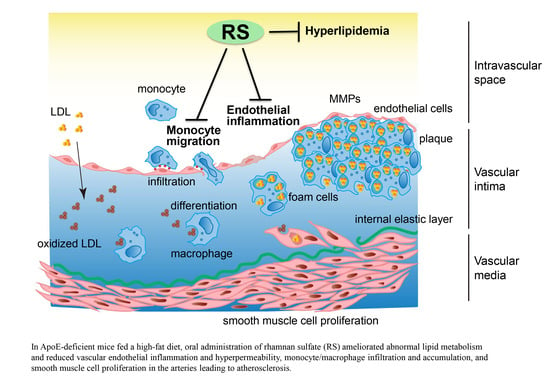
Oral Administration of Rhamnan Sulfate from Monostroma nitidum Suppresses Atherosclerosis in ApoE-Deficient Mice Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification and Molecular Weight Measurement of RS
2.2. Quantification of RS Components
2.2.1. Standard Materials
2.2.2. Measurement of Constituent Sugars
2.2.3. Measurement of Sulfate Groups
2.2.4. Measurement of Sulfate-Bound Cations
2.3. Animal Experiments
2.4. Quantitative Real-Time PCR
2.5. Immunohistochemistry
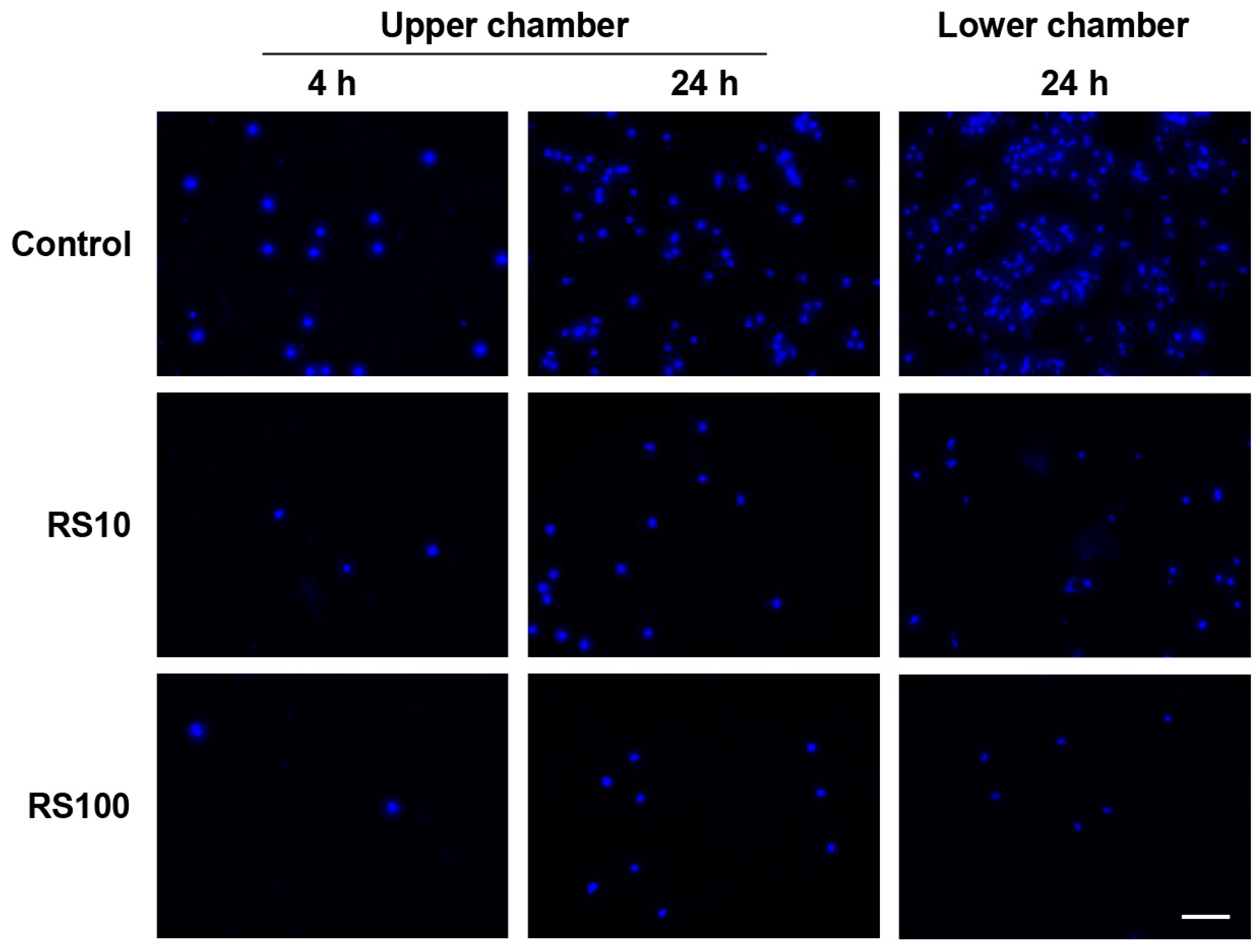
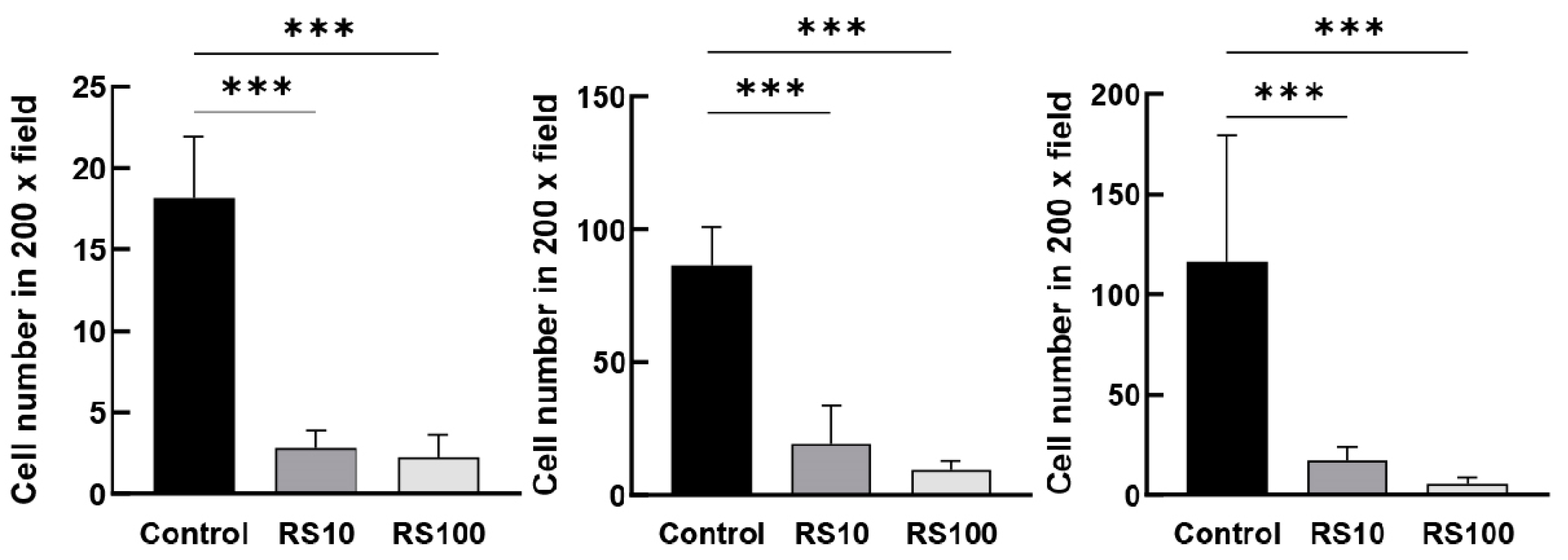
2.6. Migration Assay
2.7. Statistical Analysis
3. Results
3.1. Chemical Characteristics of RS Isolated from M. nitidum
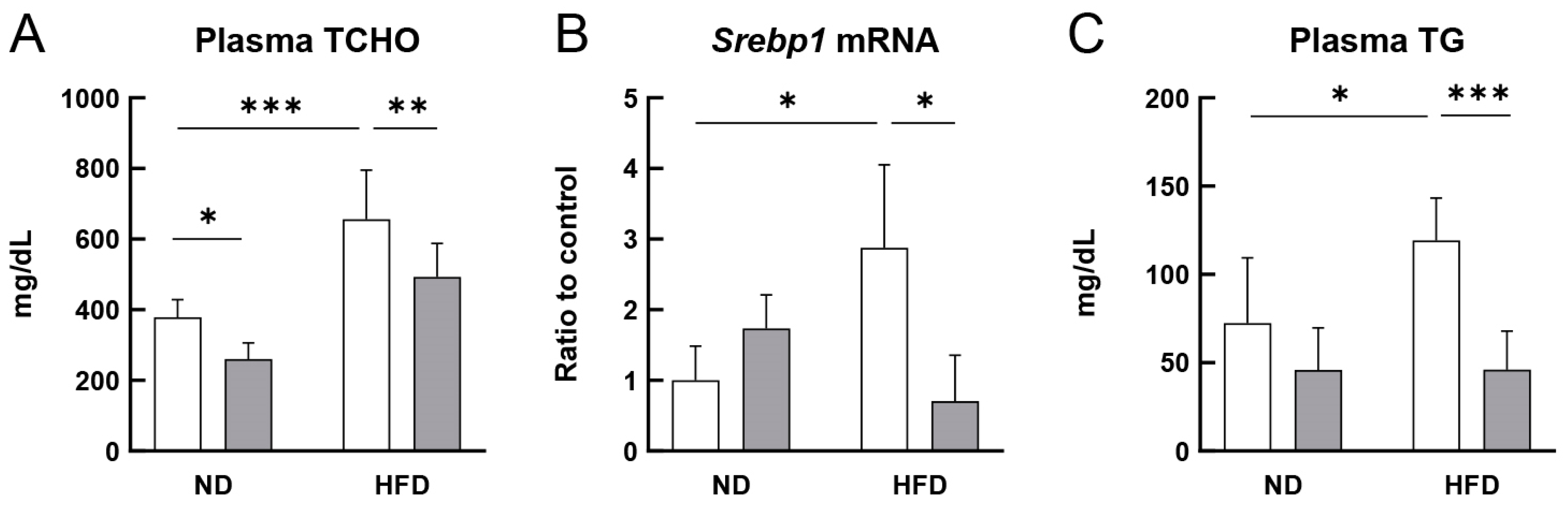
3.2. RS Improved the Lipid Profile of HFD-Fed ApoE−/− Mice
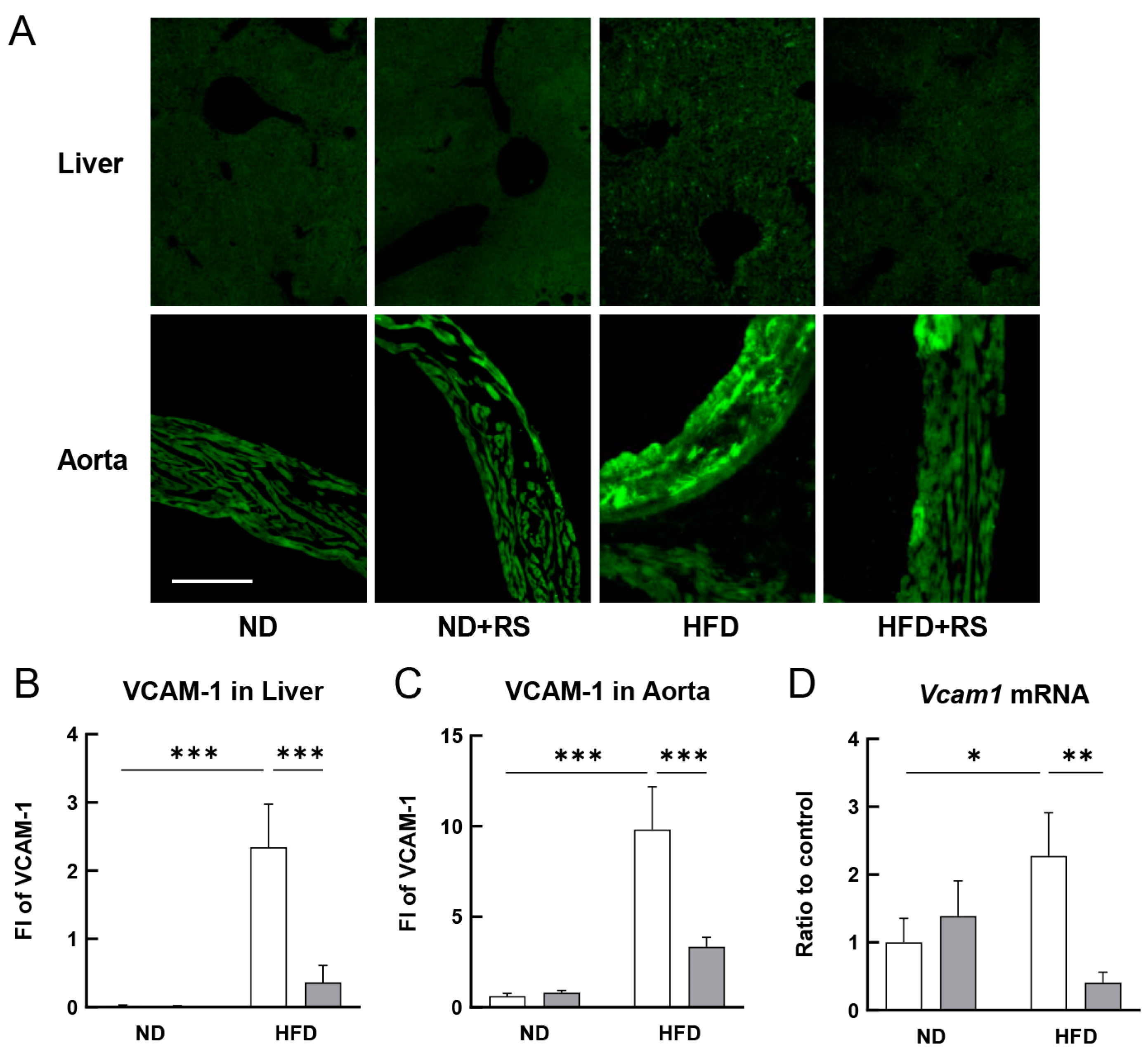
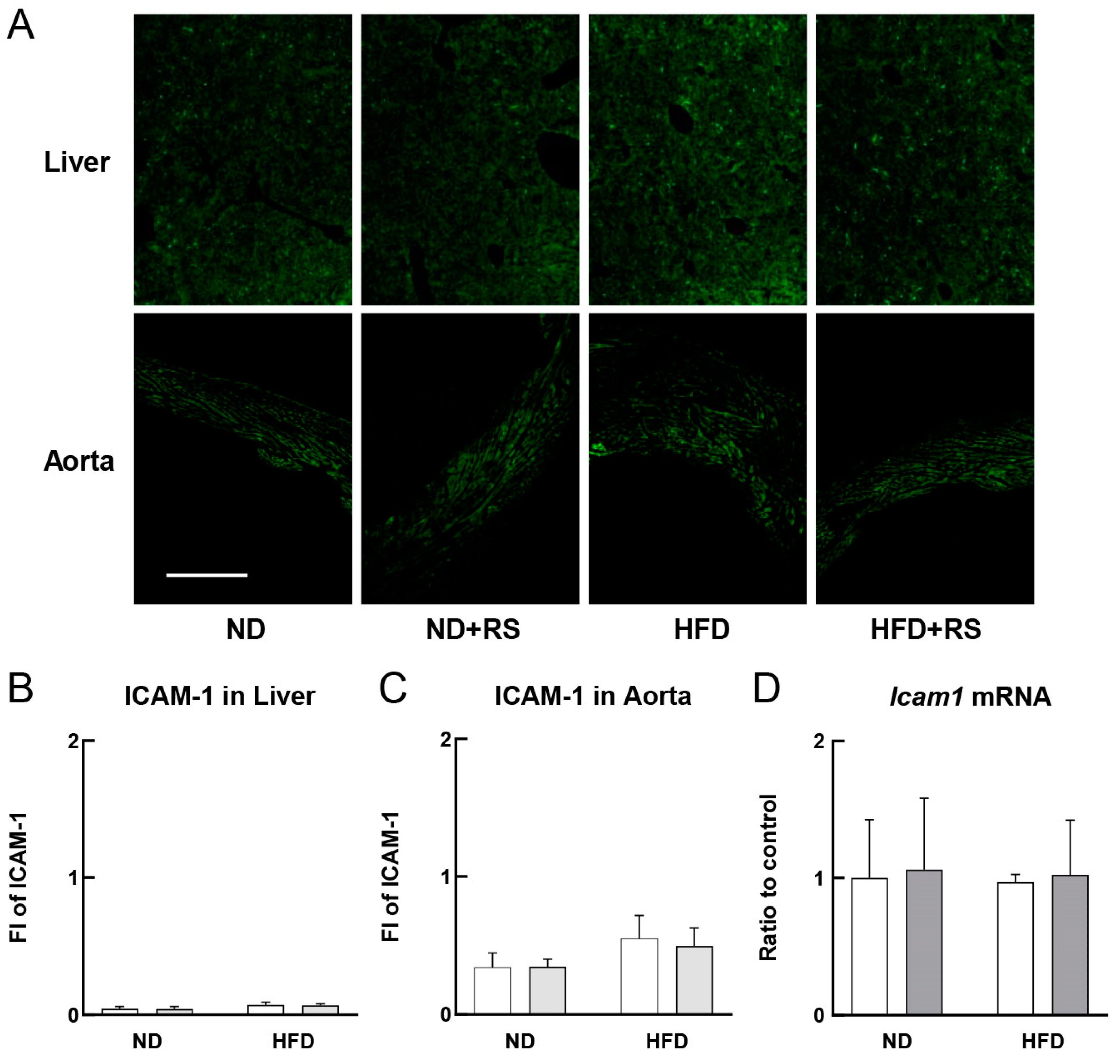
3.3. RS Reduced VCAM-1 Expression in the Liver and Aorta of HFD-Fed ApoE−/− Mice
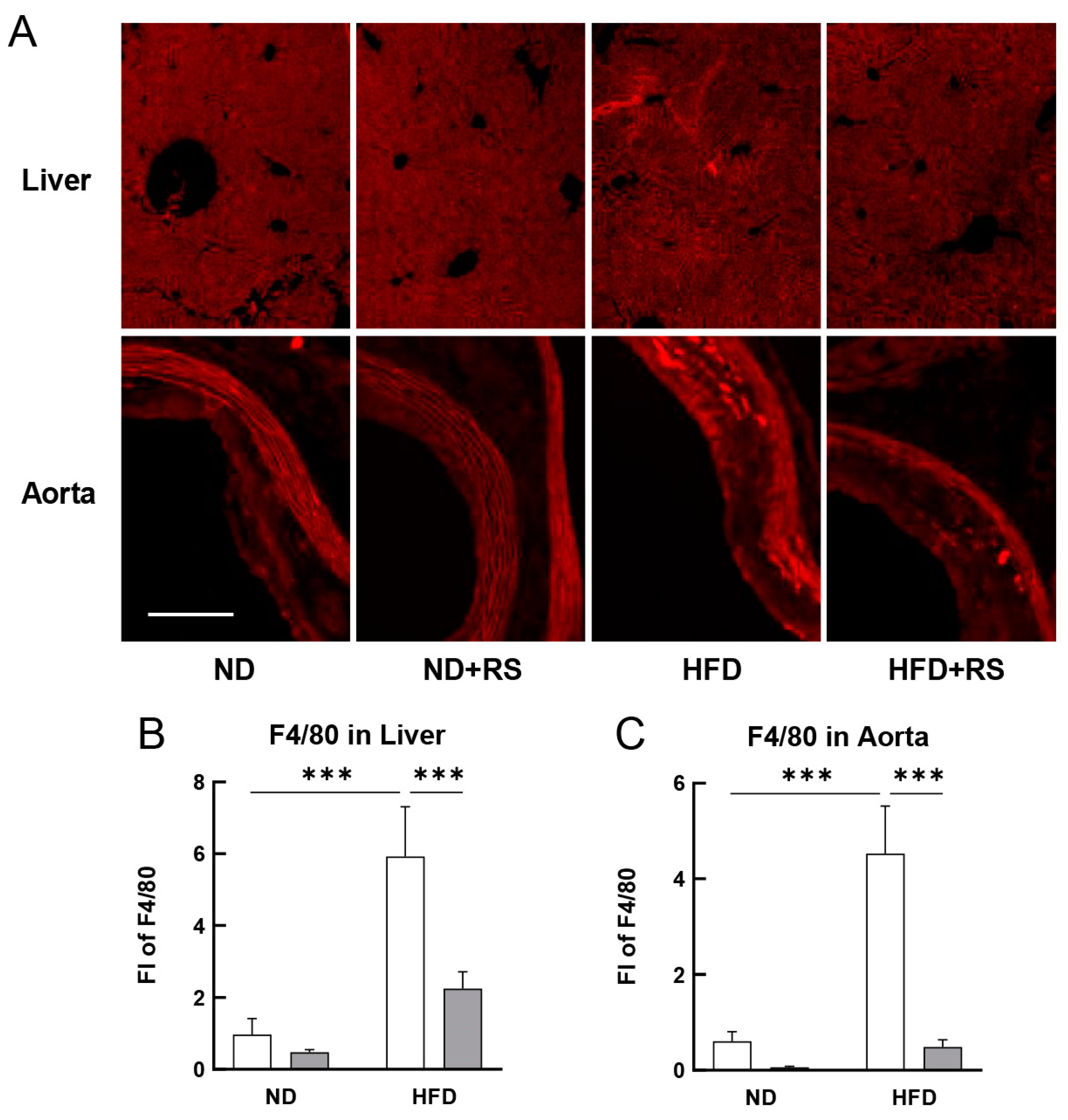
3.4. RS Reduced Macrophage Accumulation in the Liver and Aorta of HFD-Fed ApoE−/− Mice
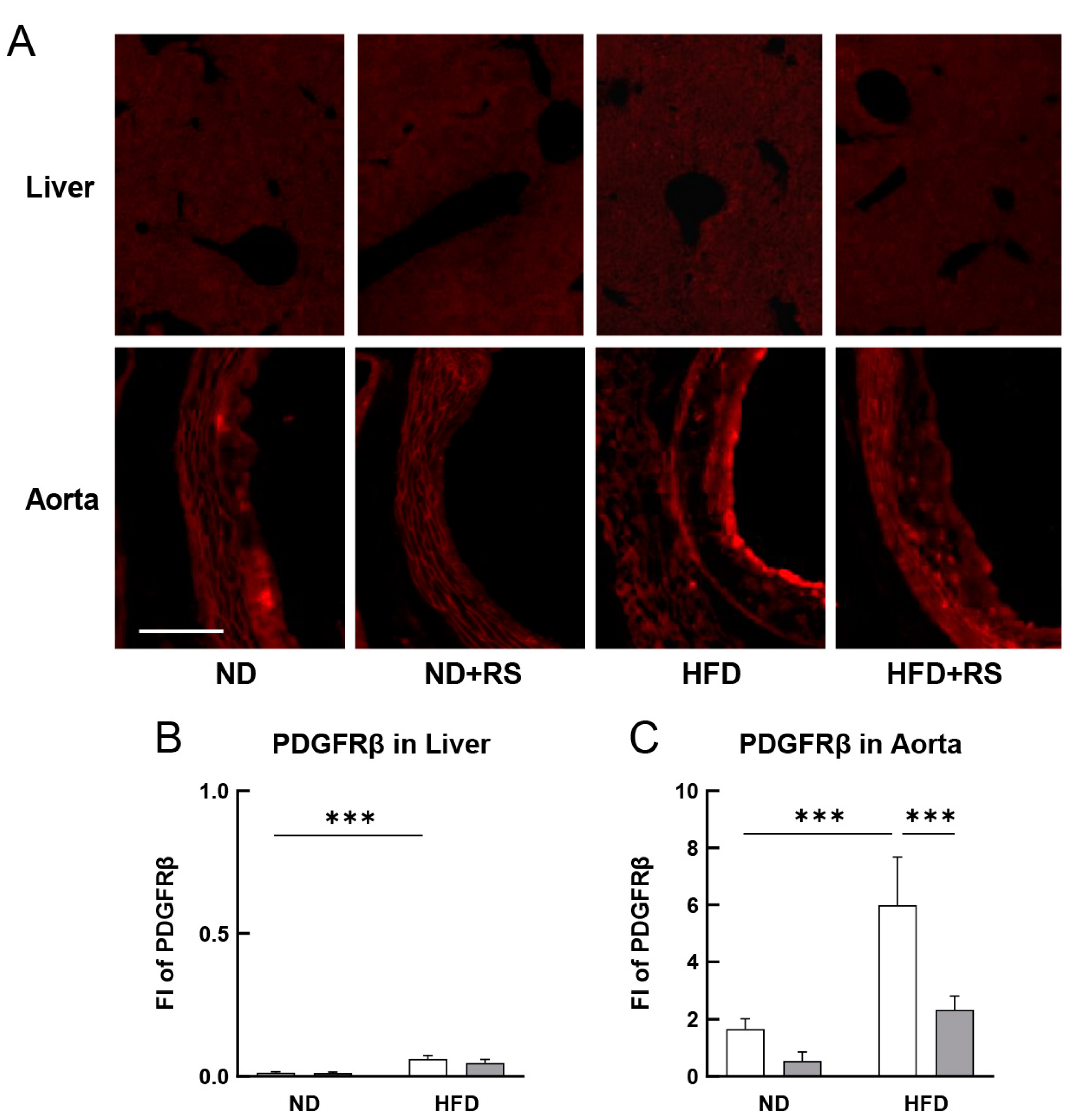
3.5. RS Reduced PDGFRβ Expression in the Aorta of HFD-Fed ApoE−/− Mice
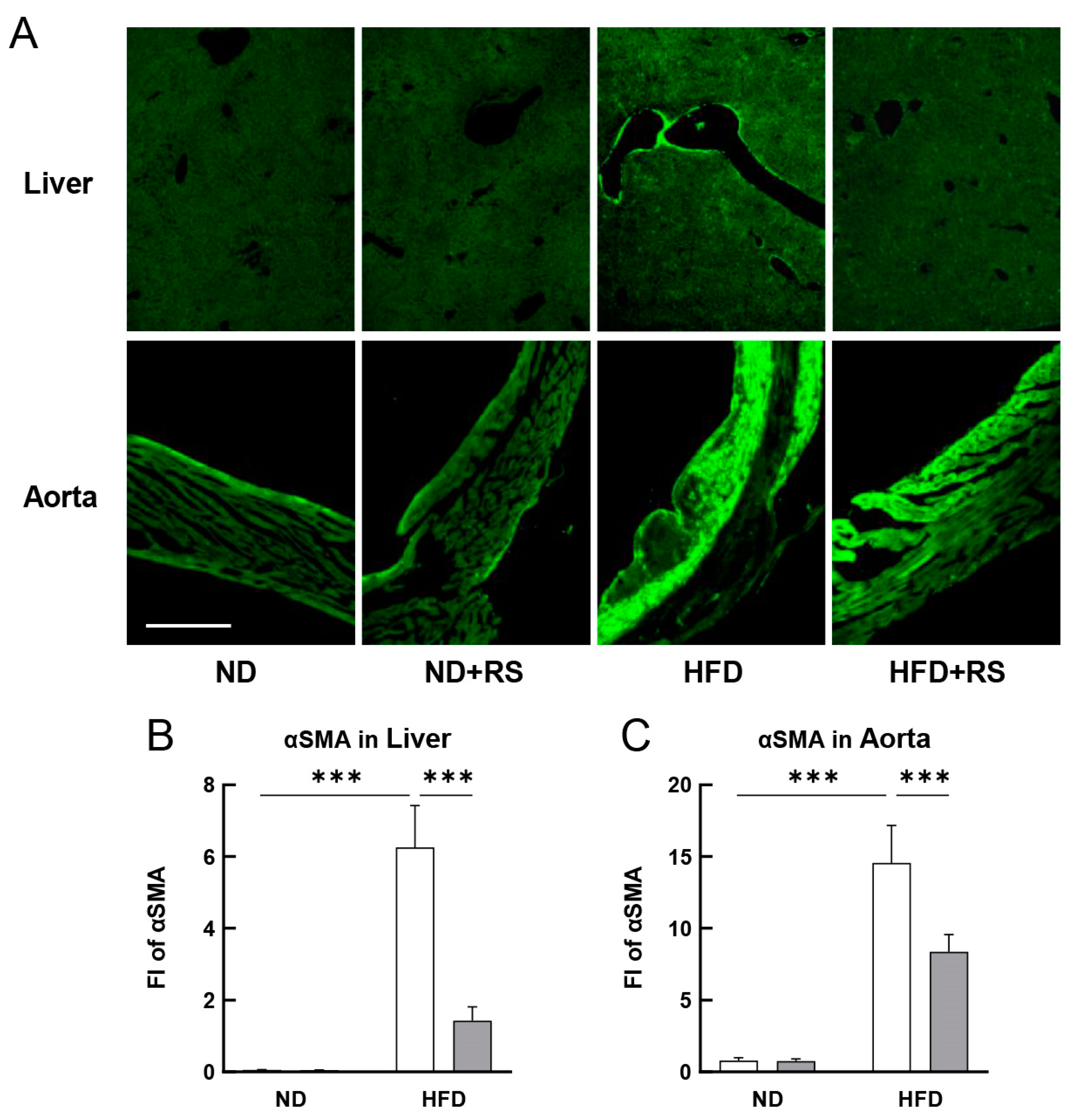
3.6. RS Reduced αSMA Expression in the Liver and Aorta of HFD-Fed ApoE−/− Mice
3.7. Correlation between Plasma TCHO or TG Levels and Aortic Atherosclerotic Molecule Levels in ND- or HFD-Fed ApoE−/− Mice with or without RS Administration
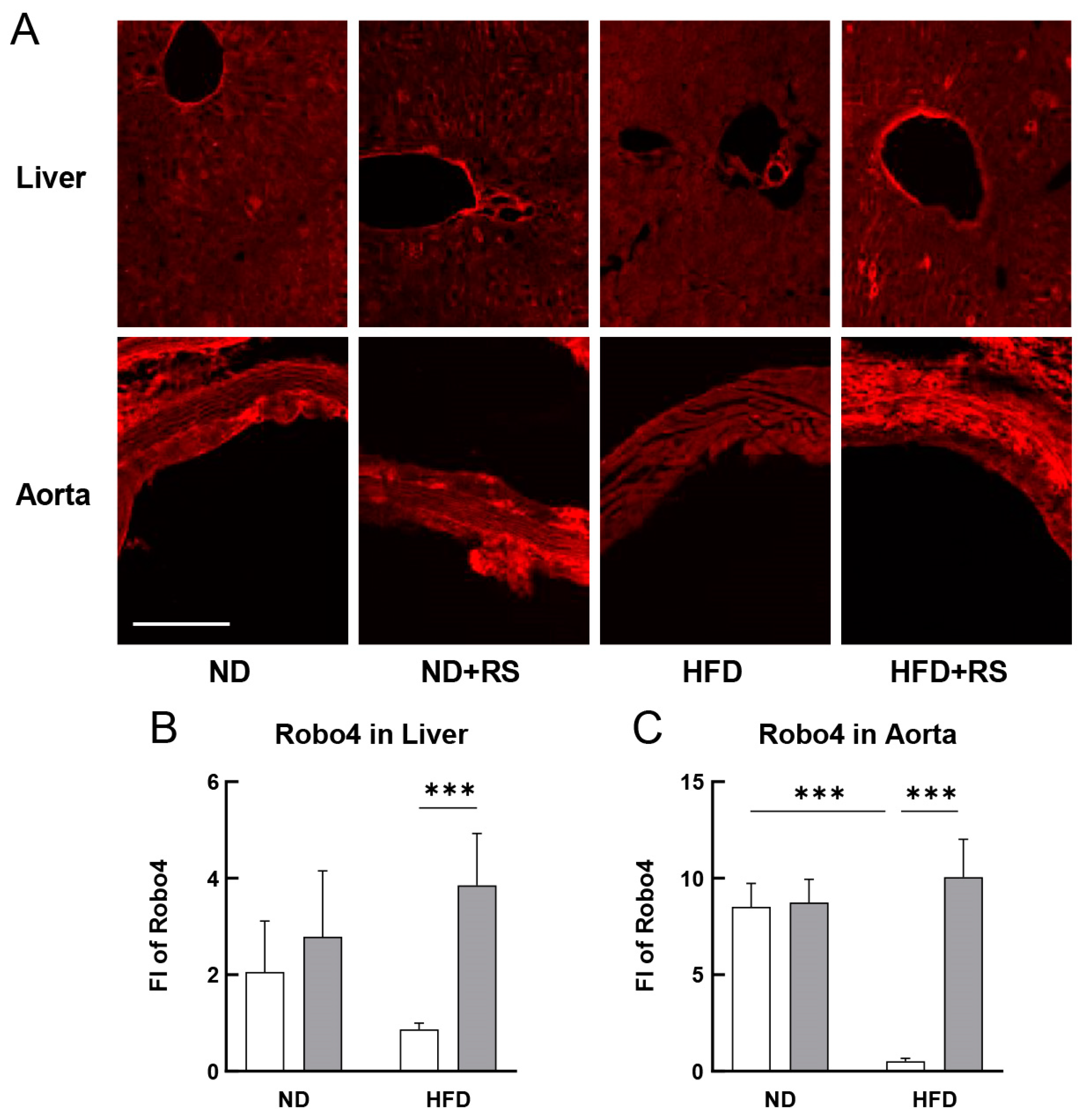
3.8. Effect of RS on the Expression of Robo4 in the Liver and Aorta of HFD ApoE−/− Mice
3.9. RS Reduced Mmp9 mRNA Expression in the Aorta of ApoE−/− Mice
