Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition
Abstract
1. Introduction
2. Materials and Methods
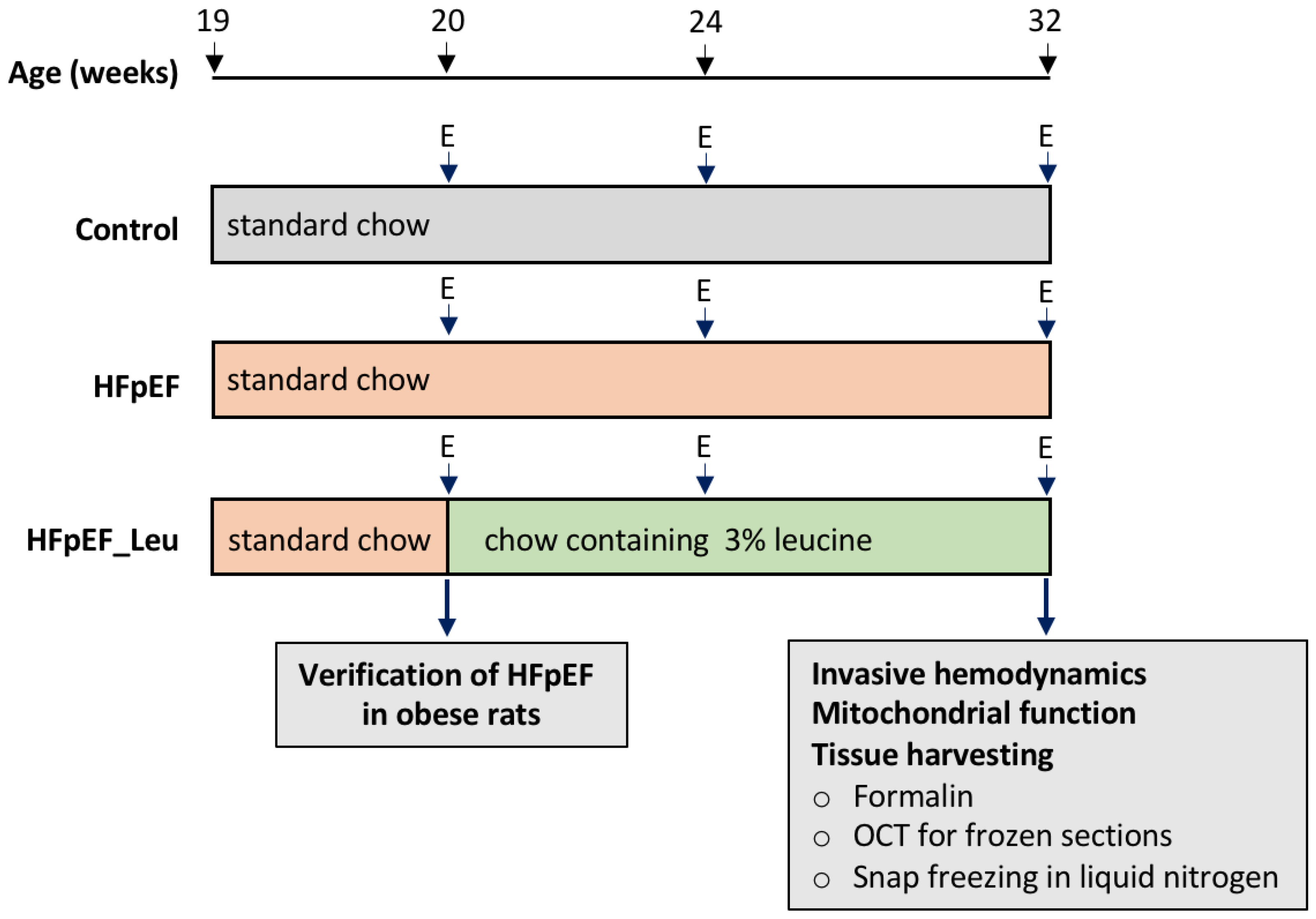
2.1. Study Design
2.2. NT-proBNP
2.3. Echocardiography
2.4. Invasive Hemodynamics
2.5. Left Ventricular Mitochondrial Respiration
2.6. Enzymes Activity
2.7. Western Blotting
2.8. RNA Extraction and Quantitative Real-Time PCR
2.9. Immunohistochemistry
2.10. Data Analysis
3. Results
3.1. Impact of Leucine Supplementation on Biometric Features, Myocardial Function and Hemodynamics
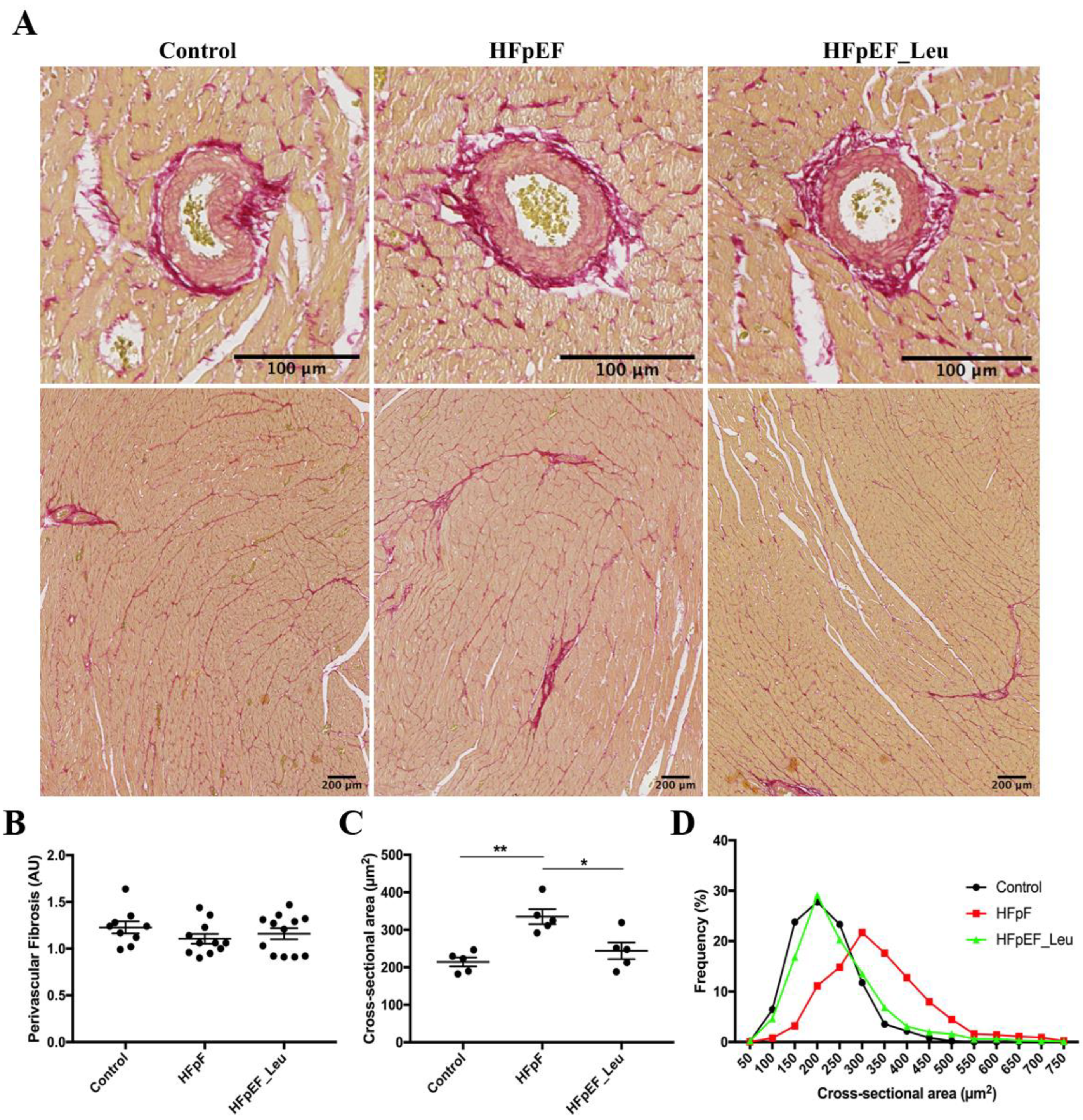
3.2. Impact of Leucine Supplementation on Myocardial Fibrosis and Fiber Size
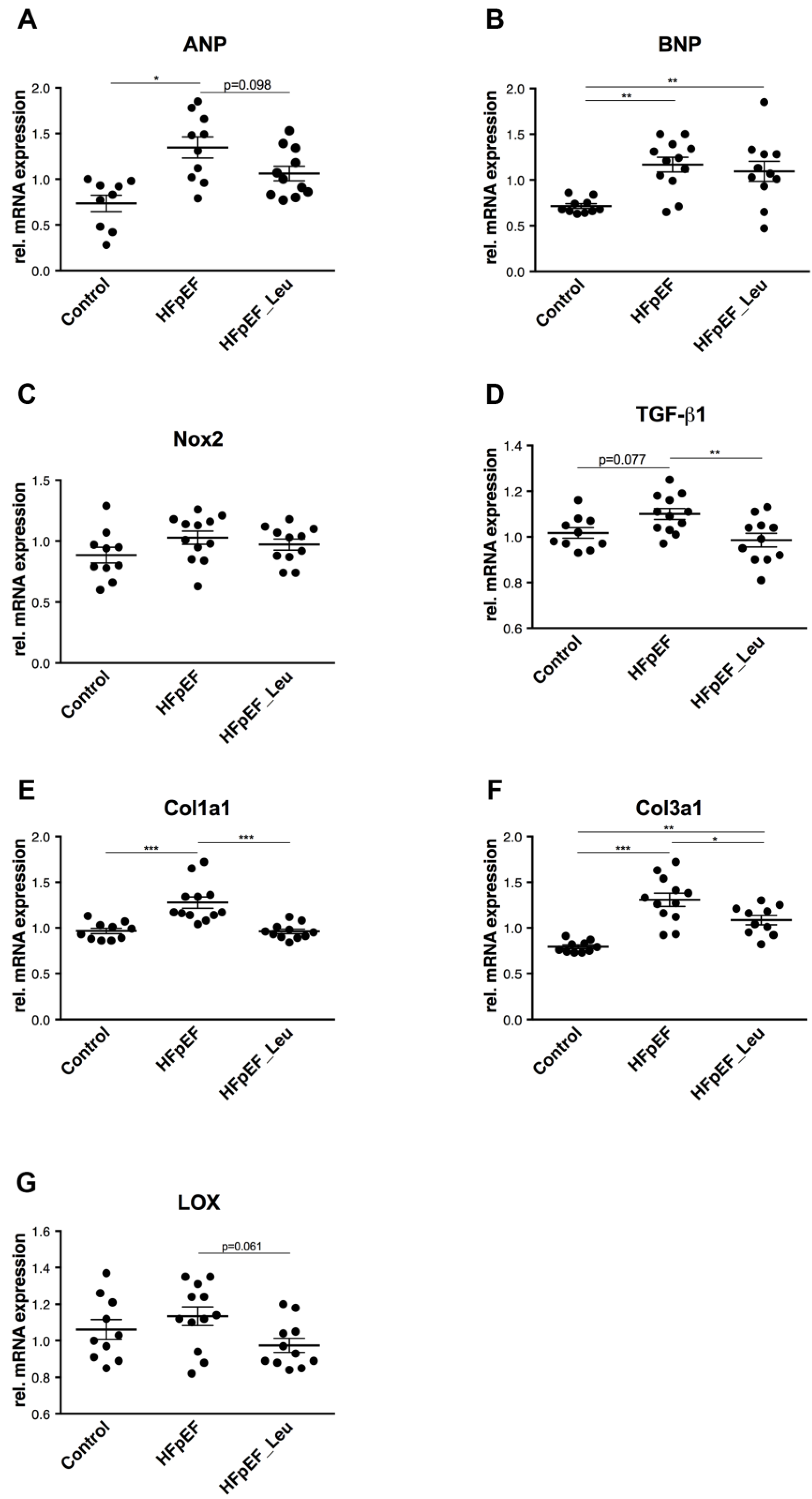
3.3. Impact of Leucine Supplementation on Myocardial Stress and Fibrosis
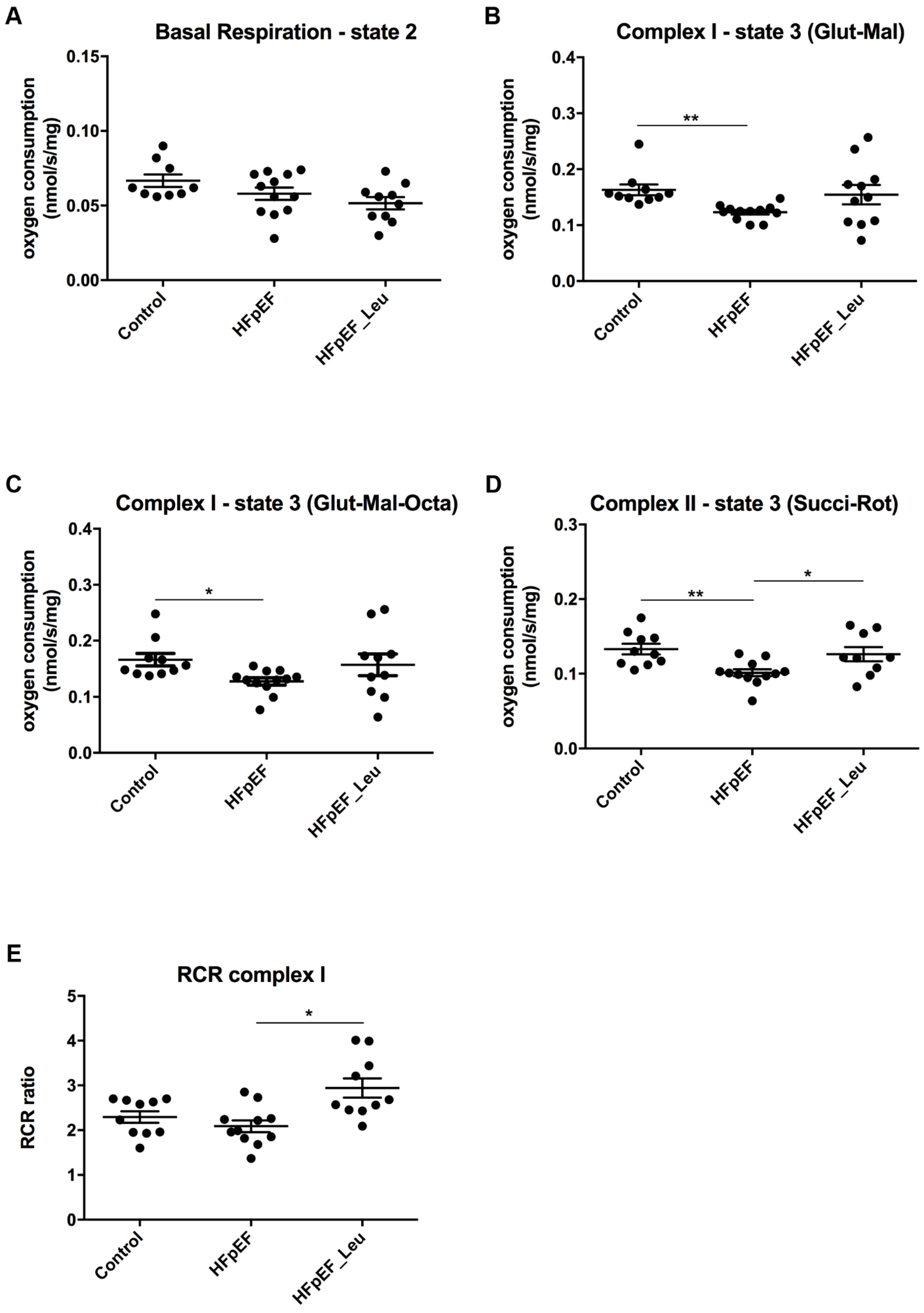
3.4. Impact of Leucine Supplementation on Mitochondrial Respiratory Function
3.5. Impact of Leucine Supplementation on Protein Expression of Mitochondrial Complexes
3.6. Impact of Leucine Supplementation on Myocardial Metabolism
3.7. Impact of Leucine Supplementation on Catabolic and Anabolic Markers
3.8. Impact of Leucine Supplementation on HDAC4 Modulation
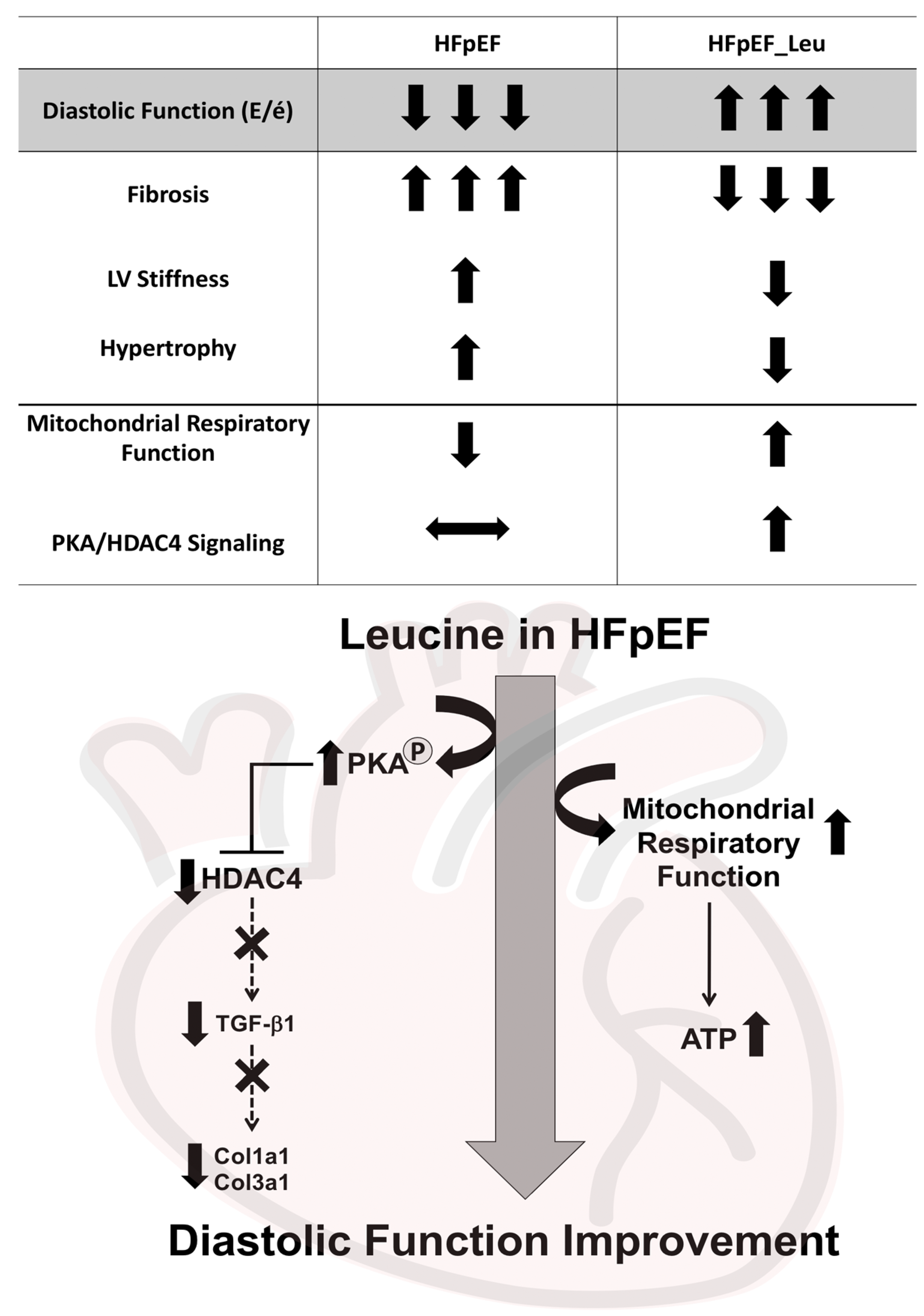
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.P.; Sato, N.; Shah, A.N.; et al. The Global Health and Economic Burden of Hospitalizations for Heart Failure: Lessons Learned from Hospitalized Heart Failure Registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; de Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Authors/Task Force Members; McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Pandey, A.; Parashar, A.; Kumbhani, D.; Agarwal, S.; Garg, J.; Kitzman, D.; Levine, B.; Drazner, M.; Berry, J. Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: A Meta-Analysis of Randomized Control Trials. Circ. Heart Fail. 2015, 8, 33–40. [Google Scholar] [CrossRef]
- Mueller, S.; Winzer, E.B.; Duvinage, A.; Gevaert, A.B.; Edelmann, F.; Haller, B.; Pieske-Kraigher, E.; Beckers, P.; Bobenko, A.; Hommel, J.; et al. Effect of High-Intensity Interval Training, Moderate Continuous Training, or Guideline-Based Physical Activity Advice on Peak Oxygen Consumption in Patients with Heart Failure with Preserved Ejection Fraction. JAMA 2021, 325, 542–551. [Google Scholar] [CrossRef]
- Taegtmeyer, H.; Young, M.E.; Lopaschuk, G.D.; Abel, E.D.; Brunengraber, H.; Darley-Usmar, V.; Des Rosiers, C.; Gerszten, R.; Glatz, J.F.; Griffin, J.L.; et al. Assessing Cardiac Metabolism: A Scientific Statement From the American Heart Association. Circ. Res. 2016, 118, 1659–1701. [Google Scholar] [CrossRef]
- Rech, M.; Barandiarán Aizpurua, A.; van Empel, V.; van Bilsen, M.; Schroen, B. Pathophysiological Understanding of HFpEF: microRNAs as Part of the Puzzle. Cardiovasc. Res. 2018, 114, 782–793. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Aviram, M. Specific Amino Acids Affect Cardiovascular Diseases and Atherogenesis via Protection against Macrophage Foam Cell Formation: Review Article. Rambam Maimonides Med. J. 2018, 9, e0022. [Google Scholar] [CrossRef]
