The Role of p53 in Nanoparticle-Based Therapy for Cancer
Abstract
:1. Introduction
2. p53 in Nanoparticle-Based Gene Therapy for Cancer
2.1. Liposomal Vectors
2.2. Polymer NPs
2.3. Metallic NPs
2.4. Other NPs
3. Impact of NPs on the p53 Protein
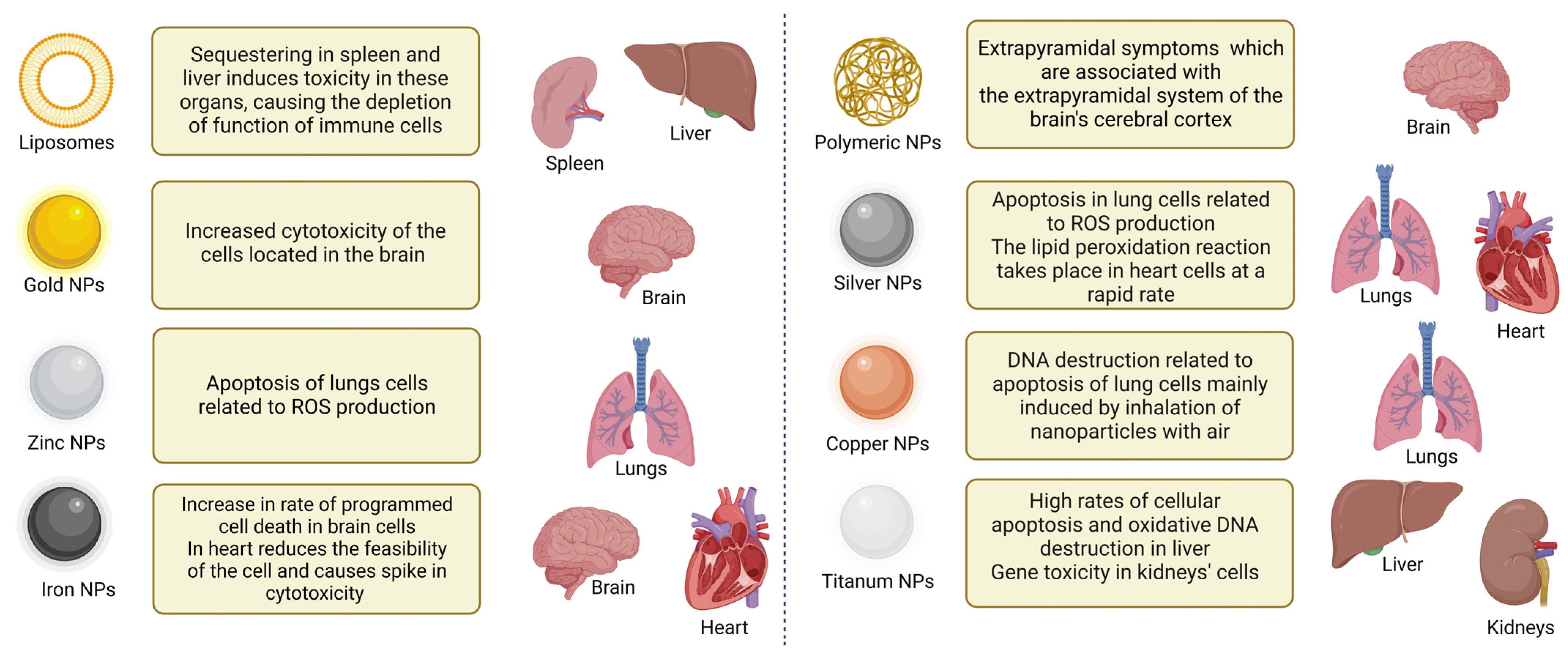
4. Toxicity of NPs
5. Limitations and Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lane, D.P. P53, Guardian of the Genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, J.M.; Zvereva, S.; Dalina, A.; Blatov, I.; Zubarev, I.; Luppov, D.; Bessmertnyi, A.; Romanishin, A.; Alsoulaiman, L.; Kumeiko, V.; et al. The P53 Family Member P73 in the Regulation of Cell Stress Response. Biol. Direct 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.F.; Kelly, G.L.; Strasser, A. Of the Many Cellular Responses Activated by TP53, which Ones Are Critical for Tumour Suppression? Cell Death Differ. 2022, 29, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Riley, T.; Sontag, E.D.; Chen, P.; Levine, A.J. Transcriptional Control of Human P53-Regulated Genes. Nat. Rev. Mol. Cell Biol. 2008, 9, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Barlev, N.A.; Sayan, B.S.; Candi, E.; Okorokov, A.L. The microRNA and P53 Families Join Forces against Cancer. Cell Death Differ. 2010, 17, 373–375. [Google Scholar] [CrossRef]
- Parfenyev, S.; Singh, A.; Fedorova, O.A.; Daks, A.; Kulshreshtha, R.; Barlev, N.A. Interplay between P53 and Non-Coding RNAs in the Regulation of EMT in Breast Cancer. Cell Death Dis. 2021, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Hermeking, H. MicroRNAs in the P53 Network: Micromanagement of Tumour Suppression. Nat. Rev. Cancer 2012, 12, 613–626. [Google Scholar] [CrossRef]
- Marouco, D.; Garabadgiu, A.V.; Melino, G.; Barlev, N.A.; Barlev, N.A. Lysine-Specific Modifications of P53: A Matter of Life and Death? Oncotarget 2013, 4, 1556–1571. [Google Scholar] [CrossRef]
- Liu, Y.; Tavana, O.; Gu, W. P53 Modifications: Exquisite Decorations of the Powerful Guardian. J. Mol. Cell Biol. 2019, 11, 564–577. [Google Scholar] [CrossRef]
- Konopleva, M.; Martinelli, G.; Daver, N.; Papayannidis, C.; Wei, A.; Higgins, B.; Ott, M.; Mascarenhas, J.; Andreeff, M. MDM2 Inhibition: An Important Step Forward in Cancer Therapy. Leukemia 2020, 34, 2858–2874. [Google Scholar] [CrossRef]
- Klein, A.M.; de Queiroz, R.M.; Venkatesh, D.; Prives, C. The Roles and Regulation of MDM2 and MDMX: It Is Not Just about P53. Genes Dev. 2021, 35, 575–601. [Google Scholar] [CrossRef]
- Morgunkova, A.; Barlev, N.A. Lysine Methylation Goes Global. Cell Cycle 2006, 5, 1308–1312. [Google Scholar] [CrossRef] [PubMed]
- Lezina, L.; Aksenova, V.; Fedorova, O.; Malikova, D.; Shuvalov, O.; Antonov, A.V.; Tentler, D.; Garabadgiu, A.V.; Melino, G.; Barlev, N.A. KMT Set7/9 Affects Genotoxic Stress Response via the Mdm2 Axis. Oncotarget 2015, 6, 25843–25855. [Google Scholar] [CrossRef] [PubMed]
- Petukhov, A.; Ag, M.; Moiseeva, T.N.; Tn, M.; Barlev, N.A. Role of Proteasomes in Transcription and Their Regulation by Covalent Modifications. Front. Biosci. 2008, 13, 7184–7192. [Google Scholar] [CrossRef]