3.10. RS Directly Inhibited the Migration of Mouse Macrophage-Like Cells In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanhoutte, P.M.; Shimokawa, H.; Tang, E.H.; Feletou, M. Endothelial dysfunction and vascular disease. Acta Physiol. 2009, 196, 193–222. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Wada, H.; Wakita, Y.; Shiku, H. Tissue factor expression in endothelial cells in health and disease Blood Coagul. Fibrinolysis 1995, 6, S26–S31. [Google Scholar] [CrossRef]
- Nemerson, Y. Tissue factor and hemostasis. Blood 1988, 71, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.S.; Aye, M.T.; Ganz, P.R.; Halpenny, M.; Hashemi, S. Adenosine nucleotides and serotonin stimulate von Willebrand factor release from cultured human endothelial cells. Thromb. Haemost. 1994, 72, 132–139. [Google Scholar] [CrossRef]
- Okamoto, T.; Akita, N.; Terasawa, M.; Hayashi, T.; Suzuki, K. Rhamnan sulfate extracted from Monostroma nitidum attenuates blood coagulation and inflammation of vascular endothelial cells. J. Nat. Med. 2019, 73, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Bochner, B.S.; Luscinskas, F.W.; Gimbrone, M.A., Jr.; Newman, W.; Sterbinsky, S.A.; Derse-Anthony, C.P.; Klunk, D.; Schleimer, R.P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: Contributions of endothelial cell adhesion molecules. J. Exp. Med. 1991, 173, 1553–1557. [Google Scholar] [CrossRef]
- Mantovani, A.; Bussolino, F.; Introna, M. Cytokine regulation of endothelial cell function: From molecular level to the bedside. Immunol. Today 1997, 18, 231–240. [Google Scholar] [CrossRef]
- Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 1994, 76, 301–314. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.; Harker, L. Response to injury and atherogenesis. Am. J. Pathol. 1977, 86, 675–684. [Google Scholar] [PubMed]
- Meir, K.S.; Leitersdorf, E. Atherosclerosis in the apolipoprotein-E-deficient mouse: A decade of progress. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1006–1014. [Google Scholar] [CrossRef]