- Morio, A.; Tsutsumi, R.; Satomi, S.; Kondo, T.; Miyoshi, H.; Kato, T.; Kuroda, M.; Kitamura, T.; Hara, K.; Saeki, N.; et al. Leucine Imparts Cardioprotective Effects by Enhancing mTOR Activity and Mitochondrial Fusion in a Myocardial Ischemia/Reperfusion Injury Murine Model. Diabetol. Metab. Syndr. 2021, 13, 139. [Google Scholar] [CrossRef]
- Toneto, A.T.; Ferreira Ramos, L.A.; Salomão, E.M.; Tomasin, R.; Aereas, M.A.; Gomes-Marcondes, M.C.C. Nutritional Leucine Supplementation Attenuates Cardiac Failure in Tumour-Bearing Cachectic Animals: Leucine-Rich Diet Attenuates Cardiac Failure. J. Cachexia Sarcopenia Muscle 2016, 7, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Fidale, T.M.; Antunes, H.K.M.; Alex dos Santos, L.; Rodrigues de Souza, F.; Deconte, S.R.; Borges Rosa de Moura, F.; Mantovani, M.M.; Alves Duarte, P.R.; Roever, L.; Resende, E.S. Increased Dietary Leucine Reduces Doxorubicin-Associated Cardiac Dysfunction in Rats. Front. Physiol. 2018, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Tannu, M.; Gadi, S.; Nayak, A.; Liu, C.; Mehta, A.; Tahhan, A.S.; Ko, Y.-A.; Uppal, K.; Jones, D.; Butler, J.; et al. L-Leucine: A Novel Biomarker that Predicts Lower Mortality in Patients with Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2020, 75, 939. [Google Scholar] [CrossRef]
- Walsh, M.E.; Van Remmen, H. Emerging Roles for Histone Deacetylases in Age-Related Muscle Atrophy. Nutr. Healthy Aging 2016, 4, 17–30. [Google Scholar] [CrossRef]
- McKinsey, T.A.; Zhang, C.-L.; Lu, J.; Olson, E.N. Signal-Dependent Nuclear Export of a Histone Deacetylase Regulates Muscle Differentiation. Nature 2000, 408, 106–111. [Google Scholar] [CrossRef]
- Zhang, L.X.; Du, J.; Zhao, Y.T.; Wang, J.; Zhang, S.; Dubielecka, P.M.; Wei, L.; Zhuang, S.; Qin, G.; Chin, Y.E.; et al. Transgenic Overexpression of Active HDAC4 in the Heart Attenuates Cardiac Function and Exacerbates Remodeling in Infarcted Myocardium. J. Appl. Physiol. 2018, 125, 1968–1978. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Zhao, Y.; Wang, J.; Dubielecka, P.M.; Zhuang, S.; Qin, G.; Chin, Y.E.; Kao, R.L.; Zhao, T.C. Myocyte-Specific Overexpressing HDAC4 Promotes Myocardial Ischemia/Reperfusion Injury. Mol. Med. 2018, 24, 37. [Google Scholar] [CrossRef]
- Alves, P.K.N.; Cruz, A.; Silva, W.J.; Labeit, S.; Moriscot, A.S. Leucine Supplementation Decreases HDAC4 Expression and Nuclear Localization in Skeletal Muscle Fiber of Rats Submitted to Hindlimb Immobilization. Cells 2020, 9, 2582. [Google Scholar] [CrossRef]
- Schauer, A.; Draskowski, R.; Jannasch, A.; Kirchhoff, V.; Goto, K.; Männel, A.; Barthel, P.; Augstein, A.; Winzer, E.; Tugtekin, M.; et al. ZSF1 Rat as Animal Model for HFpEF: Development of Reduced Diastolic Function and Skeletal Muscle Dysfunction. ESC Heart Fail. 2020, 7, 2123–2134. [Google Scholar] [CrossRef]
