andrade et al. propose that autocitrullination regulates the production of citrullinated proteins during cellular activation, and polymorphisms influence this process in PAD4, which plays a pivotal role in both its structural configuration and the immune response. They identified multiple citrullination sites in PAD4, including Arg-372 and Arg-374, located within the substrate recognition sites. These sites serve as potential targets for autocitrullination, leading to enzyme inactivation, thereby altering the structure of PAD4 and increasing its recognition by human antibodies. This alteration subsequently affects enzyme–substrate interaction [16,82].
Single-nucleotide variants (SNVs) found in PADI4 have been identified as being associated with susceptibility to RA. Additionally, interactions between individuals homozygous for the GTG haplotype of PADI4 and the HLA-DRB1 SE have been linked to the production of anti-CCP antibodies, tobacco smoking, and erosive disease in these patients [83].
PADI1, PADI2, PADI4, and PADI6 genes have SNVs associated with RA worldwide, as shown in Table 3. Since identification of its role in the cellular nucleus, PADI4 is the most evaluated gene, mainly in Caucasians (British, North American, Swedish, French, German, and Dutch populations); Asians report associations in Japanese, Chinese, Korean, Indian, and Iranian populations, and in Latin America a single study was carried out in the Mexican population. PADI2 has had fewer studies associating SNV with RA, only in Chinese, Malayan, and Indian populations. PADI6 has also had scarce studies, including Chinese and Malayan populations; PADI1 only has an association report in the Chinese population.

Table 3.
Association studies of polymorphisms in PADI genes in rheumatoid arthritis.
Polymorphisms located in the intergenic region of PADI3/PADI4, combined with anti-cyclic citrullinated peptide (anti-CCP) antibodies, have been identified as factors associated with the onset of RA. These genetic variations have been linked to the citrullination process of histones, underscoring their significant role in deimination and subsequent ACPA generation [68]. Citrullination occurring at the Arg-372 and Arg-374 sites can be elucidated as the cause of PAD4 inactivation. This modification preserves the lysine amino acid while removing the guanidine group during citrullination, altering the enzyme’s binding affinity [16].
The SNVs associated with RA and most commonly observed in distinct populations are PADI2, particularly the rs1005753 in populations from China, Malaysia, and India, linked to decreased susceptibility to RA (OR < 1). While in PADI4, rs1748033, in Japan, Korea, India, and Iran, was linked to an increased RA risk (OR > 1), and rs11203366, in populations from Korea, Germany, France, and Mexico, was linked to susceptibility to RA (OR > 1.5).
4.2. Lung Diseases
The involvement of PAD and various citrullinated proteins has been identified in lung conditions, including cystic fibrosis, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and autoimmune-associated interstitial disease, such as RA (RA-ILD). These individuals were subjected to biological sample analysis of serum, bronchoalveolar lavage fluids, and sputum, which could reflect the particular damaged site environment (see Table 4).
The participation of PAD has been most frequently reported in interstitial lung diseases (ILD), a condition encompassing numerous subacute and chronic respiratory diseases characterized by a diffuse commitment of lung parenchyma that affects mainly alveolar interstitium and space. According to the ILD classification, we can distinguish primary and secondary types. It has been described that ILD can affect inflammation processes, like fibrosis in the lung parenchyma interstitium [96,97,98,99,100].
Pulmonary damage of collagen vascular diseases can affect almost every region in the lung, including the pleural cavity, alveoli, interstitium, blood vessels, lymphatic tissue, and the upper and lower respiratory tract [101]. Interstitial pneumonia patients associated with vascular collagen diseases have a better prognosis than other ILD patients, considering that collagen is a target for PAD enzymes [102,103,104,105].
4.2.1. Role of PADs in Lung Diseases
Several mechanisms have been proposed to explain autoantibody development and antigen response in the lung and other organs. Paulin et al. propose two possible pathways. First, an immune response against citrullinated peptides starts at joints and spreads to the lungs, resulting in pulmonary interstitium inflammation. Second, usual interstitial pneumonia patients carrying some genetic susceptibility to RA originate an immune response against citrullinated peptides by autoantibodies (ACPAs) in the lung, starting an inflammatory process that affects secondary joints [104,106]. Harlow et al. suggest that the immune response against citrullinated proteins is initiated in the lungs [107].
ACPAs have been strongly associated with smoking and SE in HLA-DRB1 presence between citrullinated vimentin and α-enolase peptide-1, suggesting that they can trigger an immune response in the lungs that increases citrullinated peptide formation shifting on ACPA response [108].
High levels of protein citrullination and PAD are primarily linked to smoking, particularly PAD2 [109]. Smoking is one of several factors that facilitate the modification of protein citrullination in the lung, potentially resulting in the production of ACPAs and contributing to the onset of autoimmune disease [81] (Table 4).

Table 4.
Association studies of PAD protein in lung diseases.
Table 4.
Association studies of PAD protein in lung diseases.
| Protein | Lung Disease | Evaluation | Clinical Finding | References |
|---|---|---|---|---|
| PAD4 | Cystic fibrosis | Autoantibody anti-PAD4 levels | Elevated levels compared with the control group. Negative correlation with pulmonary function. | [110] |
| PAD4 | Cystic fibrosis | Autoantibody anti-PAD4 levels | Increased levels were observed, compared to patients with rheumatoid arthritis. A negative correlation was found between lung function and increased P. aeruginosa lung infection. | [111] |
| PAD2 PAD4 | COPD | LL-37 Citrullination | Infiltration of airway cells with PAD4 and Neutrophils. PADI2 in bronchial epithelial cells and leukocytes. PADI2 and PADI4 citrullinated LL-37 at three arginine sites (7, 29, and 34). This led to changes in the production of proinflammatory cytokines, including TNF-α. | [112] |
| PAD2 | IPF | Fibrosis | Citrullinated vimentin in lung macrophages, a significant increase in IPF and IPF-smokers. | [113] |
| PAD4 | IPF RA-ILD | Protein expression in granulocytes and macrophages | Citrullinated peptide increased in IPF and ILD | [114] |
| PAD4 | IPF RA-ILD | Autoantibody production | Anti-PAD4- patients have a higher DTA Fibrosis Score (fibrosis on HRCT was quantified using a data-driven texture analysis DTA fibrosis score). Anti-PAD4+ better lung function (FVC%) | [115] |
| CEP-1 | RA-ILD | Autoantibody production | The presence of anti-CCP/CEP-1+ is associated with ILD and erosive disease. | [116] |
| ACPA | RA-ILD | Reactivity of peptides Citrullinated proteins | >3-fold increase in reactivity. | [117] |
| PAD2 PAD4 | RA-ILD | Protein levels and SNV in gene PADI | Increased protein PAD4 levels in RA-ILD patients and PADI4 SNV risk genotype carriers. | [118] |
Anti-citrullinated alpha-enolase (CEP-1); anti-citrullinated peptide antibody (ACPA); chronic obstructive pulmonary disease (COPD); idiopathic pulmonary fibrosis (IPF); rheumatoid arthritis associated with interstitial lung disease (RA-ILD); high-resolution computed tomography (HRCT); forced vital capacity (FVC); single-nucleotide variants (SNVs).