- Suzuki, K.; Terasawa, M. Biological Activities of Rhamnan Sulfate Extract from the Green Algae Monostroma nitidum (Hitoegusa). Mar. Drugs 2020, 18, 228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Koizumi, S.; Hayashi, K.; Hayashi, T. Structure of rhamnan sulfate from the green alga Monostroma nitidum and its anti-herpetic effect. Carbohydr. Polym. 2010, 81, 572–577. [Google Scholar] [CrossRef]
- Tako, M.; Yamashiro, Y.; Teruya, T.; Uechi, S. Structure-Function Relationship of Rhamnan Sulfate Isolated from Commercially Cultured Edible Green Seaweed, Monostroma nitidum. Am. J. Appl. Chem. 2017, 5, 38–44. [Google Scholar] [CrossRef]
- Li, H.; Mao, W.; Hou, Y.; Gao, Y.; Qi, X.; Zhao, C.; Chen, Y.; Chen, Y.; Li, N.; Wang, C. Preparation, structure and anticoagulant activity of a low molecular weight fraction produced by mild acid hydrolysis of sulfated rhamnan from Monostroma latissimum. Bioresour. Technol. 2012, 114, 414–418. [Google Scholar] [CrossRef]
- Harada, N.; Maeda, M. Chemical structure of antithrombin-active Rhamnan sulfate from Monostrom nitidum. Biosci. Biotechnol. Biochem. 1998, 62, 1647–1652. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, S.; He, X.; Qin, L.; He, M.; Yang, Y.; Hao, J.; Mao, W. Structural Characteristics and Anticoagulant Property In Vitro and In Vivo of a Seaweed Sulfated Rhamnan. Mar. Drugs 2018, 16, 243. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Nakamura, M.; Yogi, T.; Teruya, T.; Konishi, T.; Uechi, S.; Tako, M. Anticoagulant Activity of Rhamnan Sulfate Isolated from Commercially Cultured Monostroma nitidum. Int. J. Biomed. Mater. Res. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- Terasawa, M.; Hiramoto, K.; Uchida, R.; Suzuki, K. Anti-Inflammatory Activity of Orally Administered Monostroma nitidum Rhamnan Sulfate against Lipopolysaccharide-Induced Damage to Mouse Organs and Vascular Endothelium. Mar. Drugs 2022, 20, 121. [Google Scholar] [CrossRef]
