Abstract
Early preimplantation mouse embryos are sensitive to increased osmolarity, which can block their development. To overcome this, they accumulate organic osmolytes to maintain cell volume. The main organic osmolyte used by early mouse embryos is glycine. Glycine is transported during the mature egg and 1-cell to 4-cell embryo stages by a transporter identified as GLYT1, encoded by the Slc6a9 gene. Here, we have produced an oocyte-specific knockout of Slc6a9 by crossing mice that have a segment of the gene flanked by LoxP elements with transgenic mice expressing iCre driven by the oocyte-specific Gdf9 promoter. Slc6a9 null oocytes failed to develop glycine transport activity during meiotic maturation. However, females with these oocytes were fertile. When enclosed in their cumulus-oocyte complex, Slc6a9 null oocytes could accumulate glycine via GLYT1 transport in their coupled cumulus cells, which may support female fertility in vivo. In vitro, embryos derived from Slc6a9 null oocytes displayed a clear phenotype. While glycine rescued complete preimplantation development of wild type embryos from increased osmolarity, embryos derived from null oocytes failed to develop past the 2-cell stage even with glycine. Thus, Slc6a9 is required for glycine transport and protection against increased osmolarity in mouse eggs and early embryos.
Keywords:
oocyte; preimplantation embryo; glycine; transport; cell volume; knockout; GLYT1; mouse; cumulus; osmolyte 1. Introduction
Early preimplantation mouse embryos are particularly susceptible to relatively small increases in external osmolarity [1,2,3]. Increases in osmolarity can result in complete blocks to development in vitro, including the classic “2-cell block” in mouse embryos [2,4]. Because mammalian cells regulate their volumes by adjusting intracellular osmotic pressure [5], such sensitivity to hypertonicity was linked to disruption of their ability to maintain cell volume within an acceptable range [1,6,7].
The acute response of mammalian cells to a decrease in cell volume is to activate a set of transporters that act to accumulate inorganic ions, mainly Na+, Cl− and K+ [5]. Such mechanisms are active in early mouse preimplantation embryos, principally functionally coupled sodium-hydrogen and bicarbonate-chloride exchangers [1,8,9,10,11]. However, accumulation of sufficient inorganic ions to balance external osmotic pressure can be damaging in the long term, which has led many somatic cells to employ mechanisms to accumulate benign “organic osmolytes” that can replace a portion of the inorganic ions while providing osmotic support [12]. Characteristic organic osmolyte transporters operate in somatic cells [13], but early embryos instead have unique organic osmolyte transporters, with the main one relying on glycine transport [1,3,7,14].
The glycine transport that is present in early embryos was attributed to “System Gly,” a classic transport activity characterized as accepting glycine and sarcosine (N-methylglycine) as high-affinity substrates and dependence on cotransport of Na+ and Cl− with the organic substrate [15,16]. System Gly activity was found to be mediated at the molecular level by the product of the Slc6a9 gene (initially named Glyt1) with the System Gly transporter then designated GLYT1 [17]. Early mouse embryos have robust glycine transport that is competitively inhibited by sarcosine and is dependent on the Na+ and Cl− gradients [15,16], consistent with GLYT1 activity.
GLYT1 activity first appears during meiotic maturation of the oocyte. Germinal vesicle stage oocytes transport little or no glycine, but glycine transport reaches its maximum rate within several hours of ovulation being triggered in vivo or the oocyte being removed from the follicle in vitro [18,19,20]. After GLYT1 is activated, intracellular soluble glycine levels increase from undetectable in the GV oocyte to very high levels of ~20–30 mM in second meiotic metaphase (MII) eggs and early preimplantation embryos, which is sufficient to provide substantial osmotic support [18,21]. The apparent GLYT1 activity is present up to about the 4-cell stage, after which it again becomes undetectable. The high intracellular glycine levels also remain through the 2-cell stage but disappear by the morula stage [18]. Thus, GLYT1-mediated glycine transport appears to be present during the stages when embryos are most susceptible to hypertonic stress and support the accumulation of large amounts of intracellular glycine to act as an organic osmolyte. Glycine transport is present in early human embryos where it may perform a similar function [22].
Glycine rescues the development of 1-cell mouse embryos to blastocysts in hypertonic media [7,14]. This ability has been attributed to GLYT1 because virtually all detectable glycine transport from the mature egg through the 4-cell stage has transport characteristics consistent with GLYT1 (above) and because the ability of glycine to rescue development at increased osmolarity was eliminated in the presence of a small molecule inhibitor that was developed to be highly selective against GLYT1 [23]. However, it has not been shown directly that Slc6a9 expression is necessary for glycine transport in early mouse embryos nor whether it is required for glycine to have a protective effect on embryo development under hypertonic conditions. Furthermore, whether lack of Slc6a9 or GLYT1 activity in oocytes affects female fertility is not known. Here, we have created a conditional knockout of Slc6a9 in oocytes to resolve these questions.
2. Materials and Methods
2.1. Chemicals and Media
Chemicals and reagents were obtained from Sigma-Aldrich (Oakville, ON, Canada) unless otherwise specified. In vitro maturation of oocytes was done in Minimal Essential Medium Alpha (MEMα; catalog #12561–056, Life Technologies, Burlington, ON, Canada) that was supplemented with cold water-soluble polyvinyl alcohol (PVA). Preimplantation embryos were cultured in modified potassium-supplemented Simplex Optimized Medium (mKSOM) with PVA instead of bovine serum albumin and omitting glutamine [24]. The mKSOM medium contains NaCl (95 mM), KCl (2.5 mM), KH2PO4 (0.35 mM), MgSO4·7H2O (0.2 mM), Na lactate (10 mM), Glucose (0.2 mM), Na pyruvate (0.2 mM), NaHCO3 (25 mM), CaCl2.2H2O) (1.7 mM), tetrasodium ethylenediaminetetraacetic acid (EDTA) (0.01 mM), K penicillin G (0.16 mM), Streptomycin SO4 (0.03 mM), and 1mg/mL PVA. mKSOM was used at 37 °C in 5% CO2 in air at 100% humidity. Collection of oocytes and 1-cell embryos was done using Hepes-mKSOM that was identical to mKSOM except 21 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Hepes) was added and NaHCO3 was reduced to 4 mM, and pH was adjusted to 7.4 at room temperature using NaOH. Components of media were embryo-tested or cell culture-tested grade. The osmolarities were 250 mOsM for mKSOM and 240 mOsM for Hepes-mKSOM (±5 mOsM). Where hypertonic mKSOM was used, its osmolarity was increased using the inert trisaccharide D(+)-raffinose as previously described [14].
2.2. Nomenclature
Genes are identified using their standard nomenclature. Mouse lines are identified by the standard nomenclature used in the literature. The shorthand designations of each mouse line and their derivatives are used throughout (see below). For most lines, the gene and mouse line designations correspond, including Gdf9-Cre, Amhr2-Cre, and Cyp19-Cre. However, where LoxP sites were inserted in the Slc6a9 gene, the designations use “Glyt1,” which was the original designation of this gene and still commonly used in the literature. To be consistent with previously published work [25,26], we have used the Glyt1 nomenclature for this line and its derivatives, while referring to the gene itself as Slc6a9.
2.3. Animals
The University of Ottawa Animal Care Committee approved the animal usage and breeding protocols (approval numbers 3347 for breeding and 3348 for experiments), which comply with the regulations of the Canadian Council on Animal Care. Mice were maintained on a 12 h light:dark cycle with unrestricted access to water and Teklad Global 18% protein rodent diet 2018 (Envigo, Indianapolis, IN, USA).
B6D2F1 (BDF1) male mice were obtained from Charles River Canada (St. Constant, QC, Canada) and used for mating with females to produce offspring for fertility testing and 1-cell stage embryos for culture.
Glyt1tm1.2fl/fl mice (shorthand: Glyt1fl/fl) have LoxP sites that flank Slc6a9 gene exons 5–11 and were produced as previously described [25,26]. They were maintained on the C57Bl6 background.
