Effect of Proton Therapy on Tumor Cell Killing and Immune Microenvironment for Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
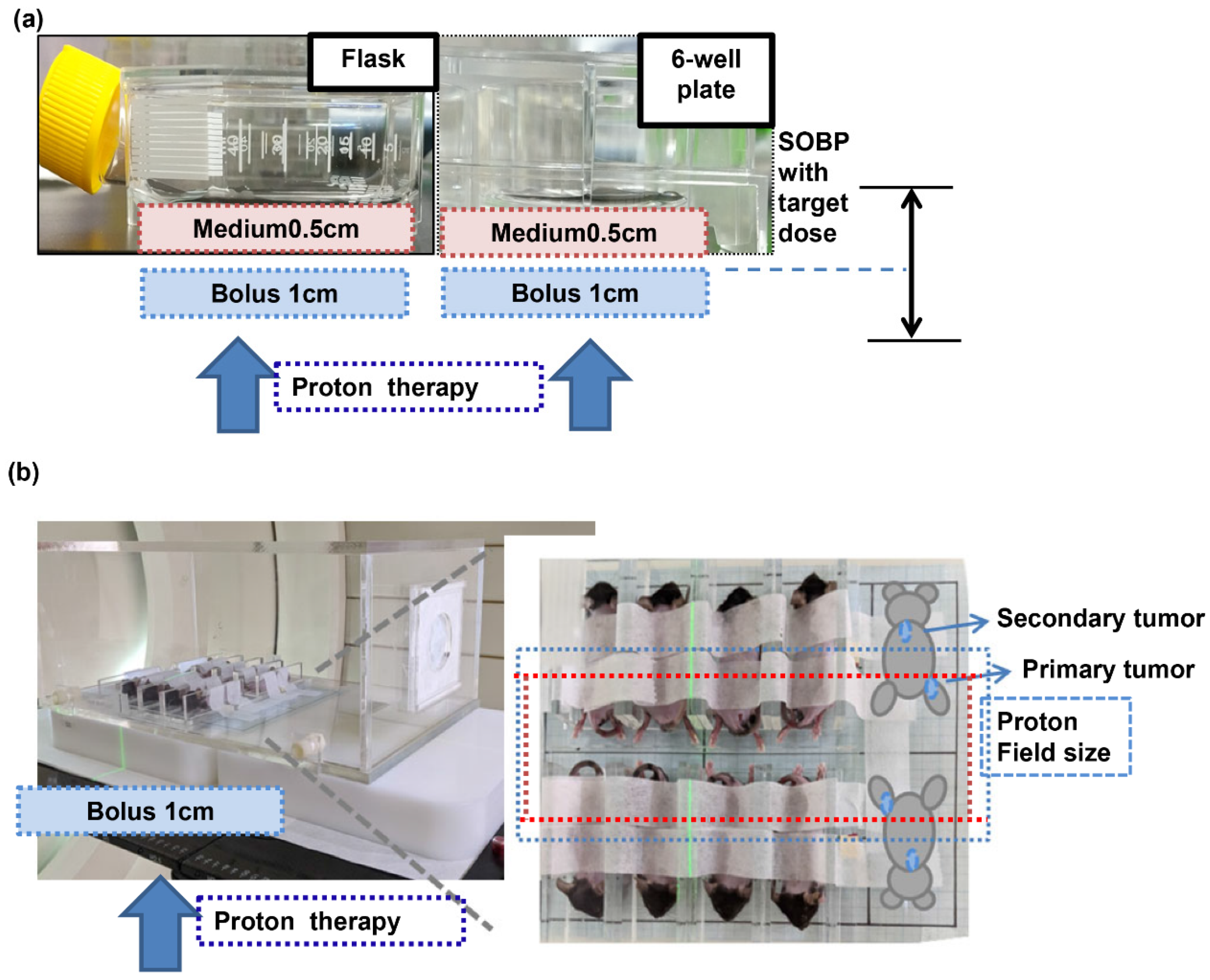
2.2. Irradiation
2.3. T-Cell Proliferation Assay
2.4. Induction of CD14+HLA-DR—From Peripheral Blood Mononuclear Cells (PBMCs)
2.5. Clonogenic Assay
2.6. Syngeneic (Ectopic and Orthotopic) Tumor Models
2.7. MDSC Flow Cytometric Analyses In Vivo
2.8. Statistical Analysis
3. Results:
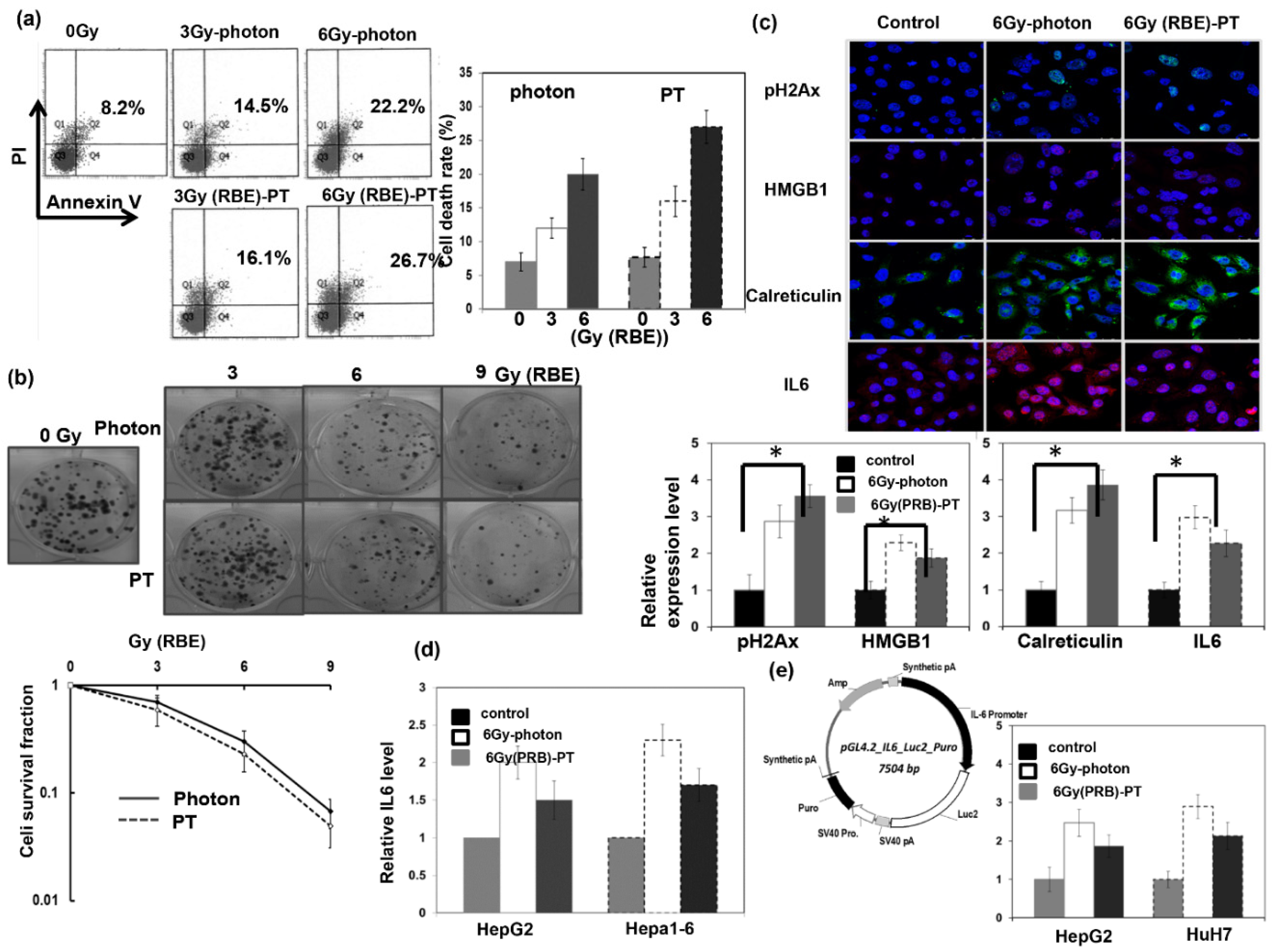
3.1. Response of HCC to PT Response of HCC
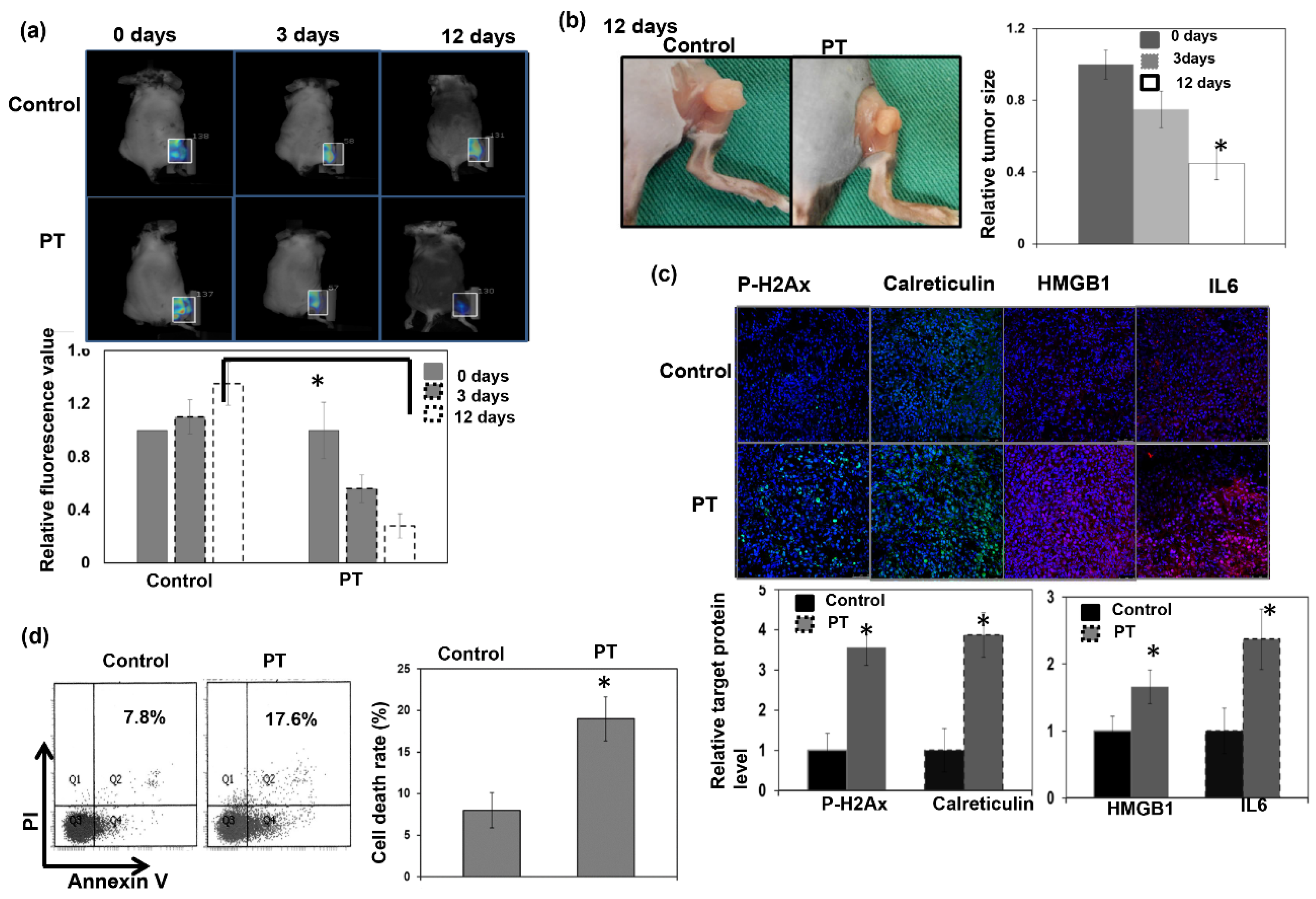
3.2. Response to PT Treatment in the Immunocompetent Host
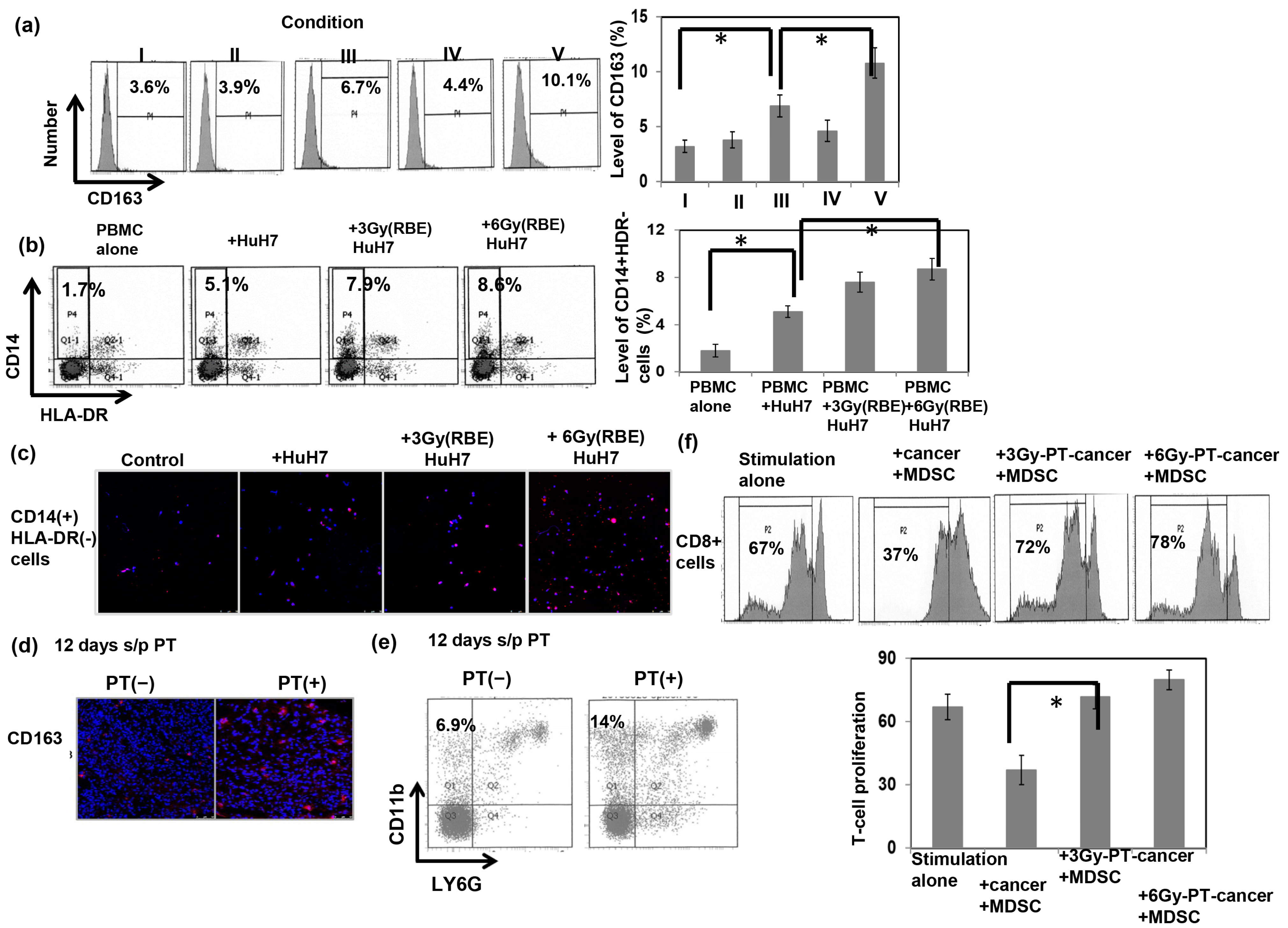
3.3. The Immunomodulatory Effects Induced by PT
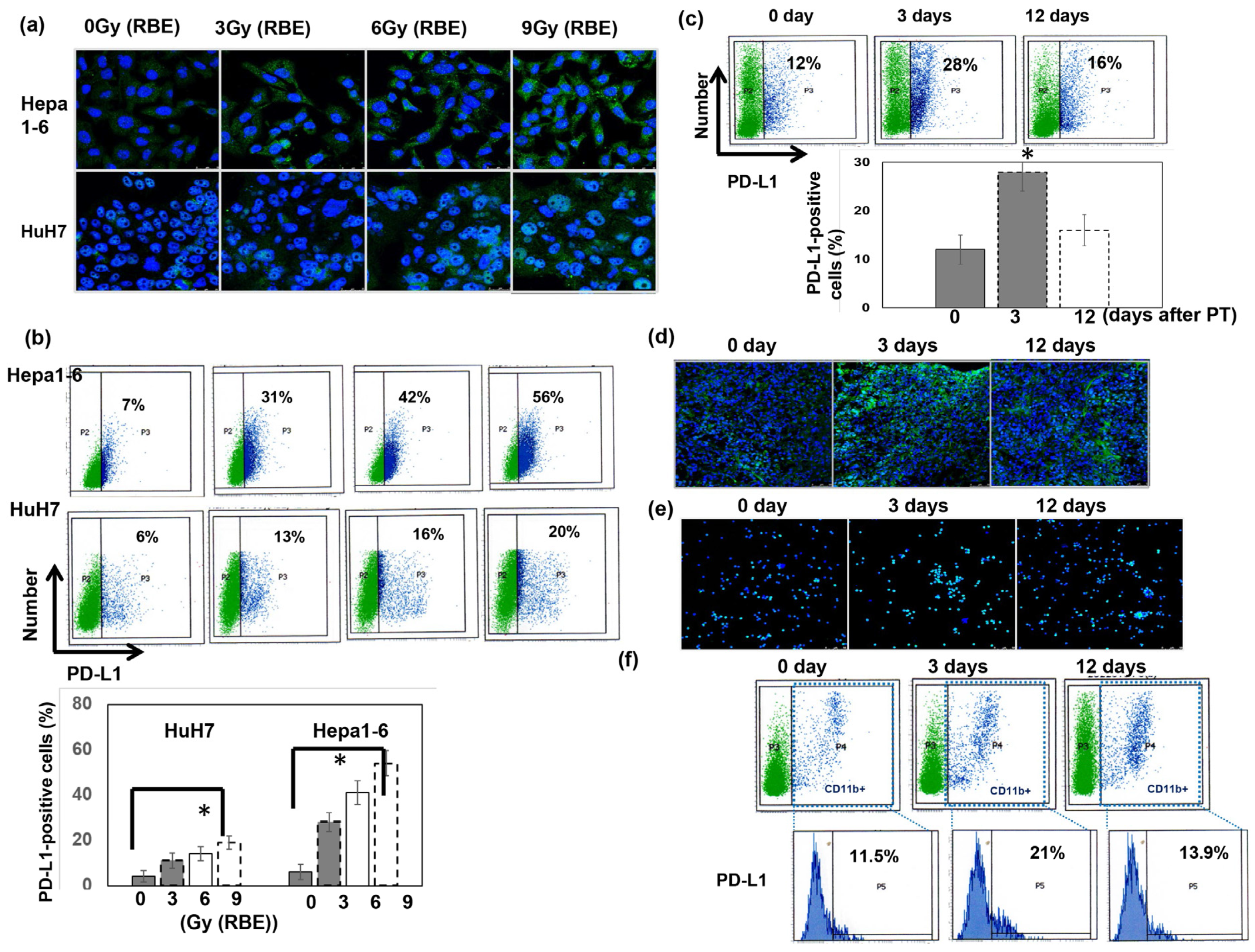
3.4. Role of PT in the Expression of Programmed Cell Death Ligand 1 (PD-L1)
3.5. Effect of PD-L1 on the Response of HCC to PT In Vivo
3.6. Blockade of PD-L1 Enhances the Abscopal Effect on Liver Cancer following PT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCC | hepatocellular carcinoma |
| RT | radiation therapy |
| PT | proton therapy |
| FACS | fluorescence-activated cell analysis |
| RBE | relative biological effectiveness dose |
| PBS | pencil beam scanning |
| SOBP | spread-out Bragg peak |
| CFSE | carboxyfluorescein succinimidyl ester |
| DMEM | Dulbecco’s Modified Eagle Medium (DMEM) |
| HMGB1 | high mobility group Box 1 |
| FMT | fluorescence molecular tomography (FMT) |
