The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis
Abstract
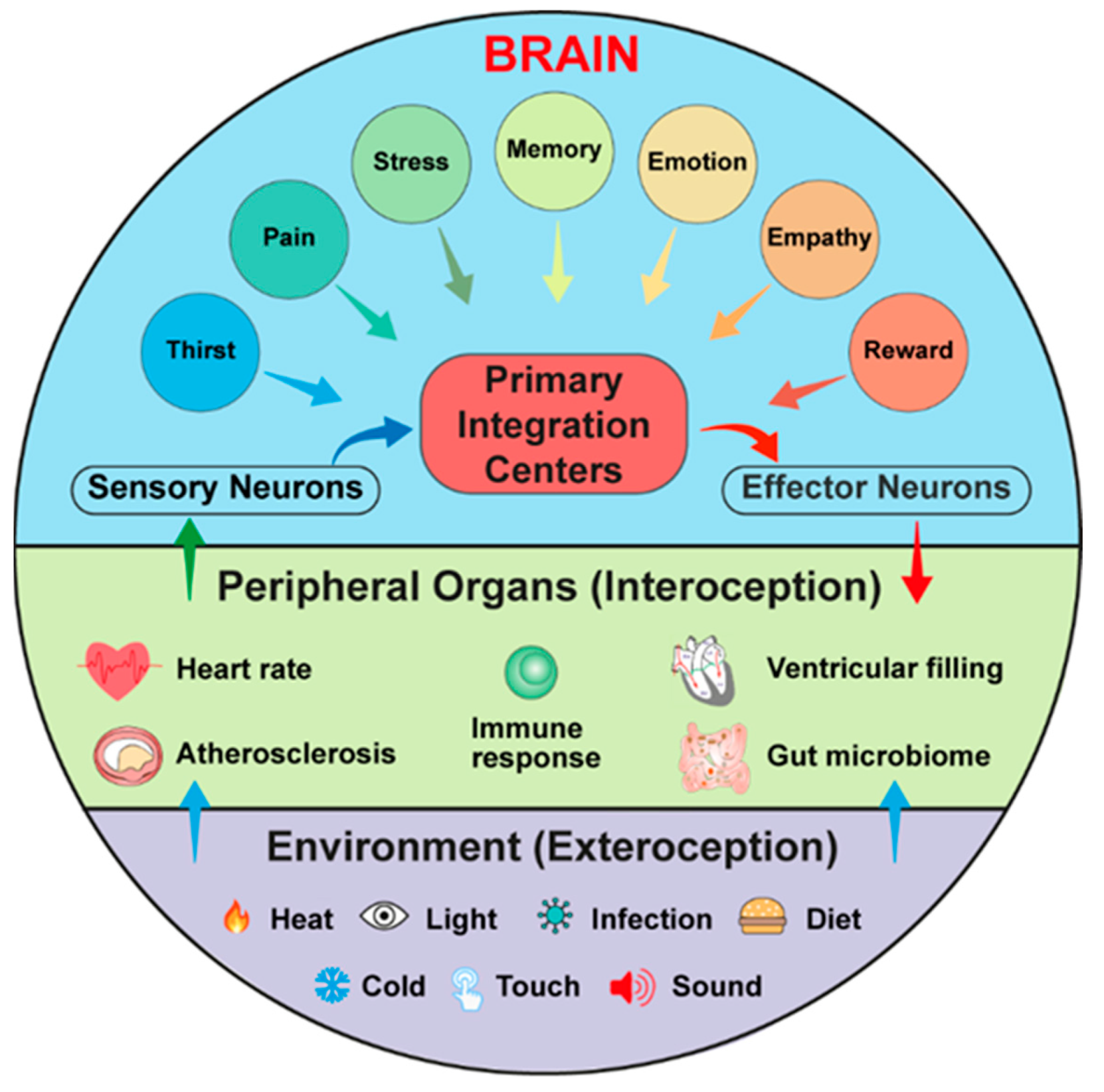
:1. Introduction
2. Tripartite Interaction between the Nervous System, the Immune System, and the Cardiovascular System Gives Rise to the Neuroimmune Cardiovascular Interface Concept
