Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis
Abstract
1. Introduction
1.1. Canonical and Non Canonical Inflammasomes
1.2. Non-LPS Mediated Activation of Non Canonical Inflammasome Pathway
1.3. Negative Regulation of Inflammasomes
1.4. Positive Regulation of Inflammasome-Mediated Inflammatory Cell Death by Caspase 3, 7, and 8
2. Human Monocytotropic Ehrlichiosis (HME)
2.1. Clinical Presentations
2.2. Pathogenesis of HME and Immunopathology
3. Role of Canonical and Non-Canonical Inflammasome Pathway in Ehrlichiosis
3.1. Canonical Inflammasome Pathway (s) in Ehrlichiosis
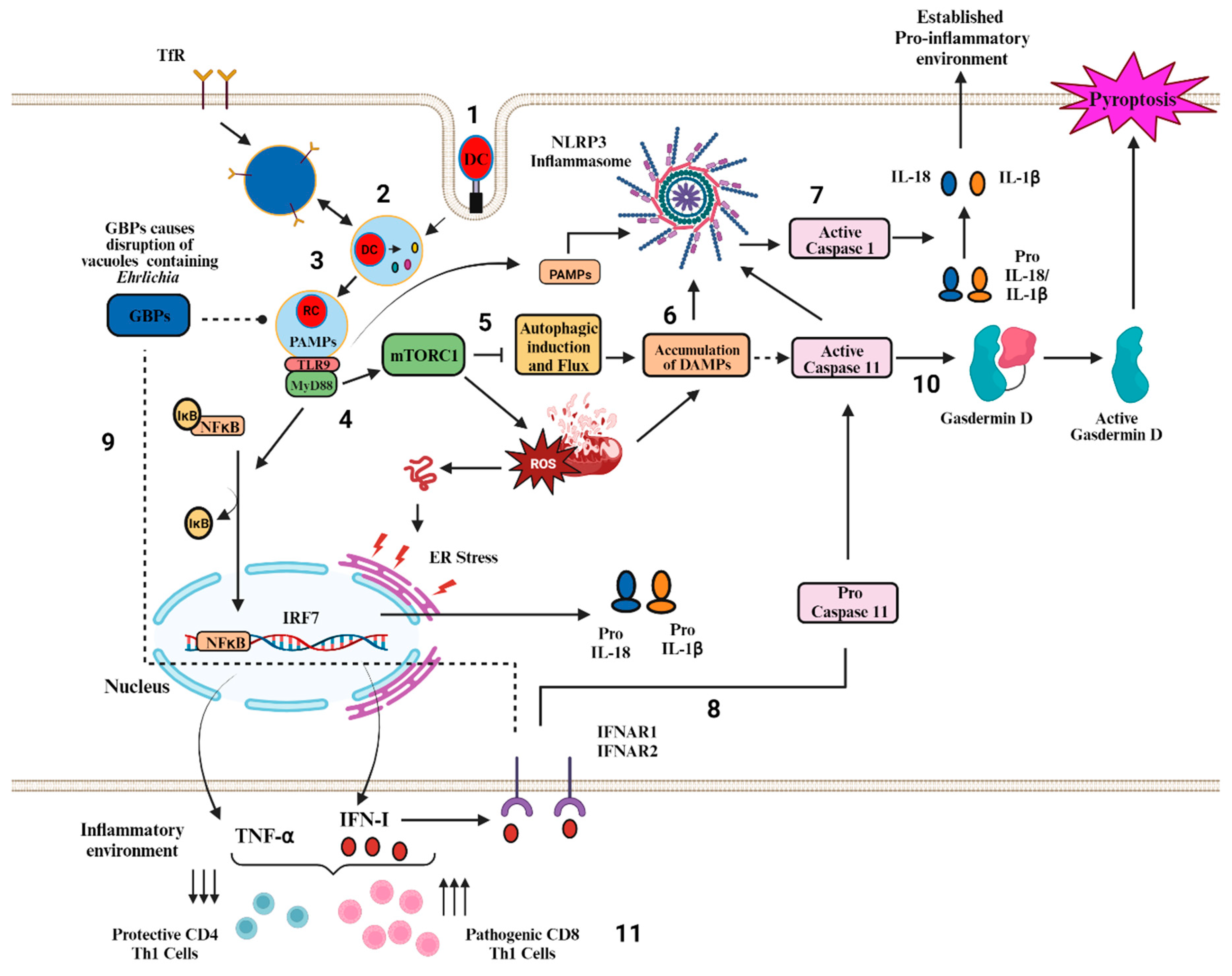
3.2. Non-Canonical Inflammasome Pathways and Their Regulation by Type I- IFN in Fatal Ehrlichiosis
3.3. Potential PAMPS and DAMPS That Trigger Inflammasome (s) Activation in Ehrlichiosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.L.; Biggs, K.E.; Rankin, B.E.; Havrda, M.C. NLRP3 inflammasome in neurodegenerative disease. Transl. Res. 2023, 252, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Ting, J.P.; O’Neill, L.A. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat. Immunol. 2012, 13, 352–357. [Google Scholar] [CrossRef]
- Lu, A.; Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 2015, 282, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Kanneganti, T.D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016, 16, 7–21. [Google Scholar] [CrossRef]
- Xia, S.; Hollingsworth, L.R.t.; Wu, H. Mechanism and Regulation of Gasdermin-Mediated Cell Death. Cold Spring Harb. Perspect. Biol. 2020, 12, a036400. [Google Scholar] [CrossRef]
- Stojkov, D.; Claus, M.J.; Kozlowski, E.; Oberson, K.; Schären, O.P.; Benarafa, C.; Yousefi, S.; Simon, H.U. NET formation is independent of gasdermin D and pyroptotic cell death. Sci. Signal 2023, 16, eabm0517. [Google Scholar] [CrossRef]
- Kambara, H.; Liu, F.; Zhang, X.; Liu, P.; Bajrami, B.; Teng, Y.; Zhao, L.; Zhou, S.; Yu, H.; Zhou, W.; et al. Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep. 2018, 22, 2924–2936. [Google Scholar] [CrossRef]
- Burgener, S.S.; Leborgne, N.G.F.; Snipas, S.J.; Salvesen, G.S.; Bird, P.I.; Benarafa, C. Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell Rep. 2019, 27, 3646–3656.e5. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Levinsohn, J.L.; Newman, Z.L.; Hellmich, K.A.; Fattah, R.; Getz, M.A.; Liu, S.; Sastalla, I.; Leppla, S.H.; Moayeri, M. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012, 8, e1002638. [Google Scholar] [CrossRef]
- Lin, J.; Cheng, A.; Cheng, K.; Deng, Q.; Zhang, S.; Lan, Z.; Wang, W.; Chen, J. New insights into the mechanisms of pyroptosis and implications for diabetic kidney disease. Int. J. Mol. Sci. 2020, 21, 7057. [Google Scholar] [CrossRef]
- Sastalla, I.; Crown, D.; Masters, S.L.; McKenzie, A.; Leppla, S.H.; Moayeri, M. Transcriptional analysis of the three Nlrp1 paralogs in mice. BMC Genom. 2013, 14, 188. [Google Scholar] [CrossRef]