PAD activity has also been studied in different pulmonary diseases and described in BAL samples retrieved from bronchial mucosa, and biopsies of smokers show an increase in PAD2 expression [81,109]. Table 4 outlines lung diseases in which PAD proteins and citrullinated peptides are linked to pulmonary clinical findings. Regarding cystic fibrosis, PAD4 levels correlate negatively with lung function. Furthermore, the presence of P. aeruginosa infection can elevate the protein levels [110,111,119].
In COPD, the citrullination by PAD2 and PAD4 on the anti-microbial, anti-inflammatory peptide LL-37 has been reported. This modification is seen to alter the activity and function of the peptide and proteins signaled during lung inflammation [112].
PAD2 and PAD4 with their substrates (vimentin, fibrinogen, and α-enolase peptide 1) have been found in the lungs, lymph nodes, spleen, and skeletal muscle of COPD patients [108]. Other citrullinated proteins involved in COPD are Fibulin-5 and cytokeratins wich PAD modifies in lung cells and are associated with lung disease in parenchymal destruction processes such as emphysema and COPD exacerbations [120,121,122].
Citrullination and generation of antibodies, such as anti-elastin and anti-CCP, have also been observed in α1-Antitrypsin deficiency. Smoking, one of the main generators of pro-inflammatory molecules, plays an essential role in this process, generating a conducive environment for peptide citrullination in the lung [123,124]. Although smoking is most related to citrullination in pathological processes, exposure to smoke from biomass burning has also been described in individuals without RA in COPD patients [125].
In the context of interstitial lung diseases, an increase in PAD proteins and citrullinated peptides such as vimentin in various cell types has been described in IPF (Table 4). Finally, RA-ILD indicates a potential synergistic interaction between smoking, the elevation of PAD protein levels, and autoantibodies (PAD3/4), which promote an immunological response-favorable environment [118,126].
One of the proteins that may regulate PAD is Gal-9, an immunomodulatory protein that enhances granulocyte activation, resulting in an increase in the expression of PAD4 and the formation of autoantigens [127]. Furthermore, citrullinated proteins have been found to increase in granulocytes and macrophages in IPF and ILD, along with other proteins. Finally, it has been observed that the generation of anti-PAD4 autoantibodies is associated with improved lung function and reduced fibrosis scores in ILD linked to RA [128].
4.2.2. NET-PAD in Lung Diseases
The involvement of PAD4 in the formation of neutrophil extracellular traps (NETs) in the lung is a topic that varies according to the lung disease in which it was described. Physiologically, PAD4 participates in histone citrullination but primarily in infections (Klebsiella pneumoniae) [72], which may be found generating new epitopes from the lung; however, not all people with lung disease have such infections.
In cystic fibrosis, there is no difference in NETs and serum-free DNA compared to controls, but increased levels of PAD4, particularly in individuals with P. aeruginosa infection, and, in turn, increased levels of ACPAs [111]; however, NETs were not associated. PAD4 was correlated with free DNA (anti-dsDNA IgA autoantibodies) [110].
In COPD, neutrophil response and NET formation have been associated with increased disease progression and decreased lung function [129], but they are also associated with disease severity, not just exacerbations [121].
In fibrotic ILD, the production of NETs in an inflammatory phase has been described, and concerning PAD4, a deficiency of the protein could prevent the development of NETs and, consequently, pulmonary fibrosis, avoiding damage and destruction of the alveolar epithelium and endothelium [130]. It has also been suggested that the NETs levels is related to other proteins, such as the HIF-1α-αMβ2 integrin axis in the pulmonary interstitium [131].
Among the limitations of our review is the lack of genetic information on PAD in lung diseases, especially among RA patients, in which susceptibility has been described in different populations with controvert results, so it is the perspective of the study to evaluate the predisposition of alleles in PADI genes and their impact on proteins, as well as involvement in lung diseases. Furthermore, an explanation of how the lung and joint work together in protein citrullination and immune response should be provided.
5. Conclusions
The role of peptidyl arginine deiminases in protein citrullination is crucial in physiological pathways. In chronic diseases, environmental factors often lead to continuous alteration of this biological mechanism. In addition, genetic factors play a specific role in their pathogenesis. Genetic variations may represent a new component in the role of PADs in chronic diseases, potentially contributing to developing new pharmacological targets and valuable biomarkers to improve diagnosis and prevention.
In rheumatoid arthritis, single-nucleotide variants in genes encoding PADs and haplotype analyses have been associated with functional changes in the protein and its enzymatic function. Anti-PAD4 autoantibodies and citrullinated proteins are essential in cellular development and physiological and pathological lung maintenance in lung diseases.
Author Contributions
Conceptualization, K.J.N.-Q., L.A.L.-F. and J.R.-S.; methodology, K.J.N.-Q., L.A.L.-F. and G.P.-R.; software, K.J.N.-Q.; validation, L.A.L.-F. and R.F.-V.; formal analysis, J.R.-S. and G.P.-R.; investigation, K.J.N.-Q. and L.A.L.-F.; resources, J.R.-S. and G.P.-R.; data curation, K.J.N.-Q. and L.A.L.-F.; writing—original draft preparation, K.J.N.-Q.; writing—review and editing, K.J.N.-Q., L.A.L.-F. and R.F.-V.; visualization, K.J.N.-Q.; supervision, J.R.-S., G.P.-R. and R.F.-V.; project administration, R.F.-V.; funding acquisition, R.F.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
