Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation
Abstract
1. Introduction
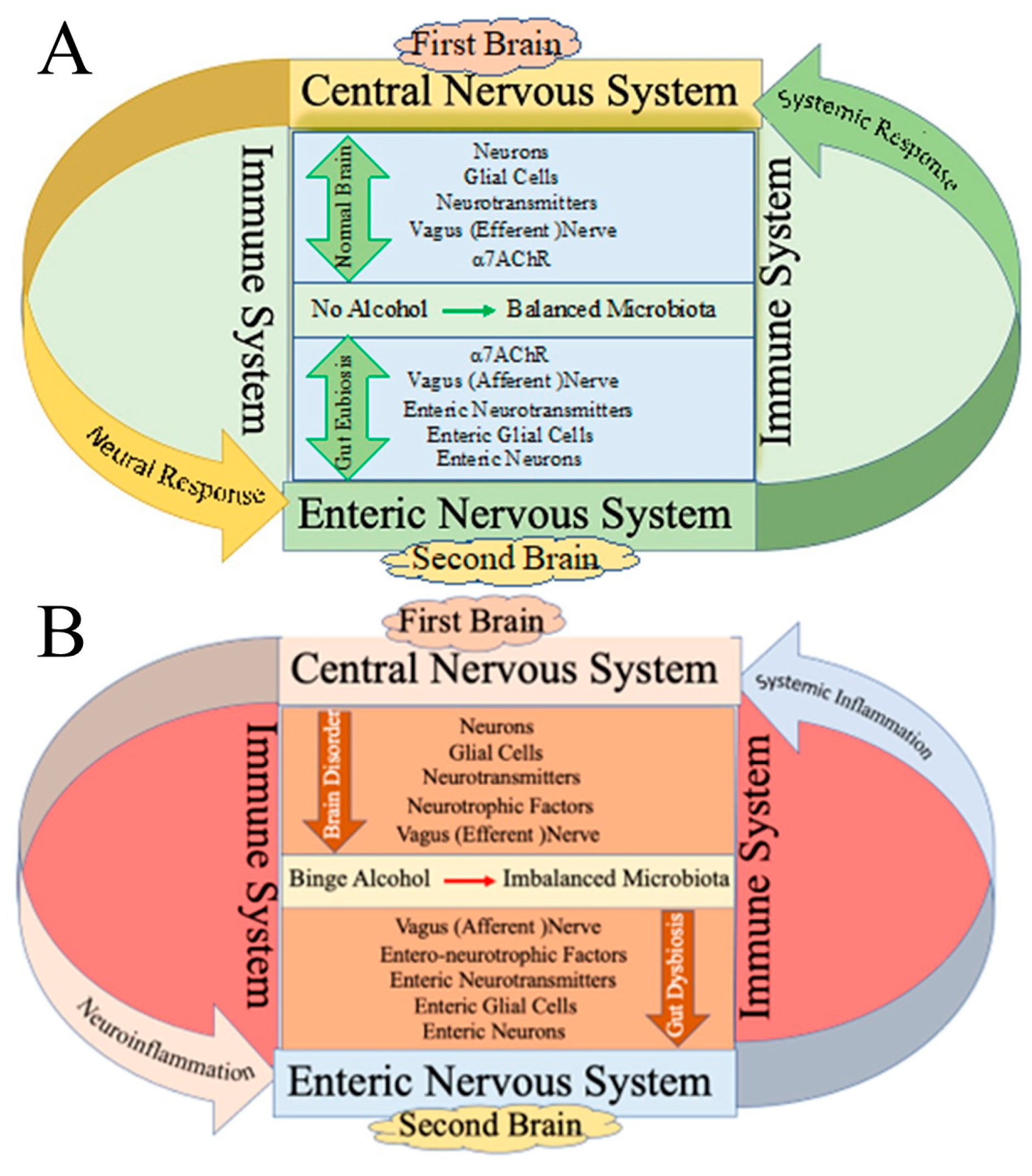
2. Effect of Alcohol on the First Brain (CNS) and Second Brain (Gut)
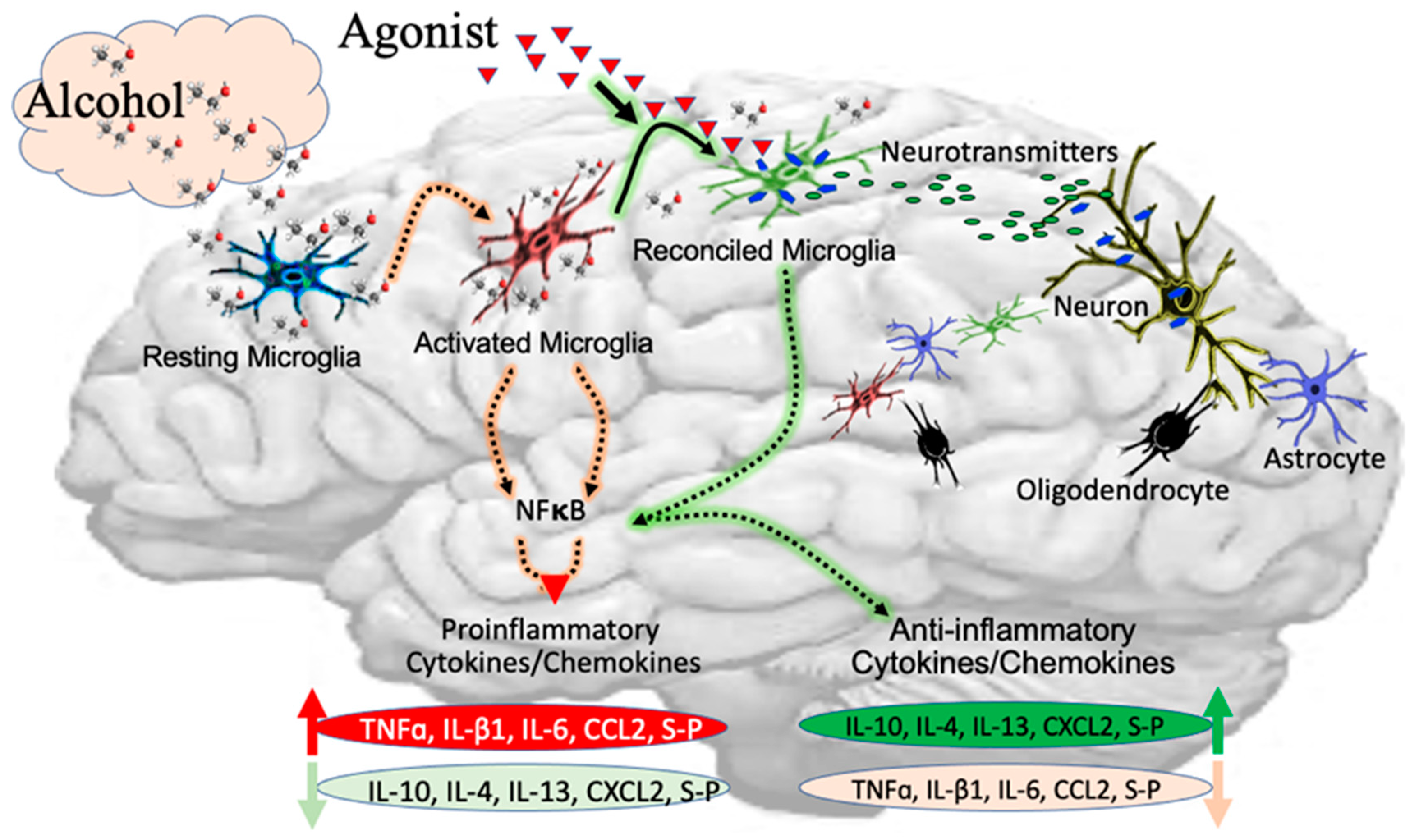
2.1. Alcohol-Induced Neurotoxicity in Microglia
2.2. Proinflammatory Mediators in Activation of Microglia
2.3. Expressions and Activation of Receptors in Microglia
2.4. Effect of Alcohol on Phenotypic Change in Microglia
2.5. Impact of Alcohol on Neurotransmitters in the First Brain (CNS)
2.6. Influence of Alcohol on Neurotrophic Factors in the First Brain
3. Alcohol-Induced Inflammation in the Second Brain (Gut)
3.1. Influence of Alcohol on Neurotransmitters in the Second Brain
3.2. Contribution of Enteric Glial Cells in Alcohol-Induced Gut Inflammation
3.3. Influence of Alcohol on Neurotrophic Factors in the Second Brain
3.4. Role of Microbiota in Brain–Gut Axis
4. Alcohol-Induced Modulation of Brain–Liver–Gut Axis
5. Effects of Alcohol on Innate and Adaptive Immune Responses
6. Synopsis and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costardi, J.V.; Nampo, R.A.; Silva, G.L.; Ribeiro, M.A.; Stella, H.J.; Stella, M.B.; Malheiros, S.V. A review on alcohol: From the central action mechanism to chemical dependency. Rev. Assoc. Med. Bras. 2015, 61, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Wilms, N.; Seitz, N.N.; Schwarzkopf, L.; Olderbak, S.; Kraus, L. Alcoholic Beverage Preference in Germany: An Age-Period-Cohort Analysis of Trends 1995–2018. Alcohol Alcohol. 2023, 58, 426–435. [Google Scholar] [CrossRef]
- Trius-Soler, M.; Pratico, G.; Gurdeniz, G.; Garcia-Aloy, M.; Canali, R.; Fausta, N.; Brouwer-Brolsma, E.M.; Andres-Lacueva, C.; Dragsted, L.O. Biomarkers of moderate alcohol intake and alcoholic beverages: A systematic literature review. Genes. Nutr. 2023, 18, 7. [Google Scholar] [CrossRef]
- Jaramillo, A.A.; Agan, V.E.; Makhijani, V.H.; Pedroza, S.; McElligott, Z.A.; Besheer, J. Functional role for suppression of the insular-striatal circuit in modulating interoceptive effects of alcohol. Addict. Biol. 2018, 23, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.A.; Randall, P.A.; Frisbee, S.; Besheer, J. Modulation of sensitivity to alcohol by cortical and thalamic brain regions. Eur. J. Neurosci. 2016, 44, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.A.; Van Voorhies, K.; Randall, P.A.; Besheer, J. Silencing the insular-striatal circuit decreases alcohol self-administration and increases sensitivity to alcohol. Behav. Brain Res. 2018, 348, 74–81. [Google Scholar] [CrossRef] [PubMed]
- de Guglielmo, G.; Simpson, S.; Kimbrough, A.; Conlisk, D.; Baker, R.; Cantor, M.; Kallupi, M.; George, O. Voluntary and forced exposure to ethanol vapor produces similar escalation of alcohol drinking but differential recruitment of brain regions related to stress, habit, and reward in male rats. Neuropharmacology 2023, 222, 109309. [Google Scholar] [CrossRef]
- Goins, A.; Ramaswamy, V.; Dirr, E.; Dulany, K.; Irby, S.; Webb, A.; Allen, J. Development of poly (1,8 octanediol-co-citrate) and poly (acrylic acid) nanofibrous scaffolds for wound healing applications. Biomed. Mater. 2017, 13, 015002. [Google Scholar] [CrossRef]
- Van Skike, C.E.; Goodlett, C.; Matthews, D.B. Acute alcohol and cognition: Remembering what it causes us to forget. Alcohol 2019, 79, 105–125. [Google Scholar] [CrossRef]
- Holloway, K.N.; Pinson, M.R.; Douglas, J.C.; Rafferty, T.M.; Kane, C.J.M.; Miranda, R.C.; Drew, P.D. Cerebellar Transcriptomic Analysis in a Chronic plus Binge Mouse Model of Alcohol Use Disorder Demonstrates Ethanol-Induced Neuroinflammation and Altered Glial Gene Expression. Cells 2023, 12, 745. [Google Scholar] [CrossRef]
- Wolfe, M.; Menon, A.; Oto, M.; Fullerton, N.E.; Leach, J.P. Alcohol and the central nervous system. Pract. Neurol. 2023, 23, 273–285. [Google Scholar] [CrossRef]
- Wong, C.X.; Tu, S.J.; Marcus, G.M. Alcohol and Arrhythmias. JACC Clin. Electrophysiol. 2023, 9, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Roerecke, M. Alcohol’s Impact on the Cardiovascular System. Nutrients 2021, 13, 3419. [Google Scholar] [CrossRef]
- Bataller, R.; Arab, J.P.; Shah, V.H. Alcohol-Associated Hepatitis. N. Engl. J. Med. 2022, 387, 2436–2448. [Google Scholar] [CrossRef]
- Becker, U.; Timmermann, A.; Ekholm, O.; Gronbaek, M.; Drewes, A.M.; Novovic, S.; Nojgaard, C.; Olesen, S.S.; Tolstrup, J.S. Alcohol Drinking Patterns and Risk of Developing Acute and Chronic Pancreatitis. Alcohol Alcohol. 2023, 58, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Qamar, N.; Castano, D.; Patt, C.; Chu, T.; Cottrell, J.; Chang, S.L. Meta-analysis of alcohol induced gut dysbiosis and the resulting behavioral impact. Behav. Brain Res. 2019, 376, 112196. [Google Scholar] [CrossRef]