- Schauer, A.; Adams, V.; Augstein, A.; Jannasch, A.; Draskowski, R.; Kirchhoff, V.; Goto, K.; Mittag, J.; Galli, R.; Männel, A.; et al. Sacubitril/Valsartan Improves Diastolic Function But Not Skeletal Muscle Function in a Rat Model of HFpEF. Int. J. Mol. Sci. 2021, 22, 3570. [Google Scholar] [CrossRef]
- Winzer, E.B.; Schauer, A.; Langner, E.; Augstein, A.; Goto, K.; Männel, A.; Barthel, P.; Jannasch, A.; Labeit, S.; Mangner, N.; et al. Empagliflozin Preserves Skeletal Muscle Function in a HFpEF Rat Model. Int. J. Mol. Sci. 2022, 23, 10989. [Google Scholar] [CrossRef]
- Adams, V.; Schauer, A.; Augstein, A.; Kirchhoff, V.; Draskowski, R.; Jannasch, A.; Goto, K.; Lyall, G.; Männel, A.; Barthel, P.; et al. Targeting MuRF1 by Small Molecules in a HFpEF Rat Model Improves Myocardial Diastolic Function and Skeletal Muscle Contractility. J. Cachexia Sarcopenia Muscle 2022, 13, 1565–1581. [Google Scholar] [CrossRef]
- Schwarzer, M.; Osterholt, M.; Lunkenbein, A.; Schrepper, A.; Amorim, P.; Doenst, T. Mitochondrial Reactive Oxygen Species Production and Respiratory Complex Activity in Rats with Pressure Overload-Induced Heart Failure. J. Physiol. 2014, 592, 3767–3782. [Google Scholar] [CrossRef]
- Osumi, T.; Hashimoto, T. Occurrence of Two 3-Hydroxyacyl-CoA Dehydrogenases in Rat Liver. Biochim. Biophys. Acta 1979, 574, 258–267. [Google Scholar]
- Dzeja, P.P.; Pucar, D.; Redfield, M.M.; Burnett, J.C.; Terzic, A. Reduced Activity of Enzymes Coupling ATP-Generating with ATP-Consuming Processes in the Failing Myocardium. Mol. Cell. Biochem. 1999, 201, 33–40. [Google Scholar] [CrossRef]
- Hiltunen, J.K.; Saukko, P.; Hirvonen, J. Correlations between Enzyme Histochemical Reactions and Respective Enzyme Activities in Global Ischaemic Rat Hearts. Br. J. Exp. Pathol. 1985, 66, 743–752. [Google Scholar]
- Turko, I.V.; Marcondes, S.; Murad, F. Diabetes-Associated Nitration of Tyrosine and Inactivation of Succinyl-CoA:3-Oxoacid CoA-Transferase. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H2289–H2294. [Google Scholar] [CrossRef]
- Dai, Z.; Aoki, T.; Fukumoto, Y.; Shimokawa, H. Coronary Perivascular Fibrosis Is Associated with Impairment of Coronary Blood Flow in Patients with Non-Ischemic Heart Failure. J. Cardiol. 2012, 60, 416–421. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Ventrucci, G.; Gomes-Marcondes, M.C.C. A Leucine-Rich Diet Modulates the mTOR Cell Signalling Pathway in the Gastrocnemius Muscle under Different Walker-256 Tumour Growth Conditions. BMC Cancer 2019, 19, 349. [Google Scholar] [CrossRef]
- Baptista, I.L.; Silva, W.J.; Artioli, G.G.; Guilherme, J.P.L.F.; Leal, M.L.; Aoki, M.S.; Miyabara, E.H.; Moriscot, A.S. Leucine and HMB Differentially Modulate Proteasome System in Skeletal Muscle under Different Sarcopenic Conditions. PLoS ONE 2013, 8, e76752. [Google Scholar] [CrossRef]
- Ke, X.; Lin, Z.; Ye, Z.; Leng, M.; Chen, B.; Jiang, C.; Jiang, X.; Li, G. Histone Deacetylases in the Pathogenesis of Diabetic Cardiomyopathy. Front. Endocrinol. 2021, 12, 679655. [Google Scholar] [CrossRef] [PubMed]