To the Programa de Doctorado en Ciencias Médicas Odontológicas y de la Salud of the Universidad Nacional Autónoma de México (UNAM). Furthermore, the support provided by the Consejo Nacional de Humanidades, Ciencia y Tecnología (National Council of Science and Technology), (CONAHCyT), CVU: 690362.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Dreyton, C.J.; Knuckley, B.; Jones, J.E.; Lewallen, D.M.; Thompson, P.R. Mechanistic Studies of Protein Arginine Deiminase 2: Evidence for a Substrate-Assisted Mechanism. Biochemistry 2014, 53, 4426–4433. [Google Scholar] [CrossRef]
- Anzilotti, C.; Pratesi, F.; Tommasi, C.; Migliorini, P. Peptidylarginine Deiminase 4 and Citrullination in Health and Disease. Autoimmun. Rev. 2010, 9, 158–160. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.W.; van Venrooij, W.J.; Pruijn, G.J.M. PAD, a Growing Family of Citrullinating Enzymes: Genes, Features and Involvement in Disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- van Gaalen, F.A.; Linn-Rasker, S.P.; van Venrooij, W.J.; de Jong, B.A.; Breedveld, F.C.; Verweij, C.L.; Toes, R.E.; Huizinga, T.W. Autoantibodies to Cyclic Citrullinated Peptides Predict Progression to Rheumatoid Arthritis in Patients with Undifferentiated Arthritis: A Prospective Cohort Study. Arthritis Rheum. 2004, 50, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Chavanas, S.; Adoue, V.; Méchin, M.-C.; Ying, S.; Dong, S.; Duplan, H.; Charveron, M.; Takahara, H.; Serre, G.; Simon, M. Long-Range Enhancer Associated with Chromatin Looping Allows AP-1 Regulation of the Peptidylarginine Deiminase 3 Gene in Differentiated Keratinocyte. PLoS ONE 2008, 3, e3408. [Google Scholar] [CrossRef] [PubMed]
- Méchin, M.C.; Enji, M.; Nachat, R.; Chavanas, S.; Charveron, M.; Ishida-Yamamoto, a.; Serre, G.; Takahara, H.; Simon, M. The Peptidylarginine Deiminases Expressed in Human Epidermis Differ in Their Substrate Specificities and Subcellular Locations. Cell. Mol. Life Sci. 2005, 62, 1984–1995. [Google Scholar] [CrossRef]
- Nachat, R.; Méchin, M.C.; Takahara, H.; Chavanas, S.; Charveron, M.; Serre, G.; Simon, M. Peptidylarginine Deiminase Isoforms 1-3 Are Expressed in the Epidermis and Involved in the Deimination of K1 and Filaggrin. J. Investig. Dermatol. 2005, 124, 384–393. [Google Scholar] [CrossRef]
- Dong, S.; Kanno, T.; Yamaki, A.; Kojima, T.; Shiraiwa, M.; Kawada, A.; Méchin, M.-C.; Chavanas, S.; Serre, G.; Simon, M.; et al. NF-Y and Sp1/Sp3 Are Involved in the Transcriptional Regulation of the Peptidylarginine Deiminase Type III Gene (PADI3) in Human Keratinocytes. Biochem. J. 2006, 397, 449–459. [Google Scholar] [CrossRef]
- Chang, X. Jinxiang Han Expression of Peptidylarginine Deiminase Type 4 (PAD4) in Various Tumors. Mol. Carcinog. 2006, 45, 183–196. [Google Scholar] [CrossRef]
- Baka, Z.; György, B.; Géher, P.; Buzás, E.I.; Falus, A.; Nagy, G. Citrullination under Physiological and Pathological Conditions. Jt. Bone Spine 2012, 79, 431–436. [Google Scholar] [CrossRef]
- Kim, B.; Kan, R.; Anguish, L.; Nelson, L.M.; Coonrod, S.A. Potential Role for MATER in Cytoplasmic Lattice Formation in Murine Oocytes. PLoS ONE 2010, 5, e12587. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Kawada, A.; Yamanouchi, J.; Yosida-Noro, C.; Yoshiki, A.; Shiraiwa, M.; Kusakabe, M.; Manabe, M.; Tezuka, T.; Takahara, H. Human Peptidylarginine Deiminase Type III: Molecular Cloning and Nucleotide Sequence of the CDNA, Properties of the Recombinant Enzyme, and Immunohistochemical Localization in Human Skin. J. Investig. Dermatol. 2000, 115, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Ishigami, A.; Maruyama, N.; Carp, R.I.; Kim, Y.-S.; Choi, E.-K. Peptidylarginine Deiminase and Protein Citrullination in Prion Diseases: Strong Evidence of Neurodegeneration. Prion 2013, 7, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Rocha-Ferreira, E.; Thei, L.; Mawjee, P.; Bennett, K.; Thompson, P.R.; Subramanian, V.; Nicholas, A.P.; Peebles, D.; Hristova, M.; et al. Peptidylarginine Deiminases: Novel Drug Targets for Prevention of Neuronal Damage Following Hypoxic Ischemic Insult (HI) in Neonates. J. Neurochem. 2014, 130, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone Hypercitrullination Mediates Chromatin Decondensation and Neutrophil Extracellular Trap Formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.; Darrah, E.; Gucek, M.; Cole, R.N.; Rosen, A.; Zhu, X. Autocitrullination of Human Peptidyl Arginine Deiminase Type 4 Regulates Protein Citrullination during Cell Activation. Arthritis Rheum. 2010, 62, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Arandjelovic, S.; McKenney, K.R.; Leming, S.S.; Mowen, K.A. ATP Induces Protein Arginine Deiminase 2-Dependent Citrullination in Mast Cells through the P2X7 Purinergic Receptor. J. Immunol. 2012, 189, 4112–4122. [Google Scholar] [CrossRef] [PubMed]
- Tarcsa, E.; Marekov, L.N.; Mei, G.; Melino, G.; Lee, S.C.; Steinert, P.M. Protein Unfolding by Peptidylarginine Deiminase: Substrate Specificity and Structural Relationships of the Natural Substrates Trichohyalin and Filaggrin. J. Biol. Chem. 1996, 271, 30709–30716. [Google Scholar] [CrossRef]
- van Venrooij, W.J.; Pruijn, G.J. Citrullination: A Small Change for a Protein with Great Consequences for Rheumatoid Arthritis. Arthritis Res. 2000, 2, 249–251. [Google Scholar] [CrossRef]
- Teo, C.Y.; Shave, S.; Chor, A.L.T.; Salleh, A.B.; Rahman, M.B.B.A.; Walkinshaw, M.D.; Tejo, B.A. Discovery of a New Class of Inhibitors for the Protein Arginine Deiminase Type 4 (PAD4) by Structure-Based Virtual Screening. BMC Bioinform. 2012, 13 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Tsai, I.-C.; Chang, C.-W.; Liao, Y.-F.; Liu, G.-Y.; Hung, H.-C. Functional Roles of the Non-Catalytic Calcium-Binding Sites in the N-Terminal Domain of Human Peptidylarginine Deiminase 4. PLoS ONE 2013, 8, e51660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dong, S.; Ying, S.; Kojima, T.; Shiraiwa, M.; Kawada, A.; Méchin, M.-C.; Adoue, V.; Chavanas, S.; Serre, G.; Simon, M.; et al. Crucial Roles of MZF1 and Sp1 in the Transcriptional Regulation of the Peptidylarginine Deiminase Type I Gene (PADI1) in Human Keratinocytes. J. Investig. Dermatol. 2008, 128, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Hagiwara, T.; Yamada, M. Nuclear Localization of Peptidylarginine Deiminase V and Histone Deimination in Granulocytes. J. Biol. Chem. 2002, 277, 49562–49568. [Google Scholar] [CrossRef] [PubMed]
