Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea
Abstract
1. Introduction
2. Basic Principles of Lymphatic Network Development & Patterning
2.1. Lymphatic Network Origin and Development
2.2. Tip and Stalk Cell Theory of Lymphangiogenesis
3. Corneal Lymphangiogenesis Guidance Mechanisms
| Molecular Family | Molecule | Target or Ligand | Effect on Lymphatic Endothelial Guidance | References |
|---|---|---|---|---|
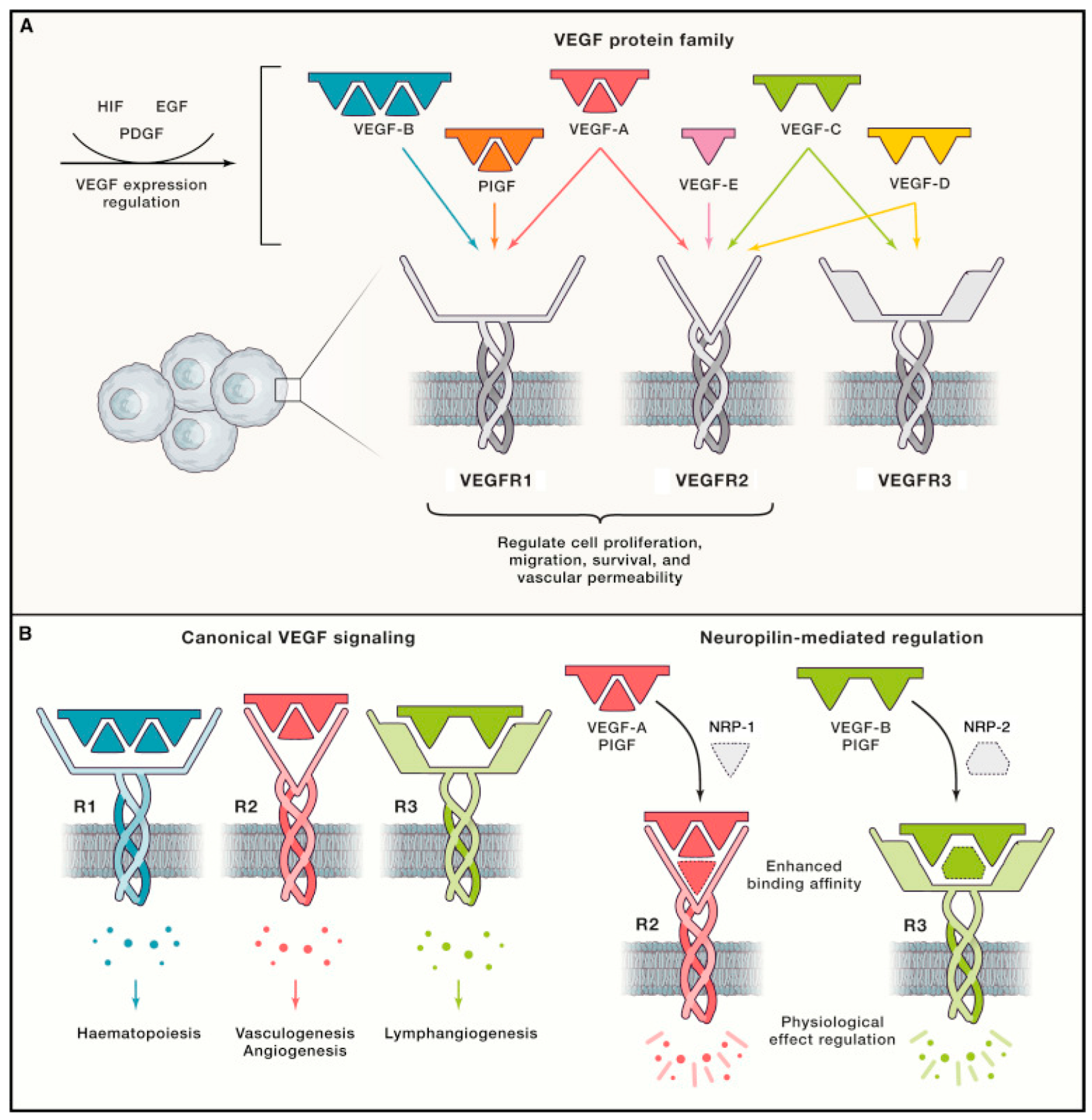
| VEGF | VEGF-A | VEGFR-1,2 Notch signaling | Induces LEC proliferation. Recruits VEGF-C/D-producing macrophages to sites of injury. Internalizes VEGF-C/VEGFR-2 complexes in vascular endothelial cells. | [18,23,36,37,38,42,43,44,45,46,47,48] |
| VEGF-C | VEGFR-2,3 | Expands the lymphatic vascular network via LEC migration, proliferation, and sprouting. Essential for lymphatic network formation from embryonic cardinal vein. Influences directional migration of lymphangioblasts. Primary driver of lymphangiogenesis. | ||
| VEGF-D | VEGFR-3 | Expands the lymphatic vascular network via LEC migration, proliferation, and sprouting. | ||
| FGF | bFGF | FGFR | Increases the secretion of VEGF-C from vascular endothelial cells. Directly binds to LECs and promotes LEC migration, proliferation, and survival. Pro-lymphangiogenic effects inhibited upon interaction with LYVE-1. | [49,50,51] |
| Neuropilin | Neuropilin-2 | VEGF-C | Acts as a coreceptor for VEGFR-3, promoting LEC migration and sprouting. Encourages lymphatic endothelial tip cell extension and prevents tip cell retraction during sprouting. Highly concentrated in cells at the leading tip of growing lymphatic sprouts. | [52,53,54,55] |
| Ephrin | EphrinB2 | EphB4 | Promotes maturation of lymphatic valves both during valvulogenesis and post-injury. Promotes VEGF-induced LEC migration and lymphatic tube formation. | [56,57,58,59,60] |
| Wnt proteins | Wnt5a | FZD3, RYK, β-catenin | Promotes maturation of lymphatic valves both during valvulogenesis and post-injury. Elongates lymphatic networks. | [61,62,63] |