| PD-L1 | programmed cell death ligand 1 |
| MDSCs | myeloid-derived suppressor cells |
| TIL | tumor infiltrating lymphocytes |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.-L.; Torre, L.-A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chuong, M.; Kaiser, A.; Molitoris, J.; Mendez Romero, A.; Apisarnthanarax, S. Proton beam therapy for liver cancers. J. Gastrointest. Oncol. 2020, 11, 157–165. [Google Scholar] [CrossRef]
- Yoo, G.-S.; Yu, J.-I.; Park, H.-C. Proton therapy for hepatocellular carcinoma: Current knowledges and future perspectives. World J. Gastroenterol. 2018, 24, 3090–3100. [Google Scholar] [CrossRef]
- Yuan, T.-Z.; Zhan, Z.-J.; Qian, C.-N. New frontiers in proton therapy: Applications in cancers. Cancer Commun. 2019, 39, 61. [Google Scholar] [CrossRef] [PubMed]
- Deycmar, S.; Faccin, E.; Kazimova, T.; Knobel, P.-A.; Telarovic, I.; Tschanz, F.; Waller, V.; Winkler, R.; Yong, C.; Zingariello, D. The relative biological effectiveness of proton irradiation in dependence of DNA damage repair. Br. J. Radiol. 2020, 93, 20190494. [Google Scholar] [CrossRef] [PubMed]
- Luhr, A.; von Neubeck, C.; Krause, M.; Troost, E.G.C. Relative biological effectiveness in proton beam therapy—Current knowledge and future challenges. Clin. Transl. Radiat. Oncol. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Girdhani, S.; Sachs, R.; Hlatky, L. Biological effects of proton radiation: What we know and don’t know. Radiat. Res. 2013, 179, 257–272. [Google Scholar] [CrossRef]