3. Neural Circuits of the Cardiovascular System
3.1. The ABC
3.2. The HBC
3.3. The CBC
4. Interoception and Exteroception Involve Participation of Risk Factors for CVDs
5. Why Propose the Neuroimmune Cardiovascular Circuit Hypothesis Now?
6. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohanta, S.K.; Yin, C.; Weber, C.; Godinho-Silva, C.; Veiga-Fernandes, H.; Xu, Q.J.; Chang, R.B.; Habenicht, A.J.R. Cardiovascular Brain Circuits. Circ. Res. 2023, 132, 1546–1565. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Dimmeler, S. A coalition to heal—The impact of the cardiac microenvironment. Science 2022, 377, eabm4443. [Google Scholar] [CrossRef] [PubMed]
- Klein Wolterink, R.G.J.; Wu, G.S.; Chiu, I.M.; Veiga-Fernandes, H. Neuroimmune Interactions in Peripheral Organs. Annu. Rev. Neurosci. 2022, 45, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Udit, S.; Blake, K.; Chiu, I.M. Somatosensory and autonomic neuronal regulation of the immune response. Nat. Rev. Neurosci. 2022, 23, 157–171. [Google Scholar] [CrossRef]
- Talbot, S.; Foster, S.L.; Woolf, C.J. Neuroimmunity: Physiology and Pathology. Annu. Rev. Immunol. 2016, 34, 421–447. [Google Scholar] [CrossRef]
- Schiller, M.; Ben-Shaanan, T.L.; Rolls, A. Neuronal regulation of immunity: Why, how and where? Nat. Rev. Immunol. 2021, 21, 20–36. [Google Scholar] [CrossRef]
- Osterhout, J.A.; Kapoor, V.; Eichhorn, S.W.; Vaughn, E.; Moore, J.D.; Liu, D.; Lee, D.; DeNardo, L.A.; Luo, L.; Zhuang, X.; et al. A preoptic neuronal population controls fever and appetite during sickness. Nature 2022, 606, 937–944. [Google Scholar] [CrossRef]
- Pavlov, V.A.; Chavan, S.S.; Tracey, K.J. Molecular and Functional Neuroscience in Immunity. Annu. Rev. Immunol. 2018, 36, 783–812. [Google Scholar] [CrossRef]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- O’Brien, C.J.O.; Haberman, E.R.; Domingos, A.I. A Tale of Three Systems: Toward a Neuroimmunoendocrine Model of Obesity. Annu. Rev. Cell Dev. Biol. 2021, 37, 549–573. [Google Scholar] [CrossRef]
- You, Z.; Liu, B.; Qi, H. Neuronal regulation of B-cell immunity: Anticipatory immune posturing? Neuron 2022, 110, 3582–3596. [Google Scholar] [CrossRef]
- Prescott, S.L.; Liberles, S.D. Internal senses of the vagus nerve. Neuron 2022, 110, 579–599. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Pavlov, V.A.; Tracey, K.J. Mechanisms and Therapeutic Relevance of Neuro-immune Communication. Immunity 2017, 46, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.M.; von Hehn, C.A.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.F.; Nakamura, K. Central Mechanisms for Thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- McMahon, S.B.; La Russa, F.; Bennett, D.L. Crosstalk between the nociceptive and immune systems in host defence and disease. Nat. Rev. Neurosci. 2015, 16, 389–402. [Google Scholar] [CrossRef]
- Matic, Z.; Platisa, M.M.; Kalauzi, A.; Bojic, T. Slow 0.1 Hz Breathing and Body Posture Induced Perturbations of RRI and Respiratory Signal Complexity and Cardiorespiratory Coupling. Front. Physiol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Goldberger, A.L. Giles f. Filley lecture. Complex systems. Proc. Am. Thorac. Soc. 2006, 3, 467–471. [Google Scholar] [CrossRef]
- Poller, W.C.; Downey, J.; Mooslechner, A.A.; Khan, N.; Li, L.; Chan, C.T.; McAlpine, C.S.; Xu, C.; Kahles, F.; He, S.; et al. Brain motor and fear circuits regulate leukocytes during acute stress. Nature 2022, 607, 578–584. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Kiss, M.G.; Rattik, S.; He, S.; Vassalli, A.; Valet, C.; Anzai, A.; Chan, C.T.; Mindur, J.E.; Kahles, F.; et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature 2019, 566, 383–387. [Google Scholar] [CrossRef]
- Gogolla, N. The brain remembers where and how inflammation struck. Cell 2021, 184, 5851–5853. [Google Scholar] [CrossRef]