- Wintrich, J.; Kindermann, I.; Ukena, C.; Selejan, S.; Werner, C.; Maack, C.; Laufs, U.; Tschöpe, C.; Anker, S.D.; Lam, C.S.P.; et al. Therapeutic Approaches in Heart Failure with Preserved Ejection Fraction: Past, Present, and Future. Clin. Res. Cardiol. 2020, 109, 1079–1098. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.; Abdel-Aty, H.; Bohl, S.; Boyé, P.; Zagrosek, A.; Dietz, R.; Schulz-Menger, J. Noninvasive Detection of Fibrosis Applying Contrast-Enhanced Cardiac Magnetic Resonance in Different Forms of Left Ventricular Hypertrophy Relation to Remodeling. J. Am. Coll. Cardiol. 2009, 53, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, M.; Winkler, J.; Ramos, I.; Do, Q.-T.; Firat, H.; McDonald, K.; González, A.; Thum, T.; Díez, J.; Jaisser, F.; et al. Myocardial Fibrosis: Biomedical Research from Bench to Bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.M.; Takawale, A.; Hulsurkar, M.; Menassa, D.A.; Antanaviciute, A.; Lahiri, S.K.; Mehta, N.; Evans, N.; Psarros, C.; Robinson, P.; et al. Paracrine Signalling by Cardiac Calcitonin Controls Atrial Fibrogenesis and Arrhythmia. Nature 2020, 587, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, X.H.; Derynck, R. Smad3 and Smad4 Cooperate with C-Jun/c-Fos to Mediate TGF-Beta-Induced Transcription. Nature 1998, 394, 909–913. [Google Scholar] [CrossRef]
- Ma, Z.-G.; Yuan, Y.-P.; Wu, H.-M.; Zhang, X.; Tang, Q.-Z. Cardiac Fibrosis: New Insights into the Pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef]
- Leivonen, S.-K.; Chantry, A.; Häkkinen, L.; Han, J.; Kähäri, V.-M. Smad3 Mediates Transforming Growth Factor-β-Induced Collagenase-3 (Matrix Metalloproteinase-13) Expression in Human Gingival Fibroblasts: EVIDENCE FOR CROSS-TALK BETWEEN Smad3 AND P38 SIGNALING PATHWAYS. J. Biol. Chem. 2002, 277, 46338–46346. [Google Scholar] [CrossRef]
- Pan, X.; Chen, Z.; Huang, R.; Yao, Y.; Ma, G. Transforming Growth Factor Β1 Induces the Expression of Collagen Type I by DNA Methylation in Cardiac Fibroblasts. PLoS ONE 2013, 8, e60335. [Google Scholar] [CrossRef]
- Hillege, M.M.G.; Galli Caro, R.A.; Offringa, C.; de Wit, G.M.J.; Jaspers, R.T.; Hoogaars, W.M.H. TGF-β Regulates Collagen Type I Expression in Myoblasts and Myotubes via Transient Ctgf and Fgf-2 Expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef]
- Bujak, M.; Ren, G.; Kweon, H.J.; Dobaczewski, M.; Reddy, A.; Taffet, G.; Wang, X.-F.; Frangogiannis, N.G. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation 2007, 116, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Alliston, T.; Delston, R.; Derynck, R. Repression of Runx2 Function by TGF-Beta through Recruitment of Class II Histone Deacetylases by Smad3. EMBO J. 2005, 24, 2543–2555. [Google Scholar] [CrossRef] [PubMed]