- Van Limbergen, E.-J.; De Ruysscher, D.-K.; Olivo Pimentel, V.; Marcus, D.; Berbee, M.; Hoeben, A.; Rekers, N.; Theys, J.; Yaromina, A.; Dubois, L.-J.; et al. Combining radiotherapy with immunotherapy: The past, the present and the future. Br. J. Radiol. 2017, 90, 20170157. [Google Scholar] [CrossRef]
- Shabason, J.-E.; Minn, A.-J. Radiation and Immune Checkpoint Blockade: From Bench to Clinic. Semin. Radiat. Oncol. 2017, 27, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: Challenges and opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef]
- Baird, J.-R.; Monjazeb, A.-M.; Shah, O.; McGee, H.; Murphy, W.-J.; Crittenden, M.-R.; Gough, M.-J. Stimulating Innate Immunity to Enhance Radiation Therapy-Induced Tumor Control. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 362–373. [Google Scholar] [CrossRef]
- Bernstein, M.-B.; Krishnan, S.; Hodge, J.-W.; Chang, J.-Y. Immunotherapy and stereotactic ablative radiotherapy (ISABR): A curative approach? Nat. Rev. Clin. Oncol. 2016, 13, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Pilones, K.-A.; Vanpouille-Box, C.; Demaria, S. Combination of radiotherapy and immune checkpoint inhibitors. Semin. Radiat. Oncol. 2015, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, D.; Pop, L.; Takeshima, T.; Iyengar, P.; Hannan, R. Rationale and evidence to combine radiation therapy and immunotherapy for cancer treatment. Cancer Immunol. Immunother. 2017, 66, 281–298. [Google Scholar] [CrossRef]