- Liu, X.; Vigorito, M.; Huang, W.; Khan, M.A.S.; Chang, S.L. The Impact of Alcohol-Induced Dysbiosis on Diseases and Disorders of the Central Nervous System. J. Neuroimmune Pharmacol. 2022, 17, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.W.; Suffoletto, B.; Zhang, T.; Chung, T.; Ozolcer, M.; Islam, M.R.; Dey, A.K. Leveraging Mobile Phone Sensors, Machine Learning, and Explainable Artificial Intelligence to Predict Imminent Same-Day Binge-drinking Events to Support Just-in-time Adaptive Interventions: Algorithm Development and Validation Study. JMIR Form. Res. 2023, 7, e39862. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Lueras, J.M.; Nagel, B.J. Effects of Binge Drinking on the Developing Brain. Alcohol Res. 2018, 39, 87–96. [Google Scholar]
- Fuchs, F.D.; Fuchs, S.C. The Effect of Alcohol on Blood Pressure and Hypertension. Curr. Hypertens. Rep. 2021, 23, 42. [Google Scholar] [CrossRef]
- Brito, E.; Gomes, E.; Fale, P.L.; Borges, C.; Pacheco, R.; Teixeira, V.; Machuqueiro, M.; Ascensao, L.; Serralheiro, M.L.M. Bioactivities of decoctions from Plectranthus species related to their traditional use on the treatment of digestive problems and alcohol intoxication. J. Ethnopharmacol. 2018, 220, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qin, Y.Y.; Chen, Q.; Jiang, H.; Chen, X.Z.; Xu, C.L.; Mao, P.J.; He, J.; Zhou, Y.H. Alcohol intake and risk of stroke: A dose-response meta-analysis of prospective studies. Int. J. Cardiol. 2014, 174, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Fuenzalida, C.; Dufeu, M.S.; Poniachik, J.; Roblero, J.P.; Valenzuela-Perez, L.; Beltran, C.J. Probiotics-Based Treatment as an Integral Approach for Alcohol Use Disorder in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 729950. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Chergui, K. Ethanol inhibits excitatory neurotransmission in the nucleus accumbens of adolescent mice through GABAA and GABAB receptors. Addict. Biol. 2013, 18, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.A.; Jerlhag, E. Alcohol: Mechanisms along the mesolimbic dopamine system. Prog. Brain Res. 2014, 211, 201–233. [Google Scholar]
- Carkaci-Salli, N.; Salli, U.; Kuntz-Melcavage, K.L.; Pennock, M.M.; Ozgen, H.; Tekin, I.; Freeman, W.M.; Vrana, K.E. TPH2 in the ventral tegmental area of the male rat brain. Brain Res. Bull. 2011, 84, 376–380. [Google Scholar] [CrossRef]
- Grace, A.A. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction 2000, 95 (Suppl. S2), S119–S128. [Google Scholar] [CrossRef]
- Buesa-Lorenzo, J.B.; Rojo-Bofill, L.M.R.; Plumed-Domingo, J.P.; Rubio-Granero, T.; Rojo-Moreno, L. Marchiafava-Bignami disease in a patient with schizophrenia and alcohol use disorder. Actas Esp. Psiquiatr. 2021, 49, 228–231. [Google Scholar]
- Peng, B.; Yang, Q.; Joshi, R.B.; Liu, Y.; Akbar, M.; Song, B.J.; Zhou, S.; Wang, X. Role of Alcohol Drinking in Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef]
- Hoshino, Y.; Ueno, Y.; Shimura, H.; Miyamoto, N.; Watanabe, M.; Hattori, N.; Urabe, T. Marchiafava-Bignami disease mimics motor neuron disease: Case report. BMC Neurol. 2013, 13, 208. [Google Scholar] [CrossRef]
- Noguchi, T.; Kakinuma, Y.; Arikawa, M.; Okazaki, K.; Hoshino, E.; Iiyama, T.; Kubo, T.; Kitaoka, H.; Doi, Y.; Sato, T. Donepezil can improve ischemic muscle atrophy by activating angiomyogenic properties of satellite cells. Circ. J. 2014, 78, 2317–2324. [Google Scholar] [CrossRef]
- Adermark, L.; Bowers, M.S. Disentangling the Role of Astrocytes in Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2016, 40, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Meschkat, M.; Ruhwedel, T.; Trevisiol, A.; Tzvetanova, I.D.; Battefeld, A.; Kusch, K.; Kole, M.H.P.; Strenzke, N.; Mobius, W.; et al. A role of oligodendrocytes in information processing. Nat. Commun. 2020, 11, 5497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Connaghan, K.P.; Wei, Y.; Yang, Z.; Li, M.D.; Chang, S.L. Involvement of the Hippocampus in Binge Ethanol-Induced Spleen Atrophy in Adolescent Rats. Alcohol. Clin. Exp. Res. 2016, 40, 1489–1500. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Kril, J.J. Human alcohol-related neuropathology. Acta Neuropathol. 2014, 127, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Hasegawa, H. Blood Vessels as a Key Mediator for Ethanol Toxicity: Implication for Neuronal Damage. Life 2022, 12, 1882. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.V.; Harris, R.A.; Pfefferbaum, A. Alcohol’s effects on brain and behavior. Alcohol Res. Health 2010, 33, 127–143. [Google Scholar] [PubMed]