- Sdek, P.; Ying, H.; Chang, D.L.F.; Qiu, W.; Zheng, H.; Touitou, R.; Allday, M.J.; Allday, M.J.; Xiao, Z.-X.J. MDM2 Promotes Proteasome-Dependent Ubiquitin-Independent Degradation of Retinoblastoma Protein. Mol. Cell 2005, 20, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Stindt, M.H.; Carter, S.A.; Vigneron, A.M.; Ryan, K.M.; Vousden, K.H. MDM2 Promotes SUMO-2/3 Modification of P53 to Modulate Transcriptional Activity. Cell Cycle 2011, 10, 3176–3188. [Google Scholar] [CrossRef]
- Abida, W.M.; Nikolaev, A.; Zhao, W.; Zhang, W.; Gu, W.; Abida, W.M.; Nikolaev, A.; Zhao, W.; Zhang, W.; Gu, W. FBXO11 Promotes the Neddylation of P53 and Inhibits Its Transcriptional Activity. J. Biol. Chem. 2007, 282, 1797–1804. [Google Scholar] [CrossRef]
- Rada, M.; Vasileva, E.; Lezina, L.; Marouco, D.; Antonov, A.V.; Macip, S.; Melino, G.; Barlev, N.A. Human EHMT2/G9a Activates P53 through Methylation-Independent Mechanism. Oncogene 2017, 36, 922–932. [Google Scholar] [CrossRef]
- Daks, A.; Shuvalov, O.; Fedorova, O.; Parfenyev, S.; Simon, H.-U.; Barlev, N.A. Barlev Methyltransferase Set7/9 as a Multifaceted Regulator of ROS Response. Int. J. Biol. Sci. 2023, 19, 2304–2318. [Google Scholar] [CrossRef]
- Ivanov, G.S.; Ivanova, T.; Kurash, J.; Ivanov, A.; Chuikov, S.; Gizatullin, F.; Herrera-Medina, E.M.; Rauscher, F.; Reinberg, D.; Barlev, N.A. Methylation-Acetylation Interplay Activates P53 in Response to DNA Damage. Mol. Cell. Biol. 2007, 27, 6756–6769. [Google Scholar] [CrossRef]
- Xu, M.; Kumar, D.; Kumar, D.; Kumar, D.; Srinivas, S.; DeTolla, L.J.; Yu, S.F.; Stass, S.A.; Mixson, A.J. Parenteral Gene Therapy with P53 Inhibits Human Breast Tumors in Vivo through a Bystander Mechanism without Evidence of Toxicity. Hum. Gene Ther. 1997, 8, 177–185. [Google Scholar] [CrossRef]
- Pfister, N.T.; Prives, C. Transcriptional Regulation by Wild-Type and Cancer-Related Mutant Forms of P53. Cold Spring Harb. Perspect. Med. 2017, 7, a026054. [Google Scholar] [CrossRef] [PubMed]
- Brady, C.A.; Attardi, L.D. P53 at a Glance. J. Cell Sci. 2010, 123, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Bellazzo, A.; Sicari, D.; Valentino, E.; Del Sal, G.; Collavin, L. Complexes Formed by Mutant P53 and Their Roles in Breast Cancer. Breast Cancer 2018, 10, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Bykov, V.J.N.; Eriksson, S.E.; Bianchi, J.; Wiman, K.G. Targeting Mutant P53 for Efficient Cancer Therapy. Nat. Rev. Cancer 2018, 18, 89–102. [Google Scholar] [CrossRef]
- Vassilev, L.T. MDM2 Inhibitors for Cancer Therapy. Trends Mol. Med. 2007, 13, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, P.; Aksenova, V.; Petrova, V.; Tentler, D.; Orlova, D.; Smirnov, S.; Gurzhiy, V.; Okorokov, A.L.; Garabadzhiu, A.; Melino, G.; et al. Discovery of Novel Isatin-Based P53 Inducers. ACS Med. Chem. Lett. 2015, 6, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Fallatah, M.M.J.; Law, F.V.; Chow, W.A.; Kaiser, P. Small-Molecule Correctors and Stabilizers to Target P53. Trends Pharmacol. Sci. 2023, 44, 274–289. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Johnson, M.L.; Tolcher, A.W.; Shapiro, G.I.; Thompson, J.A.; El-Khoueiry, A.B.; Vandross, A.L.; Kummar, S.; Parikh, A.R.; Munster, P.N.; et al. First-in-Human Study of PC14586, a Small Molecule Structural Corrector of Y220C Mutant P53, in Patients with Advanced Solid Tumors Harboring a TP53 Y220C Mutation. J. Clin. Oncol. 2022, 40 (Suppl. S16), 3003. [Google Scholar] [CrossRef]