- Lee, Y.-H.; Tai, D.; Yip, C.; Choo, S.-P.; Chew, V. Combinational Immunotherapy for Hepatocellular Carcinoma: Radiotherapy, Immune Checkpoint Blockade and Beyond. Front. Immunol. 2020, 11, 568759. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Yoo, G.-S.; Cho, W.-K.; Park, H.-C. Optimizing radiotherapy with immune checkpoint blockade in hepatocellular carcinoma. World J. Gastroenterol. 2019, 25, 2416–2429. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Feng, Y.-C.; Zhao, G.; Zhang, R.; Cheng, Z.-Z.; Kong, W.-N.; Wu, H.-L.; Xu, B.; Lv, X.; Ma, X.M. Tumor-Associated CD163(+) M2 Macrophage Infiltration is Highly Associated with PD-L1 Expression in Cervical Cancer. Cancer Manag. Res. 2020, 12, 5831–5843. [Google Scholar] [CrossRef]
- Cassetta, L.; Noy, R.; Swierczak, A.; Sugano, G.; Smith, H.; Wiechmann, L.; Pollard, J.-W. Isolation of Mouse and Human Tumor-Associated Macrophages. Adv. Exp. Med. Biol. 2016, 899, 211–229. [Google Scholar]
- Chikamatsu, K.; Sakakura, K.; Toyoda, M.; Takahashi, K.; Yamamoto, T.; Masuyama, K. Immunosuppressive activity of CD14+ HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci. 2012, 103, 976–983. [Google Scholar] [CrossRef]
- Chen, M.-F.; Kuan, F.-C.; Yen, T.-C.; Lu, M.-S.; Lin, P.-Y.; Chung, Y.-H.; Chen, W.-C.; Lee, K.-D. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget 2014, 5, 8716–8728. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.-P.; Frey, A.-B.; Greten, T.-F.; Mandruzzato, S.; Murray, P.-J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Curtis, M.