FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. siRNA Transfection
2.3. Western Blot Analysis
2.4. Antibody Array
2.5. Viability Assay
2.6. Neurosphere Formation Assay
2.7. Orthotopic Xenotransplantation of GBMNS
2.8. Statistical Analysis
3. Results
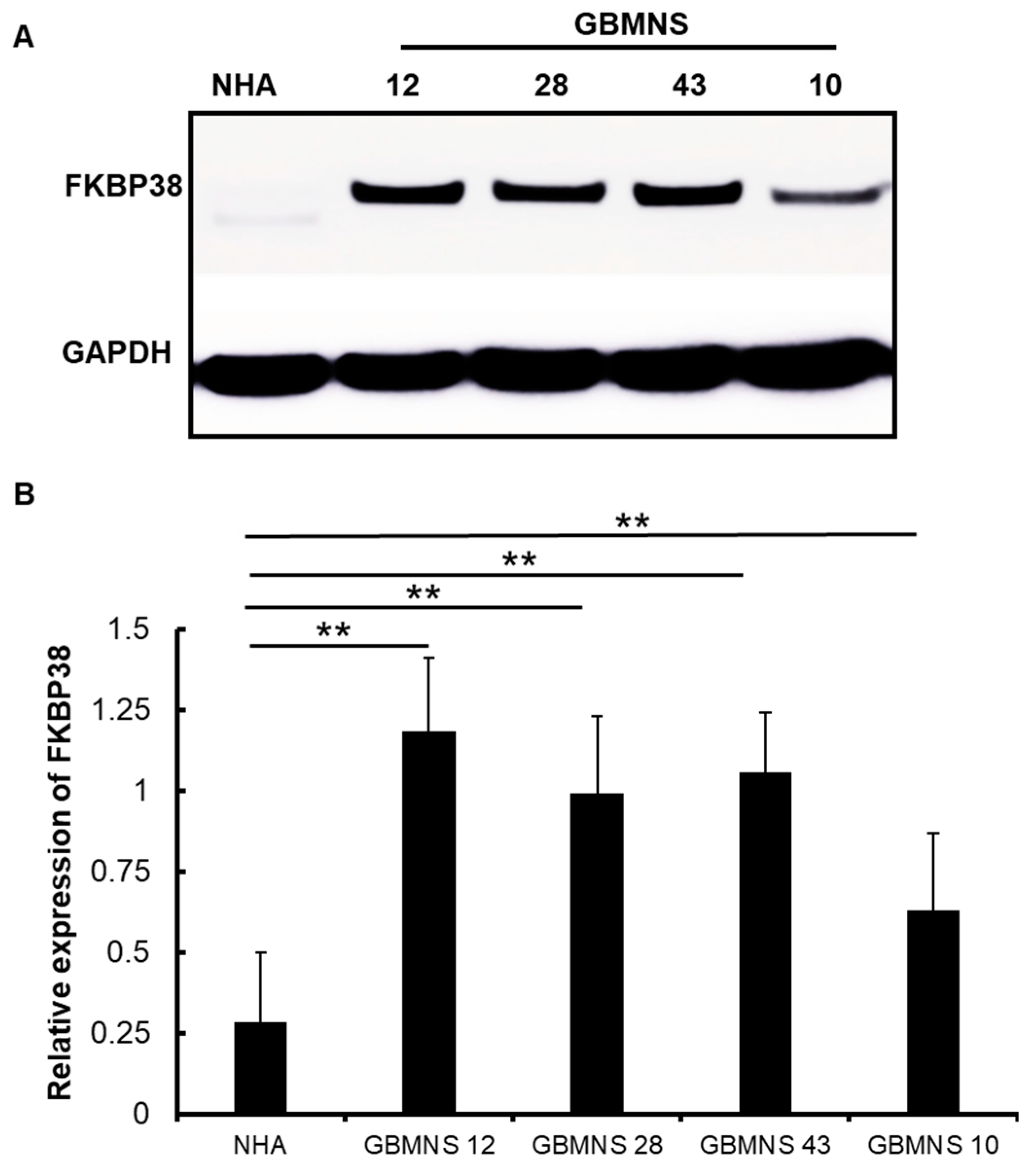
3.1. Expression of FKBP38 Is Higher in Patient-Derived Primary GBMNS
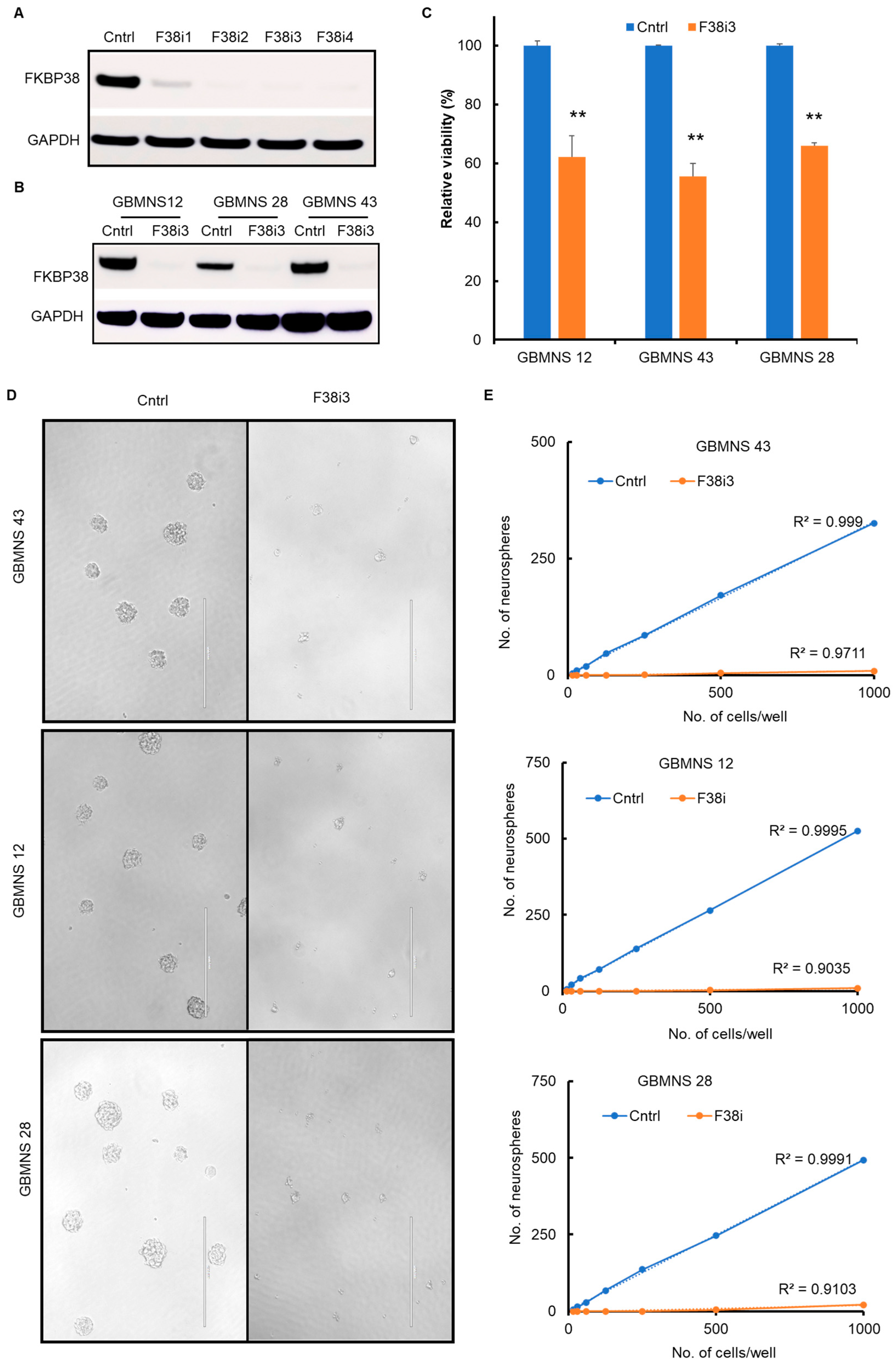
3.2. FKBP38 Knockdown Decreases the Viability and Self-Renewal Capacity of GBMNSs
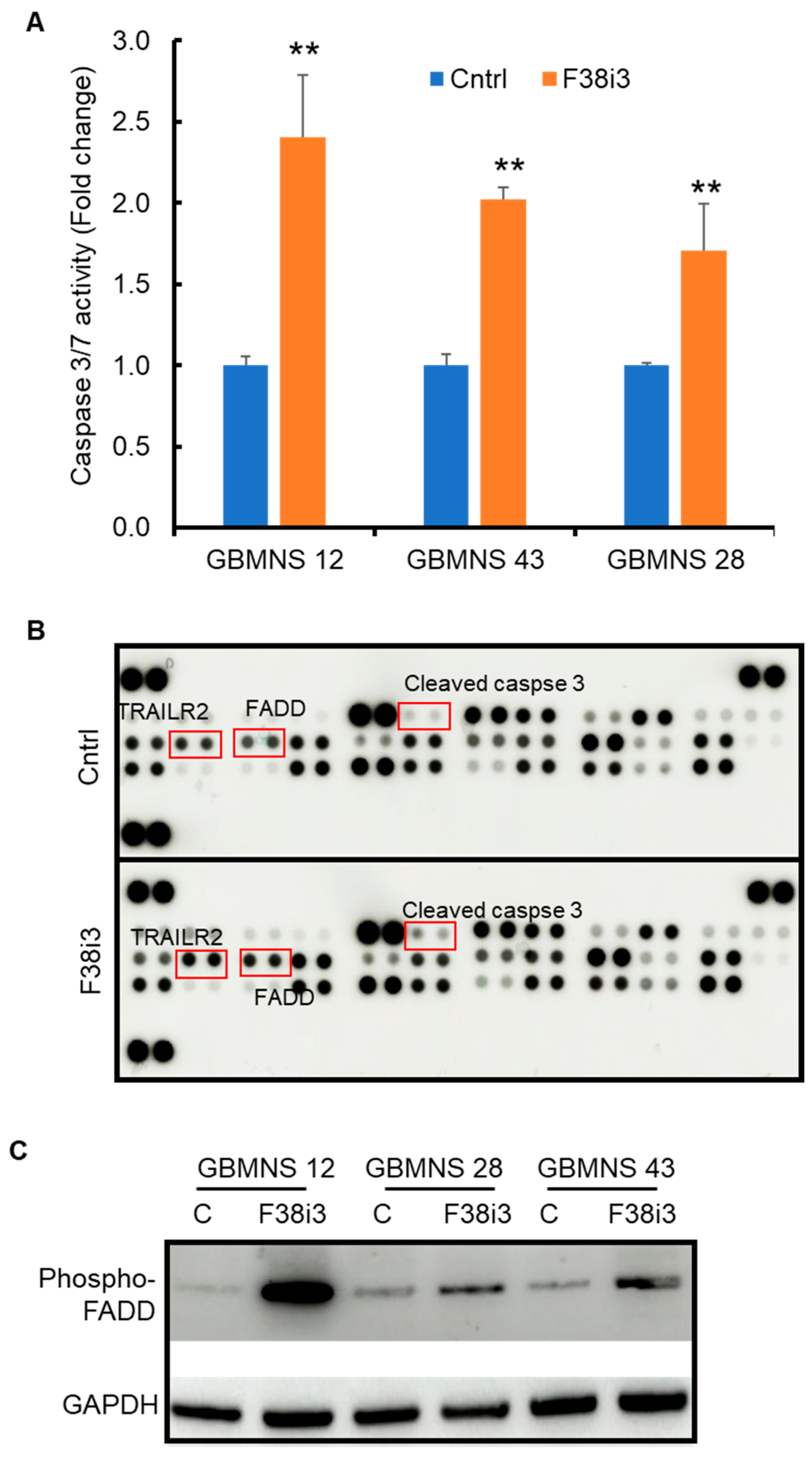
3.3. Depletion of FKBP38 Induces Apoptosis in GBMNS
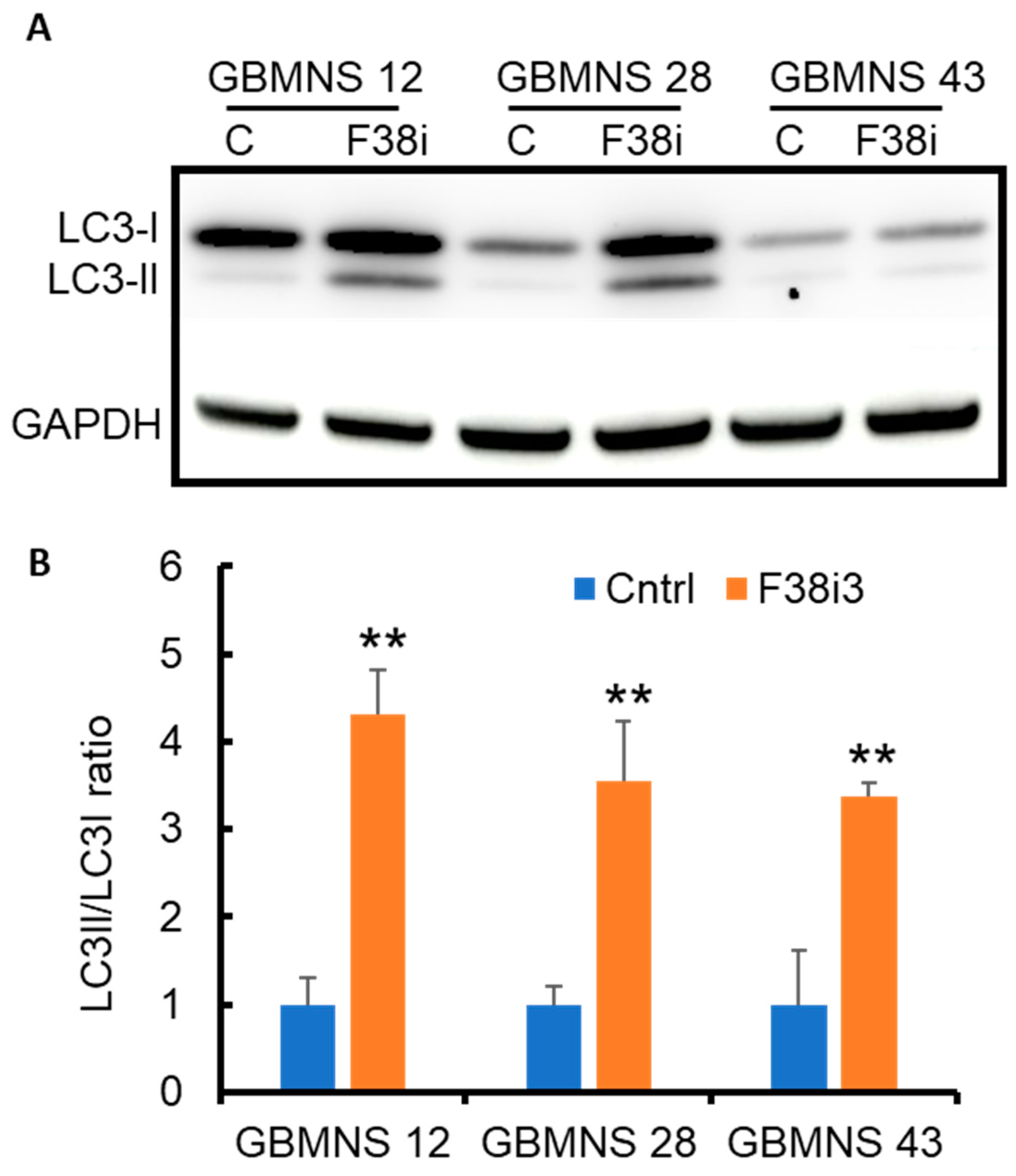
3.4. FKBP38 Depletion Drives GBMNS toward Autophagy
3.5. PI3K/AKT Mediates FKBP38-Regulated Autophagy in GBMNS
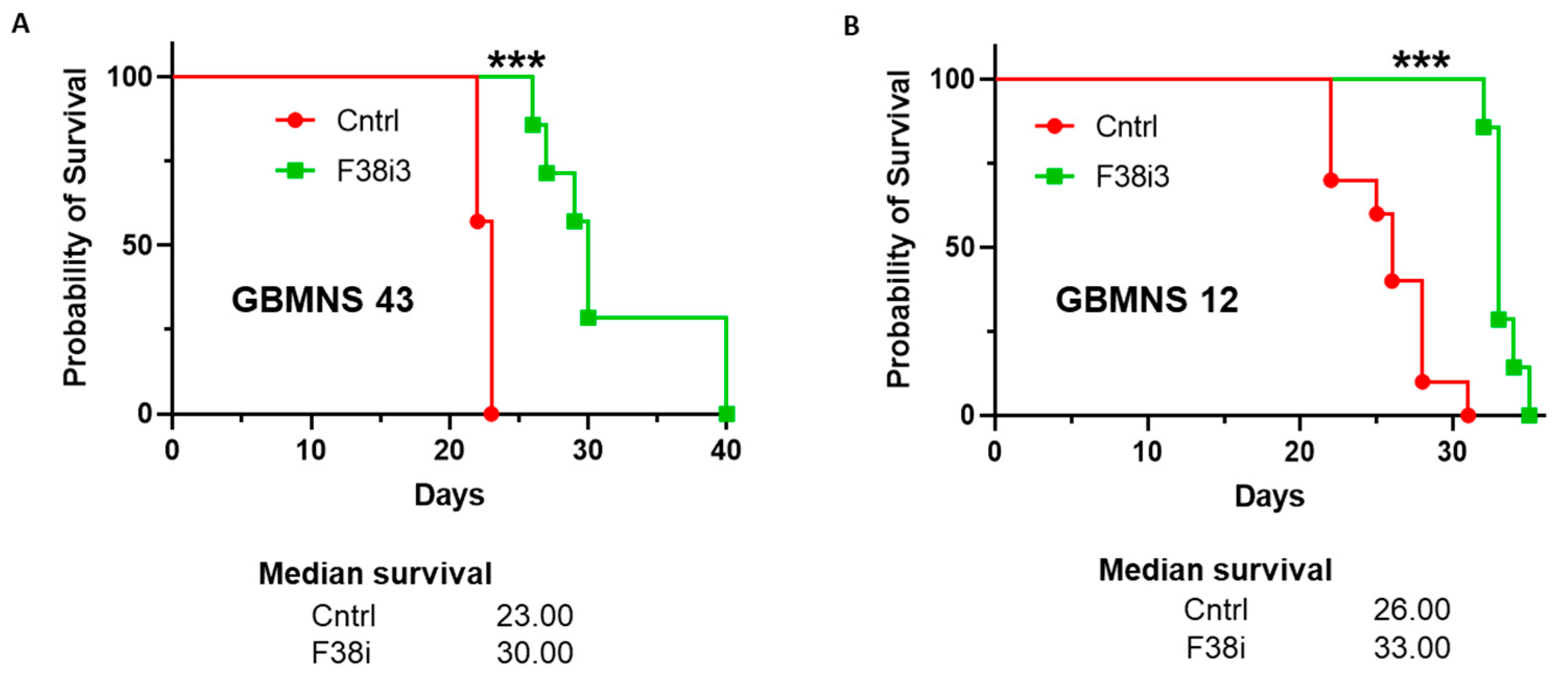
3.6. FKBP38 Depletion Extends the Survival of Tumor-Bearing Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, M.E. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20 (Suppl. S5), S2–S8. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncology 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Harikishore, A.; Yoon, H.S. Immunophilins: Structures, Mechanisms and Ligands. Curr. Mol. Pharmacol. 2015, 9, 37–47. [Google Scholar] [CrossRef]
- Hausch, F. FKBPs and their role in neuronal signaling. Biochim. Biophys. Acta 2015, 1850, 2035–2040. [Google Scholar] [CrossRef]