- Gounder, M.; Bauer, T.; Schwartz, G.; Weise, A.; LoRusso, P.; Kumar, P.; Tao, B.; Hong, Y.; Patel, P.; Lu, Y.; et al. A First-in-Human Phase I Study of Milademetan, an MDM2 Inhibitor, in Patients With Advanced Liposarcoma, Solid Tumors, or Lymphomas. J. Clin. Oncol. 2023, 41, 1714–1724. [Google Scholar] [CrossRef]
- Mahfoudhi, E.; Lordier, L.; Marty, C.; Pan, J.; Roy, A.; Roy, L.; Rameau, P.; Abbes, S.; Debili, N.; Raslova, H.; et al. P53 Activation Inhibits All Types of Hematopoietic Progenitors and All Stages of Megakaryopoiesis. Oncotarget 2016, 7, 31980–31992. [Google Scholar] [CrossRef]
- Khurana, A.; Shafer, D.A. MDM2 Antagonists as a Novel Treatment Option for Acute Myeloid Leukemia: Perspectives on the Therapeutic Potential of Idasanutlin (RG7388). OncoTargets Ther. 2019, 12, 2903–2910. [Google Scholar] [CrossRef]
- Daks, A.; Petukhov, A.; Fedorova, O.; Shuvalov, O.; Merkulov, V.; Vasileva, E.; Antonov, A.; Barlev, N.A. E3 Ubiquitin Ligase Pirh2 Enhances Tumorigenic Properties of Human Non-Small Cell Lung Carcinoma Cells. Genes Cancer 2016, 7, 383–393. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023? Viruses 2023, 15, 698. [Google Scholar] [CrossRef]
- Zeimet, A.G.; Marth, C. Why Did P53 Gene Therapy Fail in Ovarian Cancer. Lancet Oncol. 2003, 4, 415–422. [Google Scholar] [CrossRef]
- Szewczyk, O.K.; Roszczenko, P.; Czarnomysy, R.; Bielawska, A.; Bielawski, K. An Overview of the Importance of Transition-Metal Nanoparticles in Cancer Research. Int. J. Mol. Sci. 2022, 23, 6688. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Esmailzadeh, A.; Shanei, A. Bystander Effect of Therapeutic Ultrasound in the Presence of Cisplatin: An in Vitro Study on Human Melanoma Cells. J. Biomed. Phys. Eng. 2023, 13, 433–442. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Yan, J.; Li, Y.; Yan, S.; Wang, S.; Hou, P.; Lu, W. Resurrecting a P53 Peptide Activator—An Enabling Nanoengineering Strategy for Peptide Therapeutics. J. Control. Release 2020, 325, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Ma, W.; Adjei, I.M.; Panyam, J.; Dimitrijevic, S.; Labhasetwar, V. Nanoparticle-Mediated P53 Gene Therapy for Tumor Inhibition. Drug Deliv. Transl. Res. 2011, 1, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk-Roszczenko, O.K.; Roszczenko, P.; Shmakova, A.; Finiuk, N.; Holota, S.; Lesyk, R.; Bielawska, A.; Vassetzky, Y.; Bielawski, K. The Chemical Inhibitors of Endocytosis: From Mechanisms to Potential Clinical Applications. Cells 2023, 12, 2312. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-K.; Choi, E.J.; Choi, E.-J.; Choi, S.-H.; Park, J.-S.; Haider, K.H.; Ahn, W.S. Enhanced P53 Gene Transfer to Human Ovarian Cancer Cells Using the Cationic Nonviral Vector, DDC. Gynecol. Oncol. 2003, 90, 265–272. [Google Scholar] [CrossRef]
- Zou, Y.; Zong, G.; Ling, Y.H.; Hao, M.M.; Lozano, G.; Hong, W.K.; Perez-Soler, R. Effective Treatment of Early Endobronchial Cancer With Regional Administration of Liposome-P53 Complexes. J. Natl. Cancer Inst. 1998, 90, 1130–1137. [Google Scholar] [CrossRef]