- Glenisson, W.; Castronovo, V.; Waltregny, D. Histone Deacetylase 4 Is Required for TGFβ1-Induced Myofibroblastic Differentiation. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2007, 1773, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.K.; Qiao, H.-Y.; Fu, M.-H.; Li, G.; Li, W.-B.; Chen, Z.; Wei, J.; Liang, B.-S. MiR-206 Attenuates Denervation-Induced Skeletal Muscle Atrophy in Rats Through Regulation of Satellite Cell Differentiation via TGF-Β1, Smad3, and HDAC4 Signaling. Med. Sci. Monit. 2016, 22, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-O.; Sampson, E.R.; Maynard, R.D.; O’Keefe, R.J.; Chen, D.; Drissi, H.; Rosier, R.N.; Hilton, M.J.; Zuscik, M.J. Ski Inhibits TGF-β/Phospho-Smad3 Signaling and Accelerates Hypertrophic Differentiation in Chondrocytes. J. Cell. Biochem. 2012, 113, 2156–2166. [Google Scholar] [CrossRef]
- Zhang, L.X.; DeNicola, M.; Qin, X.; Du, J.; Ma, J.; Tina Zhao, Y.; Zhuang, S.; Liu, P.Y.; Wei, L.; Qin, G.; et al. Specific Inhibition of HDAC4 in Cardiac Progenitor Cells Enhances Myocardial Repairs. Am. J. Physiol.—Cell Physiol. 2014, 307, C358–C372. [Google Scholar] [CrossRef]
- Yang, J.; He, J.; Ismail, M.; Tweeten, S.; Zeng, F.; Gao, L.; Ballinger, S.; Young, M.; Prabhu, S.D.; Rowe, G.C.; et al. HDAC Inhibition Induces Autophagy and Mitochondrial Biogenesis to Maintain Mitochondrial Homeostasis during Cardiac Ischemia/Reperfusion Injury. J. Mol. Cell. Cardiol. 2019, 130, 36–48. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, L.; Niu, F.; Li, Q.; Wang, C.; Yang, H.; Gao, C. Histone Deacetylase HDAC4 Participates in the Pathological Process of Myocardial Ischemia-Reperfusion Injury via MEKK1/JNK Pathway by Binding to miR-206. Cell Death Discov. 2021, 7, 240. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Prosperi, S.; Fanisio, F.; Birtolo, L.I.; Costi, B.; Netti, L.; Chimenti, C.; Lavalle, C.; Maestrini, V.; et al. Myocardial Tissue Characterization in Heart Failure with Preserved Ejection Fraction: From Histopathology and Cardiac Magnetic Resonance Findings to Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 7650. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Murach, K.A. Chapter 24—Anabolic and Catabolic Signaling Pathways That Regulate Skeletal Muscle Mass. In Nutrition and Enhanced Sports Performance, 2nd ed.; Bagchi, D., Nair, S., Sen, C.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 275–290. ISBN 978-0-12-813922-6. [Google Scholar]
- Gielen, S.; Sandri, M.; Kozarez, I.; Kratzsch, J.; Teupser, D.; Thiery, J.; Erbs, S.; Mangner, N.; Lenk, K.; Hambrecht, R.; et al. Exercise Training Attenuates MuRF-1 Expression in the Skeletal Muscle of Patients with Chronic Heart Failure Independent of Age. Circulation 2012, 125, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-T.; Huang, S.-M.; Ho, C.-L.; Yen, L.-C.; Huang, C.-J.; Lin, W.-S.; Chan, J.Y.-H. The Regulatory Mechanisms of Myogenin Expression in Doxorubicin-Treated Rat Cardiomyocytes. Oncotarget 2015, 6, 37443–37457. [Google Scholar] [CrossRef] [PubMed]
- Martinez, P.F.; Okoshi, K.; Zornoff, L.A.M.; Carvalho, R.F.; Oliveira Junior, S.A.; Lima, A.R.R.; Campos, D.H.S.; Damatto, R.L.; Padovani, C.R.; Nogueira, C.R.; et al. Chronic Heart Failure-Induced Skeletal Muscle Atrophy, Necrosis, and Changes in Myogenic Regulatory Factors. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2010, 16, BR374–BR383. [Google Scholar]