| Netrin | Netrin-4 | Unc5 Neogenin | Promotes LEC migration, proliferation, adhesion, and tube formation. | [64,65] |
| Slit | Slit2 | Robo1 | Stimulates LEC migration and tube formation. | [66,67] |
| Robo4 | Induces VEGFR-3 internalization in LECs. Inhibits the activation of LECs by VEGF-C. | |||
| CXCL | CXCL12 | CXCR4, VEGF-C | Induces LEC migration and tube formation. Directs early trunk lymphatic network assembly. | [68,69,70] |
| Sphingolipids | S1P | S1PR1 | Influences inward LEC migration in response to wall stress and directional LEC migration in response to fluid flow stimulus. When absent, induces VEGF-C expression. | [71,72,73,74,75,76] |
| Glycosaminoglycans | Hyaluronan (LMW, HMW) | LYVE-1, LYVE-2, S1P-3, ERK-1/2 | Promotes lymphatic vessel sprouting and proliferation in both healthy and pathological states. Organizes lymphatic endothelium into vessel-like cell sheets, promoting lymphatic tube formation. Synergistically increases lymphatic tube formation and sprouting when administered with VEGF-C. | [8,77,78] |
| Integrins | α9β1 | VEGF-A/C/D, fibronectin EDA, emilin-1, polydom | Promotes LEC migration, vessel sprouting, and both developmental and pathological valvulogenesis. | [79,80,81,82,83] |
| α5β1 | Promotes LEC sprouting and VEGF-C-mediated guidance. | |||
| α6β1 | Promotes LEC adhesion and migration to netrin-4. | |||
| BMP | BMP4 | ALK | Inhibits guidance and neovascularization by decreasing VEGF-C/VEGFR-3 signaling. | [84,85] |
| BMP9 | Directs early lymphatic endothelial tip cell expansion. Low concentration enhances LYVE-1-positive LECs; high concentration enhances LYVE-1-negative LECs. Activates VEGF-A at high concentrations. | |||
| Angiopoietin | Ang-1 | Tie-1 Tie-2 | Ang-2 guides sprouting of lymphatics around blood vessels. Ang-2 sensitizes LECs to inflammatory stimuli post-injury. Tie-1 facilitates early stages of developmental LEC proliferation and LEC survival. | [86,87,88] |
| Ang-2 | ||||