- Marvalim, C.; Datta, A.; Lee, S.C. Role of P53 in Breast Cancer Progression: An Insight into P53 Targeted Therapy. Theranostics 2023, 13, 1421–1442. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Labhasetwar, V. Nanoparticle-Mediated Wild-Type P53 Gene Delivery Results in Sustained Antiproliferative Activity in Breast Cancer Cells. Mol. Pharm. 2004, 1, 211–219. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, Y.; Wang, C.-H.; Pack, D.W. Monodisperse Double-Walled Microspheres Loaded with Chitosan-P53 Nanoparticles and Doxorubicin for Combined Gene Therapy and Chemotherapy. J. Control. Release 2012, 163, 130–135. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Vydiam, K.; Srinivasan, D.J.; Mukherjee, S.; Roy, A.; Kuncha, M.; Rao, T.N.; Sistla, R.; Gopal, V.; Patra, C.R. Restoration of P53 Function in Ovarian Cancer Mediated by Gold Nanoparticle-Based EGFR Targeted Gene Delivery System. ACS Biomater. Sci. Eng. 2019, 5, 3631–3644. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiar, A.; Neah, A.S.; Ng, K.Y.; Chowdhury, E.H. In Vivo Evaluation of Biodistribution and Toxicity of pH-Responsive Strontium Nanoparticles for Gene Delivery. J. Pharm. Investig. 2021, 52, 95–107. [Google Scholar] [CrossRef]
- Gaspar, V.M.; Correia, I.J.; Sousa, Â.; Silva, F.; Paquete, C.M.; Queiroz, J.A.; Sousa, F. Nanoparticle Mediated Delivery of Pure P53 Supercoiled Plasmid DNA for Gene Therapy. J. Control. Release 2011, 156, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Divita, G.; Czuba, E.; Grunenberger, A.; Guidetti, M.; Josserand, V.; Desai, N. P53 mRNA Rescue of Tumor Suppressor Function Prevents Tumor Growth and Restores PARPi Sensitivity in P53-Deficient Cancers in Vitro and in Vivo. Eur. J. Cancer 2022, 174, S21–S22. [Google Scholar] [CrossRef]
- Li, F.; Chen, D.; Sun, Q.; Wu, J.; Gan, Y.; Leong, K.W.; Liang, X. MDM2-Targeting Reassembly Peptide (TRAP) Nanoparticles for p53-Based Cancer Therapy. Adv. Mater. 2023, 35, 2305164. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, W.; Fang, Y.; Yang, H.; Tian, L.; Li, K.; Lai, W.; Bian, L.; Lin, B.; Liu, X.; et al. Neurotoxicity of Aluminum Oxide Nanoparticles and Their Mechanistic Role in Dopaminergic Neuron Injury Involving P53-Related Pathways. J. Hazard. Mater. 2020, 392, 122312. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, J.; Zhou, S.; Zhang, T.; Cai, J.; Liu, Y. Ag Nanoparticles Green-Mediated by Scrophularia Striata Aqueous Extract Induce Apoptosis via P53 and Signal Transducer and Activator of Transcription 3 Signaling Pathways in Gastric Cancer Cells. Inorg. Chem. Commun. 2023, 155, 110942. [Google Scholar] [CrossRef]
- Satapathy, S.R.; Mohapatra, P.; Preet, R.; Das, D.; Sarkar, B.; Choudhuri, T.; Wyatt, M.D.; Kundu, C.N. Silver-Based Nanoparticles Induce Apoptosis in Human Colon Cancer Cells Mediated through P53. Nanomed. Nanotechnol. Biol. Med. 2013, 8, 1307–1322. [Google Scholar] [CrossRef] [PubMed]
- Shafagh, M.; Rahmani, F.; Delirezh, N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran. J. Basic Med. Sci. 2015, 18, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Akhtar, M.J. Selective Killing of Cancer Cells by Iron Oxide Nanoparticles Mediated through Reactive Oxygen Species via P53 Pathway. J. Nanopart. Res. 2013, 15, 1225. [Google Scholar] [CrossRef]