- Nakayama-Hamada, M.; Suzuki, A.; Kubota, K.; Takazawa, T.; Ohsaka, M.; Kawaida, R.; Ono, M.; Kasuya, A.; Furukawa, H.; Yamada, R.; et al. Comparison of Enzymatic Properties between HPADI2 and HPADI4. Biochem. Biophys. Res. Commun. 2005, 327, 192–200. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Tóth, E.; Tarcsa, E.; Falus, A.; Buzás, E.I. Citrullination: A Posttranslational Modification in Health and Disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Knuckley, B.; Bhatia, M.; Thompson, P.R. Protein Arginine Deiminase 4: Evidence for a Reverse Protonation Mechamism. Biochemistry 2007, 46, 6578–6587. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, S.B.; Stitt, B.L.; Ash, D.E. Cysteine 351 Is an Essential Nucleophile in Catalysis by Porphyromonas Gingivalis Peptidylarginine Deiminase. Arc. Biochem. Biophys. 2011, 504, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Causey, C.P.; Knuckley, B.; Slack-Noyes, J.L.; Thompson, P.R. Protein Arginine Deiminase 4 (PAD4): Current Understanding and Future Therapeutic Potential. Curr. Opin. Drug Discov. Devel. 2009, 12, 616–627. [Google Scholar]
- Leitman, D.C.; Paruthiyil, S.; Vivar, O.I.; Saunier, E.F.; Herber, C.B.; Cohen, I.; Tagliaferri, M.; Speed, T.P. Regulation of Specific Target Genes and Biological Responses by Estrogen Receptor Subtype Agonists. Curr. Opin. Pharmacol. 2011, 10, 629–636. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Després, N.; Lapointe, E.; van der Heijden, A.; Lora, M.; Senshu, T.; van Venrooij, W.J.; Ménard, H.A. Rheumatoid Arthritis Specific Anti-Sa Antibodies Target Citrullinated Vimentin. Arthritis Res. Ther. 2004, 6, 142–150. [Google Scholar] [CrossRef]
- Nakashima, K.; Arai, S.; Suzuki, A.; Nariai, Y.; Urano, T.; Nakayama, M.; Ohara, O.; Yamamura, K.; Yamamoto, K.; Miyazaki, T. PAD4 Regulates Proliferation of Multipotent Haematopoietic Cells by Controlling C-Myc Expression. Nat. Commun. 2013, 4, 1836. [Google Scholar] [CrossRef] [PubMed]
- Too, C.L.; Murad, S.; Dhaliwal, J.S.; Larsson, P.T.; Jiang, X.; Ding, B.; Alfredsson, L.; Klareskog, L.; Padyukov, L. Polymorphisms in Peptidylarginine Deiminase (PADI) Associate with Rheumatoid Arthritis in Diverse Asian Populations: Evidence from MyEIRA Study and Meta-Analysis. Arthritis Res. Ther. 2012, 14, R250. [Google Scholar] [CrossRef] [PubMed]
- Iida, A.; Nakamura, Y. Identification of 45 Novel SNPs in the 83-Kb Region Containing Peptidylarginine Deiminase Types 1 and 3 Loci on Chromosomal Band 1p36.13. J. Hum. Genet. 2004, 49, 387–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zerbino, D.R.; Achuthan, P.; Akanni, W.; Amode, M.R.; Barrell, D.; Bhai, J.; Billis, K.; Cummins, C.; Gall, A.; Girón, C.G.; et al. Ensembl 2018. Nucleic Acids Res. 2017, 46, D754–D761. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Bello, J.; Vargas-Alarcón, G.; Tovilla-Zárate, C.; Fragoso, J.M. Polimorfismos de Un Solo Nucleótido (SNP): Implicaciones Funcionales de Los SNP Reguladores (RSNP) y de Los SNP-ARN Estructurales (SrSNP) En Enfermedades Complejas. Gac. Med. Mex. 2013, 149, 220–228. [Google Scholar] [PubMed]
- Lim, M.-K.; Shim, T.S.; Park, M.; Lee, S.-K.; Sohn, Y.-H.; Sheen, D.-H.; Shim, S.-C. Heterozygote Genotypes for PADI4_89 Were Protectively Associated with Susceptibility to Tuberculosis in Koreans. Rheumatol. Int. 2014, 35, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Moscarello, M.A.; Wood, D.D.; Ackerley, C.; Boulias, C. Myelin in Multiple Sclerosis Is Developmentally Immature. J. Clin. Investig. 1994, 94, 146–154. [Google Scholar] [CrossRef]
- Moscarello, M.A.; Mastronardi, F.G.; Wood, D.D. The Role of Citrullinated Proteins Suggests a Novel Mechanism in the Pathogenesis of Multiple Sclerosis. Neurochem. Res. 2007, 32, 251–256. [Google Scholar] [CrossRef]
- Mastronardi, F.G.; Wood, D.D.; Mei, J.; Raijmakers, R.; Tseveleki, V.; Dosch, H.-M.; Probert, L.; Casaccia-Bonnefil, P.; Moscarello, M.A. Increased Citrullination of Histone H3 in Multiple Sclerosis Brain and Animal Models of Demyelination: A Role for Tumor Necrosis Factor-Induced Peptidylarginine Deiminase 4 Translocation. J. Neurosci. 2006, 26, 11387–11396. [Google Scholar] [CrossRef]
- Asaga, H.; Ishigami, A. Protein Deimination in the Rat Brain after Kainate Administration: Citrulline-Containing Proteins as a Novel Marker of Neurodegeneration. Neurosci. Lett. 2001, 299, 5–8. [Google Scholar] [CrossRef]
- Lange, S.; Gögel, S.; Leung, K.-Y.; Vernay, B.; Nicholas, A.P.; Causey, C.P.; Thompson, P.R.; Greene, N.D.E.; Ferretti, P. Protein Deiminases: New Players in the Developmentally Regulated Loss of Neural Regenerative Ability. Dev. Biol. 2011, 355, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, A.; Ohsawa, T.; Hiratsuka, M.; Taguchi, H.; Kobayashi, S.; Saito, Y.; Murayama, S.; Asaga, H.; Toda, T.; Kimura, N.; et al. Abnormal Accumulation of Citrullinated Proteins Catalyzed by Peptidylarginine Deiminase in Hippocampal Extracts from Patients with Alzheimer’s Disease. J. Neurosci. Res. 2005, 80, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nunokawa, A.; Kaneko, N.; Arinami, T.; Ujike, H.; Inada, T.; Iwata, N.; Kunugi, H.; Itokawa, M.; Otowa, T.; et al. A Two-Stage Case-Control Association Study of PADI2 with Schizophrenia. J. Hum. Genet. 2009, 54, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, H.J.; Sun, N.B.; Chang, X.T.; Xu, B.; Wang, Y.; Cao, M.; Zhu, Q.; Zang, Q.I.; Jiang, Z.M. B-Cell Specific Moloney Leukemia Virus Insert Site 1 and Peptidyl Arginine Deiminase IV Positively Regulate Carcinogenesis and Progression of Esophageal Squamous Cell Carcinoma. Oncol. Lett. 2017, 13, 4349–4356. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Han, J.; Pang, L.; Zhao, Y.; Yang, Y.; Shen, Z. Increased PADI4 Expression in Blood and Tissues of Patients with Malignant Tumors. BMC Cancer 2009, 9, 40. [Google Scholar] [CrossRef]