| TGFBIp | Integrins | Promotes LEC sprouting, migration, adhesion, and tube formation. Synergistically enhances stimulatory effect of VEGF-C. | [89] | |
| Semaphorins | Sema3A | NRP1, plexinA1 | Contributes to lymphatic vessel and valve morphology during development. | [90,91,92,93,94,95,96,97,98,99,100,101] |
| Sema3F | NRP2, plexinA3, plexinA1 | Globally suppresses LEC proliferation and sprouting in low concentrations. Overexpression causes a chemorepulsive effect on LECs. | ||
| Sema3G | NRP2, plexinD1, plexinA2 | Locally suppresses LEC proliferation and sprouting in high concentrations. Repels LECs away from arteries and induces lymphatic vascular branching. | ||
| Sema7A | β1-integrin receptor | Promotes lymphatic vessel invasion, including LEC tube formation. | ||
| Delta-like ligands | Dll4 | Notch | Suppresses LEC migration and lymphatic vessel sprouting. Suppresses lymphangiogenesis via effects on VEGF-A and VEGF-C signaling. Suppresses Prox1+ LECs during embryonic development. | [32,44] |
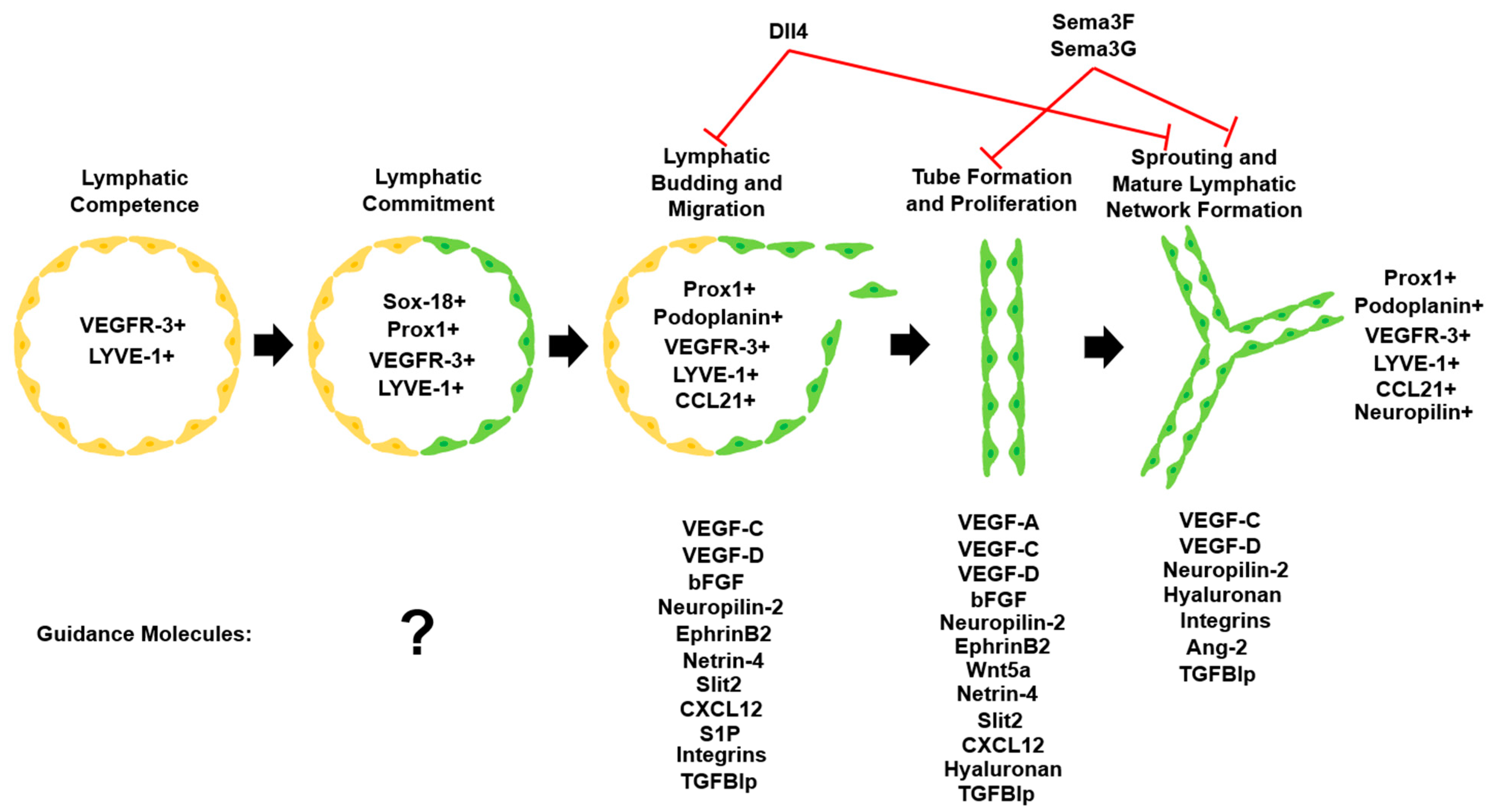
3.1. VEGF Family
3.2. bFGF
3.3. Neuropilin-2
3.4. Ephrin–Eph Signaling
3.5. Wnt/β-Catenin
3.6. Netrins
3.7. Slit/Robo
3.8. Chemokines
3.9. Sphingosine 1-Phosphate
3.10. Hyaluronan
3.11. Integrin Family
3.12. BMP Family
3.13. Angiopoietin/Tie Receptors
3.14. TGFBIp
3.15. Semaphorins
3.16. Notch Proteins
3.17. Other Guidance Molecules
4. Pathologies Involving Lymphatic Endothelial Guidance
4.1. Dry Eye Disease
4.2. Corneal Graft Rejection
4.3. Infectious Keratitis
4.4. Alkali Burn
5. Therapeutic Strategies Targeting Lymphangiogenesis Guidance Mechanisms
6. Occurrence of Lymphangiogenesis without Hemangiogenesis
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ACKR | atypical chemokine receptor |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| AKT | protein kinase B (PKB) |
| ALK | activin receptor-like kinase |
| Ang | angiopoietin |
| APC | antigen-presenting cell |
| AS1 | hydrocinnamoyl-L-valylpyrrolidine |
| bFGF | basic fibroblast growth factor |
| BMP | bone morphogenic protein |