- D’Osualdo, A.; Anania, V.G.; Yu, K.; Lill, J.R.; Kaufman, R.J.; Matsuzawa, S.; Reed, J.C. Transcription Factor ATF4 Induces NLRP1 Inflammasome Expression during Endoplasmic Reticulum Stress. PLoS ONE 2015, 10, e0130635. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Jiang, Z.; Waggoner, S.N.; Sharma, S.; Cole, L.E.; Waggoner, L.; Vanaja, S.K.; Monks, B.G.; Ganesan, S.; Latz, E.; et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 2010, 11, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wallet, P.; Benaoudia, S.; Mosnier, A.; Lagrange, B.; Martin, A.; Lindgren, H.; Golovliov, I.; Michal, F.; Basso, P.; Djebali, S.; et al. IFN-gamma extends the immune functions of Guanylate Binding Proteins to inflammasome-independent antibacterial activities during Francisella novicida infection. PLoS Pathog. 2017, 13, e1006630. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Sasai, M.; Place, D.E.; Kesavardhana, S.; Temirov, J.; Frase, S.; Zhu, Q.; Malireddi, R.K.S.; Kuriakose, T.; et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell 2016, 167, 382–396.e317. [Google Scholar] [CrossRef] [PubMed]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Miura, M.; Jung, Y.-k.; Zhu, H.; Li, E.; Yuan, J. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 1998, 92, 501–509. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Loughran, P.A.; Yang, M.; Lin, M.; Yang, C.; Gao, W.; Jin, S.; Li, S.; Cai, J.; et al. LPS Induces Active HMGB1 Release from Hepatocytes into Exosomes Through the Coordinated Activities of TLR4 and Caspase-11/GSDMD Signaling. Front. Immunol. 2020, 11, 229. [Google Scholar] [CrossRef] [PubMed]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-canonical inflammasome activation targets caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- von Moltke, J.; Trinidad, N.J.; Moayeri, M.; Kintzer, A.F.; Wang, S.B.; van Rooijen, N.; Brown, C.R.; Krantz, B.A.; Leppla, S.H.; Gronert, K.; et al. Rapid induction of inflammatory lipid mediators by the inflammasome in vivo. Nature 2012, 490, 107–111. [Google Scholar] [CrossRef]
- de Carvalho, R.V.H.; Andrade, W.A.; Lima-Junior, D.S.; Dilucca, M.; de Oliveira, C.V.; Wang, K.; Nogueira, P.M.; Rugani, J.N.; Soares, R.P.; Beverley, S.M.; et al. Leishmania Lipophosphoglycan Triggers Caspase-11 and the Non-canonical Activation of the NLRP3 Inflammasome. Cell Rep. 2019, 26, 429–437.e5. [Google Scholar] [CrossRef]
- Gabrielli, E.; Pericolini, E.; Luciano, E.; Sabbatini, S.; Roselletti, E.; Perito, S.; Kasper, L.; Hube, B.; Vecchiarelli, A. Induction of caspase-11 by aspartyl proteinases of Candida albicans and implication in promoting inflammatory response. Infect. Immun. 2015, 83, 1940–1948. [Google Scholar] [CrossRef]
- Elizagaray, M.L.; Gomes, M.T.R.; Guimaraes, E.S.; Rumbo, M.; Hozbor, D.F.; Oliveira, S.C.; Moreno, G. Canonical and Non-canonical Inflammasome Activation by Outer Membrane Vesicles Derived from Bordetella pertussis. Front. Immunol. 2020, 11, 1879. [Google Scholar] [CrossRef]