- Baka, Z.; Barta, P.; Losonczy, G.; Krenács, T.; Pápay, J.; Szarka, E.; Sármay, G.; Babos, F.; Magyar, A.; Géher, P.; et al. Specific Expression of PAD4 and Citrullinated Proteins in Lung Cancer Is Not Associated with Anti-CCP Antibody Production. Int. Immunol. 2011, 23, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Cherrington, B.D.; Zhang, X.; McElwee, J.L.; Morency, E.; Anguish, L.J.; Coonrod, S.A. Potential Role for PAD2 in Gene Regulation in Breast Cancer Cells. PLoS ONE 2012, 7, e41242. [Google Scholar] [CrossRef] [PubMed]
- McElwee, J.L.; Mohanan, S.; Griffith, O.L.; Breuer, H.C.; Anguish, L.J.; Cherrington, B.D.; Palmer, A.M.; Howe, L.R.; Subramanian, V.; Causey, C.P.; et al. Identification of PADI2 as a Potential Breast Cancer Biomarker and Therapeutic Target. BMC Cancer 2012, 12, 500. [Google Scholar] [CrossRef]
- Zhang, X.; Gamble, M.J.; Stadler, S.; Cherrington, B.D.; Causey, C.P.; Thompson, P.R.; Roberson, M.S.; Kraus, W.L.; Coonrod, S.A. Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate c-Fos Expression in Breast Cancer Cells. PLoS Genet. 2011, 7, e1002112. [Google Scholar] [CrossRef]
- McNee, G.; Eales, K.L.; Wei, W.; Williams, D.S.; Barkhuizen, A.; Bartlett, D.B.; Essex, S.; Anandram, S.; Filer, A.; Moss, P.A.H.; et al. Citrullination of Histone H3 Drives IL-6 Production by Bone Marrow Mesenchymal Stem Cells in MGUS and Multiple Myeloma. Leukemia 2017, 31, 373–381. [Google Scholar] [CrossRef]
- Jiang, Z.; Cui, Y.; Wang, L.; Zhao, Y.; Yan, S.; Chang, X. Investigating Citrullinated Proteins in Tumour Cell Lines. World J. Surg. Oncol. 2013, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, M.; Simon, M.; Vincent, C.; Masson-Bessière, C.; Girbal, E.; Durieux, J.-J.; Serre, G. The Antiperinuclear Factor and the So-Called Antikeratin Antibodies Are the Same Rheumatoid Arthritis-Specific Autoantibodies. J. Clin. Investig. 1995, 95, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Firestein, G.S. Evolving Concepts of Rheumatoid Arthritis. Nature 2003, 423, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Radstake, T.R.D.; Van Der Heijden, A.; Van Mansum, M.A.M.; Dieteren, C.; De Rooij, D.J.; Barrera, P.; Zendman, A.J.W.; Van Venrooij, W.J. Expression and Activity of Citrullinating Peptidylarginine Deiminase Enzymes in Monocytes and Macrophages. Ann. Rheum. Dis. 2004, 63, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, S.; Chen, B.; Zhu, Q.; Xu, L.; Li, F. Serum Anti-Citrullinated Protein Antibodies and Rheumatoid Factor Increase the Risk of Rheumatoid Arthritis–Related Interstitial Lung Disease: A Meta-Analysis. Clin. Rheumatol. 2021, 40, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Shoda, H.; Fujio, K.; Shibuya, M.; Okamura, T.; Sumitomo, S.; Okamoto, A.; Sawada, T.; Yamamoto, K. Detection of Autoantibodies to Citrullinated BiP in Rheumatoid Arthritis Patients and Pro-Inflammatory Role of Citrullinated BiP in Collagen-Induced Arthritis. Arthritis Res. Ther. 2011, 13, R191. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kochi, Y.; Shoda, H.; Seri, Y.; Fujio, K.; Sawada, T.; Yamada, R.; Yamamoto, K. Decreased Severity of Experimental Autoimmune Arthritis in Peptidylarginine Deiminase Type 4 Knockout Mice. BMC Musculoskelet. Disord. 2016, 17, 205. [Google Scholar] [CrossRef]
- Damgaard, D.; Senolt, L.; Nielsen, M.F.; Pruijn, G.J.; Nielsen, C.H. Demonstration of Extracellular Peptidylarginine Deiminase (PAD) Activity in Synovial Fluid of Patients with Rheumatoid Arthritis Using a Novel Assay for Citrullination of Fibrinogen. Arthritis Res. Ther. 2014, 16, 498. [Google Scholar] [CrossRef]
- Ikari, K.; Kuwahara, M.; Nakamura, T.; Momohara, S.; Hara, M.; Yamanaka, H.; Tomatsu, T.; Kamatani, N. Association between PADI4 and Rheumatoid Arthritis: A Replication Study. Arthritis Rheum. 2005, 52, 3054–3057. [Google Scholar] [CrossRef]
- Kang, C.P.; Lee, H.S.; Ju, H.; Cho, H.; Kang, C.; Bae, S.C. A Functional Haplotype of the PADI4 Gene Associated with Increased Rheumatoid Arthritis Susceptibility in Koreans. Arthritis Rheum. 2006, 54, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Soto, M.A.; Rodríguez-Rodríguez, M.; Pérez-Pérez, M.E.; Daza-Benitez, L.; Bollain-y-Goytia, J.J.; Carrillo-Jiménez, M.A.; Avalos-Díaz, E.; Herrera-Esparza, R. Potential Protein Targets of the Peptidylarginine Deiminase 2 and Peptidylarginine Deiminase 4 Enzymes in Rheumatoid Synovial Tissue and Its Possible Meaning. Eur. J. Rheumatol. 2016, 3, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, D.; Senolt, L.; Nielsen, C.H. Increased Levels of Peptidylarginine Deiminase 2 in Synovial Fluid from Anti-CCP-Positive Rheumatoid Arthritis Patients: Association with Disease Activity and Inflammatory Markers. Rheumatology 2016, 55, 918–927. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Konig, M.F.; Gelber, A.C.; Iii, C.O.B.; Darrah, E. Smoking Is Not Linked to the Development of Anti-Peptidylarginine Deiminase 4 Autoantibodies in Rheumatoid Arthritis. Arthritis Res. Ther. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kolfenbach, J.R.; Deane, K.D.; Derber, L.A.; Colin, I.; Donnell, O.; Gilliland, W.R.; Edison, J.D.; Rosen, A.; Darrah, E.; Norris, J.M.; et al. Autoimmunity to Peptidyl Arginine Deiminase Type 4 Precedes Clinical Onset of Rheumatoid Arthritis. Arthritis Rheum. 2010, 62, 2633–2639. [Google Scholar] [CrossRef]