- Nielsen, J.V.; Mitchelmore, C.; Pedersen, K.M.; Kjaerulff, K.M.; Finsen, B.; Jensen, N.A. Fkbp8: Novel isoforms, genomic organization, and characterization of a forebrain promoter in transgenic mice. Genomics 2004, 83, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F.; Weiwad, M.; Erdmann, F.; Fanghanel, J.; Jarczowski, F.; Rahfeld, J.U.; Fischer, G. Bcl-2 regulator FKBP38 is activated by Ca2+/calmodulin. EMBO J. 2005, 24, 2688–2699. [Google Scholar] [CrossRef]
- Shirane, M.; Nakayama, K.I. Inherent calcineurin inhibitor FKBP38 targets Bcl-2 to mitochondria and inhibits apoptosis. Nat. Cell Biol. 2003, 5, 28–37. [Google Scholar] [CrossRef]
- Germain, M.; Shore, G.C. Cellular distribution of Bcl-2 family proteins. Sci. STKE 2003, 2003, pe10. [Google Scholar] [CrossRef]
- Rosner, M.; Hofer, K.; Kubista, M.; Hengstschlager, M. Cell size regulation by the human TSC tumor suppressor proteins depends on PI3K and FKBP38. Oncogene 2003, 22, 4786–4798. [Google Scholar] [CrossRef][Green Version]
- Bulgakov, O.V.; Eggenschwiler, J.T.; Hong, D.H.; Anderson, K.V.; Li, T. FKBP8 is a negative regulator of mouse sonic hedgehog signaling in neural tissues. Development 2004, 131, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Ma, D.; Liu, A.; Shen, X.; Wang, Q.J.; Liu, Y.; Jiang, Y. Rheb activates mTOR by antagonizing its endogenous inhibitor, FKBP38. Science 2007, 318, 977–980. [Google Scholar] [CrossRef]