- Chu, L.H.; Indramohan, M.; Ratsimandresy, R.A.; Gangopadhyay, A.; Morris, E.P.; Monack, D.M.; Dorfleutner, A.; Stehlik, C. The oxidized phospholipid oxPAPC protects from septic shock by targeting the non-canonical inflammasome in macrophages. Nat. Commun. 2018, 9, 996. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Vanaja, S.K.; Waggoner, L.; Sokolovska, A.; Becker, C.; Stuart, L.M.; Leong, J.M.; Fitzgerald, K.A. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 2012, 150, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Lupfer, C.R.; Anand, P.K.; Liu, Z.; Stokes, K.L.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Reactive oxygen species regulate caspase-11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. PLoS Pathog. 2014, 10, e1004410. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Daily, K.; Estfanous, S.; Hamilton, K.; Badr, A.; Abu Khweek, A.; Hegazi, R.; Anne, M.N.; Klamer, B.; Zhang, X.; et al. Caspase-11 counteracts mitochondrial ROS-mediated clearance of Staphylococcus aureus in macrophages. EMBO Rep. 2019, 20, e48109. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zeng, L.; Zhu, S.; Liu, J.; Zeh, H.J.; Kroemer, G.; Wang, H.; Billiar, T.R.; Jiang, J.; Tang, D.; et al. cAMP metabolism controls caspase-11 inflammasome activation and pyroptosis in sepsis. Sci. Adv. 2019, 5, eaav5562. [Google Scholar] [CrossRef]
- Mishra, B.B.; Rathinam, V.A.; Martens, G.W.; Martinot, A.J.; Kornfeld, H.; Fitzgerald, K.A.; Sassetti, C.M. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1β. Nat. Immunol. 2013, 14, 52–60. [Google Scholar] [CrossRef]
- Wei, M.; Wang, L.; Wu, T.; Xi, J.; Han, Y.; Yang, X.; Zhang, D.; Fang, Q.; Tang, B. NLRP3 Activation Was Regulated by DNA Methylation Modification during Mycobacterium tuberculosis Infection. Biomed. Res. Int. 2016, 2016, 4323281. [Google Scholar] [CrossRef]
- Moreira, J.D.; Iakhiaev, A.; Vankayalapati, R.; Jung, B.G.; Samten, B. Histone deacetylase-2 controls IL-1β production through the regulation of NLRP3 expression and activation in tuberculosis infection. iScience 2022, 25, 104799. [Google Scholar] [CrossRef]
- Neudecker, V.; Haneklaus, M.; Jensen, O.; Khailova, L.; Masterson, J.C.; Tye, H.; Biette, K.; Jedlicka, P.; Brodsky, K.S.; Gerich, M.E.; et al. Myeloid-derived miR-223 regulates intestinal inflammation via repression of the NLRP3 inflammasome. J. Exp. Med. 2017, 214, 1737–1752. [Google Scholar] [CrossRef]
- Houshmandfar, S.; Saeedi-Boroujeni, A.; Rashno, M.; Khodadadi, A.; Mahmoudian-Sani, M.R. miRNA-223 as a regulator of inflammation and NLRP3 inflammasome, the main fragments in the puzzle of immunopathogenesis of different inflammatory diseases and COVID-19. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 2187–2195. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, M.; Du, R.-H.; Qiao, C.; Jiang, C.-Y.; Zhang, K.-Z.; Ding, J.-H.; Hu, G. MicroRNA-7 targets Nod-like receptor protein 3 inflammasome to modulate neuroinflammation in the pathogenesis of Parkinson’s disease. Mol. Neurodegener. 2016, 11, 28. [Google Scholar] [CrossRef]
- Han, Q.Q.; Le, W. NLRP3 Inflammasome-Mediated Neuroinflammation and Related Mitochondrial Impairment in Parkinson’s Disease. Neurosci. Bull. 2023, 39, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.N.; Kim, D.; Melia, T.; Karri, K.; Velenosi, T.J.; Takahashi, S.; Aibara, D.; Bonzo, J.A.; Levi, M.; Waxman, D.J.; et al. Long non-coding RNA Gm15441 attenuates hepatic inflammasome activation in response to PPARA agonism and fasting. Nat. Commun. 2020, 11, 5847. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Chen, S.; Chen, M.; Ma, Y.; Wang, Y.; Huang, B.; He, Z.; Zeng, Y.; Hu, Y.; Sun, S.; et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res. 2013, 23, 201–212. [Google Scholar] [CrossRef]