- Asharani, P.V.; Xinyi, N.; Hande, M.P.; Valiyaveettil, S. DNA Damage and P53-Mediated Growth Arrest in Human Cells Treated with Platinum Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 5, 51–64. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J. Endothelial Cells Dysfunction Induced by Silica Nanoparticles through Oxidative Stress via JNK/P53 and NF-κB Pathways. Biomaterials 2010, 31, 8198–8209. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, B.M.; Lee, Y.-J.; Chung, H.W. Titanium Dioxide Nanoparticles Trigger P53-Mediated Damage Response in Peripheral Blood Lymphocytes. Environ. Mol. Mutagen. 2008, 49, 399–405. [Google Scholar] [CrossRef]
- Wu, J.; Sun, J.; Xue, Y. Involvement of JNK and P53 Activation in G2/M Cell Cycle Arrest and Apoptosis Induced by Titanium Dioxide Nanoparticles in Neuron Cells. Toxicol. Lett. 2010, 199, 269–276. [Google Scholar] [CrossRef]
- Xi, W.; Tang, H.; Liu, Y.; Liu, C.; Gao, Y.; Cao, A.; Liu, Y.; Chen, Z.; Wang, H. Cytotoxicity of Vanadium Oxide Nanoparticles and Titanium Dioxide-coated Vanadium Oxide Nanoparticles to Human Lung Cells. J. Appl. Toxicol. 2020, 40, 567–577. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Kumar, A.; Dhawan, A. Zinc Oxide Nanoparticles Induce Oxidative DNA Damage and ROS-Triggered Mitochondria Mediated Apoptosis in Human Liver Cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Roszczenko, P.; Szewczyk, O.K.; Czarnomysy, R.; Bielawski, K.; Bielawska, A. Biosynthesized Gold, Silver, Palladium, Platinum, Copper, and Other Transition Metal Nanoparticles. Pharmaceutics 2022, 14, 2286. [Google Scholar] [CrossRef]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef]
- Inglut, C.T.; Sorrin, A.J.; Kuruppu, T.; Vig, S.; Cicalo, J.; Ahmad, H.; Huang, H.-C. Immunological and Toxicological Considerations for the Design of Liposomes. Nanomaterials 2020, 10, 190. [Google Scholar] [CrossRef]
- Mendonça, M.C.P.; Radaic, A.; Garcia-Fossa, F.; Da Cruz-Höfling, M.A.; Vinolo, M.A.R.; De Jesus, M.B. The in Vivo Toxicological Profile of Cationic Solid Lipid Nanoparticles. Drug Deliv. Transl. Res. 2020, 10, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, L.; Meng, X.; Liu, T.; Hu, Q.; Miao, L. Cytotoxicity of Silver Nanoparticles on Human Periodontal Ligament Fibroblasts. Nanosci. Nanotechnol. Lett. 2017, 9, 1015–1022. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zou, Z.; Wang, B.; Xu, G.; Wu, Q.; Zhang, Y.; Yuan, Z.; Yang, X.; Yu, C.; et al. Lysosomal Deposition of Copper Oxide Nanoparticles Triggers HUVEC Cells Death. Biomaterials 2018, 161, 228–239. [Google Scholar] [CrossRef]
- Singh, S. Zinc Oxide Nanoparticles Impacts: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity. Toxicol. Mech. Methods 2019, 29, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-Specific Toxicity of Magnetic Iron Oxide-Based Nanoparticles. Nanotoxicology 2020, 15, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Sairam, A.; Sanmugam, A.; Pushparaj, A.; Kumar, G.M.; Sundarapandian, N.; Balaji, S.; Nallal, M.; Park, K. Toxicity of Polymeric Nanodrugs as Drug Carriers. ACS Chem. Health Saf. 2023, 30, 236–250. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural Parameters of Nanoparticles Affecting Their Toxicity for Biomedical Applications: A Review. J. Nanopart. Res. 2023, 25, 43. [Google Scholar] [CrossRef] [PubMed]