- Song, Y.; He, P.; Rodrigues, A.L.; Datta, P.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Chen, C.; Dordick, J.; Zhang, F.; et al. Anti-SARS-CoV-2 Activity of Rhamnan Sulfate from Monostroma nitidum. Mar. Drugs 2021, 19, 685. [Google Scholar] [CrossRef]
- Terasawa, M.; Hayashi, K.; Lee, J.B.; Nishiura, K.; Matsuda, K.; Hayashi, T.; Kawahara, T. Anti-Influenza A Virus Activity of Rhamnan Sulfate from Green Algae Monostroma nitidum in Mice with Normal and Compromised Immunity. Mar. Drugs 2020, 18, 254. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hayashi, K.; Hayashi, T.; Sankawa, U.; Maeda, M. Antiviral activities against HSV-1, HCMV, and HIV-1 of rhamnan sulfate from Monostroma latissimum. Planta Med. 1999, 65, 439–441. [Google Scholar] [CrossRef]
- Shimada, Y.; Terasawa, M.; Okazaki, F.; Nakayama, H.; Zang, L.; Nishiura, K.; Matsuda, K.; Nishimura, N. Rhamnan sulphate from green algae Monostroma nitidum improves constipation with gut microbiome alteration in double-blind placebo-controlled trial. Sci. Rep. 2021, 11, 13384. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.P.; Gomez-Hernandez, A.; Zhang, F.; Cancel, L.; Feng, X.; Yan, L.; Xia, K.; Takematsu, E.; Yang, E.Y.; Le, V.; et al. Rhamnan sulfate reduces atherosclerotic plaque formation and vascular inflammation. Biomaterials 2022, 291, 121865. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, T.J.; Loiselle, J.J.; Sutherland, L.C. Assessment of reference genes for real-time quantitative PCR gene expression normalization during C2C12 and H9c2 skeletal muscle differentiation. Mol. Biotechnol. 2014, 56, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, M.; Maeda, T.; Moriwaki, S.; Nomura, A.; Kato, Y.; Niida, S.; Kruger, M.C.; Suzuki, K. Petunidin, a B-ring 5’-O-Methylated Derivative of Delphinidin, Stimulates Osteoblastogenesis and Reduces sRANKL-Induced Bone Loss. Int. J. Mol. Sci. 2019, 20, 2795. [Google Scholar] [CrossRef]