- Gao, Y.; Tu, D.; Yang, R.; Chu, C.H.; Hong, J.S.; Gao, H.M. Through Reducing ROS Production, IL-10 Suppresses Caspase-1-Dependent IL-1β Maturation, thereby Preventing Chronic Neuroinflammation and Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 465. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Zhu, J.; Zhang, M.; Li, C.; Zhou, X.; Zhou, M.; Ke, M.; Busuttil, R.W.; Ying, Q.L.; Kupiec-Weglinski, J.W.; et al. The myeloid heat shock transcription factor 1/β-catenin axis regulates NLR family, pyrin domain-containing 3 inflammasome activation in mouse liver ischemia/reperfusion injury. Hepatology 2016, 64, 1683–1698. [Google Scholar] [CrossRef]
- Talty, A.; Deegan, S.; Ljujic, M.; Mnich, K.; Naicker, S.D.; Quandt, D.; Zeng, Q.; Patterson, J.B.; Gorman, A.M.; Griffin, M.D.; et al. Inhibition of IRE1α RNase activity reduces NLRP3 inflammasome assembly and processing of pro-IL1β. Cell Death Dis. 2019, 10, 622. [Google Scholar] [CrossRef]
- Green, D.R. The Mitochondrial Pathway of Apoptosis: Part I: MOMP and Beyond. Cold Spring Harb. Perspect. Biol. 2022, 14, a041038. [Google Scholar] [CrossRef]
- Rogers, C.; Fernandes-Alnemri, T.; Mayes, L.; Alnemri, D.; Cingolani, G.; Alnemri, E.S. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017, 8, 14128. [Google Scholar] [CrossRef]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef]
- Gou, X.; Xu, W.; Liu, Y.; Peng, Y.; Xu, W.; Yin, Y.; Zhang, X. IL-6 Prevents Lung Macrophage Death and Lung Inflammation Injury by Inhibiting GSDME- and GSDMD-Mediated Pyroptosis during Pneumococcal Pneumosepsis. Microbiol. Spectr. 2022, 10, e0204921. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.-C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A., Jr.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, H.; Hou, Y.; Li, Z.; Li, L.; Song, X.; Ding, J.; Shao, F.; Xu, Y. Calmodulin Binding Activates Chromobacterium CopC Effector to ADP-Riboxanate Host Apoptotic Caspases. mBio 2022, 13, e0069022. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.K.; Hagar, J.A.; Kanneganti, T.D.; Franchi, L.; Nuñez, G.; O’Riordan, M.X. Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog. 2012, 8, e1002628. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Maltez, V.I.; Rayamajhi, M.; Tubbs, A.L.; Mitchell, J.E.; Lacey, C.A.; Harvest, C.K.; Li, L.; Nash, W.T.; Larson, H.N.; et al. Caspase-7 activates ASM to repair gasdermin and perforin pores. Nature 2022, 606, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, M.H.; Munn, R.G.K.; Fearnhead, H.O. Non-Canonical Roles of Apoptotic Caspases in the Nervous System. Front. Cell Dev. Biol. 2022, 10, 840023. [Google Scholar] [CrossRef]
- Yanumula, A.; Cusick, J.K. Biochemistry, Extrinsic Pathway of Apoptosis. In StatPearls; Disclosure: John Cusick Declares no Relevant Financial Relationships with Ineligible Companies; Treasure Island (FL) Ineligible Companies: Treasure Island, FL, USA, 2023. [Google Scholar]