Tg(Gdf9-icre)5092Coo/J (shorthand: Gdf9-iCre) mice express iCre driven by the mouse Gdf9 gene promoter, restricting iCre exclusively to the oocyte [27]. They were a gift from Dr. Barbara Vanderhyden (Ottawa, ON, Canada) and were maintained on the C57Bl6 background.
Amhr2tm3(cre)Bhr (shorthand: Amhr2-Cre) mice express Cre driven by the Amhr2 promoter [28]. Amhr2 is highly expressed in the granulosa cells of preantral and antral follicles as well as other reproductive tissues of Müllerian duct origin [29,30]. They were a gift from Dr. Barbara Vanderhyden (Ottawa, ON) and were maintained on the C57Bl6 background.
Tg(CYP19A1-cre)1Jri (shorthand: Cyp19-Cre) mice express iCre driven by the Cyp19a1 promoter. Cyp19a1 is highly expressed in granulosa cells of preantral and antral follicles [31,32]. Cyp19-Cre mice that also had the Smarca4 (a.k.a. Brg1) gene flanked by LoxP sites were a gift from Dr. Barbara Vanderhyden (Ottawa, ON, Canada). These were first crossed with C57Bl6/J mice to eliminate Smarca4fl/fl and then were maintained on the C57Bl6 background.
2.4. Production of Conditional Knockouts
The Gdf9-iCre:Glyt1fl/fl line was produced by crossing Gdf9-iCre with Glyt1fl/fl mice. Because Gdf9-iCre is expressed in oocytes, this transgene should normally be transmitted only through males to prevent heterozygous loss of Glyt1 that occurs in offspring of GDF9-iCre:Glyt1fl/fl females mated with wild type males [33]. However, in the initial cycles of breeding, the Gdf9-iCre transgene was also transmitted through females to more rapidly increase the breeding stock. In this case, only Glyt1fl/fl offspring were retained and those that were Glyt1fl/− were not bred further (except for producing Amhr2-Cre:Glyt1fl/− mice; see below). Generally, Gdf9-iCre:Glyt1fl/+ males from the initial crosses were mated to Glyt1fl/fl females to provide Gdf9-iCre:Glyt1fl/fl males, which were paired with Glyt1fl/fl females and maintained as breeding stock. Since the males carry one copy of the iCre transgene, the offspring were either Gdf9-iCre:Glyt1fl/fl or Glyt1fl/fl. Females of these genotypes were used as the experimental animals (Glyt1 knockout oocytes) and controls (Glyt1 wild type oocytes), respectively.
The Amhr2-Cre:Glyt1fl/fl line was produced by crossing Amhr2-Cre with Glyt1fl/fl mice to produce Amhr2-Cre:Glyt1fl/+ offspring. These were mated to Glyt1fl/fl to produce Amhr2-Cre:Glyt1fl/fl offspring, which were then paired with Glyt1fl/fl and maintained as breeding pairs. Because there was no oocyte expression of Cre, both males and females of the Amhr2-Cre:Glyt1fl/fl genotype could be used for breeding to produce offspring that were either Amhr2-Cre:Glyt1fl/fl or Glyt1fl/fl. Females of these genotypes were used as the experimental animals (Cre positive) and controls (Cre negative), respectively.
The Cyp19-Cre:Glyt1fl/fl line was produced using the same breeding scheme as the Amhr2-Cre:Glyt1fl/fl line. Offspring of the breeding pairs were either Cyp19-Cre:Glyt1fl/fl (experimental) or Glytfl/fl (control).
Amhr2-Cre:Glyt1fl/− mice were produced by crossing Glyt1+/− mice with Amhr2-Cre:Glyt1fl/fl mice. The Glyt1+/− mice were produced from mating Gdf9-iCre:Glyt1fl/fl females with BDF1 males. Since Gdf9-iCre:Glyt1fl/fl females have both copies of Glyt1 inactivated in their oocytes, they produce offspring with one copy of Glyt1 inactivated (Glyt1+/−) when mated with wild type males. Those Glyt1+/− offspring that did not inherit Gdf9-Cre were selected and mated with Amhr2-Cre:Glyt1fl/fl mice to produce offspring with Amhr2-Cre:Glyt1fl/+ and Amhr2-Cre:Glyt1fl/− genotypes as well as the corresponding Amhr2-Cre negative genotypes (Glyt1fl/+ and Glyt1fl/−).
2.5. Genotyping
Genotyping was done using ear notch samples. PCR products were visualized on 1.4% agarose gels with ethidium bromide. Band sizes were estimated from DNA ladders (ThermoFisher Scientific #SM0403). The lower set of resolved marker bands range from 100 bp to 900 bp at 100 bp intervals and then 1031 bp; the upper set was 1500 bp, 2000 bp, and 2500 bp, then 3000 bp to 10,000 bp at 1000 bp intervals, the upper range of which was generally not fully resolved. Glyt1fl/fl genotyping of offspring was done using the 17854 sense primer (5′-TGG CAC CTC TCT GAG TGT GC-3′) and 17672 antisense primer (5′- TTC CAG GAC ATC CAG ATG ATG C - 3′) that yield bands of 258 bp for Glyt1fl/fl and 182 bp for wild type.
Determinations of whether Cre-mediated deletion of a segment of Slc6a9 had occurred in Glyt1fl/fl tissues was done using the o250 sense primer (5′-CCC ATG CCC AGA TCC ATG C-3′) targeted 5′ to the LoxP site and the o228 antisense primer targeting Slc6a9 sequence 3′ to the PGK-neomycin cassette (5′-GTC AAC CTG ACT CCT AGC CCT GTA CC-3′) as previously described [25]. In the absence of Cre, this yields a product of ~6600 bp. With successful Cre-mediated deletion, a band of ~450 bp is produced instead. This was performed on ovary and liver samples to assess the effects of Cre targeted to granulosa cells.
The Gdf9-iCre transgene was detected using the primer sets specified by The Jackson Laboratory (https://www.jax.org/Protocol?stockNumber=011062&protocolID=17814 (accessed on 4 September 2023)). The iCre transgene was detected with forward primer 25494 (5′-GGC ATG CTT GAG GTC TGA TTA C-3′) and reverse primer 21218 (5′-CAG GTT TTG GTG CAC AGT CA-3′), which were multiplexed with the Internal Positive Control (targeting the mouse Il2 gene) forward primer oIMR7338 (5′-CTA GGC CAC AGA ATT GAA AGA TCT-3′) and reverse primer oIMR7339 (5′-GTA GGT GGA AAT TCT AGC ATC ATC C-3′). The Cre transgene primers yield a 200 bp product and the positive control primers yield 324 bp.
The Amhr2-Cre transgene was detected with the forward primer AMHR2Cre FWD (5′-CTC TGG TGT AGC TGA TGA TC-3′) and reverse primer AMHR2Cre REV (5′-TAA TCG CCA TCT TCC AGC AG -3′), which were multiplexed with the Internal Positive Control primer IMR0015 (5′-CAA ATG TTG CTT GTC TGG TG-3′) and reverse primer IMR0016 (5′-GTC AGT CGA GTG CAC AGT TT-3′). The Cre transgene was indicated by a 340 bp product and the positive control product was 206 bp.
The Cyp19-Cre transgene was detected with the forward primer Cyp19CreF (5′-ACTTGGTCAAAGTCAGTGCG-3′) and reverse primer Cyp19CreR (5′-CCTGGTGCAAGCTGAACAAC-3′), which yields a 290 bp product. No internal control was used for Cyp19-Cre, so these primers were always paired with the Glyt1fl/fl primers (above) to ensure DNA quality.
2.6. GV Oocytes and 1-Cell Stage Embryos
To obtain germinal vesicle stage (GV) oocytes, females were superovulated with 5 IU equine chorionic gonadotropin (eCG, Prospec, Sturgeon County, AB, Canada) administered by intraperitoneal (IP) injection. Ovaries were excised and minced in Hepes-KSOM at 44–46 h post-eCG. Cumulus-oocyte complexes (COCs) were collected, and cumulus cells were removed by repeated pipetting to obtain denuded oocytes.