- Barth, S.; Nesper, J.; Hasgall, P.A.; Wirthner, R.; Nytko, K.J.; Edlich, F.; Katschinski, D.M.; Stiehl, D.P.; Wenger, R.H.; Camenisch, G. The peptidyl prolyl cis/trans isomerase FKBP38 determines hypoxia-inducible transcription factor prolyl-4-hydroxylase PHD2 protein stability. Mol. Cell Biol. 2007, 27, 3758–3768. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Nishimura, Y.; Ichimura, T.; Suzuki, K.; Miyamura, T.; Suzuki, T.; Moriishi, K.; Matsuura, Y. Hepatitis C virus RNA replication is regulated by FKBP8 and Hsp90. EMBO J. 2006, 25, 5015–5025. [Google Scholar] [CrossRef] [PubMed]
- Banasavadi-Siddegowda, Y.K.; Mai, J.; Fan, Y.; Bhattacharya, S.; Giovannucci, D.R.; Sanchez, E.R.; Fischer, G.; Wang, X. FKBP38 peptidylprolyl isomerase promotes the folding of cystic fibrosis transmembrane conductance regulator in the endoplasmic reticulum. J. Biol. Chem. 2011, 286, 43071–43080. [Google Scholar] [CrossRef]
- Aguilera, M.O.; Robledo, E.; Melani, M.; Wappner, P.; Colombo, M.I. FKBP8 is a novel molecule that participates in the regulation of the autophagic pathway. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119212. [Google Scholar] [CrossRef]