- Philip, N.H.; Dillon, C.P.; Snyder, A.G.; Fitzgerald, P.; Wynosky-Dolfi, M.A.; Zwack, E.E.; Hu, B.; Fitzgerald, L.; Mauldin, E.A.; Copenhaver, A.M.; et al. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 7385–7390. [Google Scholar] [CrossRef]
- Demarco, B.; Grayczyk, J.P.; Bjanes, E.; Le Roy, D.; Tonnus, W.; Assenmacher, C.A.; Radaelli, E.; Fettrelet, T.; Mack, V.; Linkermann, A.; et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 2020, 6, eabc3465. [Google Scholar] [CrossRef]
- Chen, M.; Xing, Y.; Lu, A.; Fang, W.; Sun, B.; Chen, C.; Liao, W.; Meng, G. Internalized Cryptococcus neoformans Activates the Canonical Caspase-1 and the Noncanonical Caspase-8 Inflammasomes. J. Immunol. 2015, 195, 4962–4972. [Google Scholar] [CrossRef]
- Shi, C.; Cao, P.; Wang, Y.; Zhang, Q.; Zhang, D.; Wang, Y.; Wang, L.; Gong, Z. PANoptosis: A Cell Death Characterized by Pyroptosis, Apoptosis, and Necroptosis. J. Inflamm. Res. 2023, 16, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Kanneganti, T.D. The regulation of the ZBP1-NLRP3 inflammasome and its implications in pyroptosis, apoptosis, and necroptosis (PANoptosis). Immunol. Rev. 2020, 297, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Rikihisa, Y. Molecular Pathogenesis of Ehrlichia chaffeensis Infection. Annu. Rev. Microbiol. 2015, 69, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Sharma, A.; Soong, L.; Walker, D.H. Review: Protective Immunity and Immunopathology of Ehrlichiosis. Zoonoses 2022, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Ismail, N. Role of Autophagy in Ehrlichia-Induced Liver Injury. Cells 2023, 12, 1334. [Google Scholar] [CrossRef]
- Sharma, A.K.; Dhasmana, N.; Arora, G. Bridging the Gap: Exploring the Connection between Animal and Human Health. Zoonotic Dis. 2023, 3, 176–178. [Google Scholar] [CrossRef]
- Ismail, N.; Soong, L.; McBride, J.W.; Valbuena, G.; Olano, J.P.; Feng, H.M.; Walker, D.H. Overproduction of TNF-alpha by CD8+ type 1 cells and down-regulation of IFN-gamma production by CD4+ Th1 cells contribute to toxic shock-like syndrome in an animal model of fatal monocytotropic ehrlichiosis. J. Immunol. 2004, 172, 1786–1800. [Google Scholar] [CrossRef]
- Dumler, J.S. Anaplasma and Ehrlichia infection. Ann. N. Y. Acad. Sci. 2005, 1063, 361–373. [Google Scholar] [CrossRef]
- Sehdev, A.E.; Dumler, J.S. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am. J. Clin. Pathol. 2003, 119, 859–865. [Google Scholar] [CrossRef]
- Okada, H.; Tajima, T.; Kawahara, M.; Rikihisa, Y. Ehrlichial proliferation and acute hepatocellular necrosis in immunocompetent mice experimentally infected with the HF strain of Ehrlichia, closely related to Ehrlichia chaffeensis. J. Comp. Pathol. 2001, 124, 165–171. [Google Scholar] [CrossRef]
- Ismail, N.; Bloch, K.C.; McBride, J.W. Human ehrlichiosis and anaplasmosis. Clin. Lab. Med. 2010, 30, 261–292. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.; Dawson, J.; Jones, D.; Wilson, K. Ehrlichia chaffeensis, a new species associated with human ehrlichiosis. J. Clin. Microbiol. 1991, 29, 2838–2842. [Google Scholar] [CrossRef]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and’HGE agent’as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar] [PubMed]