- Carvalho, J.K.F.; Pereira-Rufino, L.D.S.; Panfilio, C.E.; Silva, R.D.A.; Cespedes, I.C. Effect of chronic alcohol intake on motor functions on the elderly. Neurosci. Lett. 2021, 745, 135630. [Google Scholar] [CrossRef]
- Vinader-Caerols, C.; Talk, A.; Montanes, A.; Duque, A.; Monleon, S. Differential Effects of Alcohol on Memory Performance in Adolescent Men and Women with a Binge Drinking History. Alcohol Alcohol. 2017, 52, 610–616. [Google Scholar] [CrossRef]
- Mitoma, H.; Manto, M.; Shaikh, A.G. Mechanisms of Ethanol-Induced Cerebellar Ataxia: Underpinnings of Neuronal Death in the Cerebellum. Int. J. Environ. Res. Public. Health 2021, 18, 8678. [Google Scholar] [CrossRef]
- Manto, M.; Perrotta, G. Toxic-induced cerebellar syndrome: From the fetal period to the elderly. Handb. Clin. Neurol. 2018, 155, 333–352. [Google Scholar]
- Kamal, H.; Tan, G.C.; Ibrahim, S.F.; Shaikh, M.F.; Mohamed, I.N.; Mohamed, R.M.P.; Hamid, A.A.; Ugusman, A.; Kumar, J. Alcohol Use Disorder, Neurodegeneration, Alzheimer’s and Parkinson’s Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity. Front. Cell Neurosci. 2020, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- White, B.A.; Ramos, G.P.; Kane, S. The Impact of Alcohol in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2022, 28, 466–473. [Google Scholar] [CrossRef]
- Harrison, N.L.; Skelly, M.J.; Grosserode, E.K.; Lowes, D.C.; Zeric, T.; Phister, S.; Salling, M.C. Effects of acute alcohol on excitability in the CNS. Neuropharmacology 2017, 122, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.; Barbosa-Luna, I.G.; Perez-Luna, J.M.; Cupo, A.; Oikawa, J. Effects of acute ethanol administration on methionine-enkephalin expression and release in regions of the rat brain. Neuropeptides 2010, 44, 413–420. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Friedman, B.A.; Dejanovic, B.; Sheng, M. Microglia in Brain Development, Homeostasis, and Neurodegeneration. Annu. Rev. Genet. 2019, 53, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, W.; Xue, T.; Zhang, D.; Lv, M.; Jiang, Y. Sphk1-induced autophagy in microglia promotes neuronal injury following cerebral ischaemia-reperfusion. Eur. J. Neurosci. 2022, 56, 4287–4303. [Google Scholar] [CrossRef]
- Portis, S.M.; Haass-Koffler, C.L. New Microglial Mechanisms Revealed in Alcohol Use Disorder: How Does That Translate? Biol. Psychiatry 2020, 88, 893–895. [Google Scholar] [CrossRef]
- Kelly, T.M.; Donovan, J.E.; Chung, T.; Bukstein, O.G.; Cornelius, J.R. Brief screens for detecting alcohol use disorder among 18–20 year old young adults in emergency departments: Comparing AUDIT-C, CRAFFT, RAPS4-QF, FAST, RUFT-Cut, and DSM-IV 2-Item Scale. Addict. Behav. 2009, 34, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; McClain, J.A.; Wooden, J.I.; Nixon, K. Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Front. Neuroanat. 2020, 14, 52. [Google Scholar] [CrossRef]
- Marshall, D.A.; Pham, T.; Faris, P.; Chen, G.; O’Donnell, S.; Barber, C.E.H.; LeClercq, S.; Katz, S.; Homik, J.; Patel, J.N.; et al. Determination of Rheumatoid Arthritis Incidence and Prevalence in Alberta Using Administrative Health Data. ACR Open Rheumatol. 2020, 2, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Calvo-Rodriguez, M.; Nunez, L.; Villalobos, C.; Urena, J.; Guerri, C. Toll-like receptors in neuroinflammation, neurodegeneration, and alcohol-induced brain damage. IUBMB Life 2021, 73, 900–915. [Google Scholar] [CrossRef] [PubMed]
- Cherry, J.D.; Meng, G.; Daley, S.; Xia, W.; Svirsky, S.; Alvarez, V.E.; Nicks, R.; Pothast, M.; Kelley, H.; Huber, B.; et al. CCL2 is associated with microglia and macrophage recruitment in chronic traumatic encephalopathy. J. Neuroinflamm. 2020, 17, 370. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Nixon, K. Microglia Phenotypes Following the Induction of Alcohol Dependence in Adolescent Rats. Alcohol. Clin. Exp. Res. 2020, 45, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Kim, S.S.; Suh, W.Y.; Seo, Y.R.; Lee, S.; Kim, H.G.; Kim, J.S.; Yoon, S.J.; Jung, J.G. Association of High-Sensitivity C-Reactive Protein and Alcohol Consumption on Metabolic Syndrome in Korean Men. Int. J. Environ. Res. Public. Health 2022, 19, 2571. [Google Scholar] [CrossRef]
- Albert, M.A.; Glynn, R.J.; Buring, J.; Ridker, P.M. C-reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study). Am. J. Cardiol. 2004, 93, 1238–1242. [Google Scholar] [CrossRef]
- Albert, M.A.; Glynn, R.J.; Ridker, P.M. Alcohol consumption and plasma concentration of C-reactive protein. Circulation 2003, 107, 443–447. [Google Scholar] [CrossRef]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, J. Role of MCP-1 and CCR2 in alcohol neurotoxicity. Pharmacol. Res. 2019, 139, 360–366. [Google Scholar] [CrossRef]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef]