- Dasarathy, S.; Muc, S.; Hisamuddin, K.; Edmison, J.M.; Dodig, M.; McCullough, A.J.; Kalhan, S.C. Altered Expression of Genes Regulating Skeletal Muscle Mass in the Portacaval Anastamosis Rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G1105–G1113. [Google Scholar] [CrossRef][Green Version]
- Hatade, T.; Takeuchi, K.; Fujita, N.; Arakawa, T.; Miki, A. Effect of Heat Stress Soon after Muscle Injury on the Expression of MyoD and Myogenin during Regeneration Process. J. Musculoskelet Neuronal Interact 2014, 14, 325–333. [Google Scholar] [PubMed]
- Ju, S.-H.; Lee, E.J.; Sim, B.C.; Nga, H.T.; Lee, H.Y.; Tian, J.; Cho, K.J.; Park, H.; Choi, D.E.; Ham, Y.R.; et al. Leucine-Enriched Amino Acid Supplementation and Exercise to Prevent Sarcopenia in Patients on Hemodialysis: A Single-Arm Pilot Study. Front. Nutr. 2023, 10, 1069651. [Google Scholar] [CrossRef]
- Massimino, E.; Izzo, A.; Castaldo, C.; Amoroso, A.P.; Rivellese, A.A.; Capaldo, B.; Della Pepa, G. Protein and Leucine Intake at Main Meals in Elderly People with Type 2 Diabetes. Nutrients 2023, 15, 1345. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Phillips, B.E.; Hill, I.; Greenhaff, P.; Lund, J.N.; Williams, J.P.; Rankin, D.; Wilkinson, D.J.; Smith, K.; Atherton, P.J. Human Skeletal Muscle Is Refractory to the Anabolic Effects of Leucine during the Postprandial Muscle-Full Period in Older Men. Clin. Sci. Lond. Engl. 2017, 131, 2643–2653. [Google Scholar] [CrossRef]
- Backx, E.M.P.; Horstman, A.M.H.; Marzuca-Nassr, G.N.; van Kranenburg, J.; Smeets, J.S.; Fuchs, C.J.; Janssen, A.A.W.; de Groot, L.C.P.G.M.; Snijders, T.; Verdijk, L.B.; et al. Leucine Supplementation Does Not Attenuate Skeletal Muscle Loss during Leg Immobilization in Healthy, Young Men. Nutrients 2018, 10, 635. [Google Scholar] [CrossRef]
- Wandrag, L.; Brett, S.J.; Frost, G.S.; To, M.; Loubo, E.A.; Jackson, N.C.; Umpleby, A.M.; Bountziouka, V.; Hickson, M. Leucine-Enriched Essential Amino Acid Supplementation in Mechanically Ventilated Trauma Patients: A Feasibility Study. Trials 2019, 20, 561. [Google Scholar] [CrossRef]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and Exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Szwiega, S.; Pencharz, P.B.; Rafii, M.; Lebarron, M.; Chang, J.; Ball, R.O.; Kong, D.; Xu, L.; Elango, R.; Courtney-Martin, G. Dietary Leucine Requirement of Older Men and Women Is Higher than Current Recommendations. Am. J. Clin. Nutr. 2021, 113, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Baptista, I.L.; Silvestre, J.G.; Silva, W.J.; Labeit, S.; Moriscot, A.S. FoxO3a Suppression and VPS34 Activity Are Essential to Anti-Atrophic Effects of Leucine in Skeletal Muscle. Cell Tissue Res. 2017, 369, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.H. Lack of Sexual Dimorphism on the Inhibitory Effect of Alcohol on Muscle Protein Synthesis in Rats under Basal Conditions and after Anabolic Stimulation. Physiol. Rep. 2018, 6, e13929. [Google Scholar] [CrossRef]