- Jonsson, M.K.; Kantyka, T.; Falkowski, K.; Aliko, A.; Aga, A.B.; Lillegraven, S.; Sexton, J.; Fevang, B.T.; Mydel, P.; Haavardsholm, E.A. Peptidylarginine Deiminase 4 (PAD4) Activity in Early Rheumatoid Arthritis. Scand. J. Rheumatol. 2020, 49, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Darrah, E.; Giles, J.T.; Davis, R.L.; Naik, P.; Wang, H.; Konig, M.F.; Cappelli, L.C.; Bingham, C.O.; Danoff, S.K.; Andrade, F. Autoantibodies to Peptidylarginine Deiminase 2 Are Associated with Less Severe Disease in Rheumatoid Arthritis. Front. Immunol. 2018, 9, 2696. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Pratesi, F.; Brink, M.; Ärlestig, L.; Amato, C.D.; Bartaloni, D.; Migliorini, P.; Rantapää-Dahlqvist, S. Antibodies Directed against Endogenous and Exogenous Citrullinated Antigens Pre-Date the Onset of Rheumatoid Arthritis. Arthritis Res. Ther. 2016, 18, 127. [Google Scholar] [CrossRef]
- Auger, I.; Balandraud, N.; Massy, E.; Hemon, M.F.; Peen, E.; Arnoux, F.; Mariot, C.; Martin, M.; Lafforgue, P.; Busnel, J.M.; et al. Peptidylarginine Deiminase Autoimmunity and the Development of Anti–Citrullinated Protein Antibody in Rheumatoid Arthritis: The Hapten–Carrier Model. Arthritis Rheumatol. 2020, 72, 903–911. [Google Scholar] [CrossRef]
- Arnoux, F.; Mariot, C.; Peen, E.; Lambert, N.C.; Balandraud, N.; Roudier, J.; Isabelle, A. Peptidyl Arginine Deiminase Immunization Induces Anticitrullinated Protein Antibodies in Mice with Particular MHC Types. Proc. Natl. Acad. Sci. USA 2017, 114, E10169–E10177. [Google Scholar] [CrossRef]
- Shelf, M.A.; Sokolove, J.; Lahey, L.J.; Wagner, C.A.; Wang, Y.; Beebe, D.J.; Robinson, W.H.; Huttenlocher, A. Peptidylarginine Deiminase 4 Contributes to Tumor Necrosis Factor α–Induced Inflammatory Arthritis. Arthritis Rheumatol. 2014, 66, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, I.; Radhakrishnan, A.; Kuppusamy, G.; Kumar Singh, S.; Dua, K. Peptidylarginine Deiminase-4: Medico-Formulative Strategy towards Management of Rheumatoid Arthritis. Biochem. Pharmacol. 2022, 200, 115040. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Pedrera, C.; Barbarroja, N.; Patiño-Trives, A.M.; Luque-Tévar, M.; Collantes-Estevez, E.; Escudero-Contreras, A.; Pérez-Sánchez, C. Molecular Sciences Effects of Biological Therapies on Molecular Features of Rheumatoid Arthritis. Int. J. Mol. Sci. 2020, 21, 9067. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A.; Costenbader, K.H. Genetics, Environment, and Gene-Environment Interactions in the Development of Systemic Rheumatic Diseases. Rheum. Dis. Clin. North Am. 2015, 40, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Kronzer, V.L.; Sparks, J.A. Occupational Inhalants, Genetics and the Respiratory Mucosal Paradigm for ACPA-Positive Rheumatoid Arthritis. Ann. Rheum. Dis. 2023, 82, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.; Kelly, C.; Eggleton, P.; De Soyza, A.; Hutchinson, D. The Lung in ACPA-Positive Rheumatoid Arthritis: An Initiating Site of Injury? Rheumatology 2014, 53, 1940–1950. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Karlson, E.W. Cigarette Smoking and Autoimmune Disease: What Can We Learn from Epidemiology? Lupus 2006, 15, 737–745. [Google Scholar] [CrossRef]
- Alsalahy, M.M.; Nasser, H.S.; Hashem, M.M.; Elsayed, S.M. Effect of Tobacco Smoking on Tissue Protein Citrullination and Disease Progression in Patients with Rheumatoid Arthritis. Saudi Pharm. J. 2010, 18, 75–80. [Google Scholar] [CrossRef]
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Erlandsson Harris, H.; Ulfgren, A.-K.; Rantapää-Dahlqvist, S.; et al. A New Model for an Etiology of Rheumatoid Arthritis: Smoking May Trigger HLA–DR (Shared Epitope)–Restricted Immune Reactions to Autoantigens Modified by Citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; Hermansson, M.; Ulfgren, A.-K.; Nicholas, A.P.; Zendman, A.J.W.; Eklund, A.; Grunewald, J.; Skold, C.M.; Klareskog, L.; Catrina, A.I. Smoking Increases Peptidylarginine Deiminase 2 Enzyme Expression in Human Lungs and Increases Citrullination in BAL Cells. Ann. Rheum. Dis. 2008, 67, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Arita, K.; Shimizu, T.; Hashimoto, H.; Hidaka, Y.; Yamada, M.; Sato, M. Structural Basis for Histone N-Terminal Recognition by Human Peptidylarginine Deiminase 4. Proc. Natl. Acad. Sci. USA 2006, 103, 5291–5296. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.-Y.; Han, T.-U.; Choi, C.-B.; Sung, Y.-K.; Bae, S.-C.; Kang, C. Peptidyl Arginine Deiminase Type IV (PADI4) Haplotypes Interact with Shared Epitope Regardless of Anti-Cyclic Citrullinated Peptide Antibody or Erosive Joint Status in Rheumatoid Arthritis: A Case Control Study. Arthritis Res. Ther. 2010, 12, R115. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Xia, Y.; Pan, J.; Meng, Q.; Zhao, Y.; Yan, X. PADI2 Is Significantly Associated with Rheumatoid Arthritis. PLoS ONE 2013, 8, e81259. [Google Scholar] [CrossRef] [PubMed]
- Kochi, Y.; Thabet, M.M.; Suzuki, A.; Okada, Y.; Daha, N.A.; Toes, R.E.M.; Huizinga, T.W.J.; Myouzen, K.; Kubo, M.; Yamada, R.; et al. PADI4 Polymorphism Predisposes Male Smokers to Rheumatoid Arthritis. Ann. Rheum. Dis. 2011, 70, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamada, R.; Chang, X.; Tokuhiro, S.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Nakayama-Hamada, M.; Kawaida, R.; Ono, M.; et al. Functional Haplotypes of PADI4, Encoding Citrullinating Enzyme Peptidylarginine Deiminase 4, Are Associated with Rheumatoid Arthritis. Nat. Genet. 2003, 34, 395–402. [Google Scholar] [CrossRef]
- Ciesla, M.; Kolarz, B.; Darmochwal-Kolarz, D. The Lack of Association between PADI4_94 or PADI4_104 Polymorphisms and RF, ACPA and Anti-PAD4 in Patients with Rheumatoid Arthritis. Sci. Rep. 2022, 12, 11882. [Google Scholar] [CrossRef]
- Panati, K.; Pal, S.; Rao, K.V.; Reddy, V.D. Association of Single Nucleotide Polymorphisms (SNPs) of PADI4 Gene with Rheumatoid Arthritis (RA) in Indian Population. Genes Genet. Syst. 2012, 87, 191–196. [Google Scholar] [CrossRef][Green Version]
- Barton, A.; Bowes, J.; Eyre, S.; Spreckley, K.; Hinks, A.; John, S.; Worthington, J. A Functional Haplotype of ThePADI4 Gene Associated with Rheumatoid Arthritis in a Japanese Population Is Not Associated in a United Kingdom Population. Arthritis Rheum. 2004, 50, 1117–1121. [Google Scholar] [CrossRef]