- Pan, S.D.; Grandgirard, D.; Leib, S.L. Adjuvant Cannabinoid Receptor Type 2 Agonist Modulates the Polarization of Microglia towards a Non-Inflammatory Phenotype in Experimental Pneumococcal Meningitis. Front. Cell Infect. Microbiol. 2020, 10, 588195. [Google Scholar] [CrossRef]
- Nixon, K.; McClain, J.A. Adolescence as a critical window for developing an alcohol use disorder: Current findings in neuroscience. Curr. Opin. Psychiatry 2010, 23, 227–232. [Google Scholar] [CrossRef]
- McClain, J.A.; Morris, S.A.; Deeny, M.A.; Marshall, S.A.; Hayes, D.M.; Kiser, Z.M.; Nixon, K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav. Immun. 2011, 25 (Suppl. S1), S120–S128. [Google Scholar] [CrossRef] [PubMed]
- Coleman, L.G., Jr.; Zou, J.; Crews, F.T. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J. Neuroinflamm. 2020, 17, 27. [Google Scholar] [CrossRef]
- Aubert, B.; Barate, R.; Boutigny, D.; Couderc, F.; Karyotakis, Y.; Lees, J.P.; Poireau, V.; Tisserand, V.; Zghiche, A.; Grauges, E.; et al. Measurements of the absolute branching fractions of B± → K±Xcc. Phys. Rev. Lett. 2006, 96, 052002. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Pascual, M.; Millan-Esteban, D.; Guerri, C. Binge-like ethanol treatment in adolescence impairs autophagy and hinders synaptic maturation: Role of TLR4. Neurosci. Lett. 2018, 682, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Alfonso-Loeches, S.; Guerri, C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol. Clin. Exp. Res. 2016, 40, 2260–2270. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Rodriguez-Arias, M.; Minarro, J.; Guerri, C. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav. Immun. 2016, 53, 159–171. [Google Scholar] [CrossRef]
- Montesinos, J.; Pascual, M.; Pla, A.; Maldonado, C.; Rodriguez-Arias, M.; Minarro, J.; Guerri, C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav. Immun. 2015, 45, 233–244. [Google Scholar] [CrossRef]
- Ibanez, F.; Montesinos, J.; Urena-Peralta, J.R.; Guerri, C.; Pascual, M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J. Neuroinflamm. 2019, 16, 136. [Google Scholar] [CrossRef]
- Pascual-Lucas, M.; Fernandez-Lizarbe, S.; Montesinos, J.; Guerri, C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J. Neurochem. 2014, 129, 448–462. [Google Scholar] [CrossRef]
- Goodwani, S.; Saternos, H.; Alasmari, F.; Sari, Y. Metabotropic and ionotropic glutamate receptors as potential targets for the treatment of alcohol use disorder. Neurosci. Biobehav. Rev. 2017, 77, 14–31. [Google Scholar] [CrossRef]
- Smart, K.; Zheng, M.Q.; Ahmed, H.; Fang, H.; Xu, Y.; Cai, L.; Holden, D.; Kapinos, M.; Haider, A.; Felchner, Z.; et al. Comparison of three novel radiotracers for GluN2B-containing NMDA receptors in non-human primates: (R)-[11C]NR2B-Me, (R)-[18F]of-Me-NB1, and (S)-[18F]of-NB1. J. Cereb. Blood Flow. Metab. 2022, 42, 1398–1409. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Angarita, G.A.; Esterlis, I.; Anderson, J.M.; Nabulsi, N.; Lim, K.; Ropchan, J.; Carson, R.E.; Krystal, J.H.; Malley, S.S.O.; et al. Longitudinal imaging of metabotropic glutamate 5 receptors during early and extended alcohol abstinence. Neuropsychopharmacology 2021, 46, 380–385. [Google Scholar] [CrossRef]
- Rodriguez, L.; Yi, C.; Chu, C.; Duriez, Q.; Watanabe, S.; Ryu, M.; Reyes, B.; Asatryan, L.; Boue-Grabot, E.; Davies, D. Cross-Talk between P2X and NMDA Receptors. Int. J. Mol. Sci. 2020, 21, 7187. [Google Scholar] [CrossRef]
- Asatryan, L.; Ostrovskaya, O.; Lieu, D.; Davies, D.L. Ethanol differentially modulates P2X4 and P2X7 receptor activity and function in BV2 microglial cells. Neuropharmacology 2018, 128, 11–21. [Google Scholar] [CrossRef]
- Suurvali, J.; Boudinot, P.; Kanellopoulos, J.; Ruutel Boudinot, S. P2X4: A fast and sensitive purinergic receptor. Biomed. J. 2017, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gofman, L.; Cenna, J.M.; Potula, R. P2X4 receptor regulates alcohol-induced responses in microglia. J. Neuroimmune Pharmacol. 2014, 9, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine modulates the motility of resting and activated microglia via different adrenergic receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef] [PubMed]
- Gyoneva, S.; Davalos, D.; Biswas, D.; Swanger, S.A.; Garnier-Amblard, E.; Loth, F.; Akassoglou, K.; Traynelis, S.F. Systemic inflammation regulates microglial responses to tissue damage in vivo. Glia 2014, 62, 1345–1360. [Google Scholar] [CrossRef]