One-cell stage embryos were obtained by inducing ovulation with an IP injection of 5 IU human chorionic gonadotropin (hCG, Prospec) at 47 h post-eCG. Females were then caged overnight with BDF1 males (Charles River Canada) following hCG injection. Embryos were obtained 21–24 h post-hCG by flushing oviducts with Hepes-KSOM medium using a blunt-end syringe.
2.7. Glycine Transport Measurements
The methods for measuring GLYT1 activity have been extensively described and validated previously [18,19,20,23,34]. [3H]glycine ([2-3H]glycine; 40–60 Ci/mmole) was obtained from Perkin-Elmer (Waltham, MA) and used where indicated. During the course of this work, [3H]glycine that passed quality control tests (see below) became unavailable from this source. After that, [3H]glycine ([2-3H]glycine; 2.4 Ci/mmole) was obtained from Movarek (Brea, CA) and used where indicated. Each new stock of [3H]-glycine was subject to a quality control test that confirmed that transport of 1 µM [3H]-glycine into mouse 1-cell embryos was inhibited by >90% in the presence of 5 mM unlabelled glycine.
Glycine transport into individual denuded oocytes was measured by incubating oocytes in 3 µM [3H]glycine (Perkin-Elmer) after they had been in vitro matured overnight in MEMα medium to MII eggs to allow GLYT1 activity to reach maximum levels. Glycine transport into COCs was measured using 1 µM [3H]glycine from Perkin-Elmer or 10 µM of the lower specific activity [3H]glycine from Movarek. It was confirmed using remaining Perkin-Elmer stock that had passed the quality control test that essentially the same results were obtained when using [3H]glycine from either source. Glycine transport measurements were carried out in 50 µL drops of mKSOM under mineral oil with a 10 min incubation with [3H]glycine.
After incubation with [3H]glycine, denuded oocytes or COCs were washed 7× in ice-cold Hepes-mKSOM and placed into scintillation vials with 4 mL Scintiverse BD scintillation fluid (Fisher Scientific, Pittsburgh, PA). An LS6500 liquid scintillation counter (Beckman Coulter, Brea, CA) set at a 5 min counting period was used to quantify the accumulated [3H]glycine. CPM were converted to molar amounts of [3H]glycine using standard curves constructed from serial dilutions of [3H]glycine stocks. Backgrounds with no added [3H]glycine were subtracted from each reading. Rates of glycine transport were reported as fmole [3H]-glycine per oocyte per min per µM [3H]-glycine in the incubation medium.
Transfer of [3H]glycine from cumulus cells into the enclosed oocyte was assessed by incubating both COCs and denuded GV oocytes for 4 h in mKSOM with 10 µM [3H]glycine (Movarek). After the incubation, cumulus cells were removed from the oocyte in COCs by repeated pipetting though a narrow bore pipette. The amount of [3H]glycine that had been accumulated in oocytes that were within COCs vs. those that were denuded oocytes during the 4 h incubation was then determined by liquid scintillation counting as described above.
2.8. Data Analysis
Data were analyzed and graphed using GraphPad Prism 9 or 10 (San Diego, CA, USA). The statistical significance of differences between means was determined by one-way ANOVA with Tukey’s multiple comparisons test for more than two groups. A t-test was used for two groups (or a Mann-Whitney test for non-normally distributed data). One-sample t-tests were used to test for significant difference of means from zero. Statistical significance was taken to be p < 0.05.
3. Results
3.1. Oocyte-Specific Glyt1 Deletion
We first confirmed that Gdf9-iCre:Glyt1fl/fl females produced Glyt1 null oocytes. To avoid genotyping individual oocytes, Gdf9-iCre:Glyt1fl/fl females were mated with wild type (BDF1) proven fertile males and their offspring were genotyped. If all oocytes in Gdf9-iCre:Glyt1fl/fl females had a disrupted Glyt1 (Slc6a9) gene, then all offspring from this mating would be heterozygous and have an Slc6a9+/− genotype. Four Gdf9-iCre:Glyt1fl/fl female x BDF1 male matings produced litters of 3, 6, 9, and 7 pups, all 25 of which had an Slc6a9+/− genotype. Three control Glyt1fl/fl females were mated with BDF1 males and produced litters of 6, 8, and 9 pups, all 23 of which had an Slc6a9+/+ genotype. Therefore, the genotypes of the offspring indicate that Gdf9-iCre:Glyt1fl/fl females only produce oocytes in which both copies of Glyt1 have been disrupted.
We next assessed glycine transport in individual denuded oocytes. Glycine transport into individual oocytes obtained from Gdf9-iCre:Glyt1fl/fl or from control Glyt1fl/fl females was measured using [3H]glycine. Breeding pairs of Gdf9-iCre:Glyt1fl/fl males mated with Glyt1fl/fl females produced litters with both Glyt1fl/fl (Cre negative control) and Gdf9-iCre:Glyt1fl/fl (Cre positive) offspring. Three different breeding pairs produced the litters used in these experiments. All females in the litters were genotyped, their ovaries excised at ~4 weeks of age, and GV oocytes collected. Since GLYT1 is maximally activated in MII eggs [18], the oocytes were cultured in MEMα medium overnight before the rate of glycine transport was measured in each individual egg. Twelve female offspring in total were obtained from the three pairs for these experiments, seven of which were Gdf9-iCre:Glyt1fl/fl and five of which were Glyt1fl/fl (Figure 1A). All oocytes from Gdf9-iCre:Glyt1fl/fl females exhibited low rates of glycine transport that were approximately the level expected for non-specific transport, while all oocytes from Glyt1fl/fl had high rates of glycine transport (Figure 1B). Thus, oocytes with Slc6a9 disrupted did not transport glycine, consistent with essentially all glycine transport in oocytes being mediated by GLYT1. This also independently confirms that all GV oocytes in females with the Gdf9-iCre:Glyt1fl/fl genotype have the expected Slc6a9−/− phenotype of no GLYT1 activity. Because of the limited number of females that could be produced and the ability to directly demonstrate the functional knockout of glycine transport in single oocytes, we did not also attempt to confirm the knockout by measuring Slc6a9 transcripts or protein, which require large pools of oocytes.
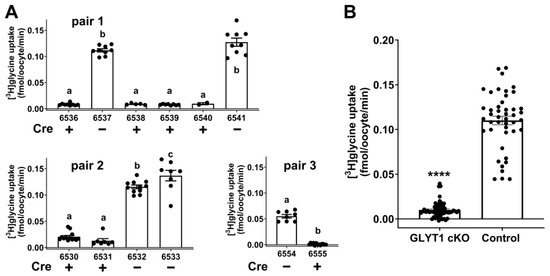
Figure 1.
Glycine transport into individual denuded oocytes from females of either Gdf9-iCre:Glyt1fl/fl or Glyt1fl/fl genotypes. (A) Gdf9-iCre:Glyt1fl/fl males were mated with Glyt1fl/fl females. Oocytes were collected from the female offspring of three such pairs (pair 1, pair2, pair 3) and the transport of [3H]glycine measured for each oocyte after in vitro maturation to MII eggs. The genotypes of the offspring were either Glyt1fl/fl (6537, 6541, 6532, 6533, 6554) or Gdf9-iCre:Glyt1fl/fl (6536, 6538, 6539, 6540, 6530, 6531, 6555). All oocytes from the Cre positive females exhibited very low glycine transport, while those from Cre negative females exhibited high levels of glycine transport. The rates of glycine transport differed significantly between oocytes from Cre positive vs. Cre negative females (p < 0.0001 for a vs. b or c within each panel by ANOVA with Tukey’s multiple comparisons test for pairs 1 and 2, or unpaired t-test for pair 3; for pair 2, uptake by the oocytes from the two Cre negative females also differed significantly, b vs. c, p = 0.026). (B) Data for all individual oocytes shown in (A) were pooled. Overall, there was a highly significant difference between the Cre positive (Glyt1 cKO) and Cre negative (control) oocytes (p < 0.0001, Mann-Whitney test). Bars indicate the mean ± s.e.m.