- Bhujabal, Z.; Birgisdottir, A.B.; Sjottem, E.; Brenne, H.B.; Overvatn, A.; Habisov, S.; Kirkin, V.; Lamark, T.; Johansen, T. FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep. 2017, 18, 947–961. [Google Scholar] [CrossRef]
- Choi, B.H.; Feng, L.; Yoon, H.S. FKBP38 protects Bcl-2 from caspase-dependent degradation. J. Biol. Chem. 2010, 285, 9770–9779. [Google Scholar] [CrossRef]
- Solassol, J.; Mange, A.; Maudelonde, T. FKBP family proteins as promising new biomarkers for cancer. Curr. Opin. Pharmacol. 2011, 11, 320–325. [Google Scholar] [CrossRef]
- Banasavadi-Siddegowda, Y.K.; Russell, L.; Frair, E.; Karkhanis, V.A.; Relation, T.; Yoo, J.Y.; Zhang, J.; Sif, S.; Imitola, J.; Baiocchi, R.; et al. PRMT5-PTEN molecular pathway regulates senescence and self-renewal of primary glioblastoma neurosphere cells. Oncogene 2017, 36, 263–274. [Google Scholar] [CrossRef]
- Otani, Y.; Sur, H.P.; Rachaiah, G.; Namagiri, S.; Chowdhury, A.; Lewis, C.T.; Shimizu, T.; Gangaplara, A.; Wang, X.; Vezina, A.; et al. Inhibiting protein phosphatase 2A increases the antitumor effect of protein arginine methyltransferase 5 inhibition in models of glioblastoma. Neuro Oncol. 2021, 23, 1481–1493. [Google Scholar] [CrossRef]