- Lina, T.T.; Farris, T.; Luo, T.; Mitra, S.; Zhu, B.; McBride, J.W. Hacker within! Ehrlichia chaffeensis Effector Driven Phagocyte Reprogramming Strategy. Front. Cell Infect. Microbiol. 2016, 6, 58. [Google Scholar] [CrossRef]
- Sotomayor, E.A.; Popov, V.L.; Feng, H.M.; Walker, D.H.; Olano, J.P. Animal model of fatal human monocytotropic ehrlichiosis. Am. J. Pathol. 2001, 158, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Pritt, B.S.; Sloan, L.M.; Johnson, D.K.; Munderloh, U.G.; Paskewitz, S.M.; McElroy, K.M.; McFadden, J.D.; Binnicker, M.J.; Neitzel, D.F.; Liu, G.; et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N. Engl. J. Med. 2011, 365, 422–429. [Google Scholar] [CrossRef]
- Allen, M.B.; Pritt, B.S.; Sloan, L.M.; Paddock, C.D.; Musham, C.K.; Ramos, J.M.; Cetin, N.; Rosenbaum, E.R. First reported case of Ehrlichia ewingii involving human bone marrow. J. Clin. Microbiol. 2014, 52, 4102–4104. [Google Scholar] [CrossRef]
- Buller, R.S.; Arens, M.; Hmiel, S.P.; Paddock, C.D.; Sumner, J.W.; Rikhisa, Y.; Unver, A.; Gaudreault-Keener, M.; Manian, F.A.; Liddell, A.M.; et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 1999, 341, 148–155. [Google Scholar] [CrossRef]
- Lin, M.; Xiong, Q.; Chung, M.; Daugherty, S.C.; Nagaraj, S.; Sengamalay, N.; Ott, S.; Godinez, A.; Tallon, L.J.; Sadzewicz, L.; et al. Comparative Analysis of Genome of Ehrlichia sp. HF, a Model Bacterium to Study Fatal Human Ehrlichiosis. BMC Genom. 2021, 22, 11. [Google Scholar] [CrossRef]
- Yang, Q.; Ghose, P.; Ismail, N. Neutrophils mediate immunopathology and negatively regulate protective immune responses during fatal bacterial infection-induced toxic shock. Infect. Immun. 2013, 81, 1751–1763. [Google Scholar] [CrossRef]
- Ismail, N.; Walker, D.H.; Ghose, P.; Tang, Y.W. Immune mediators of protective and pathogenic immune responses in patients with mild and fatal human monocytotropic ehrlichiosis. BMC Immunol. 2012, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Tominello, T.R.; Oliveira, E.R.A.; Hussain, S.S.; Elfert, A.; Wells, J.; Golden, B.; Ismail, N. Emerging Roles of Autophagy and Inflammasome in Ehrlichiosis. Front. Immunol. 2019, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, H.L.; Estes, M.D.; Thirumalapura, N.R.; Walker, D.H.; Ismail, N. Natural killer cells promote tissue injury and systemic inflammatory responses during fatal Ehrlichia-induced toxic shock-like syndrome. Am. J. Pathol. 2010, 177, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Ghose, P.; Ali, A.Q.; Fang, R.; Forbes, D.; Ballard, B.; Ismail, N. The interaction between IL-18 and IL-18 receptor limits the magnitude of protective immunity and enhances pathogenic responses following infection with intracellular bacteria. J. Immunol. 2011, 187, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.; Alaoui-EL-Azher, M.; Vorhauer, J.; Kode, B.B.; Wells, J.Z.; Stolz, D.; Michalopoulos, G.; Wells, A.; Scott, M.; Ismail, N. MyD88-dependent inflammasome activation and autophagy inhibition contributes to Ehrlichia-induced liver injury and toxic shock. PLoS Pathog. 2017, 13, e1006644. [Google Scholar] [CrossRef]