- Nasiri-Ansari, Ν.; Dimitriadis, G.K.; Agrogiannis, G.; Perrea, D.; Kostakis, I.D.; Kaltsas, G.; Papavassiliou, A.G.; Randeva, H.S.; Kassi, E. Canagliflozin attenuates the progression of atherosclerosis and inflammation process in APOE knockout mice. Cardiovasc. Diabetol. 2018, 17, 106. [Google Scholar] [CrossRef]
- Duivenvoorde, L.P.; van Schothorst, E.M.; Bunschoten, A.; Keijer, J. Dietary restriction of mice on a high-fat diet induces substrate efficiency and improves metabolic health. J. Mol. Endocrinol. 2011, 47, 81–97. [Google Scholar] [CrossRef]
- Fath-Bayati, L.; Ai, J. Assessment of mesenchymal stem cell effect on foreign body response induced by intraperitoneally implanted alginate spheres. J. Biomed. Mater. Res. A 2020, 108, 94–102. [Google Scholar] [CrossRef]
- Yokoyama, S.; Hiramoto, K.; Koyama, M.; Ooi, K. Skin disruption is associated with indomethacin-induced small intestinal injury in mice. Exp. Dermatol. 2014, 23, 659–663. [Google Scholar] [CrossRef]
- Geng, Y.; Xing, L.; Sun, M.; Su, F. Immunomodulatory effects of sulfated polysaccharides of pine pollen on mouse macrophages. Int. J. Biol. Macromol. 2016, 91, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Yamagaki, T.; Maeda, M.; Nakanishi, H. Rhamnan sulfate from cell walls of Monostroma latissimum. Phytochemistry 1998, 48, 921–925. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Gorini, F.; Vassalle, C. Lipids in Atherosclerosis: Pathophysiology and the Role of Calculated Lipid Indices in Assessing Cardiovascular Risk in Patients with Hyperlipidemia. Int. J. Mol. Sci. 2022, 24, 75. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Tanaka, T.; Nishimura, N. Rhamnan sulphate from Monostroma nitidum attenuates hepatic steatosis by suppressing lipogenesis in a diet-induced obesity zebrafish model. J. Funct. Foods 2015, 17, 364–370. [Google Scholar] [CrossRef]