- Maity, S.; Farrell, K.; Navabpour, S.; Narayanan, S.N.; Jarome, T.J. Epigenetic Mechanisms in Memory and Cognitive Decline Associated with Aging and Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 12280. [Google Scholar] [CrossRef] [PubMed]
- Josselyn, S.A.; Tonegawa, S. Memory engrams: Recalling the past and imagining the future. Science 2020, 367, eaaw4325. [Google Scholar] [CrossRef] [PubMed]
- Brea, D.; Veiga-Fernandes, H. Inflammation in the gut is encoded by neurons in the brain. Nature 2022, 602, 217–218. [Google Scholar] [CrossRef]
- Willems, T.; Henke, K. Imaging human engrams using 7 Tesla magnetic resonance imaging. Hippocampus 2021, 31, 1257–1270. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915. [Google Scholar] [CrossRef]
- Klein, A.S.; Dolensek, N.; Weiand, C.; Gogolla, N. Fear balance is maintained by bodily feedback to the insular cortex in mice. Science 2021, 374, 1010–1015. [Google Scholar] [CrossRef]
- Cardoso, F.; Klein Wolterink, R.G.J.; Godinho-Silva, C.; Domingues, R.G.; Ribeiro, H.; da Silva, J.A.; Mahu, I.; Domingos, A.I.; Veiga-Fernandes, H. Neuro-mesenchymal units control ILC2 and obesity via a brain-adipose circuit. Nature 2021, 597, 410–414. [Google Scholar] [CrossRef]
- Zeng, W.; Pirzgalska, R.M.; Pereira, M.M.; Kubasova, N.; Barateiro, A.; Seixas, E.; Lu, Y.H.; Kozlova, A.; Voss, H.; Martins, G.G.; et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 2015, 163, 84–94. [Google Scholar] [CrossRef]
- Min, S.; Chang, R.B.; Prescott, S.L.; Beeler, B.; Joshi, N.R.; Strochlic, D.E.; Liberles, S.D. Arterial Baroreceptors Sense Blood Pressure through Decorated Aortic Claws. Cell Rep. 2019, 29, 2192–2201.e2193. [Google Scholar] [CrossRef]
- Li, L.; Huang, C.; Ai, J.; Yan, B.; Gu, H.; Ma, Z.; Li, A.Y.; Xinyan, S.; Harden, S.W.; Hatcher, J.T.; et al. Structural remodeling of vagal afferent innervation of aortic arch and nucleus ambiguus (NA) projections to cardiac ganglia in a transgenic mouse model of type 1 diabetes (OVE26). J. Comp. Neurol. 2010, 518, 2771–2793. [Google Scholar] [CrossRef]
- Hainsworth, R. Reflexes from the heart. Physiol. Rev. 1991, 71, 617–658. [Google Scholar] [CrossRef] [PubMed]
- Bin, N.R.; Prescott, S.L.; Horio, N.; Wang, Y.; Chiu, I.M.; Liberles, S.D. An airway-to-brain sensory pathway mediates influenza-induced sickness. Nature 2023, 615, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.; Bloom, J.R.; Kraemer, H.C.; Gottheil, E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet 1989, 2, 888–891. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, S.K.; Peng, L.; Li, Y.; Lu, S.; Sun, T.; Carnevale, L.; Perrotta, M.; Ma, Z.; Forstera, B.; Stanic, K.; et al. Neuroimmune cardiovascular interfaces control atherosclerosis. Nature 2022, 605, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, B.; Chen, R.; Jo, Y.; Tang, D.; Raffiee, M.; Kim, Y.S.; Inoue, M.; Randles, S.; Ramakrishnan, C.; Patel, S.; et al. Cardiogenic control of affective behavioural state. Nature 2023, 615, 292–299. [Google Scholar] [CrossRef]
- Wagner, J.U.G.; Tombor, L.S.; Malacarne, P.F.; Kettenhausen, L.M.; Panthel, J.; Kujundzic, H.; Manickam, N.; Schmitz, K.; Cipca, M.; Stilz, K.A.; et al. Aging impairs the neurovascular interface in the heart. Science 2023, 381, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, D. Neuroimmune axis of cardiovascular control: Mechanisms and therapeutic implications. Nat. Rev. Cardiol. 2022, 19, 379–394. [Google Scholar] [CrossRef]
- Carmeliet, P.; Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 2005, 436, 193–200. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Brain, S.D. A historical perspective on the role of sensory nerves in neurogenic inflammation. Semin. Immunopathol. 2018, 40, 229–236. [Google Scholar] [CrossRef]