- Hettinger, K.; Vikhanskaya, F.; Poh, M.K.; Lee, M.K.; de Belle, I.; Zhang, J.T.; Reddy, S.A.; Sabapathy, K. c-Jun promotes cellular survival by suppression of PTEN. Cell Death Differ. 2007, 14, 218–229. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Dunn, G.P.; Rinne, M.L.; Wykosky, J.; Genovese, G.; Quayle, S.N.; Dunn, I.F.; Agarwalla, P.K.; Chheda, M.G.; Campos, B.; Wang, A.; et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes. Dev. 2012, 26, 756–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, H.; Wang, Z.L.; Li, W.H.; Liu, H.; Zhao, Y.X. The PI3K/AKT/mTOR pathway regulates autophagy to induce apoptosis of alveolar epithelial cells in chronic obstructive pulmonary disease caused by PM2.5 particulate matter. J. Int. Med. Res. 2020, 48, 300060520927919. [Google Scholar] [CrossRef] [PubMed]
- Karberg, S. Switching on epigenetic therapy. Cell 2009, 139, 1029–1031. [Google Scholar] [CrossRef]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.M.; Gallia, G.L.; et al. An integrated genomic analysis of human glioblastoma multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Lam, E.; Martin, M.; Wiederrecht, G. Isolation of a cDNA encoding a novel human FK506-binding protein homolog containing leucine zipper and tetratricopeptide repeat motifs. Gene 1995, 160, 297–302. [Google Scholar] [CrossRef]
- Weiwad, M.; Edlich, F.; Erdmann, F.; Jarczowski, F.; Kilka, S.; Dorn, M.; Pechstein, A.; Fischer, G. A reassessment of the inhibitory capacity of human FKBP38 on calcineurin. FEBS Lett. 2005, 579, 1591–1596. [Google Scholar] [CrossRef]