- Shchelkunova, T.A.; Morozov, I.A.; Rubtsov, P.M.; Bobryshev, Y.V.; Sobenin, I.A.; Orekhov, A.N.; Andrianova, I.V.; Smirnov, A.N. Lipid regulators during atherogenesis: Expression of LXR, PPAR, and SREBP mRNA in the human aorta. PLoS ONE 2013, 8, e63374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cybulsky, M.I.; Iiyama, K.; Li, H.; Zhu, S.; Chen, M.; Iiyama, M.; Davis, V.; Gutierrez-Ramos, J.C.; Connelly, P.W.; Milstone, D.S. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 2001, 107, 1255–1262. [Google Scholar] [CrossRef]
- Dansky, H.M.; Barlow, C.B.; Lominska, C.; Sikes, J.L.; Kao, C.; Weinsaft, J.; Cybulsky, M.I.; Smith, J.D. Adhesion of Monocytes to Arterial Endothelium and Initiation of Atherosclerosis Are Critically Dependent on Vascular Cell Adhesion Molecule-1 Gene Dosage. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1662–1667. [Google Scholar] [CrossRef]
- Nakashima, Y.; Raines, E.W.; Plump, A.S.; Breslow, J.L.; Ross, R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 842–851. [Google Scholar] [CrossRef]
- He, C.; Medley, S.C.; Hu, T.; Hinsdale, M.E.; Lupu, F.; Virmani, R.; Olson, L.E. PDGFRbeta signalling regulates local inflammation and synergizes with hypercholesterolaemia to promote atherosclerosis. Nat. Commun. 2015, 6, 7770. [Google Scholar] [CrossRef]
- Saja, M.F.; Baudino, L.; Jackson, W.D.; Cook, H.T.; Malik, T.H.; Fossati-Jimack, L.; Ruseva, M.; Pickering, M.C.; Woollard, K.J.; Botto, M. Triglyceride-Rich Lipoproteins Modulate the Distribution and Extravasation of Ly6C/Gr1(low) Monocytes. Cell Rep. 2015, 12, 1802–1815. [Google Scholar] [CrossRef] [PubMed]