-J.; Bond, R.-A.; Spina, D.; Ahluwalia, A.; Alexander, S.P.A.; Giembycz, M.-A.; Gilchrist, A.; Hoyer, D.; Insel, P.-A.; Izzo, A.-A. Experimental design and analysis and their reporting: New guidance for publication in BJP. Br. J. Pharmacol. 2015, 172, 3461–3471. [Google Scholar] [CrossRef]
- Haikerwal, S.-J.; Hagekyriakou, J.; MacManus, M.; Martin, O.-A.; Haynes, N.-M. Building immunity to cancer with radiation therapy. Cancer Lett. 2015, 368, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Liu, Z.; Xu, Z.; Liu, J.; Zhang, J. High mobility group box 1 (HMGB1): A pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 2020, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Mimura, K.; Yoshimoto, Y.; Watanabe, M.; Ohkubo, Y.; Izawa, S.; Murata, K.; Fujii, H.; Nakano, T.; Kono, K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer. Res. 2012, 72, 3967–3976. [Google Scholar] [CrossRef]
- Chen, M.-F.; Hsieh, C.-C.; Chen, W.-C.; Lai, C.-H. Role of interleukin-6 in the radiation response of liver tumors. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e621–e630. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Liu, S.; Fu, S.; Wu, J. HMGB1 in Radiotherapy: A Two Headed Signal Regulating Tumor Radiosensitivity and Immunity. Oncol. Targets Ther. 2020, 13, 6859–6871. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef]
- Afreen, S.; Dermime, S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: Killing many birds with one stone. Hematol. Oncol. Stem. Cell. Ther. 2014, 7, 1–17. [Google Scholar] [CrossRef]