3.2. Fertility of Gdf9-iCre:Glyt1fl/fl Females
To determine if Gdf9-iCre:Glyt1fl/fl females, whose eggs lacked GLYT1 activity, were fertile, those females or control Glyt1fl/fl genotype females (N = 3 each) were paired with wild type BDF1 males until they had three litters. The Glyt1fl/fl females had three litters in 68, 78, and 78 days, while the Gdf9-iCre:Glyt1fl/fl had three litters in 96, 64, and 89 days (mean ± s.e.m. = 74.7 ± 3.3 and 83.0 ± 9.7 days, respectively). The mean times to produce three litters were not significantly different (p = 0.46, t-test). The mean number of pups per litter were 7.56 ± 0.63 for Glyt1fl/fl and 7.67 ± 0.97 for Gdf9-iCre:Glyt1fl/fl, which was not significantly different (p = 0.92, t-test). This indicated that oocytes that lacked GLYT1 activity had normal developmental potential in vivo.
3.3. Attempts to Delete GLYT1 Activity from Cumulus Cells
Because the deletion of GLYT1 activity from oocytes did not affect female fertility, we asked whether oocytes could be accumulating glycine through other routes. The cumulus cells surrounding oocytes also transport glycine mainly via GLYT1 and can supply glycine to the enclosed oocyte via the gap junctions that couple the oocyte to the cumulus [35]. Glycine transport into COCs becomes active when ovulation is triggered or the COC is removed from the follicle, similar to denuded oocytes [18,19]. Therefore, it was possible that oocytes that lacked their own GLYT1 activity could be supplied with glycine in vivo via cumulus cells.
We therefore attempted to create a mouse model in which glycine transport into granulosa cells was deleted by interbreeding Amhr2-Cre (which is expressed in cumulus cells [29,30]) and Glyt1fl/fl lines to produce Amhr2-Cre:Glyt1fl/fl mice. Detection of the Glyt1fl/fl deletion of a segment of Slc6a9 was confirmed in whole ovaries (Figure 2). We then isolated COCs from Amhr2-Cre:Glyt1fl/fl females and Glyt1fl/fl controls and compared the rates of glycine transport. Despite the presence in ovaries of the Slc6a9 gene in which the region flanked by LoxP sequences had been deleted, the rates of glycine transport into COCs from these females was not significantly lower than transport into COCs from Glyt1fl/fl controls (Figure 3A).
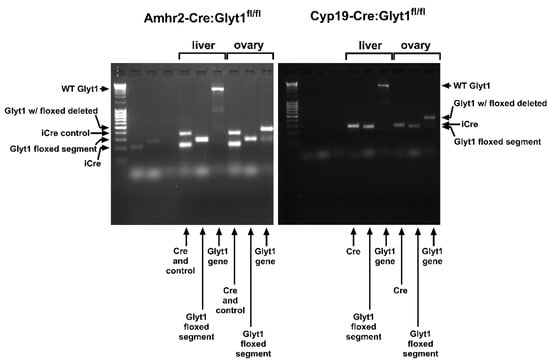
Figure 2.
Assessing the deletion of the floxed segment of Slc6a9 in Amhr2-Cre:Glyt1fl/fl or Cyp19-Cre:Glyt1fl/fl ovaries. Whole ovaries from Amhr2-Cre:Glyt1fl/fl or Cyp19-Cre:Glyt1fl/fl female mice (as indicated above gels) were assessed by PCR. MassRuler DNA ladders are shown at left. Liver from the same females was used as a control. Amhr2-Cre:Glyt1fl/fl: For both liver and ovary, the presence of the Amhr2-Cre transgene was confirmed by the iCre band at 200 bp obtained with Jackson Laboratory Cre primers (see Materials and Methods) multiplexed with their internal control primers (oIMR7338 and oIMR7339; 324 bp; labeled “Cre and control”). As expected, the Amhr2-Cre transgene was present in both liver and ovary (“iCre” arrow at left). The floxed segment of Slc6a9 was confirmed using the 17854 and 17672 primers that yield a 258 bp product for Glyt1fl/fl (labeled “Glyt1 floxed segment” and corresponding arrow at left). Deletion of the floxed segment of the Slc6a9 gene was assessed using the o250 and o228 primer pair (labeled “Glyt1 gene”). In liver, only the ~6,600 bp product corresponding to the full-length gene was amplified (“Glyt1 WT” arrow at left). In contrast, only a ~450 bp band corresponding to the Slc6a9 gene with the floxed segment deleted (“Glyt1 w/ floxed deleted” arrow at left) was amplified from ovary. Full-length Slc6a9 would also be present in ovary but cannot be detected here due to the overwhelming preferential amplification of the ~450 bp product. Together, this confirms that the Amhr2-Cre transgene is present, that the tissues are Glyt1fl/fl, and that no detectable Slc6a9 deletion occurred in liver but was present in ovary. The extent of the deletion in ovary is unknown, however. Cyp19-Cre:Glyt1fl/fl: Lanes are labeled as for Amhr2-Cre:Glyt1fl/fl. The Cyp19-Cre transgene was confirmed in both liver and ovary with the Cp19CreF and Cp19CreR primer pair that yields a 290 bp product. No control gene was used. Primers for Glyt1fl/fl and detecting deletion of the floxed segment of Slc6a9 are as for Amhr2-Cre. The presence of the Cyp19-Cre transgene was confirmed (labeled “Cre” and “iCre” arrow at right). As for Amhr2-Cre, both tissues were Glyt1fl/fl (labeled “Glyt1 floxed segment” and corresponding arrow at right). No detectable Slc6a9 deletion occurred in liver but was present in ovary (labeled “Glyt1 gene” with “WT Glyt1” and Glyt1 w/ floxed deleted” arrows at right). As for Amhr2-Cre, the extent of deletion in ovary is not known (see above). In each panel, the first three lanes are ear notch samples that failed to yield a sufficient signal.
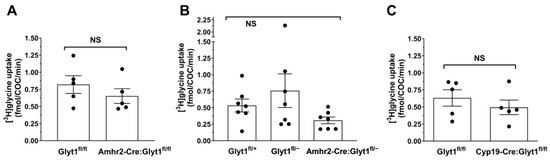
Figure 3.
Glycine transport into cumulus-oocyte complexes. (A) Glycine transport was measured in COCs from females of Glyt1fl/fl or Amhr2-Cre: Glyt1fl/fl genotypes. There was no significant difference in the rates of glycine transport between genotypes (p = 0.35 by unpaired t-test). Each point represents the uptake from 4–10 pooled COCs from one female (expressed as rate per COC; numbers vary since all intact COCs from a female were used). The bars indicate the mean ± s.e.m. for 5 females in each group. (B) Glycine transport was measured in COCs from females of Glyt1fl/fl or Amhr2-Cre: Glyt1fl/fl genotypes as in (A) except that they were on an Slc6a9+/˗ background as an attempt to obtain a more complete knockout in cumulus cells. There was no significant difference between the rates of glycine transport between genotypes (p = 0.17 by one-way ANOVA comparing all three groups). Each point represents the uptake from 4–23 pooled COCs from one female as in (A). The bars indicate the mean ± s.e.m. for 7 females in each group. (C) Glycine transport was measured in COCs from females of Glyt1fl/fl or Cyp19-Cre: Glyt1fl/fl genotypes as in (A,B). There was no significant difference between the rates of glycine transport between genotypes (p = 0.42 by unpaired t-test). Each point represents the uptake from 2–13 pooled COCs from one female as in (A,B). The bars indicate the mean ± s.e.m. for 5 females in each group.