- Dolma, L.; Muller, P.A.J. GOF Mutant P53 in Cancers: A Therapeutic Challenge. Cancers 2022, 14, 5091. [Google Scholar] [CrossRef] [PubMed]
- Munisamy, M.; Mukherjee, N.; Thomas, L.; Pham, A.T.; Shakeri, A.; Zhao, Y.; Kolesar, J.; Rao, P.P.N.; Rangnekar, V.M.; Rao, M. Therapeutic Opportunities in Cancer Therapy: Targeting the P53-MDM2/MDMX Interactions. Am. J. Cancer Res. 2021, 11, 5762–5781. [Google Scholar] [PubMed]
- Zhang, C.; Liu, J.; Xu, D.; Zhang, T.; Hu, W.; Feng, Z. Gain-of-Function Mutant P53 in Cancer Progression and Therapy. J. Mol. Cell Biol. 2020, 12, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Matissek, K.J.; Mossalam, M.; Okal, A.; Lim, C.S. The DNA Binding Domain of P53 Is Sufficient To Trigger a Potent Apoptotic Response at the Mitochondria. Mol. Pharm. 2013, 10, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Okal, A.; Matissek, K.J.; Matissek, S.J.; Price, R.; Salama, M.E.; Janát-Amsbury, M.M.; Lim, C.S. Re-Engineered P53 Activates Apoptosis in Vivo and Causes Primary Tumor Regression in a Dominant Negative Breast Cancer Xenograft Model. Gene Ther. 2014, 21, 903–912. [Google Scholar] [CrossRef]
- Matissek, K.J.; Okal, A.; Mossalam, M.; Lim, C.S. Delivery of a Monomeric P53 Subdomain with Mitochondrial Targeting Signals from Pro-Apoptotic Bak or Bax. Pharm. Res. 2014, 31, 2503–2515. [Google Scholar] [CrossRef]
- Lu, P.; Redd Bowman, K.E.; Brown, S.M.; Joklik-Mcleod, M.; Vander Mause, E.R.; Nguyen, H.T.N.; Lim, C.S. P53-Bad: A Novel Tumor Suppressor/Proapoptotic Factor Hybrid Directed to the Mitochondria for Ovarian Cancer Gene Therapy. Mol. Pharm. 2019, 16, 3386–3398. [Google Scholar] [CrossRef]
- Waterman, M.J.; Waterman, J.L.; Halazonetis, T.D. An Engineered Four-Stranded Coiled Coil Substitutes for the Tetramerization Domain of Wild-Type P53 and Alleviates Transdominant Inhibition by Tumor-Derived P53 Mutants. Cancer Res. 1996, 56, 158–163. [Google Scholar]
- Okal, A.; Mossalam, M.; Matissek, K.J.; Dixon, A.S.; Moos, P.J.; Lim, C.S. A Chimeric P53 Evades Mutant P53 Transdominant Inhibition in Cancer Cells. Mol. Pharm. 2013, 10, 3922–3933. [Google Scholar] [CrossRef] [PubMed]
- Wallis, B.; Bowman, K.R.; Lu, P.; Lim, C.S. The Challenges and Prospects of P53-Based Therapies in Ovarian Cancer. Biomolecules 2023, 13, 159. [Google Scholar] [CrossRef]
- Ditto, A.J.; Shah, P.N.; Yun, Y.H. Non-Viral Gene Delivery Using Nanoparticles. Expert Opin. Drug Deliv. 2009, 6, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Choi, J.W.; Yun, C.-O.; Kim, S.W. Efficient Lung Orthotopic Tumor-Growth Suppression of Oncolytic Adenovirus Complexed with RGD-Targeted Bioreducible Polymer. Gene Ther. 2014, 21, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.; Zhivotovsky, B. Caspases and Cancer. Cell Death Differ. 2011, 18, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Jang, J.; Park, Y.G.; Park, W.S.; Nam, S.W.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. CASPASE-8 Gene Is Inactivated by Somatic Mutations in Gastric Carcinomas. Cancer Res. 2005, 65, 815–821. [Google Scholar] [CrossRef]