- Edlich, F.; Lucke, C. From cell death to viral replication: The diverse functions of the membrane-associated FKBP38. Curr. Opin. Pharmacol. 2011, 11, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.; Mounkes, L.; Liu, Y.; Maibaum, M.; Alonzo, E.; Desprez, P.Y.; Thor, A.D.; Kashani-Sabet, M.; Debs, R.J. Functional identification of distinct sets of antitumor activities mediated by the FKBP gene family. Proc. Natl. Acad. Sci. USA 2003, 100, 14253–14258. [Google Scholar] [CrossRef] [PubMed]
- Edlich, F.; Weiwad, M.; Wildemann, D.; Jarczowski, F.; Kilka, S.; Moutty, M.C.; Jahreis, G.; Lucke, C.; Schmidt, W.; Striggow, F.; et al. The specific FKBP38 inhibitor N-(N′,N′-dimethylcarboxamidomethyl)cycloheximide has potent neuroprotective and neurotrophic properties in brain ischemia. J. Biol. Chem. 2006, 281, 14961–14970. [Google Scholar] [CrossRef] [PubMed]
- Jawhari, S.; Ratinaud, M.H.; Verdier, M. Glioblastoma, hypoxia and autophagy: A survival-prone ‘menage-a-trois’. Cell Death Dis. 2016, 7, e2434. [Google Scholar] [CrossRef]
- Codogno, P.; Meijer, A.J. Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 2005, 12 (Suppl. S2), 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, Z.; Dai, S.; Qian, L.; Sun, L.; Gong, Z. Targeting autophagy to sensitive glioma to temozolomide treatment. J. Exp. Clin. Cancer Res. 2016, 35, 23. [Google Scholar] [CrossRef]