- Wagsater, D.; Zhu, C.; Bjorkegren, J.; Skogsberg, J.; Eriksson, P. MMP-2 and MMP-9 are prominent matrix metalloproteinases during atherosclerosis development in the Ldlr(-/-)Apob(100/100) mouse. Int. J. Mol. Med. 2011, 28, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Khachigian, L.M.; Resnick, N.; Gimbrone, M.A., Jr.; Collins, T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J. Clin. Invest. 1995, 96, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Cybulsky, M.I. NF-kappaB: Pivotal mediator or innocent bystander in atherogenesis? J. Clin. Invest. 2001, 107, 255–264. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chang, L.S. NFκB- and AP-1-mediated DNA looping regulates matrix metalloproteinase-9 transcription in TNF-α-treated human leukemia U937 cells. Biochim. Biophys. Acta 2015, 1849, 1248–1259. [Google Scholar] [CrossRef]
- Alam, S.; Liu, Q.; Liu, S.; Liu, Y.; Zhang, Y.; Yang, X.; Liu, G.; Fan, K.; Ma, J. Up-regulated cathepsin C induces macrophage M1 polarization through FAK-triggered p38 MAPK/NF-κB pathway. Exp. Cell Res. 2019, 382, 111472. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Zhang, X.; Li, Z.; Wang, L.; Sun, Y.; Liu, Z.; Ma, X. Elevated levels of plasma TNF-α are associated with microvascular endothelial dysfunction in patients with sepsis through activating the NF-κB and p38 mitogen-activated protein kinase in endothelial cells. Shock 2014, 41, 275–281. [Google Scholar] [CrossRef]
- Shirakura, K.; Ishiba, R.; Kashio, T.; Funatsu, R.; Tanaka, T.; Fukada, S.I.; Ishimoto, K.; Hino, N.; Kondoh, M.; Ago, Y.; et al. The Robo4-TRAF7 complex suppresses endothelial hyperpermeability in inflammation. J. Cell Sci. 2019, 132, jcs220228. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Laeliocattleya, R.A.; Yunianta, Y.; Risjani, Y.; Wulan, S.N. In silico molecular docking, molecular dynamics, ADMET analysis of fucoidan against receptor frizzled-8 and coreceptor LRP6 in Wnt/β-Catenin pathway and in vitro analysis of fucoidan extract from Sargassum echinocarpum as β-catenin inhibitor in breast cancer cell line (MCF-7). J. Biomol. Struct. Dyn. 2023, 1–16. [Google Scholar] [CrossRef]