- Porta, A.; Bassani, T.; Bari, V.; Tobaldini, E.; Takahashi, A.C.; Catai, A.M.; Montano, N. Model-based assessment of baroreflex and cardiopulmonary couplings during graded head-up tilt. Comput. Biol. Med. 2012, 42, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, N.N.; Pavlovic, S.U.; Milasinovic, G.; Kircanski, B.; Platisa, M.M. Bidirectional Cardio-Respiratory Interactions in Heart Failure. Front. Physiol. 2018, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.Z.; Marshall, K.L.; Min, S.; Daou, I.; Chapleau, M.W.; Abboud, F.M.; Liberles, S.D.; Patapoutian, A. PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 2018, 362, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Sakakura, K.; Ladich, E.; Cheng, Q.; Otsuka, F.; Yahagi, K.; Fowler, D.R.; Kolodgie, F.D.; Virmani, R.; Joner, M. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J. Am. Coll. Cardiol. 2014, 64, 635–643. [Google Scholar] [CrossRef]
- Ai, J.; Wurster, R.D.; Harden, S.W.; Cheng, Z.J. Vagal afferent innervation and remodeling in the aortic arch of young-adult fischer 344 rats following chronic intermittent hypoxia. Neuroscience 2009, 164, 658–666. [Google Scholar] [CrossRef]
- Hinterdobler, J.; Schott, S.; Jin, H.; Meesmann, A.; Steinsiek, A.L.; Zimmermann, A.S.; Wobst, J.; Muller, P.; Mauersberger, C.; Vilne, B.; et al. Acute mental stress drives vascular inflammation and promotes plaque destabilization in mouse atherosclerosis. Eur. Heart J. 2021, 42, 4077–4088. [Google Scholar] [CrossRef]
- Hu, D.; Mohanta, S.K.; Yin, C.; Peng, L.; Ma, Z.; Srikakulapu, P.; Grassia, G.; MacRitchie, N.; Dever, G.; Gordon, P.; et al. Artery Tertiary Lymphoid Organs Control Aorta Immunity and Protect against Atherosclerosis via Vascular Smooth Muscle Cell Lymphotoxin beta Receptors. Immunity 2015, 42, 1100–1115. [Google Scholar] [CrossRef]
- Armour, J.A. Potential clinical relevance of the ‘little brain’ on the mammalian heart. Exp. Physiol. 2008, 93, 165–176. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. Basic Clin. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Kaufmann, H.; Norcliffe-Kaufmann, L.; Palma, J.A. Baroreflex Dysfunction. N. Engl. J. Med. 2020, 382, 163–178. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, C.D.; Wang, R.; Xu, Q.J.; Dai Pra, R.; Zhang, L.; Chang, R.B. A multidimensional coding architecture of the vagal interoceptive system. Nature 2022, 603, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Wake, E.; Brack, K. Characterization of the intrinsic cardiac nervous system. Auton. Neurosci. Basic Clin. 2016, 199, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Schloss, M.J.; Swirski, F.K.; Nahrendorf, M. Modifiable Cardiovascular Risk, Hematopoiesis, and Innate Immunity. Circ. Res. 2020, 126, 1242–1259. [Google Scholar] [CrossRef]
- Vasamsetti, S.B.; Florentin, J.; Coppin, E.; Stiekema, L.C.A.; Zheng, K.H.; Nisar, M.U.; Sembrat, J.; Levinthal, D.J.; Rojas, M.; Stroes, E.S.G.; et al. Sympathetic Neuronal Activation Triggers Myeloid Progenitor Proliferation and Differentiation. Immunity 2018, 49, 93–106. [Google Scholar] [CrossRef]
- Vinik, A.I. Clinical Practice. Diabetic Sensory and Motor Neuropathy. N. Engl. J. Med. 2016, 374, 1455–1464. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 2013, 369, 362–372. [Google Scholar] [CrossRef]
- Pirzgalska, R.M.; Seixas, E.; Seidman, J.S.; Link, V.M.; Sanchez, N.M.; Mahu, I.; Mendes, R.; Gres, V.; Kubasova, N.; Morris, I.; et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 2017, 23, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, A.; Yung, A.R.; Liu, Y.; Krasnow, M.A. Molecularly defined circuits for cardiovascular and cardiopulmonary control. Nature 2022, 606, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Custodis, F.; Schirmer, S.H.; Baumhakel, M.; Heusch, G.; Bohm, M.; Laufs, U. Vascular pathophysiology in response to increased heart rate. J. Am. Coll. Cardiol. 2010, 56, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Hofman, A.; Ott, A.; Breteler, M.M.; Bots, M.L.; Slooter, A.J.; van Harskamp, F.; van Duijn, C.N.; Van Broeckhoven, C.; Grobbee, D.E. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet 1997, 349, 151–154. [Google Scholar] [CrossRef]