- Azad, M.B.; Chen, Y.; Henson, E.S.; Cizeau, J.; McMillan-Ward, E.; Israels, S.J.; Gibson, S.B. Hypoxia induces autophagic cell death in apoptosis-competent cells through a mechanism involving BNIP3. Autophagy 2008, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Baig, M.H.; Mahfooz, S.; Rahim, M.; Karacam, B.; Elbasan, E.B.; Ulasov, I.; Dong, J.J.; Hatiboglu, M.A. Deciphering the Role of Autophagy in Treatment of Resistance Mechanisms in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 1318. [Google Scholar] [CrossRef]
- Vomero, M.; Manganelli, V.; Barbati, C.; Colasanti, T.; Capozzi, A.; Finucci, A.; Spinelli, F.R.; Ceccarelli, F.; Perricone, C.; Truglia, S.; et al. Reduction of autophagy and increase in apoptosis correlates with a favorable clinical outcome in patients with rheumatoid arthritis treated with anti-TNF drugs. Arthritis Res. Ther. 2019, 21, 39. [Google Scholar] [CrossRef]
- Pistollato, F.; Rampazzo, E.; Abbadi, S.; Della Puppa, A.; Scienza, R.; D’Avella, D.; Denaro, L.; Te Kronnie, G.; Panchision, D.M.; Basso, G. Molecular mechanisms of HIF-1α modulation induced by oxygen tension and BMP2 in glioblastoma derived cells. PLoS ONE 2009, 4, e6206. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Gibson, S.B. Role of BNIP3 in proliferation and hypoxia-induced autophagy: Implications for personalized cancer therapies. Ann. N. Y. Acad. Sci. 2010, 1210, 8–16. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dowling, A.L.; Walbridge, S.; Ertekin, C.; Namagiri, S.; Camacho, K.; Chowdhury, A.; Bryant, J.-P.; Kohut, E.; Heiss, J.D.; Brown, D.A.; et al. FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres. Cells 2023, 12, 2562. https://doi.org/10.3390/cells12212562
Dowling AL, Walbridge S, Ertekin C, Namagiri S, Camacho K, Chowdhury A, Bryant J-P, Kohut E, Heiss JD, Brown DA, et al. FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres. Cells. 2023; 12(21):2562. https://doi.org/10.3390/cells12212562
Chicago/Turabian StyleDowling, Aimee L., Stuart Walbridge, Celine Ertekin, Sriya Namagiri, Krystal Camacho, Ashis Chowdhury, Jean-Paul Bryant, Eric Kohut, John D. Heiss, Desmond A. Brown, and et al. 2023. "FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres" Cells 12, no. 21: 2562. https://doi.org/10.3390/cells12212562
APA StyleDowling, A. L., Walbridge, S., Ertekin, C., Namagiri, S., Camacho, K., Chowdhury, A., Bryant, J.-P., Kohut, E., Heiss, J. D., Brown, D. A., Kumbar, S. G., & Banasavadi-Siddegowda, Y. K. (2023). FKBP38 Regulates Self-Renewal and Survival of GBM Neurospheres. Cells, 12(21), 2562. https://doi.org/10.3390/cells12212562