We reasoned that this might be due to the need to delete the LoxP-flanked segments of both copies of the Slc6a9 gene from the majority of cumulus granulosa cells surrounding each oocyte to eliminate glycine transport into the COC. To increase the chance of a complete knockout of both copies of the gene, we produced Amhr2-Cre:Glyt1fl/− mice that had one copy of the Slc6a9 gene disrupted and the other floxed. These were mated with Amhr2-Cre:Glyt1fl/fl mice to produce Amhr2-Cre:Glyt1fl/− females and also Glyt1fl/- and Glyt1fl/+ control females. COCs from Slc6a9 heterozygous (Glyt1fl/−) and wild type (Glyt1fl/fl) females exhibited similar rates of glycine transport into their COCs (Figure 3B). There was some trend towards lower glycine transport into COCs from Amhr2-Cre:Glyt1fl/− females, which was not significant, and there was still considerable glycine transport into these COCs (Figure 3B). This indicated that expression of Amhr2-Cre was not sufficient to eliminate glycine transport even from Glyt1fl/− cumulus cells.
We next attempted to eliminate glycine transport into cumulus cells using Cyp19-Cre, which is also expressed in cumulus cells [31,32]. Cyp19-Cre:Glyt1fl/fl females were produced and glycine transport into their COCs was measured. Detection of the Glyt1fl/fl deletion was confirmed in whole ovaries (Figure 2). However, similar to the results with Amhr2-Cre, glycine transport into COCs from Cyp19-Cre:Glyt1fl/fl females was not significantly decreased compared to Glyt1fl/fl controls (Figure 3C). Thus, it did not prove possible to produce a substantial decrease in glycine transport into cumulus cells, in contrast to oocytes.
3.4. Transport of Glycine from Cumulus Cells into Oocytes
As described above, we were not able to knock out glycine transport into cumulus cells, which would have allowed the production of a double knockout of glycine transport in oocytes and cumulus. However, we could nonetheless test the hypothesis that wild type cumulus cells can supply glycine to enclosed oocytes that themselves lack glycine transport. We collected COCs from Gdf9-iCre:Glyt1fl/fl females, which contain oocytes that cannot transport glycine and from Glyt1fl/fl females with oocytes that can transport glycine (see Figure 1). COCs and denuded oocytes were incubated for 4 h with [3H]glycine. The relevant comparison is between the amount of glycine that was accumulated in the conditional knockout oocytes from Gdf9-iCre:Glyt1fl/fl females that were within cumulus cells vs. those that were cultured as denuded oocytes. As predicted from the results above, denuded oocytes from Gdf9-iCre:Glyt1fl/fl females accumulated glycine to a very low level (not significantly different than zero, one-factor t-test, p = 0.093). However, those that were connected to cumulus cells during culture accumulated significantly more glycine (Figure 4A; p = 0.017 by unpaired t-test). Thus, oocytes which lack GLYT1 activity can accumulate glycine when they are within COCs. In contrast, there was no difference in glycine accumulation when COCs and oocytes from wild type Glyt1fl/fl females were used (Figure 4B; p = 0.10 by unpaired t-test).
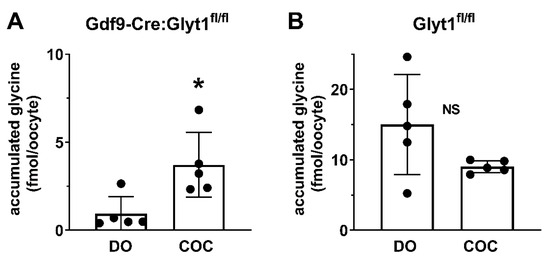
Figure 4.
Transfer of glycine to oocytes with cumulus-oocyte complexes. (A) COCs and denuded oocytes from Gdf9-Cre:Glyt1fl/fl females were incubated with [3H]glycine for 4 h, after which the oocytes were removed from the COC and the amount of [3H]glycine in oocytes was determined. Denuded oocytes from Gdf9-Cre:Glyt1fl/fl females accumulated low levels of glycine, while oocytes that had been within COCs accumulated significantly higher amounts of glycine (* p = 0.017, unpaired t-test), indicating that glycine was transferred into the enclosed oocyte from the cumulus. (B) Glycine accumulation in oocytes was determined as in (A) except the oocytes and COCs were from Glyt1fl/fl females. As expected, denuded oocytes accumulated glycine. The amount of glycine accumulated was not significantly different between oocytes that had been enclosed in COCs and denuded oocytes (p = 0.10, unpaired t-test). In both panels, each point represents a pool of 6–18 oocytes with the bars indicating the mean ± s.e.m.
3.5. Effect of Increased Osmolarity on Embryos from Oocytes of Gdf9-iCre:Glyt1fl/fl Females
It appears that oocytes lacking functional GLYT1 can likely accumulate some glycine in vivo via transport by coupled cumulus cells. However, in vitro, oocytes lacking GLYT1 activity would be predicted to be more sensitive to increased osmolarity. We first confirmed that the loss of functional GLYT1 had no effect on development in vitro at the normal osmolarity of mKSOM medium, 250 mOsM. There was no effect on development to the 2-cell, morula, or blastocyst stages at 250 mOsM (Figure 5A). There was, however, a significant decrease in development to the 4-cell stage, although this was a small effect of uncertain biological significance.
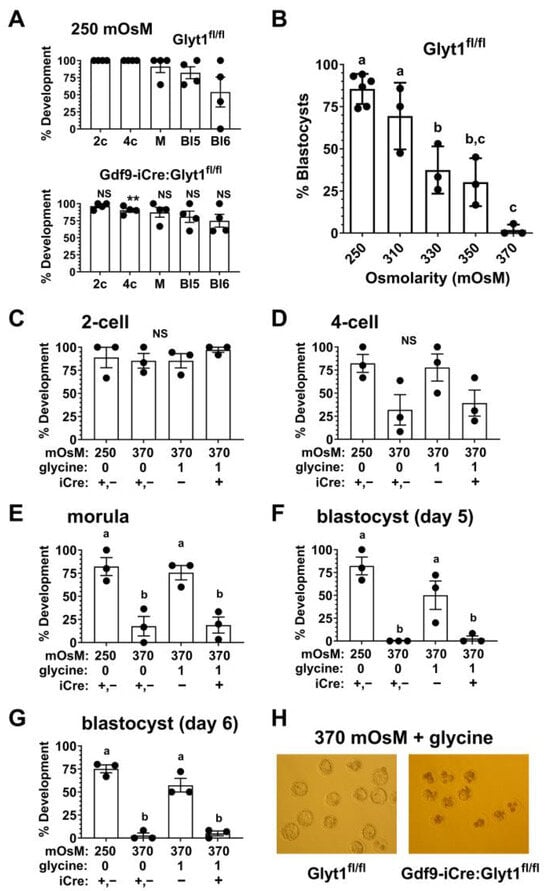
Figure 5.