- Ely, I.A.; Phillips, B.E.; Smith, K.; Wilkinson, D.J.; Piasecki, M.; Breen, L.; Larsen, M.S.; Atherton, P.J. A Focus on Leucine in the Nutritional Regulation of Human Skeletal Muscle Metabolism in Ageing, Exercise and Unloading States. Clin. Nutr. 2023, 42, 1849–1865. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Bukhari, S.S.I.; Phillips, B.E.; Limb, M.C.; Cegielski, J.; Brook, M.S.; Rankin, D.; Mitchell, W.K.; Kobayashi, H.; Williams, J.P.; et al. Effects of Leucine-Enriched Essential Amino Acid and Whey Protein Bolus Dosing upon Skeletal Muscle Protein Synthesis at Rest and after Exercise in Older Women. Clin. Nutr. Edinb. Scotl. 2018, 37, 2011–2021. [Google Scholar] [CrossRef]
- Takata, M.; Amiya, E.; Watanabe, M.; Hosoya, Y.; Nakayama, A.; Fujiwara, T.; Taya, M.; Oguri, G.; Hyodo, K.; Takayama, N.; et al. An Exploratory Study on the Efficacy and Safety of a BCAA Preparation Used in Combination with Cardiac Rehabilitation for Patients with Chronic Heart Failure. BMC Cardiovasc. Disord. 2017, 17, 205. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Rom, O.; Hamoud, S.; Volkova, N.; Hayek, T.; Abu-Saleh, N.; Aviram, M. Leucine Supplementation Attenuates Macrophage Foam-Cell Formation: Studies in Humans, Mice, and Cultured Macrophages. BioFactors 2018, 44, 245–262. [Google Scholar] [CrossRef]
- Ragni, M.; Greco, C.M.; Felicetta, A.; Ren, S.V.; Kunderfranco, P.; Ruocco, C.; Carullo, P.; Larcher, V.; Tedesco, L.; Severi, I.; et al. Dietary Essential Amino Acids for the Treatment of Heart Failure with Reduced Ejection Fraction. Cardiovasc. Res. 2023, 119, 982–997. [Google Scholar] [CrossRef]
- Hahn, V.S.; Petucci, C.; Kim, M.-S.; Bedi, K.C.; Wang, H.; Mishra, S.; Koleini, N.; Yoo, E.J.; Margulies, K.B.; Arany, Z.; et al. Myocardial Metabolomics of Human Heart Failure with Preserved Ejection Fraction. Circulation 2023, 147, 1147–1161. [Google Scholar] [CrossRef]


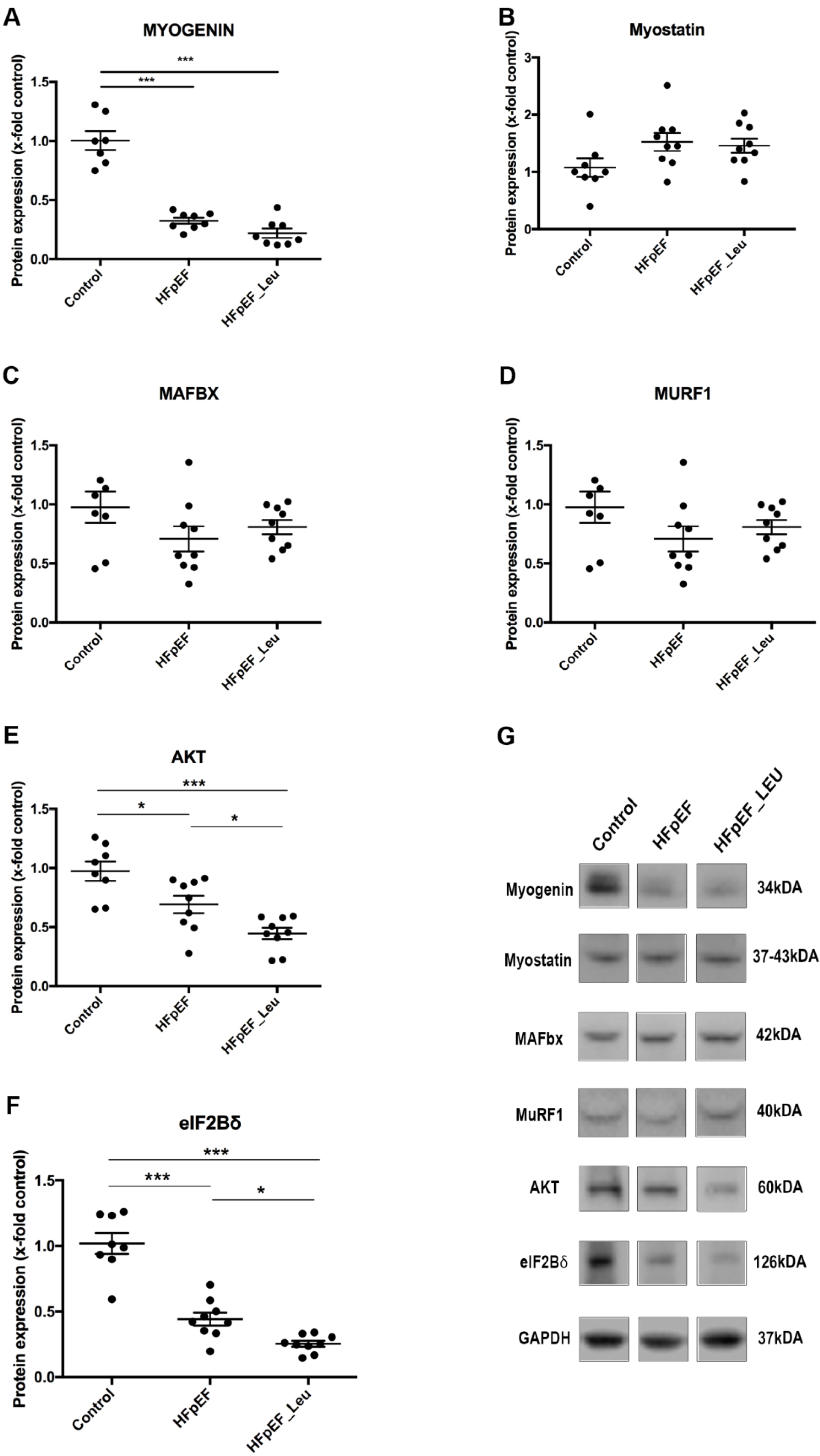
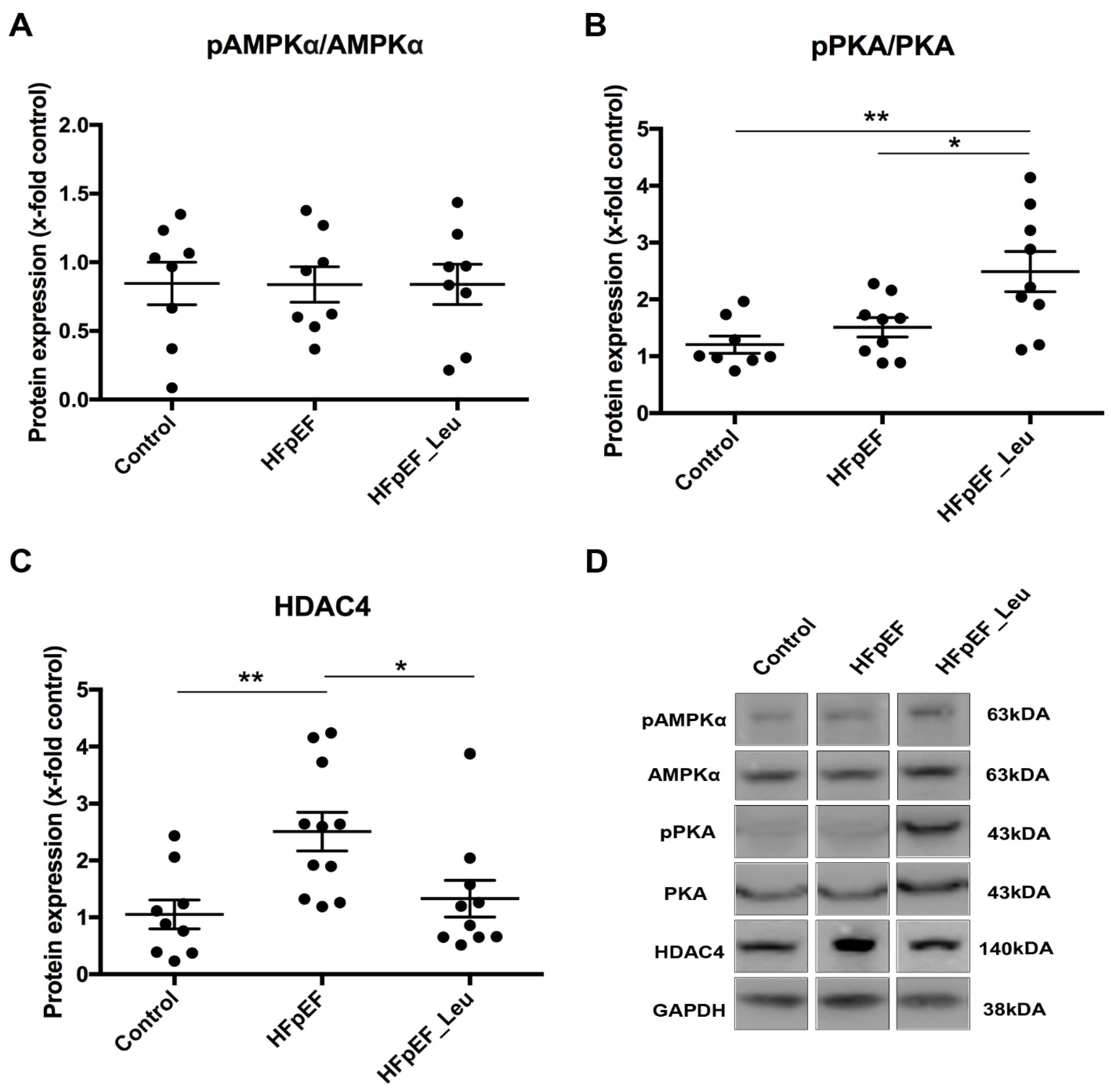
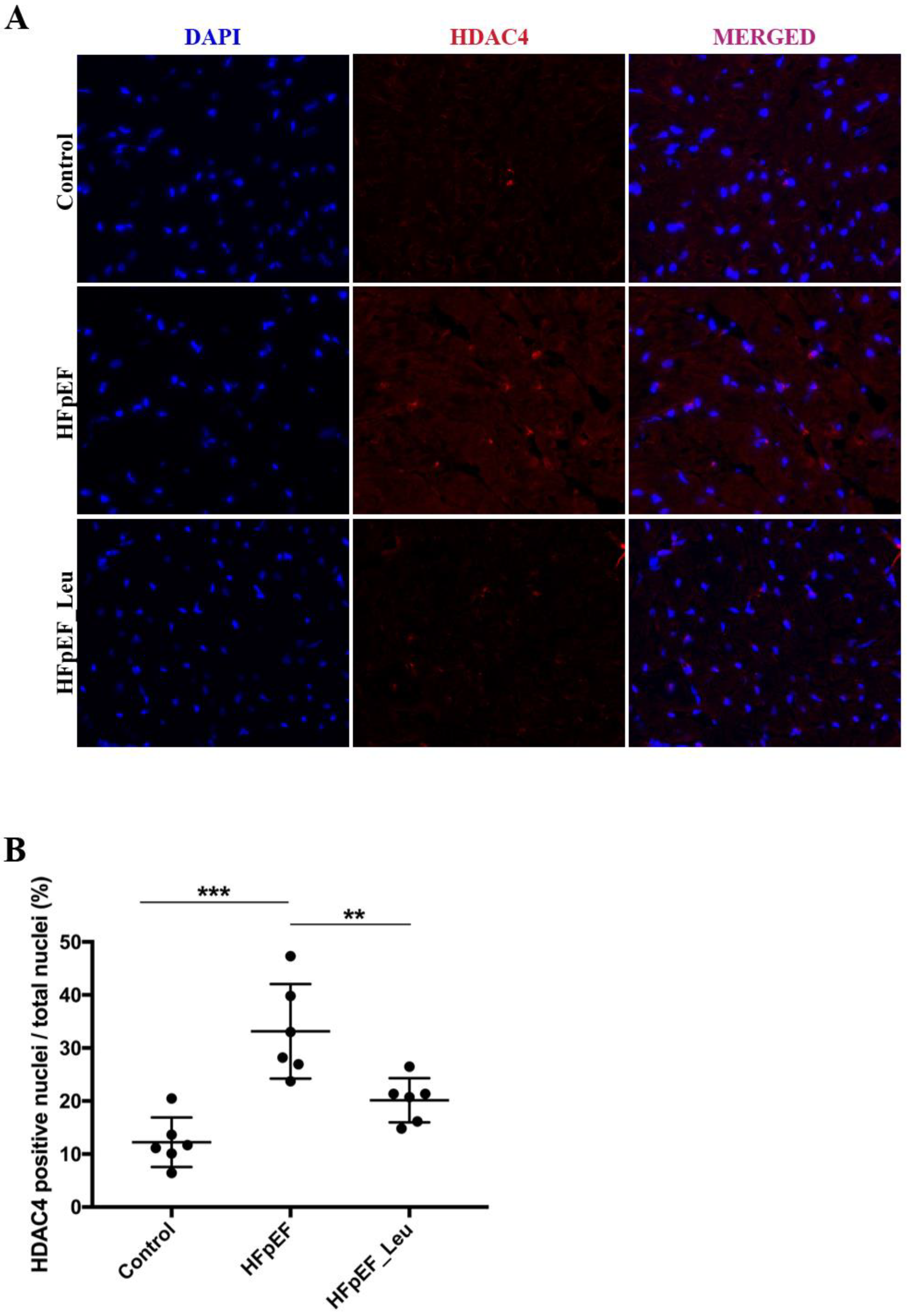
| Biometric Feature | |||
|---|---|---|---|
| Parameter | Control (n = 10) | HFpEF (n = 12) | HFpEF_Leu (n = 12) |
| Body Weight (g) | 259.6 ± 2.81 | 522.5 ± 11.85 *** | 485.8 ± 5.47 ***## |
| Tibia Length (TL, mm) | 35.31 ± 0.07 | 35.50 ± 0.08 | 35.38 ± 0.17 |
| Heart Weight/TL (mg/mm) | 26.52 ± 0.35 | 37.78 ± 0.74 *** | 37.68 ± 1.03 *** |
| Lung Wet Weight/TL (mg/mm) | 11.07 ± 0.21 | 12.63 ± 0.28 ** | 11.88 ± 0.29 |
| Kidney Weight/TL (mg/mm) | 27.71 ± 0.75 | 45.50 ± 1.49 *** | 46.75 ± 1.38 *** |
| Serum and blood parameter | |||
| Lactate | 1.36 ± 0.10 | 2.67 ± 0.17 *** | 2.55 ± 0.13 *** |
| Serum NT-proBNP (pg/mL) | 89.88 ± 8.02 | 203 ± 25.11 ** | 108.9 ± 26.2 # |
| Echocardiography | |||
| LV mass (mg) | 818.6 ± 38.32 | 1274 ± 50.95 *** | 1110 ± 37.05 ***# |
| LVEF (%) | 69.02 ± 1.40 | 67.87 ± 0.97 | 71.06 ± 0.96 |