- Kader, M.; El Andaloussi, A.; Vorhaour, J.; Tamama, K.; Nieto, N.; Scott, M.J.; Ismail, N. Interferon Type I Regulates Inflammasome Activation and High Mobility Group Box 1 Translocation in Hepatocytes during Ehrlichia-Induced Acute Liver Injury. Hepatol. Commun. 2021, 5, 33–51. [Google Scholar] [CrossRef]
- Chattoraj, P.; Yang, Q.; Khandai, A.; Al-Hendy, O.; Ismail, N. TLR2 and Nod2 mediate resistance or susceptibility to fatal intracellular Ehrlichia infection in murine models of ehrlichiosis. PLoS ONE 2013, 8, e58514. [Google Scholar] [CrossRef]
- Yang, Q.; Stevenson, H.L.; Scott, M.J.; Ismail, N. Type I interferon contributes to noncanonical inflammasome activation, mediates immunopathology, and impairs protective immunity during fatal infection with lipopolysaccharide-negative ehrlichiae. Am. J. Pathol. 2015, 185, 446–461. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Kobayashi, S.D.; Malachowa, N.; DeLeo, F.R. Neutrophils and Bacterial Immune Evasion. J. Innate Immun. 2018, 10, 432–441. [Google Scholar] [CrossRef]
- Aziz, M.; Jacob, A.; Wang, P. Revisiting caspases in sepsis. Cell Death Dis. 2014, 5, e1526. [Google Scholar] [CrossRef] [PubMed]
- Wickstrum, J.R.; Bokhari, S.M.; Fischer, J.L.; Pinson, D.M.; Yeh, H.W.; Horvat, R.T.; Parmely, M.J. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 2009, 77, 4827–4836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Thai, V.; McCabe, A.; Jones, M.; MacNamara, K.C. Type I interferons promote severe disease in a mouse model of lethal ehrlichiosis. Infect. Immun. 2014, 82, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.N.P.; Zhang, Y.; Li, J.J.; McCabe, A.; Jo, H.J.; Maloney, J.; MacNamara, K.C. Type I IFNs drive hematopoietic stem and progenitor cell collapse via impaired proliferation and increased RIPK1-dependent cell death during shock-like ehrlichial infection. PLoS Pathog. 2018, 14, e1007234. [Google Scholar] [CrossRef]
- Eltobgy, M.M.; Zani, A.; Kenney, A.D.; Estfanous, S.; Kim, E.; Badr, A.; Carafice, C.; Daily, K.; Whitham, O.; Pietrzak, M.; et al. Caspase-4/11 exacerbates disease severity in SARS-CoV-2 infection by promoting inflammation and immunothrombosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202012119. [Google Scholar] [CrossRef]
- Gillespie, J.J.; Brayton, K.A.; Williams, K.P.; Diaz, M.A.; Brown, W.C.; Azad, A.F.; Sobral, B.W. Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect. Immun. 2010, 78, 1809–1823. [Google Scholar] [CrossRef]
- Li, B.w.; Liu, Y.; Zhang, L.; Guo, X.q.; Wen, C.; Zhang, F.; Luo, X.y.; Xia, Y.p. Cytotoxin-associated gene A (CagA) promotes aortic endothelial inflammation and accelerates atherosclerosis through the NLRP3/caspase-1/IL-1β axis. FASEB J. 2021, 35, e21942. [Google Scholar] [CrossRef]
- Zhu, W.; Hammad, L.A.; Hsu, F.; Mao, Y.; Luo, Z.Q. Induction of caspase 3 activation by multiple Legionella pneumophila Dot/Icm substrates. Cell Microbiol. 2013, 15, 1783–1795. [Google Scholar] [CrossRef]
- Krause, K.; Amer, A.O. Caspase Exploitation by Legionella pneumophila. Front. Microbiol. 2016, 7, 515. [Google Scholar] [CrossRef]
- Abu Khweek, A.; Amer, A.O. Pyroptotic and non-pyroptotic effector functions of caspase-11. Immunol. Rev. 2020, 297, 39–52. [Google Scholar] [CrossRef]