Development of 1-cell embryos from Gdf9-Cre:Glyt1fl/fl females at increased osmolarity. (A) One-cell embryos from either Glyt1fl/fl or Gdf9-Cre:Glyt1fl/fl females developed similarly in normal mKSOM medium whose osmolarity is 250 mOsM. The stages of development are indicated as 2-cell (2c), 4-cell (4c), morula (M), blastocyst on day 5 of culture (Bl5), and blastocyst on day 6 (Bl6). The difference between development at each stage was tested between genotypes. The difference at the 4-cell stage reached significance (** p = 0.015, unpaired t-test), while all others were not significantly different. This indicated that the genotype did not affect normal preimplantation development. In all panels, each point represents the development of a group of ~10 embryos and the bars indicate the mean ± s.e.m. (B) The dependence of preimplantation development from 1-cell embryos to blastocysts was assessed as a function of osmolarity. Development decreased with osmolarity with a significant decrease observed by 330 mOsM and almost no development at 370 mOsM (p < 0.0001 by one-way ANOVA with Tukey’s multiple comparisons test; bars that do not share the same letter are significantly different, p < 0.05). There were three independent repeats at each osmolarity except for 250 mOsM (N = 5). (C) Development from the 1-cell to 2-cell stages was assessed at 250 mOsM and at 370 mOsM in the absence of glycine (0) or with 1 mM glycine (1) in the medium. The genotypes of the females from which the embryos were derived were either pooled Glyt1fl/fl and Gdf9-iCre:Glyt1fl/fl (iCre +,-), Gdf9-iCre:Glyt1fl/fl (+), or Glyt1fl/fl (-). Development was not significantly different between groups (p = 0.69 by one-way ANOVA). (D) Development from the 1-cell to 4-cell stages was assessed. Groups are as in (C). The differences in development to the 4-cell stage did not reach significance (p = 0.07 by one-way ANOVA). Development from the 1-cell to the morula stage (E) and blastocyst stages on day 5 (F) and day 6 (G) was assessed. Groups are as in (C). Development to each stage was significantly decreased at 370 mOsM (p < 0.005 by one-way ANOVA with Tukey’s multiple comparisons test). Glycine in the culture medium completely rescued development in embryos from Glyt1fl/fl females (−) to each stage. Development was not significantly different than at 250 mOsM (p = 0.95 for morula, 0.15 for blastocysts on day 5, and p = 0.10 on day 6). However, glycine did not rescue development of embryos derived from Gdf9-iCre:Glyt1fl/fl females (+), which were significantly different from those at 250 mOsM (p = 0.006 for morula, p = 0.001 for blastocysts on day 5, p < 0.0001 day 6) and from Glyt1fl/fl at 370 mOsM in the presence of glycine (p = 0.01 for morula, p = 0.03 for blastocysts on day 5, p = 0.0002 day 6). For E-G, bars that do not share the same letter are significantly different, p < 0.05 or as specified above. (H) Examples of blastocysts developed from 1-cell embryos derived from Glyt1fl/fl or Gdf9-iCre:Glyt1fl/fl females (as indicated at bottom) cultured at 370 mOsM with 1 mM glycine. Glycine rescued development to the blastocyst stage of Glyt1fl/fl embryos with GLYT1 activity. However, Gdf9-iCre:Glyt1fl/fl embryos lacking GLYT1 activity degenerated under the same conditions. For scale, expanded blastocysts are ~100 µm in diameter.
Previously, we had shown that strains of mice differed in their sensitivity to hypertonic stress [2], and the response of embryos from C57Bl/6 females to increased osmolarity was not known. We therefore tested the ability of 1-cell embryos from Glyt1fl/fl females on the C57Bl/6 background to develop to blastocysts in vitro in media with increased osmolarities. Embryos developed to the blastocyst stage similarly at the normal osmolarity of mKSOM (250 mOsM) and at 310 mOsM. However, development decreased significantly between 310 and 330 mOsM and very few embryos developed to blastocysts at 370 mOsM (Figure 5B). To most effectively reveal any ability of glycine to rescue development, we carried out subsequent experiments at 370 mOsM.
We then assessed the development of 1-cell embryos to the 2-cell stage (day 2 of culture), 4-cell or greater (day 3), morula (day 4), and to the blastocyst stage on days 5 or 6 (Figure 5C–G). At 250 mOsM, embryos developed to the blastocyst stage in the absence of added glycine, while little or no development to the 4-cell stage and beyond occurred in 370 mOsM medium without glycine. Due to the need to conserve embryos, we pooled 1-cell embryos derived from Glyt1fl/fl and Gdf9-iCre:Glyt1fl/fl females (indicated by iCre +,−; Figure 5C–G), since these developed similarly in culture in the absence of added glycine and the presence of the iCre transgene had no effect by itself (Figure 5A). This confirmed the expected developmental arrest after the 2-cell stage at high osmolarity. The presence of glycine in the culture medium rescued development to the blastocyst stage of 1-cell embryos derived from oocytes of Glyt1fl/fl females (iCre −), which had active GLYT1 (Figure 5C–G). However, even in the presence of glycine, 1-cell embryos derived from oocytes of Gdf9-iCre:Glyt1fl/fl females (iCre +), which lack GLYT1 activity, failed to develop beyond the 4-cell stage, and very few reached the blastocyst stage (Figure 5D–G). These embryos instead degenerated in culture (Figure 5H). Thus, GLYT1 is required in oocytes for subsequent embryo development to be rescued from hypertonic stress by glycine.
4. Discussion
Glycine is the major organic osmolyte in preimplantation mouse embryos, where it plays a key role in maintaining cell volume [7,15,23,36]. It likely has a similar role in the embryos of other mammals including humans [22]. In the absence of organic osmolytes supplied from the external environment, relatively minor increases in osmolarity can result in developmental arrest in early embryogenesis [1,2,4,14,16]. It had been established that glycine is transported into mouse embryos during the 1-cell to 4-cell stages by a transporter that had the characteristics of the classic System Gly that is mediated by the GLYT1 transporter encoded by Slc6a9. The evidence for this included competitive inhibition by sarcosine, dependence on both Na+ and Cl˗, and inhibition by a small molecule inhibitor, ORG23798, that is selective for GLYT1 [15,16,23]. However, it had not been shown that a functional Slc6a9 gene is required for glycine transport into early preimplantation embryos. Here, we were able to create an oocyte-specific knockout of Slc6a9 by crossing mice in which a segment of the gene had been flanked by LoxP sites [25,26] with transgenic mice expressing iCRE driven by the Gdf9 promoter [27]. Offspring of Gdf9-iCre:Glyt1fl/fl females mated to wild type males were all heterozygous for Slc6a9, indicating that all oocytes that gave rise to these offspring were Slc6a9−/−. Furthermore, when matured, such oocytes all failed to transport glycine, confirming that Slc6a9 in necessary for the robust glycine transport found in eggs and persisting in early preimplantation embryos.
Unexpectedly, female Gdf9-Cre:Glyt1fl/fl females were fertile, producing litters whose sizes and frequency were indistinguishable from those of Glyt1fl/fl females. There are several possible explanations for this result. The first is that glycine is not required by oocytes and early preimplantation embryos in vivo. There is considerable evidence that glycine is used by mouse embryos as an organic osmolyte and rescues development from the developmental block that occurs when osmolarity is increased in vitro [2,7,14,15,23,36]. However, it had not been shown that glycine is similarly required for preimplantation development in vivo. It is possible that it is used by embryos only under conditions of osmotic stress (e.g., dehydration), but this would not explain why embryos contain such high levels of endogenous glycine [18] when mice were housed under normal laboratory conditions with ad libitum access to water.
A second possibility is that glycine is supplied to embryos through another route that compensates for the loss of GLYT1 activity in the embryos themselves. We showed here that glycine taken up by cumulus cells can supply glycine to the enclosed oocyte within a COC, which supports the hypothesis that cumulus-mediated glycine transport can compensate at least partly for the loss of GLYT1 in the egg and early preimplantation embryo. The large majority of glycine transport by the cumulus is also via GLYT1 [35]. However, attempts to eliminate GLYT1 activity in cumulus cells by disrupting the Slc6a9 gene in granulosa cells using Cre expression driven by the Amhr2 promoter in either homozygous Glyt1fl/fl females or heterozygous Glyt1fl/− females lacking one copy of the Slc6a9 gene were unsuccessful, as were attempts using Cre expression driven by the Cyp19a1 promoter. Therefore, it could not be determined whether eliminating both routes of glycine accumulation via GLYT1 would affect female fertility. However, the accumulation of glycine in Slc6a9−/− oocytes within COCs supports the hypothesis that GLYT1 in cumulus cells compensates for its loss in eggs and early embryos and that it may contribute to maintaining female fertility.
A third possibility is that organic osmolytes other than glycine contribute to volume regulation of eggs and early embryos. In addition to glycine, betaine (N,N,N-trimethylglycine) is a major osmolyte in mouse eggs and preimplantation embryos. It is synthesized in oocytes during meiotic maturation by the enzyme choline dehydrogenase (CHDH) where it is accumulated to very high levels in the mature egg [37]. Its intracellular concentration is then regulated in 1-cell and 2-cell embryos by the SIT1 (Slc6a20a) transporter [38,39]. Thus, increased betaine synthesis or transport could compensate for decreased glycine in embryos. Whether knocking out the betaine pathways in conjunction with knocking out GLYT1 activity affects female fertility remains to be determined, however.