- Maduna, T.; Audouard, E.; Dembele, D.; Mouzaoui, N.; Reiss, D.; Massotte, D.; Gaveriaux-Ruff, C. Microglia Express Mu Opioid Receptor: Insights From Transcriptomics and Fluorescent Reporter Mice. Front. Psychiatry 2018, 9, 726. [Google Scholar] [CrossRef]
- Reiss, D.; Ceredig, R.A.; Secher, T.; Boue, J.; Barreau, F.; Dietrich, G.; Gaveriaux-Ruff, C. Mu and delta opioid receptor knockout mice show increased colonic sensitivity. Eur. J. Pain. 2017, 21, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Reiss, D.; Maduna, T.; Maurin, H.; Audouard, E.; Gaveriaux-Ruff, C. Mu opioid receptor in microglia contributes to morphine analgesic tolerance, hyperalgesia, and withdrawal in mice. J. Neurosci. Res. 2022, 100, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Leon, R.; Lopez, M.G. Anti-inflammatory role of microglial alpha7 nAChRs and its role in neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Parada, E.; Egea, J.; Buendia, I.; Negredo, P.; Cunha, A.C.; Cardoso, S.; Soares, M.P.; Lopez, M.G. The microglial alpha7-acetylcholine nicotinic receptor is a key element in promoting neuroprotection by inducing heme oxygenase-1 via nuclear factor erythroid-2-related factor 2. Antioxid. Redox Signal 2013, 19, 1135–1148. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.; Stevens, B. Microglia: The Brain’s First Responders. Cerebrum 2017, 2017, cer-14-17. [Google Scholar] [PubMed]
- Catalin, B.; Cupido, A.; Iancau, M.; Albu, C.V.; Kirchhoff, F. Microglia: First responders in the central nervous system. Rom. J. Morphol. Embryol. 2013, 54, 467–472. [Google Scholar] [PubMed]
- Wendimu, M.Y.; Hooks, S.B. Microglia Phenotypes in Aging and Neurodegenerative Diseases. Cells 2022, 11, 2091. [Google Scholar] [CrossRef]
- Marshall, S.A.; McClain, J.A.; Kelso, M.L.; Hopkins, D.M.; Pauly, J.R.; Nixon, K. Microglial activation is not equivalent to neuroinflammation in alcohol-induced neurodegeneration: The importance of microglia phenotype. Neurobiol. Dis. 2013, 54, 239–251. [Google Scholar] [CrossRef]
- Peng, H.; Geil Nickell, C.R.; Chen, K.Y.; McClain, J.A.; Nixon, K. Increased expression of M1 and M2 phenotypic markers in isolated microglia after four-day binge alcohol exposure in male rats. Alcohol 2017, 62, 29–40. [Google Scholar] [CrossRef]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Cho, Y.; Ambade, A.; Szabo, G. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J. Neuroinflamm. 2018, 15, 298. [Google Scholar] [CrossRef]
- Cruz, C.; Meireles, M.; Silva, S.M. Chronic ethanol intake induces partial microglial activation that is not reversed by long-term ethanol withdrawal in the rat hippocampal formation. Neurotoxicology 2017, 60, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kucic, N.; Racki, V.; Sverko, R.; Vidovic, T.; Grahovac, I.; Mrsic-Pelcic, J. Immunometabolic Modulatory Role of Naltrexone in BV-2 Microglia Cells. Int. J. Mol. Sci. 2021, 22, 8429. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, K.B.; Mangieri, R.A.; Roberts, A.J.; Lopez, M.F.; Firsick, E.J.; Townsley, K.G.; Beneze, A.; Bess, J.; Eisenstein, T.K.; Meissler, J.J.; et al. Preclinical and clinical evidence for suppression of alcohol intake by apremilast. J. Clin. Investig. 2023, 133, e159103. [Google Scholar] [CrossRef]
- Meredith, L.R.; Burnette, E.M.; Grodin, E.N.; Irwin, M.R.; Ray, L.A. Immune treatments for alcohol use disorder: A translational framework. Brain Behav. Immun. 2021, 97, 349–364. [Google Scholar] [CrossRef]
- Kalinin, S.; Gonzalez-Prieto, M.; Scheiblich, H.; Lisi, L.; Kusumo, H.; Heneka, M.T.; Madrigal, J.L.M.; Pandey, S.C.; Feinstein, D.L. Transcriptome analysis of alcohol-treated microglia reveals downregulation of beta amyloid phagocytosis. J. Neuroinflamm. 2018, 15, 141. [Google Scholar] [CrossRef]
- Amtul, Z.; Randhawa, J.; Najdat, A.N.; Hill, D.J.; Arany, E.J. Role of Delayed Neuroglial Activation in Impaired Cerebral Blood Flow Restoration Following Comorbid Injury. Cell Mol. Neurobiol. 2020, 40, 369–380. [Google Scholar] [CrossRef]
- Rangaraju, S.; Raza, S.A.; Li, N.X.; Betarbet, R.; Dammer, E.B.; Duong, D.; Lah, J.J.; Seyfried, N.T.; Levey, A.I. Differential Phagocytic Properties of CD45low Microglia and CD45 high Brain Mononuclear Phagocytes—Activation and Age-Related Effects. Front. Immunol. 2018, 9, 405. [Google Scholar] [CrossRef]
- Beck, A.; Wustenberg, T.; Genauck, A.; Wrase, J.; Schlagenhauf, F.; Smolka, M.N.; Mann, K.; Heinz, A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch. Gen. Psychiatry 2012, 69, 842–852. [Google Scholar] [CrossRef]
- Molina-Martinez, L.M.; Juarez, J. Differential expression of mu-opioid receptors in the nucleus accumbens, amygdala and VTA depends on liking for alcohol, chronic alcohol intake and estradiol treatment. Behav. Brain Res. 2020, 378, 112255. [Google Scholar] [CrossRef]