- Mandruzzato, S.; Brasseur, F.; Andry, G.; Boon, T.; Bruggen, P.V.D. A CASP-8 Mutation Recognized by Cytolytic T Lymphocytes on a Human Head and Neck Carcinoma. J. Exp. Med. 1997, 186, 785–793. [Google Scholar] [CrossRef]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.-M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.-H.; et al. Down-Regulation of Caspase 3 in Breast Cancer: A Possible Mechanism for Chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef]
- Tian, T. MCF-7 Cells Lack the Expression of Caspase-3. Int. J. Biol. Macromol. 2023, 231, 123310. [Google Scholar] [CrossRef]
- Liu, X.; Gonzalez, G.; Dai, X.; Miao, W.; Yuan, J.; Huang, M.; Bade, D.; Li, L.; Sun, Y.; Wang, Y. Adenylate Kinase 4 Modulates the Resistance of Breast Cancer Cells to Tamoxifen through an m6A-Based Epitranscriptomic Mechanism. Mol. Ther. 2020, 28, 2593–2604. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, B.; Lu, H. Negative Auto-Regulators Trap P53 in Their Web. J. Mol. Cell Biol. 2017, 9, 62–68. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. Role of P53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef]
- Zhang, S.; Carlsen, L.; Hernandez Borrero, L.; Seyhan, A.A.; Tian, X.; El-Deiry, W.S. Advanced Strategies for Therapeutic Targeting of Wild-Type and Mutant P53 in Cancer. Biomolecules 2022, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Chen, J.; Zhou, H.; Zeng, X.; Ruan, Z.; Pu, Z.; Jiang, X.; Matsui, A.; Zhu, L.; Amoozgar, Z.; et al. Combining P53 mRNA Nanotherapy with Immune Checkpoint Blockade Reprograms the Immune Microenvironment for Effective Cancer Therapy. Nat. Commun. 2022, 13, 758. [Google Scholar] [CrossRef]
- Malekzadeh, P.; Yossef, R.; Cafri, G.; Paria, B.C.; Lowery, F.J.; Jafferji, M.; Good, M.L.; Sachs, A.; Copeland, A.R.; Kim, S.P.; et al. Antigen Experienced T Cells from Peripheral Blood Recognize P53 Neoantigens. Clin. Cancer Res. 2020, 26, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Synnott, N.C.; McGowan, P.M.; Crown, J.; O’Connor, D.; Gallagher, W.M. P53 as a Target for the Treatment of Cancer. Cancer Treat. Rev. 2014, 40, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Senzer, N.; Nemunaitis, J.; Nemunaitis, M.; Lamont, J.; Gore, M.; Gabra, H.; Eeles, R.; Sodha, N.; Lynch, F.J.; Zumstein, L.A.; et al. P53 Therapy in a Patient with Li-Fraumeni Syndrome. Mol. Cancer Ther. 2007, 6, 1478–1482. [Google Scholar] [CrossRef]
- Swisher, S.G.; Roth, J.A.; Komaki, R.; Gu, J.; Lee, J.J.; Hicks, M.; Ro, J.Y.; Hong, W.K.; Merritt, J.A.; Ahrar, K.; et al. Induction of P53-Regulated Genes and Tumor Regression in Lung Cancer Patients after Intratumoral Delivery of Adenoviral P53 (INGN 201) and Radiation Therapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 93–101. [Google Scholar]
- Atencio, I.A.; Grace, M.; Bordens, R.; Fritz, M.; Horowitz, J.A.; Hutchins, B.; Indelicato, S.; Jacobs, S.; Kolz, K.; Maneval, D.; et al. Biological Activities of a Recombinant Adenovirus P53 (SCH 58500) Administered by Hepatic Arterial Infusion in a Phase 1 Colorectal Cancer Trial. Cancer Gene Ther. 2006, 13, 169–181. [Google Scholar] [CrossRef]