- Formenti, S.-C.; Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 879–880. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, M.-E.; Vitale, I.; Harrington, K.-J.; Melero, I.; Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 2020, 21, 120–134. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.-P. The changing paradigm of tumour response to irradiation. Br. J. Radiol. 2017, 90, 20160474. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chakraborty, A. Radiation-induced bystander phenomenon: Insight and implications in radiotherapy. Int. J. Radiat. Biol. 2019, 95, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.-R.; Malamas, A.-S.; Bernstein, M.-B.; Tsang, K.-Y.; Vassantachart, A.; Sahoo, N.; Tailor, R.; Pidikiti, R.; Guha, C.-P.; Hahn, S.-M.; et al. Tumor Cells Surviving Exposure to Proton or Photon Radiation Share a Common Immunogenic Modulation Signature, Rendering Them More Sensitive to T Cell-Mediated Killing. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 120–130. [Google Scholar] [CrossRef]
- Sun, Y.; Nelson, P.-S. Molecular pathways: Involving microenvironment damage responses in cancer therapy resistance. Clin. Cancer Res. 2012, 18, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Kozin, S.-V.; Kamoun, W.-S.; Huang, Y.; Dawson, M.-R.; Jain, R.-K.; Duda, D.-G. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010, 70, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Kao, J.; Ko, E.-C.; Eisenstein, S.; Sikora, A.-G.; Fu, S.; Chen, S.-H. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit. Rev. Oncol. Hematol. 2011, 77, 12–19. [Google Scholar] [CrossRef]