- Ciafre, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Ceccanti, M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2021, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.M.; Francati, S.; Ferraguti, G.; Coriale, G.; Ciccarelli, R.; Minni, A.; Greco, A.; Musacchio, A.; De Persis, S.; Vitali, M.; et al. Behavioral dysregulations by chronic alcohol abuse. Motivational enhancement therapy and cognitive behavioral therapy outcomes. Riv. Psichiatr. 2022, 57, 1–9. [Google Scholar]
- Pierce, R.C.; Kumaresan, V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev. 2006, 30, 215–238. [Google Scholar] [CrossRef]
- Kamens, H.M.; Andersen, J.; Picciotto, M.R. Modulation of ethanol consumption by genetic and pharmacological manipulation of nicotinic acetylcholine receptors in mice. Psychopharmacology 2010, 208, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Chen, A.L.; Braverman, E.R.; Comings, D.E.; Chen, T.J.; Arcuri, V.; Blum, S.H.; Downs, B.W.; Waite, R.L.; Notaro, A.; et al. Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr. Dis. Treat. 2008, 4, 893–918. [Google Scholar] [PubMed]
- Schulteis, G.; Koob, G.F. Reinforcement processes in opiate addiction: A homeostatic model. Neurochem. Res. 1996, 21, 1437–1454. [Google Scholar] [CrossRef]
- Avegno, E.M.; Kasten, C.R.; Snyder, W.B., 3rd; Kelley, L.K.; Lobell, T.D.; Templeton, T.J.; Constans, M.; Wills, T.A.; Middleton, J.W.; Gilpin, N.W. Alcohol dependence activates ventral tegmental area projections to central amygdala in male mice and rats. Addict. Biol. 2021, 26, e12990. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Koob, G.F. Neurobiology of alcohol dependence: Focus on motivational mechanisms. Alcohol Res. Health 2008, 31, 185–195. [Google Scholar]
- Cacialli, P.; Palladino, A.; Lucini, C. Role of brain-derived neurotrophic factor during the regenerative response after traumatic brain injury in adult zebrafish. Neural Regen. Res. 2018, 13, 941–944. [Google Scholar]
- Heberlein, A.; Muschler, M.; Wilhelm, J.; Frieling, H.; Lenz, B.; Groschl, M.; Kornhuber, J.; Bleich, S.; Hillemacher, T. BDNF and GDNF serum levels in alcohol-dependent patients during withdrawal. Prog. Neuropsychopharmacol. Biol. Psychiatry 2010, 34, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, A.; Dursteler-MacFarland, K.M.; Lenz, B.; Frieling, H.; Grosch, M.; Bonsch, D.; Kornhuber, J.; Wiesbeck, G.A.; Bleich, S.; Hillemacher, T. Serum levels of BDNF are associated with craving in opiate-dependent patients. J. Psychopharmacol. 2011, 25, 1480–1484. [Google Scholar] [CrossRef] [PubMed]
- Peregud, D.I.; Panchenko, L.F.; Gulyaeva, N.V. Elevation of BDNF exon I-specific transcripts in the frontal cortex and midbrain of rat during spontaneous morphine withdrawal is accompanied by enhanced pCreb1 occupancy at the corresponding promoter. Neurochem. Res. 2015, 40, 130–138. [Google Scholar] [CrossRef]
- Peregud, D.I.; Yakovlev, A.A.; Stepanichev, M.Y.; Onufriev, M.V.; Panchenko, L.F.; Gulyaeva, N.V. Expression of BDNF and TrkB Phosphorylation in the Rat Frontal Cortex during Morphine Withdrawal are NO Dependent. Cell Mol. Neurobiol. 2016, 36, 839–849. [Google Scholar] [CrossRef]
- Peregud, D.I.; Baronets, V.Y.; Terebilina, N.N.; Gulyaeva, N.V. Role of BDNF in Neuroplasticity Associated with Alcohol Dependence. Biochemistry 2023, 88, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Slonimsky, J.D.; Yang, B.; Hinterneder, J.M.; Nokes, E.B.; Birren, S.J. BDNF and CNTF regulate cholinergic properties of sympathetic neurons through independent mechanisms. Mol. Cell Neurosci. 2003, 23, 648–660. [Google Scholar] [CrossRef]
- Yang, B.; Slonimsky, J.D.; Birren, S.J. A rapid switch in sympathetic neurotransmitter release properties mediated by the p75 receptor. Nat. Neurosci. 2002, 5, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Bujanda, L. The effects of alcohol consumption upon the gastrointestinal tract. Am. J. Gastroenterol. 2000, 95, 3374–3382. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, H.; Huo, J.R. Alcohol consumption and corresponding factors: A novel perspective on the risk factors of esophageal cancer. Oncol. Lett. 2016, 11, 3231–3239. [Google Scholar] [CrossRef]
- Lou, Z.; Xing, H.; Li, D. Alcohol consumption and the neoplastic progression in Barrett’s esophagus: A systematic review and meta-analysis. PLoS ONE 2014, 9, e105612. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Alcohol or Gut Microbiota: Who Is the Guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Wang, S.C.; Chen, Y.C.; Chen, S.J.; Lee, C.H.; Cheng, C.M. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.Y.; Zhang, J.; Gobejishvili, L.; Barve, S.S.; McClain, C.J.; Joshi-Barve, S. Elevated Fructose and Uric Acid through Aldose Reductase Contribute to Experimental and Human Alcoholic Liver Disease. Hepatology 2020, 72, 1617–1637. [Google Scholar] [CrossRef] [PubMed]