- Rikihisa, Y. The “Biological Weapons” of Ehrlichia chaffeensis: Novel Molecules and Mechanisms to Subjugate Host Cells. Front. Cell Infect. Microbiol. 2021, 11, 830180. [Google Scholar] [CrossRef] [PubMed]
- Tyrkalska, S.D.; Candel, S.; Pérez-Oliva, A.B.; Valera, A.; Alcaraz-Pérez, F.; García-Moreno, D.; Cayuela, M.L.; Mulero, V. Identification of an evolutionarily conserved ankyrin domain-containing protein, caiap, which regulates inflammasome-dependent resistance to bacterial infection. Front. Immunol. 2017, 8, 1375. [Google Scholar] [CrossRef]
- Byerly, C.D.; Patterson, L.L.; McBride, J.W. Ehrlichia TRP effectors: Moonlighting, mimicry and infection. Pathog. Dis. 2021, 79, ftab026. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Zhu, B.; Yu, X.-j.; McBride, J.W. New insights into molecular Ehrlichia chaffeensis-host interactions. Microbes Infect. 2010, 12, 337–345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kibler, C.E.; Milligan, S.L.; Farris, T.R.; Zhu, B.; Mitra, S.; McBride, J.W. Ehrlichia chaffeensis TRP47 enters the nucleus via a MYND-binding domain-dependent mechanism and predominantly binds enhancers of host genes associated with signal transduction, cytoskeletal organization, and immune response. PLoS ONE 2018, 13, e0205983. [Google Scholar] [CrossRef]
- Pittner, N.A.; Solomon, R.N.; Bui, D.-C.; McBride, J.W. Ehrlichia effector SLiM-icry: Artifice of cellular subversion. Front. Cell. Infect. Microbiol. 2023, 13, 237. [Google Scholar] [CrossRef]
- Dunphy, P.S.; Luo, T.; McBride, J.W. Ehrlichia chaffeensis exploits host SUMOylation pathways to mediate effector-host interactions and promote intracellular survival. Infect. Immun. 2014, 82, 4154–4168. [Google Scholar] [CrossRef]
- Luo, T.; Dunphy, P.S.; McBride, J.W. Ehrlichia chaffeensis tandem repeat effector targets differentially influence infection. Front. Cell. Infect. Microbiol. 2017, 7, 178. [Google Scholar] [CrossRef]
- Zhu, B.; Das, S.; Mitra, S.; Farris, T.R.; McBride, J.W. Ehrlichia chaffeensis TRP120 moonlights as a HECT E3 ligase involved in self-and host ubiquitination to influence protein interactions and stability for intracellular survival. Infect. Immun. 2017, 85, e00290-17. [Google Scholar] [CrossRef]
- Lina, T.T.; Dunphy, P.S.; Luo, T.; McBride, J.W. Ehrlichia chaffeensis TRP120 activates canonical Notch signaling to downregulate TLR2/4 expression and promote intracellular survival. MBio 2016, 7, e00672-16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, A.K.; Ismail, N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells 2023, 12, 2597. https://doi.org/10.3390/cells12222597
Sharma AK, Ismail N. Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells. 2023; 12(22):2597. https://doi.org/10.3390/cells12222597
Chicago/Turabian StyleSharma, Aditya Kumar, and Nahed Ismail. 2023. "Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis" Cells 12, no. 22: 2597. https://doi.org/10.3390/cells12222597
APA StyleSharma, A. K., & Ismail, N. (2023). Non-Canonical Inflammasome Pathway: The Role of Cell Death and Inflammation in Ehrlichiosis. Cells, 12(22), 2597. https://doi.org/10.3390/cells12222597