- Harney, S.M.J.; Meisel, C.; Sims, A.-M.; Woon, P.Y.; Wordsworth, B.P.; Brown, M.A. Genetic and Genomic Studies of PADI4 in Rheumatoid Arthritis. Rheumatology 2005, 44, 869–872. [Google Scholar] [CrossRef]
- Plenge, R.M.; Padyukov, L.; Remmers, E.F.; Purcell, S.; Lee, A.T.; Karlson, E.W.; Wolfe, F.; Kastner, D.L.; Alfredsson, L.; Altshuler, D.; et al. Replication of Putative Candidate-Gene Associations with Rheumatoid Arthritis in >4000 Samples from North America and Sweden: Association of Susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 2005, 77, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Häupl, T.; Gruber, R.; Kiesewetter, H.; Burmester, G.R.; Salama, A.; Dörner, T. Detailed Analysis of the Variability of Peptidylarginine Deiminase Type 4 in German Patients with Rheumatoid Arthritis: A Case-Control Study. Arthritis Res. Ther. 2006, 8, R34. [Google Scholar] [CrossRef] [PubMed]
- Gandjbakhch, F.; Fajardy, I.; Ferré, B.; Dubucquoi, S.; Flipo, R.-M.; Roger, N.; Solau-Gervais, E. A Functional Haplotype of PADI4 Gene in Rheumatoid Arthritis: Positive Correlation in a French Population. J. Rheumatol. 2009, 36, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Zakeri, Z.; Taheri, H.; Bahari, G.; Taheri, M. Association between Peptidylarginine Deiminase Type 4 Rs1748033 Polymorphism and Susceptibility to Rheumatoid Arthritis in Zahedan, Southeast Iran. Iran. J. Allergy. Asthma. Immunol. 2015, 14, 255–260. [Google Scholar] [PubMed]
- Zavala-Cerna, M.G.; Gonzalez-Montoya, N.G.; Nava, A.; Gamez-Nava, J.I.; Moran-Moguel, M.C.; Rosales-Gomez, R.C.; Gutierrez-Rubio, S.A.; Sanchez-Corona, J.; Gonzalez-Lopez, L.; Davalos-Rodriguez, I.P.; et al. PADI4 Haplotypes in Association with RA Mexican Patients, a New Prospect for Antigen Modulation. Clin. Dev. Immunol. 2013, 2013, 383681. [Google Scholar] [CrossRef] [PubMed]
- Verea Hernando, H.; Otero González, I. Neumopatías Por Fármacos; Elsevier B.V.: Amsterdam, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Iturbe Fernández, D.; Peris Sánchez, R.; Ferreira Moreno, A.; Fernández, F.E. Aspectos Relevantes En El Manejo de La Enfermedad Pulmonar Intersticial Difusa. Arch. Bronconeumol. 2009, 45, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Disdier, C.; Pérez-Negrín, L.; Morales, P.; Cordovilla, R. Revista Del Año 2009 En Neumología Intervencionista, Enfermedades Intersticiales y Trasplante. Arch. Bronconeumol. 2010, 46, 33–37. [Google Scholar] [CrossRef]
- Cano-Jiménez, E.; Molina-Molina, M.; Ramírez, J.; Aliaga, J.L.; Sánchez, M.; Xaubet, A. Diffuse Interstitial Lung Disease Related to Peribronchiolar Metaplasia. Arch. Bronconeumol. 2009, 45, 57–59. [Google Scholar] [CrossRef]
- Furukawa, H.; Oka, S.; Shimada, K.; Masuo, K.; Nakajima, F.; Funano, S.; Tanaka, Y.; Komiya, A.; Fukui, N.; Sawasaki, T.; et al. Autoantibody Profiles in Collagen Disease Patients with Interstitial Lung Disease (ILD): Antibodies to Major Histocompatibility Complex Class I-Related Chain A (MICA) as Markers of ILD. Biomark. Insights 2015, 10, 63–73. [Google Scholar] [CrossRef]
- Lynch, D.A. Lung Disease Related to Collagen Vascular Disease. J. Thorac. Imaging 2009, 24, 299–309. [Google Scholar] [CrossRef]
- Joo, H.P.; Dong, S.K.; Park, I.N.; Se, J.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of Fibrotic Interstitial Pneumonia: Idiopathic versus Collagen Vascular Disease-Related Subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef]
- Kim, E.J.; Elicker, B.M.; Maldonado, F.; Webb, W.R.; Ryu, J.H.; Van Uden, J.H.; Lee, J.S.; King, T.E.; Collard, H.R. Usual Interstitial Pneumonia in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Eur. Respir. J. 2010, 35, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Paulin, F.; Doyle, T.J.; Fletcher, E.A.; Ascherman, D.P.; Rosas, I.O. Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis: Shared Mechanistic and Phenotypic Traits Suggest Overlapping Disease Mechanisms. Rev. Investig. Clin. 2015, 67, 280–286. [Google Scholar]
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid Arthritis. Lancet 2009, 373, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.; Collins, B.F.; Ho, L.A.; Raghu, G. Rheumatoid Arthritis-Associated Lung Disease. Eur. Respir. Rev. 2015, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Harlow, L.; Gochuico, B.R.; Rosas, I.O.; Doyle, T.J.; Osorio, J.C.; Travers, T.S.; Camacho, C.C.; Oddis, C.V.; Ascherman, D.P.; Immunol, C. Anti-Citrullinated Heat Shock Protein 90 Antibodies Identified in Bronchoalveolar Lavage Fluid Are a Marker of Lung-Specific Immune Responses. Clin. Immunol. 2015, 155, 60–70. [Google Scholar] [CrossRef]
- Lugli, E.B.; Correia, R.E.S.M.; Fischer, R.; Lundberg, K.; Bracke, K.R.; Montgomery, A.B.; Kessler, B.M.; Brusselle, G.G.; Venables, P.J. Expression of Citrulline and Homocitrulline Residues in the Lungs of Non-Smokers and Smokers: Implications for Autoimmunity in Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, D.; Nielsen, M.F.B.; Quisgaard Gaunsbaek, M.; Palarasah, Y.; Svane-Knudsen, V.; Nielsen, C.H.; Friberg, M.; Gaunsbaek, M.Q.; Palarasah, Y.; Svane-Knudsen, V.; et al. Smoking Is Associated with Increased Levels of Extra- Cellular Peptidylarginine Deiminase 2 (PAD2) in the Lungs. Clin. Exp. Rheumatol. 2015, 33, 405–408. [Google Scholar]
- Linnemann, R.W.; Yadav, R.; Zhang, C.; Sarr, D.; Rada, B.; Stecenko, A.A. Serum Anti-PAD4 Autoantibodies Are Present in Cystic Fibrosis Children and Increase with Age and Lung Disease Severity. Autoimmunity 2022, 55, 109–117. [Google Scholar] [CrossRef]
- Yadav, R.; Yoo, D.G.; Kahlenberg, J.M.; Bridges, S.L.; Oni, O.; Huang, H.; Stecenko, A.; Rada, B. Systemic Levels of Anti-PAD4 Autoantibodies Correlate with Airway Obstruction in Cystic Fibrosis. J. Cyst. Fibros. 2019, 18, 636–645. [Google Scholar] [CrossRef]