| LVFS (%) | 24.19 ± 0.16 | 25.01 ± 0.36 | 23.95 ± 0.21 # |
| LVSV (µL) | 290.1 ± 12.41 | 424.4 ± 21.3 *** | 393.6 ± 12.56 *** |
| LVEDV (µL) | 419.4 ± 13.06 | 627.3 ± 34.38 *** | 554.7 ± 18.48 ** |
| E/é | 17.59 ± 0.74 | 24.62 ± 0.25 *** | 19.69 ± 1.04 ### |
| E/A | 1.15 ± 0.02 | 1.14 ± 0.02 | 1.41 ± 0.13 |
| LVAW; d (mm) | 1.70 ± 0.03 | 2.12 ± 0.03 *** | 2.17 ± 0.09 *** |
| LVPW; d (mm) | 1.52 ± 0.04 | 2.01 ± 0.06 *** | 2.08 ± 0.10 *** |
| LVID; d (mm) | 6.79 ± 0.09 | 7.73 ± 0.19 ** | 7.97 ± 0.18 *** |
| Septum; d (mm) | 1.67 ± 0.05 | 2.25 ± 0.04 *** | 1.94 ± 0.05 **### |
| Invasive Hemodynamics | |||
| LVEDP (mmHg) | 5.18 ± 0.56 | 10.54 ± 1.81 * | 6.55 ± 1.19 # |
| LVESP (mmHg) | 100.3 ± 4.88 | 158.4 ± 6.06 *** | 154 ± 5.68 *** |
| MAP in asc. Aorta (mmHg) | 80.13 ± 3.09 | 107.3 ± 3.50 *** | 110.6 ± 3.30 *** |
| LVEDV(µL) | 379 ± 14.48 | 516 ± 33.30 ** | 462.8 ± 29.79 |
| LVESV (µL) | 164.2 ± 13.22 | 239.4 ± 21.29 * | 189.6 ± 15.07 |
| SW (mmHg x µL) | 24,480 ± 1654 | 47,800 ± 2887 *** | 47,933 ± 2974 *** |
| PVA (mmHg x µL) | 97,767 ± 14,838 | 105,725 ± 16,540 | 97,767 ± 14,838 |
| dV/dt max (µL/s) | 5474 ± 447.7 | 5049 ± 524.4 | 5388 ± 791.8 |
| dV/dt min (µL/s) | −5228 ± 430.5 | −5551 ± 505.1 | −6486 ± 551.3 |
| Tau (ms) | 19 ± 0.66 | 17.8 ± 0.55 | 18.37 ± 0.58 |
| Slope LV-Ees (mmHg/µL) | 0.17 ± 0.03 | 0.33 ± 0.07 | 0.36 ± 0.09 |
| LV-stiffness constant βw | 0.29 ± 0.08 | 0.74 ± 0.13 * | 0.43 ± 0.07 # |
| Enzyme | Control (n = 10) | HFpEF (n = 12) | HFpEF_Leu (n = 12) |
|---|---|---|---|
| Citrate synthase (CS; mU/mg) | 254.2 ± 4.28 | 252.7 ± 6.56 | 260.1 ± 8.76 |
| Lactate dehydrogenase (LDH; mU/mg) | 877.9 ± 23.81 | 792.5 ± 19.18 * | 890.8 ± 27.97 # |
| Pyruvate kinase (PK; mU/mg) | 35.77 ± 0.95 | 32.26 ± 0.67 * | 31.54 ± 1.20 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, P.K.N.; Schauer, A.; Augstein, A.; Männel, A.; Barthel, P.; Joachim, D.; Friedrich, J.; Prieto, M.-E.; Moriscot, A.S.; Linke, A.; et al. Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition. Cells 2023, 12, 2561. https://doi.org/10.3390/cells12212561
Alves PKN, Schauer A, Augstein A, Männel A, Barthel P, Joachim D, Friedrich J, Prieto M-E, Moriscot AS, Linke A, et al. Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition. Cells. 2023; 12(21):2561. https://doi.org/10.3390/cells12212561
Chicago/Turabian StyleAlves, Paula Ketilly Nascimento, Antje Schauer, Antje Augstein, Anita Männel, Peggy Barthel, Dirk Joachim, Janet Friedrich, Maria-Elisa Prieto, Anselmo Sigari Moriscot, Axel Linke, and et al. 2023. "Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition" Cells 12, no. 21: 2561. https://doi.org/10.3390/cells12212561
APA StyleAlves, P. K. N., Schauer, A., Augstein, A., Männel, A., Barthel, P., Joachim, D., Friedrich, J., Prieto, M.-E., Moriscot, A. S., Linke, A., & Adams, V. (2023). Leucine Supplementation Improves Diastolic Function in HFpEF by HDAC4 Inhibition. Cells, 12(21), 2561. https://doi.org/10.3390/cells12212561