Another consideration is that different strains of mice have very different sensitivities to increased osmolarity. Mice of different strains exhibit widely varying susceptibility to the classic “2-cell block” to development, ranging from those that suffer a near-total block in traditional embryo culture media to classic “non-blocking” strains that developed to blastocysts in vitro at high rates [40,41]. We had previously shown that the block that occurred when osmolarity was increased was identical to the 2-cell block, with some strains becoming blocked at near-oviductal osmolarities, while others only became blocked at much higher osmolarities [2,4]. The C57Bl6 mice used in the current investigations become blocked at intermediate osmolarities beginning around 330 mOsM, between sensitive strains that become blocked at <310 mOsM and resistant strains that only become blocked at >350 mOsM. It is possible, therefore, that the fertility of more sensitive strains might be affected by lack of GLYT1 activity in oocytes. This remains to be investigated.
A requirement for GLYT1 activity in preimplantation embryos for glycine to mitigate against the deleterious effects of increased osmolarity was confirmed. As was previously shown, 1-cell stage embryos would not develop at increased osmolarity, but development could be rescued with glycine added to the medium [2,23]. The ability of glycine to rescue development was lost, however, in embryos in which Slc6a9 was disrupted, conclusively demonstrating the requirement for GLYT1. Although we used 370 mOsM medium here to obtain a maximum difference between development with and without glycine, a significant decrease in development was evident between 310 and 330 mOsM. This is nearer to the in vivo osmolarity of oviductal fluid, which has been measured to be ~300 mOsM [42,43,44], although calculations have indicated it may reach as high as 350 mOsM during early preimplantation embryo development [3]. This is supportive of a possible role for glycine in vivo, since relatively small increases in oviductal fluid osmolarity would have an impact on embryo development even at the lower values proposed for oviductal fluid. A role for glycine in vivo is also implied by the large intracellular concentration of glycine in the egg through 2-cell embryo stages [18,21], which can be maintained against a steep outward gradient only at considerable and continuous metabolic cost.
Ample evidence suggests that organic osmolytes such as glycine are beneficial to early preimplantation embryos in vitro [1]. This likely includes human embryos, since they transport glycine via GLYT1 similarly to mouse embryos [22]. Current embryo culture media in use in the clinic include glycine and other amino acids and, therefore, support cell volume regulation that requires organic osmolytes. The results presented here indicate the need for glycine in media used for in vitro oocyte maturation as well, to support the accumulation of glycine in the mature egg.
In summary, we have shown conclusively that a functional Slc6a9 gene is required for GLYT1 activity in mouse oocytes. Additionally, it is required for the rescue by glycine of development at increased osmolarities in vitro. At least in the C57Bl6 strain, GLYT1 in oocytes and preimplantation embryos is not required for female fertility. We propose that this is likely due to redundant mechanisms that include glycine transport into oocytes via cumulus cells and compensation by other organic osmolytes such as betaine. Future studies will be needed to develop a method of also knocking out glycine transport in cumulus cells, to determine whether this would decrease fertility. Also, it remains to be determined whether preventing the use of other organic osmolytes such as betaine in oocytes in conjunction with knocking out GLYT1 activity results in impaired fertility.
Author Contributions
Conceptualization, A.K.T. and J.M.B.; methodology, A.K.T.; formal analysis, A.K.T. and J.M.B.; investigation, A.K.T., T.M. and G.K.; resources, D.B.; data curation, A.K.T. and J.M.B.; writing—original draft preparation, A.K.T. and J.M.B.; writing—review and editing, A.K.T., T.M., G.K., D.B. and J.M.B.; visualization, A.K.T. and J.M.B.; supervision, A.K.T. and J.M.B.; project administration, J.M.B.; funding acquisition, J.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Canadian Institutes of Health Research grant PJT152991 and by a Lalor Foundation Fellowship award to A.K.T.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care Committee of the University of Ottawa Faculty of Medicine (approval numbers 3347 for breeding and 3348 for experiments).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data generated and analyzed for this study are included in this article. Unprocessed original data are available upon reasonable request to the corresponding author (J.M.B.).
Acknowledgments
The authors thank Barbara Vanderhyden for the gift of Gdf9-iCre, Amhr2-Cre, and Cyp19-Cre mice used to establish breeding colonies, and Vanderhyden and Elizabeth Macdonald for helpful advice on breeding conditional knockout mice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tscherner, A.K.; Macaulay, A.D.; Ortman, C.S.; Baltz, J.M. Initiation of cell volume regulation and unique cell volume regulatory mechanisms in mammalian oocytes and embryos. J. Cell. Physiol. 2021, 236, 7117–7133. [Google Scholar] [CrossRef]
- Hadi, T.; Hammer, M.A.; Algire, C.; Richards, T.; Baltz, J.M. Similar effects of osmolarity, glucose, and phosphate on cleavage past the 2-cell stage in mouse embryos from outbred and F1 hybrid females. Biol. Reprod. 2005, 72, 179–187. [Google Scholar] [CrossRef]
- Van Winkle, L.J. Perspective: One-Cell and Cleavage-Stage Mouse Embryos Thrive in Hyperosmotic Oviductal Fluid Through Expression of a Glycine Neurotransmitter Transporter and a Glycine-Gated Chloride Channel: Clinical and Transgenerational Implications. Front. Physiol. 2020, 11, 613840. [Google Scholar] [CrossRef]
- Wang, F.; Kooistra, M.; Lee, M.; Liu, L.; Baltz, J.M. Mouse embryos stressed by physiological levels of osmolarity become arrested in the late 2-cell stage before entry into M phase. Biol. Reprod. 2011, 85, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, E.K.; Lambert, I.H.; Pedersen, S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009, 89, 193–277. [Google Scholar] [CrossRef] [PubMed]
- Lawitts, J.A.; Biggers, J.D. Joint effects of sodium chloride, glutamine, and glucose in mouse preimplantation embryo culture media. Mol. Reprod. Dev. 1992, 31, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Haghighat, N.; Campione, A.L. Glycine protects preimplantation mouse conceptuses from a detrimental effect on development of the inorganic ions in oviductal fluid. J. Exp. Zool. 1990, 253, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Baltz, J.M. Bicarbonate/chloride exchange and intracellular pH throughout preimplantation mouse embryo development. Am. J. Physiol. 1996, 271, C1512–C1520. [Google Scholar] [CrossRef]
- Zhao, Y.; Chauvet, P.J.; Alper, S.L.; Baltz, J.M. Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J. Biol. Chem. 1995, 270, 24428–24434. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, C.; Meredith, M.; Baltz, J.M. Acute cell volume regulation by Janus kinase 2-mediated sodium/hydrogen exchange activation develops at the late one-cell stage in mouse preimplantation embryos. Biol. Reprod. 2017, 96, 542–550. [Google Scholar] [CrossRef]