- Hoechst, B.; Ormandy, L.A.; Ballmaier, M.; Lehner, F.; Krüger, C.; Manns, M.P.; Greten, T.F.; Korangy, F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008, 135, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Lin, Y.-C.; Chang, L.-Y.; Huang, S.-K.; Huang, C.-H.; Yang, C.-K.; Huang, C.-T.; Lin, C.-Y. Therapeutic Role of Inducible Nitric Oxide Synthase Expressing Myeloid-Derived Suppressor Cells in Acetaminophen-Induced Murine Liver Failure. Front. Immunol. 2020, 11, 574839. [Google Scholar] [CrossRef]
- Pollard, J.-W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Zhang, S.-X.; Wu, H.-J.; Rong, X.-L.; Guo, J. M2b macrophage polarization and its roles in diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-S.; Fu, S.-Y.; Wang, S.-C.; Yu, C.-F.; Chen, F.-H.; Lin, C.-M.; Hong, J.-H. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front. Oncol. 2012, 2, 89. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Lu, D.; Ni, Z.; Liu, X.; Feng, S.; Dong, X.; Shi, X.; Zhai, J.; Mai, S.; Jiang, J.; Wang, Z.; et al. Beyond T Cells: Understanding the Role of PD-1/PD-L1 in Tumor-Associated Macrophages. J. Immunol. Res. 2019, 2019, 1919082. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Hsu, C.; Chan, S.-L.; Choo, S.-P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef]