- Ciocan, D.; Spatz, M.; Trainel, N.; Hardonniere, K.; Domenichini, S.; Mercier-Nome, F.; Desmons, A.; Humbert, L.; Durand, S.; Kroemer, G.; et al. Modulation of the Bile Acid Enterohepatic Cycle by Intestinal Microbiota Alleviates Alcohol Liver Disease. Cells 2022, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Spatz, M.; Ciocan, D.; Merlen, G.; Rainteau, D.; Humbert, L.; Gomes-Rochette, N.; Hugot, C.; Trainel, N.; Mercier-Nome, F.; Domenichini, S.; et al. Bile acid-receptor TGR5 deficiency worsens liver injury in alcohol-fed mice by inducing intestinal microbiota dysbiosis. JHEP Rep. 2021, 3, 100230. [Google Scholar] [CrossRef] [PubMed]
- Christovich, A.; Luo, X.M. Gut Microbiota, Leaky Gut, and Autoimmune Diseases. Front. Immunol. 2022, 13, 946248. [Google Scholar] [CrossRef]
- Maccioni, L.; Gao, B.; Leclercq, S.; Pirlot, B.; Horsmans, Y.; De Timary, P.; Leclercq, I.; Fouts, D.; Schnabl, B.; Starkel, P. Intestinal permeability, microbial translocation, changes in duodenal and fecal microbiota, and their associations with alcoholic liver disease progression in humans. Gut Microbes 2020, 12, 1782157. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Warner, J.B.; Song, Y.L.; Rouchka, E.C.; McClain, C.J.; Warner, D.R.; Kirpich, I.A. Ileum Gene Expression in Response to Acute Systemic Inflammation in Mice Chronically Fed Ethanol: Beneficial Effects of Elevated Tissue n-3 PUFAs. Int. J. Mol. Sci. 2021, 22, 1582. [Google Scholar] [CrossRef]
- Zafari, N.; Velayati, M.; Fahim, M.; Maftouh, M.; Pourali, G.; Khazaei, M.; Nassiri, M.; Hassanian, S.M.; Ghayour-Mobarhan, M.; Ferns, G.A.; et al. Role of gut bacterial and non-bacterial microbiota in alcohol-associated liver disease: Molecular mechanisms, biomarkers, and therapeutic prospective. Life Sci. 2022, 305, 120760. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Fox, B.W.; Chao, P.H.; Schroeder, F.C.; Sengupta, P. A neurotransmitter produced by gut bacteria modulates host sensory behaviour. Nature 2020, 583, 415–420. [Google Scholar] [CrossRef]
- Verdu, E.F.; Bercik, P.; Bergonzelli, G.E.; Huang, X.X.; Blennerhasset, P.; Rochat, F.; Fiaux, M.; Mansourian, R.; Corthesy-Theulaz, I.; Collins, S.M. Lactobacillus paracasei normalizes muscle hypercontractility in a murine model of postinfective gut dysfunction. Gastroenterology 2004, 127, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and their functions in the gastrointestinal tract. Cell Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Leven, P.; Glowka, T.; Kuzmanov, I.; Lysson, M.; Schneiker, B.; Miesen, A.; Baqi, Y.; Spanier, C.; Grants, I.; et al. A novel P2X2-dependent purinergic mechanism of enteric gliosis in intestinal inflammation. EMBO Mol. Med. 2021, 13, e12724. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Young, H.M.; Bergner, A.J.; Muller, T. Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J. Comp. Neurol. 2003, 456, 1–11. [Google Scholar] [CrossRef]
- Heanue, T.A.; Pachnis, V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells 2011, 29, 128–140. [Google Scholar] [CrossRef]
- Belkind-Gerson, J.; Graham, H.K.; Reynolds, J.; Hotta, R.; Nagy, N.; Cheng, L.; Kamionek, M.; Shi, H.N.; Aherne, C.M.; Goldstein, A.M. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 2017, 7, 2525. [Google Scholar] [CrossRef]
- Ferri, G.L.; Probert, L.; Cocchia, D.; Michetti, F.; Marangos, P.J.; Polak, J.M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature 1982, 297, 409–410. [Google Scholar] [CrossRef]
- Yu, Y.B.; Li, Y.Q. Enteric glial cells and their role in the intestinal epithelial barrier. World J. Gastroenterol. 2014, 20, 11273–11280. [Google Scholar] [CrossRef] [PubMed]
- Konturek, T.J.; Martinez, C.; Niesler, B.; van der Voort, I.; Monnikes, H.; Stengel, A.; Goebel-Stengel, M. The Role of Brain-Derived Neurotrophic Factor in Irritable Bowel Syndrome. Front. Psychiatry 2020, 11, 531385. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Flemming, S.; Burkard, N.; Bergauer, L.; Metzger, M.; Germer, C.T.; Schlegel, N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G613–G624. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Flemming, S.; Burkard, N.; Wagner, J.; Germer, C.T.; Schlegel, N. The glial cell-line derived neurotrophic factor: A novel regulator of intestinal barrier function in health and disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G1118–G1123. [Google Scholar] [CrossRef]