- Yin, C.; Ackermann, S.; Ma, Z.; Mohanta, S.K.; Zhang, C.; Li, Y.; Nietzsche, S.; Westermann, M.; Peng, L.; Hu, D.; et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat. Med. 2019, 25, 496–506. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef]
- Schaid, D.J.; Chen, W.; Larson, N.B. From genome-wide associations to candidate causal variants by statistical fine-mapping. Nat. Rev. Genet. 2018, 19, 491–504. [Google Scholar] [CrossRef]
- Walsh, R.; Jurgens, S.J.; Erdmann, J.; Bezzina, C.R. Genome-wide association studies of cardiovascular disease. Physiol. Rev. 2023, 103, 2039–2055. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Hahad, O.; Sorensen, M.; Lelieveld, J.; Duerr, G.D.; Nieuwenhuijsen, M.; Daiber, A. Environmental risk factors and cardiovascular diseases: A comprehensive expert review. Cardiovasc. Res. 2022, 118, 2880–2902. [Google Scholar] [CrossRef] [PubMed]
- Everson-Rose, S.A.; Lewis, T.T. Psychosocial factors and cardiovascular diseases. Annu. Rev. Public Health 2005, 26, 469–500. [Google Scholar] [CrossRef]
- Ross, R.; Glomset, J.A. The pathogenesis of atherosclerosis (first of two parts). N. Engl. J. Med. 1976, 295, 369–377. [Google Scholar] [CrossRef]
- Deftereos, S.G.; Beerkens, F.J.; Shah, B.; Giannopoulos, G.; Vrachatis, D.A.; Giotaki, S.G.; Siasos, G.; Nicolas, J.; Arnott, C.; Patel, S.; et al. Colchicine in Cardiovascular Disease: In-Depth Review. Circulation 2022, 145, 61–78. [Google Scholar]
- Kwon, D. Your brain could be controlling how sick you get—And how you recover. Nature 2023, 614, 613–615. [Google Scholar] [CrossRef]
- Olofsson, P.S.; Steinberg, B.E.; Sobbi, R.; Cox, M.A.; Ahmed, M.N.; Oswald, M.; Szekeres, F.; Hanes, W.M.; Introini, A.; Liu, S.F.; et al. Blood pressure regulation by CD4+ lymphocytes expressing choline acetyltransferase. Nat. Biotechnol. 2016, 34, 1066–1071. [Google Scholar] [CrossRef]
- Schloss, M.J.; Hulsmans, M.; Rohde, D.; Lee, I.H.; Severe, N.; Foy, B.H.; Pulous, F.E.; Zhang, S.; Kokkaliaris, K.D.; Frodermann, V.; et al. B lymphocyte-derived acetylcholine limits steady-state and emergency hematopoiesis. Nat. Immunol. 2022, 23, 605–618. [Google Scholar] [CrossRef]
- Zhao, B.; Li, T.; Fan, Z.; Yang, Y.; Shu, J.; Yang, X.; Wang, X.; Luo, T.; Tang, J.; Xiong, D.; et al. Heart-brain connections: Phenotypic and genetic insights from magnetic resonance images. Science 2023, 380, abn6598. [Google Scholar] [CrossRef]
- Siedlinski, M.; Carnevale, L.; Xu, X.; Carnevale, D.; Evangelou, E.; Caulfield, M.J.; Maffia, P.; Wardlaw, J.; Samani, N.J.; Tomaszewski, M.; et al. Genetic analyses identify brain structures related to cognitive impairment associated with elevated blood pressure. Eur. Heart J. 2023, 44, 2114–2125. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanta, S.K.; Sun, T.; Lu, S.; Wang, Z.; Zhang, X.; Yin, C.; Weber, C.; Habenicht, A.J.R. The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis. Cells 2023, 12, 2485. https://doi.org/10.3390/cells12202485
Mohanta SK, Sun T, Lu S, Wang Z, Zhang X, Yin C, Weber C, Habenicht AJR. The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis. Cells. 2023; 12(20):2485. https://doi.org/10.3390/cells12202485
Chicago/Turabian StyleMohanta, Sarajo K., Ting Sun, Shu Lu, Zhihua Wang, Xi Zhang, Changjun Yin, Christian Weber, and Andreas J. R. Habenicht. 2023. "The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis" Cells 12, no. 20: 2485. https://doi.org/10.3390/cells12202485
APA StyleMohanta, S. K., Sun, T., Lu, S., Wang, Z., Zhang, X., Yin, C., Weber, C., & Habenicht, A. J. R. (2023). The Impact of the Nervous System on Arteries and the Heart: The Neuroimmune Cardiovascular Circuit Hypothesis. Cells, 12(20), 2485. https://doi.org/10.3390/cells12202485