- Hack, S.-P.; Spahn, J.; Chen, M.; Cheng, A.-L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 975–989. [Google Scholar] [CrossRef]
- Park, S.-S.; Dong, H.; Liu, X.; Harrington, S.-M.; Krco, C.-J.; Grams, M.-P.; Mansfield, A.-S.; Furutani, K.-M.; Olivier, K.-R.; Kwon, E.-D. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol. Res. 2015, 3, 610–619. [Google Scholar] [CrossRef]
- Demaria, S.; Ng, B.; Devitt, M.-L.; Babb, J.-S.; Kawashima, N.; Liebes, L.; Formenti, S.-C. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-F.; Chen, P.-T.; Hsieh, C.-C.; Wang, C.-C. Effect of Proton Therapy on Tumor Cell Killing and Immune Microenvironment for Hepatocellular Carcinoma. Cells 2023, 12, 332. https://doi.org/10.3390/cells12020332
Chen M-F, Chen P-T, Hsieh C-C, Wang C-C. Effect of Proton Therapy on Tumor Cell Killing and Immune Microenvironment for Hepatocellular Carcinoma. Cells. 2023; 12(2):332. https://doi.org/10.3390/cells12020332
Chicago/Turabian StyleChen, Miao-Fen, Ping-Tsung Chen, Ching-Chuan Hsieh, and Chih-Chi Wang. 2023. "Effect of Proton Therapy on Tumor Cell Killing and Immune Microenvironment for Hepatocellular Carcinoma" Cells 12, no. 2: 332. https://doi.org/10.3390/cells12020332
APA StyleChen, M.-F., Chen, P.-T., Hsieh, C.-C., & Wang, C.-C. (2023). Effect of Proton Therapy on Tumor Cell Killing and Immune Microenvironment for Hepatocellular Carcinoma. Cells, 12(2), 332. https://doi.org/10.3390/cells12020332