- Meir, M.; Burkard, N.; Ungewiss, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef]
- Legay, C.; Saffrey, M.J.; Burnstock, G. Coexistence of immunoreactive substance P and serotonin in neurones of the gut. Brain Res. 1984, 302, 379–382. [Google Scholar] [CrossRef]
- Hellmich, H.L.; Kos, L.; Cho, E.S.; Mahon, K.A.; Zimmer, A. Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial-mesenchymal interactions. Mech. Dev. 1996, 54, 95–105. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Brewer, K.C.; Doyle, A.M.; Nagy, N.; Roberts, D.J. BMP signaling is necessary for neural crest cell migration and ganglion formation in the enteric nervous system. Mech. Dev. 2005, 122, 821–833. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, C.; Dong, X.; Hu, T.; Wang, L.; Zhao, W.; Zhu, S.; Li, G.; Hu, Y.; Gao, Q.; et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors 2019, 45, 187–199. [Google Scholar] [CrossRef]
- Benomar, Y.; Berthou, F.; Vacher, C.M.; Bailleux, V.; Gertler, A.; Djiane, J.; Taouis, M. Leptin but not ciliary neurotrophic factor (CNTF) induces phosphotyrosine phosphatase-1B expression in human neuronal cells (SH-SY5Y): Putative explanation of CNTF efficacy in leptin-resistant state. Endocrinology 2009, 150, 1182–1191. [Google Scholar] [CrossRef][Green Version]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus Nerve Stimulation at the Interface of Brain-Gut Interactions. Cold Spring Harb. Perspect. Med. 2019, 9, a034199. [Google Scholar] [CrossRef]
- Link, C.D. Is There a Brain Microbiome? Neurosci. Insights 2021, 16, 26331055211018709. [Google Scholar] [CrossRef]
- Lynch, J.B.; Hsiao, E.Y. Toward understanding links between the microbiome and neurotransmitters. Ann. N. Y. Acad. Sci. 2023, 1524, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, T.; Kusumanchi, P.; Han, S.; Yang, Z.; Liangpunsakul, S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines 2020, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- García-Suástegui, W.A.; Ramos-Chávez, L.A.; Rubio-Osornio, M.; Calvillo-Velasco, M.; Atzin-Méndez, J.A.; Guevara, J.; Silva-Adaya, D. The Role of CYP2E1 in the Drug Metabolism or Bioactivation in the Brain. Oxid. Med. Cell Longev. 2017, 2017, 4680732. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.K.; Meena, A.S.; Dalal, K.; Canelas, C.; Samak, G.; Pierre, J.F.; Rao, R. Chronic stress and corticosterone exacerbate alcohol-induced tissue injury in the gut-liver-brain axis. Sci. Rep. 2021, 11, 826. [Google Scholar] [CrossRef]
- Fernandez-Checa, J.C. Alcohol-induced liver disease: When fat and oxidative stress meet. Ann. Hepatol. 2003, 2, 69–75. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef]
- Lee, J.S.; O’Connell, E.M.; Pacher, P.; Lohoff, F.W. PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. J. Clin. Med. 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Rosoff, D.; Luo, A.; Longley, M.; Phillips, M.; Charlet, K.; Muench, C.; Jung, J.; Lohoff, F.W. PCSK9 is Increased in Cerebrospinal Fluid of Individuals with Alcohol Use Disorder. Alcohol. Clin. Exp. Res. 2019, 43, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.L.; Roodsari, S.K.; Cheng, Y.; Dempsey, R.E.; Hu, W. Microglia NLRP3 Inflammasome and Neuroimmune Signaling in Substance Use Disorders. Biomolecules 2023, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Rungratanawanich, W.; Lin, Y.; Wang, X.; Kawamoto, T.; Chidambaram, S.B.; Song, B.J. ALDH2 deficiency increases susceptibility to binge alcohol-induced gut leakiness, endotoxemia, and acute liver injury in mice through the gut-liver axis. Redox Biol. 2023, 59, 102577. [Google Scholar] [CrossRef]
- Meena, A.S.; Shukla, P.K.; Bell, B.; Giorgianni, F.; Caires, R.; Fernández-Peña, C.; Beranova, S.; Aihara, E.; Montrose, M.H.; Chaib, M.; et al. TRPV6 channel mediates alcohol-induced gut barrier dysfunction and systemic response. Cell Rep. 2022, 39, 110937. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.S.; Chang, S.L. Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation. Cells 2023, 12, 2475. https://doi.org/10.3390/cells12202475
Khan MAS, Chang SL. Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation. Cells. 2023; 12(20):2475. https://doi.org/10.3390/cells12202475
Chicago/Turabian StyleKhan, Mohammed A. S., and Sulie L. Chang. 2023. "Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation" Cells 12, no. 20: 2475. https://doi.org/10.3390/cells12202475
APA StyleKhan, M. A. S., & Chang, S. L. (2023). Alcohol and the Brain–Gut Axis: The Involvement of Microglia and Enteric Glia in the Process of Neuro-Enteric Inflammation. Cells, 12(20), 2475. https://doi.org/10.3390/cells12202475