- Hassin, O.; Oren, M. Drugging P53 in Cancer: One Protein, Many Targets. Nat. Rev. Drug Discov. 2023, 22, 127–144. [Google Scholar] [CrossRef]
- Rejeeth, C.; Kannan, S. P53 Gene Therapy of Human Breast Carcinoma: Using a Transferrin-Modified Silica Nanoparticles. Breast Cancer 2016, 23, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.B.; Raimundo, L.; Calheiros, J.; Carvalho, C.; Barcherini, V.; Lima, N.R.; Gomes, C.; Almeida, M.I.; Alves, M.G.; Costa, J.L.; et al. Targeting P53 for Melanoma Treatment: Counteracting Tumour Proliferation, Dissemination and Therapeutic Resistance. Cancers 2021, 13, 1648. [Google Scholar] [CrossRef] [PubMed]
- Vlašić, I.; Horvat, A.; Tadijan, A.; Slade, N. P53 Family in Resistance to Targeted Therapy of Melanoma. Int. J. Mol. Sci. 2022, 24, 65. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.R.; Fane, M.E.; Alicea, G.M.; Basu, S.; Kossenkov, A.V.; Marino, G.E.; Douglass, S.M.; Kaur, A.; Ecker, B.L.; Gnanapradeepan, K.; et al. Paradoxical Role for Wild-Type P53 in Driving Therapy Resistance in Melanoma. Mol. Cell 2020, 77, 633–644.e5. [Google Scholar] [CrossRef]

| Type of NPs | In Vitro/ In Vivo | Tissue/Cell Line | Effect | Reference |
|---|---|---|---|---|
| Al2O3 | In vivo | Sub-brain regions of rats | Decreased expression of cyclin D1, bcl-2, Mdm2, and phospho-Rb and increased expression of p53, p21, Bax, and Rb | [51] |
| Ag | In vitro | GC1415, NCI-N87, and MKN45 | Increased p53 expression, inhibition of STAT3 | [52] |
| In vitro | HCT116 | Increased transcription of p53, p21, and caspases (3,8,9), decreased amount of AKT and NF-κB | [53] | |
| CuO | In vitro/ Ex vivo | K562 and peripheral blood mononuclear cell | Increase in Bax/Bcl-2 ratio, upregulation of p53, and ROS production | [54] |
| Fe3O4 | In vitro | HepG2, A549, IMR-90 | Induction of ROS, upregulation of p53, and caspases 3 and 9 | [55] |
| Pt | In vitro | IMR-90, U251 | Upregulation of p53 and p21, DNA damage | [56] |
| Si | In vitro | HUVECs | Activation of c-Jun, p53, caspase-3, and NF-κB, increased Bax expression and suppression Bcl-2 | [57] |
| TiO2 | Ex vivo | peripheral blood lymphocytes | Accumulation of p53 and activation of DNA damage checkpoint kinases | [58] |
| In vitro | PC12 | ROS and JNK/p53 mediated apoptosis and causing. G2/M arrest by the activation of p53/p21 pathway | [59] | |
| V2O5 | In vitro | B16F10, A549, and PANC1 | Impaired angiogenesis, increased ROS, overexpression of p53 | [60] |
| Zn | In vitro | HepG2 | ROS generation, DNA damage, activation of p53 and p38 | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczyk-Roszczenko, O.; Barlev, N.A. The Role of p53 in Nanoparticle-Based Therapy for Cancer. Cells 2023, 12, 2803. https://doi.org/10.3390/cells12242803
Szewczyk-Roszczenko O, Barlev NA. The Role of p53 in Nanoparticle-Based Therapy for Cancer. Cells. 2023; 12(24):2803. https://doi.org/10.3390/cells12242803
Chicago/Turabian StyleSzewczyk-Roszczenko, Olga, and Nikolai A. Barlev. 2023. "The Role of p53 in Nanoparticle-Based Therapy for Cancer" Cells 12, no. 24: 2803. https://doi.org/10.3390/cells12242803
APA StyleSzewczyk-Roszczenko, O., & Barlev, N. A. (2023). The Role of p53 in Nanoparticle-Based Therapy for Cancer. Cells, 12(24), 2803. https://doi.org/10.3390/cells12242803