- Zhou, C.; Baltz, J.M. JAK2 mediates the acute response to decreased cell volume in mouse preimplantation embryos by activating NHE1. J. Cell. Physiol. 2013, 228, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water stress: Evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Kwon, H.M.; Handler, J.S. Cell volume regulated transporters of compatible osmolytes. Curr. Opin. Cell Biol. 1995, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.M.; Baltz, J.M. Organic osmolytes and embryos: Substrates of the Gly and beta transport systems protect mouse zygotes against the effects of raised osmolarity. Biol. Reprod. 1997, 56, 1550–1558. [Google Scholar] [CrossRef]
- Dawson, K.M.; Collins, J.L.; Baltz, J.M. Osmolarity-dependent glycine accumulation indicates a role for glycine as an organic osmolyte in early preimplantation mouse embryos. Biol. Reprod. 1998, 59, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, L.J.; Haghighat, N.; Campione, A.L.; Gorman, J.M. Glycine transport in mouse eggs and preimplantation conceptuses. Biochim. Biophys. Acta 1988, 941, 241–256. [Google Scholar] [CrossRef]
- Guastella, J.; Brecha, N.; Weigmann, C.; Lester, H.A.; Davidson, N. Cloning, expression, and localization of a rat brain high-affinity glycine transporter. Proc. Natl. Acad. Sci. USA 1992, 89, 7189–7193. [Google Scholar] [CrossRef]
- Tartia, A.P.; Rudraraju, N.; Richards, T.; Hammer, M.A.; Talbot, P.; Baltz, J.M. Cell volume regulation is initiated in mouse oocytes after ovulation. Development 2009, 136, 2247–2254. [Google Scholar] [CrossRef]
- Richard, S.; Baltz, J.M. Preovulatory suppression of mouse oocyte cell volume-regulatory mechanisms is via signalling that is distinct from meiotic arrest. Sci. Rep. 2017, 7, 702. [Google Scholar] [CrossRef]
- Richard, S.; Tartia, A.P.; Boison, D.; Baltz, J.M. Mouse oocytes acquire mechanisms that permit independent cell volume regulation at the end of oogenesis. J. Cell. Physiol. 2017, 232, 2436–2446. [Google Scholar] [CrossRef]
- Li, L.; Zhu, S.; Shu, W.; Guo, Y.; Guan, Y.; Zeng, J.; Wang, H.; Han, L.; Zhang, J.; Liu, X.; et al. Characterization of Metabolic Patterns in Mouse Oocytes during Meiotic Maturation. Mol. Cell 2020, 80, 525–540.e529. [Google Scholar] [CrossRef]
- Hammer, M.A.; Kolajova, M.; Leveille, M.; Claman, P.; Baltz, J.M. Glycine transport by single human and mouse embryos. Hum. Reprod. 2000, 15, 419–426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steeves, C.L.; Hammer, M.A.; Walker, G.B.; Rae, D.; Stewart, N.A.; Baltz, J.M. The glycine neurotransmitter transporter GLYT1 is an organic osmolyte transporter regulating cell volume in cleavage-stage embryos. Proc. Natl. Acad. Sci. USA 2003, 100, 13982–13987. [Google Scholar] [CrossRef] [PubMed]
- Lawitts, J.A.; Biggers, J.D. Culture of preimplantation embryos. Methods Enzym. 1993, 225, 153–164. [Google Scholar]
- Yee, B.K.; Balic, E.; Singer, P.; Schwerdel, C.; Grampp, T.; Gabernet, L.; Knuesel, I.; Benke, D.; Feldon, J.; Mohler, H.; et al. Disruption of Glycine Transporter 1 Restricted to Forebrain Neurons Is Associated with a Procognitive and Antipsychotic Phenotypic Profile. J. Neurosci. 2006, 26, 3169–3181. [Google Scholar] [CrossRef] [PubMed]
- Gabernet, L.; Pauly-Evers, M.; Schwerdel, C.; Lentz, M.; Bluethmann, H.; Vogt, K.; Alberati, D.; Mohler, H.; Boison, D. Enhancement of the NMDA receptor function by reduction of glycine transporter-1 expression. Neurosci. Lett. 2005, 373, 79–84. [Google Scholar] [CrossRef]
- Lan, Z.-J.; Xu, X.; Cooney, A.J. Differential Oocyte-Specific Expression of Cre Recombinase Activity in GDF-9-iCre, Zp3cre, and Msx2Cre Transgenic Mice1. Biol. Reprod. 2004, 71, 1469–1474. [Google Scholar] [CrossRef]
- St-Jean, G.; Tsoi, M.; Abedini, A.; Levasseur, A.; Rico, C.; Morin, M.; Djordjevic, B.; Miinalainen, I.; Kaarteenaho, R.; Paquet, M.; et al. Lats1 and Lats2 are required for the maintenance of multipotency in the Müllerian duct mesenchyme. Development 2019, 146, dev180430. [Google Scholar] [CrossRef]
- Jorgez, C.J.; Klysik, M.; Jamin, S.P.; Behringer, R.R.; Matzuk, M.M. Granulosa cell-specific inactivation of follistatin causes female fertility defects. Mol. Endocrinol. 2004, 18, 953–967. [Google Scholar] [CrossRef]
- Arango, N.A.; Kobayashi, A.; Wang, Y.; Jamin, S.P.; Lee, H.-H.; Orvis, G.D.; Behringer, R.R. A mesenchymal perspective of müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol. Reprod. Dev. 2008, 75, 1154–1162. [Google Scholar] [CrossRef]
- Lapointe, E.; Boyer, A.; Rico, C.; Paquet, M.; Franco, H.L.; Gossen, J.; DeMayo, F.J.; Richards, J.S.; Boerboom, D. FZD1 regulates cumulus expansion genes and is required for normal female fertility in mice. Biol. Reprod. 2012, 87, 104. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Shimada, M.; Liu, Z.; Cahill, N.; Noma, N.; Wu, Y.; Gossen, J.; Richards, J.S. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 2008, 135, 2127–2137. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Liu, K.; Kikuchi, K. Oocyte-Specific Knockout: A Novel In Vivo Approach for Studying Gene Functions During Folliculogenesis, Oocyte Maturation, Fertilization, and Embryogenesis. Biol. Reprod. 2008, 79, 1014–1020. [Google Scholar] [CrossRef]
- Baltz, J.M.; Corbett, H.E.; Richard, S. Measuring transport and accumulation of radiolabeled substrates in oocytes and embryos. Methods Mol. Biol. 2013, 957, 163–178. [Google Scholar] [PubMed]
- Haghighat, N.; Van Winkle, L.J. Developmental change in follicular cell-enhanced amino acid uptake into mouse oocytes that depends on intact gap junctions and transport system Gly. J. Exp. Zool. 1990, 253, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Steeves, C.L.; Baltz, J.M. Regulation of intracellular glycine as an organic osmolyte in early preimplantation mouse embryos. J. Cell. Physiol. 2005, 204, 273–279. [Google Scholar] [CrossRef]
- McClatchie, T.; Meredith, M.; Ouédraogo, M.O.; Slow, S.; Lever, M.; Mann, M.R.W.; Zeisel, S.H.; Trasler, J.M.; Baltz, J.M. Betaine is accumulated via transient choline dehydrogenase activation during mouse oocyte meiotic maturation. J. Biol. Chem. 2017, 292, 13784–13794. [Google Scholar] [CrossRef]
- Anas, M.K.; Hammer, M.A.; Lever, M.; Stanton, J.A.; Baltz, J.M. The organic osmolytes betaine and proline are transported by a shared system in early preimplantation mouse embryos. J. Cell. Physiol. 2007, 210, 266–277. [Google Scholar] [CrossRef]
- Anas, M.K.; Lee, M.B.; Zhou, C.; Hammer, M.A.; Slow, S.; Karmouch, J.; Liu, X.J.; Broer, S.; Lever, M.; Baltz, J.M. SIT1 is a betaine/proline transporter that is activated in mouse eggs after fertilization and functions until the 2-cell stage. Development 2008, 135, 4123–4130. [Google Scholar] [CrossRef]
- Suzuki, O.; Asano, T.; Yamamoto, Y.; Takano, K.; Koura, M. Development in vitro of preimplantation embryos from 55 mouse strains. Reprod. Fertil. Dev. 1996, 8, 975–980. [Google Scholar] [CrossRef]
- Biggers, J.D. Reflections on the culture of the preimplantation embryo. Int. J. Dev. Biol. 1998, 42, 879–884. [Google Scholar] [PubMed]
- Collins, J.L.; Baltz, J.M. Estimates of mouse oviductal fluid tonicity based on osmotic responses of embryos. Biol. Reprod. 1999, 60, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Fiorenza, M.T.; Bevilacqua, A.; Canterini, S.; Torcia, S.; Pontecorvi, M.; Mangia, F. Early transcriptional activation of the hsp70.1 gene by osmotic stress in one-cell embryos of the mouse. Biol. Reprod. 2004, 70, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Pogorelova, M.A.; Golichenkov, V.A.; Pogorelova, V.N.; Kornienko, E.V.; Panait, A.I.; Pogorelov, A.G. Estimation of isotonic point of incubation medium for two-cell mouse embryo. Bull. Exp. Biol. Med. 2011, 152, 142–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).