| Aortic Molecules | Plasma TCHO | Plasma TG | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
| VCAM-1 | 0.927 | 0.073 | 0.836 | 0.164 |
| ICAM-1 | 0.932 | 0.068 | 0.562 | 0.438 |
| F4/80 | 0.869 | 0.131 | 0.955 | 0.046 * |
| PDGFRβ | 0.96 | 0.040 * | 0.903 | 0.098 |
| αSMA | 0.958 | 0.042 * | 0.692 | 0.308 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terasawa, M.; Zang, L.; Hiramoto, K.; Shimada, Y.; Mitsunaka, M.; Uchida, R.; Nishiura, K.; Matsuda, K.; Nishimura, N.; Suzuki, K. Oral Administration of Rhamnan Sulfate from Monostroma nitidum Suppresses Atherosclerosis in ApoE-Deficient Mice Fed a High-Fat Diet. Cells 2023, 12, 2666. https://doi.org/10.3390/cells12222666
Terasawa M, Zang L, Hiramoto K, Shimada Y, Mitsunaka M, Uchida R, Nishiura K, Matsuda K, Nishimura N, Suzuki K. Oral Administration of Rhamnan Sulfate from Monostroma nitidum Suppresses Atherosclerosis in ApoE-Deficient Mice Fed a High-Fat Diet. Cells. 2023; 12(22):2666. https://doi.org/10.3390/cells12222666
Chicago/Turabian StyleTerasawa, Masahiro, Liqing Zang, Keiichi Hiramoto, Yasuhito Shimada, Mari Mitsunaka, Ryota Uchida, Kaoru Nishiura, Koichi Matsuda, Norihiro Nishimura, and Koji Suzuki. 2023. "Oral Administration of Rhamnan Sulfate from Monostroma nitidum Suppresses Atherosclerosis in ApoE-Deficient Mice Fed a High-Fat Diet" Cells 12, no. 22: 2666. https://doi.org/10.3390/cells12222666
APA StyleTerasawa, M., Zang, L., Hiramoto, K., Shimada, Y., Mitsunaka, M., Uchida, R., Nishiura, K., Matsuda, K., Nishimura, N., & Suzuki, K. (2023). Oral Administration of Rhamnan Sulfate from Monostroma nitidum Suppresses Atherosclerosis in ApoE-Deficient Mice Fed a High-Fat Diet. Cells, 12(22), 2666. https://doi.org/10.3390/cells12222666