- Kilsgård, O.; Andersson, P.; Malmsten, M.; Nordin, S.L.; Linge, H.M.; Eliasson, M.; Sörenson, E.; Erjefält, J.S.; Bylund, J.; Olin, A.I.; et al. Peptidylarginine Deiminases Present in the Airways during Tobacco Smoking and Inflammation Can Citrullinate the Host Defense Peptide LL-37, Resulting in Altered Activities. Am. J. Respir. Cell Mol. Biol. 2012, 46, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Jun, F.; Surolia, R.; Li, H.; Wang, Z.; Liu, G.; Kulkarni, T.; Massicano, A.V.F.; Mobley, J.A.; Mondal, S.; De Andrade, J.A.; et al. Citrullinated Vimentin Mediates Development and Progression of Lung Fibrosis. Sci. Transl. Med. 2021, 13, eaba2927. [Google Scholar] [CrossRef]
- Samara, K.D.; Trachalaki, A.; Tsitoura, E.; Koutsopoulos, A.V.; Lagoudaki, E.D.; Lasithiotaki, I.; Margaritopoulos, G.; Pantelidis, P.; Bibaki, E.; Siafakas, N.M.; et al. Upregulation of Citrullination Pathway: From Autoimmune to Idiopathic Lung Fibrosis. Respir. Res. 2017, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.M.; Solomon, J.J.; Humphries, S.M.; Swigris, J.J.; Ahmed, F.; Wang, H.; Darrah, E.; Demoruelle, M.K. Serum Antibodies to Peptidylarginine Deiminase-4 in Rheumatoid Arthritis Associated-Interstitial Lung Disease Are Associated with Decreased Lung Fibrosis and Improved Survival. Am. J. Med. Sci. 2023, 365, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Bistoni, O.; Pratesi, F.; La Paglia, G.M.C.; Puxeddu, I.; Migliorini, P.; Gerli, R. Anti-Citrullinated Alpha Enolase Antibodies, Interstitial Lung Disease and Bone Erosion in Rheumatoid Arthritis. Rheumatology 2018, 57, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Danoff, S.K.; Sokolove, J.; Wagner, C.A.; Winchester, R.; Pappas, D.A.; Siegelman, S.; Connors, G.; Robinson, W.H.; Bathon, J.M. Association of Fine Specificity and Repertoire Expansion of Anticitrullinated Peptide Antibodies with Rheumatoid Arthritis Associated Interstitial Lung Disease. Ann. Rheum. Dis. 2014, 73, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Nava-Quiroz, K.J.; Rojas-Serrano, J.; Perez-Rubio, G.; Buendia-Roldan, I.; Mejía, M.; Fernández-López, J.C.; Rodríguez-Henriquez, P.; Ayala-Alcantar, N.; Ramos-Martínez, E.; López-Flores, L.A.; et al. Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients. Cells 2023, 12, 2235. [Google Scholar] [CrossRef]
- Boon, L.; Ugarte-Berzal, E.; Martens, E.; Fiten, P.; Vandooren, J.; Janssens, R.; Blanter, M.; Yu, K.; Boon, M.; Struyf, S.; et al. Citrullination as a Novel Posttranslational Modification of Matrix Metalloproteinases. Matrix Biol. 2021, 95, 68–83. [Google Scholar] [CrossRef]
- Kulvinskiene, I.; Raudoniute, J.; Bagdonas, E.; Ciuzas, D.; Poliakovaite, K.; Stasiulaitiene, I.; Zabulyte, D.; Bironaite, D.; Rimantas Venskutonis, P.; Martuzevicius, D.; et al. Lung Alveolar Tissue Destruction and Protein Citrullination in Diesel Exhaust-Exposed Mouse Lungs. Basic Clin. Pharmacol. Toxicol. 2019, 125, 166–177. [Google Scholar] [CrossRef]
- Grabcanovic-Musija, F.; Obermayer, A.; Stoiber, W.; Krautgartner, W.D.; Steinbacher, P.; Winterberg, N.; Bathke, C.A.; Klappacher, M.; Studnicka, M. Neutrophil Extracellular Trap (NET) Formation Characterises Stable and Exacerbated COPD and Correlates with Airflow Limitation. Respir. Res. 2015, 16, 59. [Google Scholar] [CrossRef]
- Sun, B.; Tomita, B.; Salinger, A.; Tilvawala, R.R.; Li, L.; Hakami, H.; Liu, T.; Tsoyi, K.; Rosas, I.O.; Reinhardt, D.P.; et al. PAD2-Mediated Citrullination of Fibulin-5 Promotes Elastogenesis. Matrix Biol. 2021, 102, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.M.; De Pablo, P.; Buckley, C.D.; Ahmad, A.; Stockley, R.A. Smoke Exposure as a Determinant of Autoantibody Titre in A1-Antitrypsin Deficiency and COPD. Eur. Respir. J. 2011, 37, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, D.; Müller, J.; McCarthy, J.E.; Gun’ko, Y.K.; Verma, N.K.; Bi, X.; Di Cristo, L.; Kickham, L.; Movia, D.; Prina-Mello, A.; et al. Cadmium Nanoparticles Citrullinate Cytokeratins within Lung Epithelial Cells: Cadmium as a Potential Cause of Citrullination in Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 441–449. [Google Scholar] [CrossRef]
- Sigari, N.; Moghimi, N.; Shahraki, F.S.; Mohammadi, S.; Roshani, D. Anti-Cyclic Citrullinated Peptide (CCP) Antibody in Patients with Wood-Smoke-Induced Chronic Obstructive Pulmonary Disease (COPD) without Rheumatoid Arthritis. Rheumatol. Int. 2015, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Darrah, E.; Danoff, S.; Johnson, C.; Andrade, F.; Rosen, A.; Bathon, J.M. Association of Cross-Reactive Antibodies Targeting Peptidyl-Arginine Deiminase 3 and 4 with Rheumatoid Arthritis-Associated Interstitial Lung Disease. PLoS ONE 2014, 9, e98794. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; Clarke, A.; Pouwels, S.D.; Perry, E.; Abdullah, T.M.; Kelly, C.; De Soyza, A.; Hutchinson, D.; Eggleton, P.; Bremer, E. Galectin-9 Is a Possible Promoter of Immunopathology in Rheumatoid Arthritis by Activation of Peptidyl Arginine Deiminase 4 (PAD-4) in Granulocytes. Int. J. Mol. Sci. 2019, 20, 4046. [Google Scholar] [CrossRef] [PubMed]
- Palterer, B.; Vitiello, G.; Del Carria, M.; D’Onofrio, B.; Martinez-Prat, L.; Mahler, M.; Cammelli, D.; Parronchi, P. Anti-Protein Arginine Deiminase Antibodies Are Distinctly Associated with Joint and Lung Involvement in Rheumatoid Arthritis. Rheumatology 2023, 62, 2410–2417. [Google Scholar] [CrossRef]
- Trivedi, A.; Khan, M.A.; Bade, G.; Talwar, A. Orchestration of Neutrophil Extracellular Traps (Nets), a Unique Innate Immune Function during Chronic Obstructive Pulmonary Disease (COPD) Development. Biomedicines 2021, 9, 53. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikari, J.; Anazawa, R.; Tanaka, N.; Katsumata, Y.; Shimada, A.; Suzuki, E.; Tatsumi, K. PAD4 Deficiency Improves Bleomycin-Induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2020, 63, 806–818. [Google Scholar] [CrossRef]
- Khawaja, A.A.; Chong, D.L.W.; Sahota, J.; Mikolasch, T.A.; Pericleous, C.; Ripoll, V.M.; Booth, H.L.; Khan, S.; Rodriguez-Justo, M.; Giles, I.P.; et al. Identification of a Novel HIF-1α-AMβ2 Integrin-NET Axis in Fibrotic Interstitial Lung Disease. Front. Immunol. 2020, 11, 2190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).