| CCBE1 | collagen- and calcium-binding EGF domain-containing protein 1 |
| CD11b+ | cluster of differentiation 11b+ |
| CD36 | cluster of differentiation 36 |
| CXCL | chemokine (C-X-C motif) ligand |
| CXCR | C-X-C chemokine receptor |
| DCC | deleted in colorectal cancer |
| DDR1 | discoidin domain receptor 1 |
| DsRed | Discosoma red fluorescent protein |
| DED | dry eye disease |
| Dll4 | Delta-like 4 Notch ligand |
| DMF | dimethylformamide |
| ECM | extracellular matrix |
| EDA | extra domain A |
| EGF | epidermal growth factor |
| Eph | ephrin receptor |
| ERK | extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FGF | fibroblast growth factor |
| FGFR | fibroblast growth factor receptor |
| Flt1 | Fms-related receptor tyrosine kinase 1 |
| FOXC2 | Forkhead box protein C2 |
| FZD3 | frizzled class receptor 3 |
| GFP | green fluorescent protein |
| HA | hyaluronan |
| HAS | hyaluronan synthase |
| HMWHA | high molecular weight hyaluronan |
| HGF | hepatocyte growth factor |
| HIF | hypoxia-inducible factor |
| HSV | herpes simplex virus |
| IL | interleukin |
| IL1R1 | interleukin 1 receptor type 1 |
| KLF | Kruppel-like factor |
| KOR | Kappa opioid receptor |
| LacZ | β-galactosidase |
| LEC | lymphatic endothelial cell |
| LYVE1 | lymphatic vessel endothelial hyaluronan receptor 1 |
| mAb | monoclonal antibody |
| MCP-1 | monocyte chemoattractant protein-1 |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| mRNA | messenger RNA |
| MyD88 | myeloid differentiation primary response gene 88 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK1R | neurokinin 1 receptor |
| Nrp | neuropilin |
| ORAI1 | calcium release-activated calcium channel protein 1 |
| PDGF | platelet-derived growth factor |
| Pdk2 | pyruvate dehydrogenase kinase isoform 2 |
| Pdl1 | programed death ligand-1 |
| PI3K | phosphoinositide 3-kinase |
| PIK3CA | phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha |
| PLDL | poly(L-lactic-co-D-lactic acid) |
| PLGA | poly(D,L-lactic-co-glycolic acid) |
| Prox1 | Prospero-related homeobox-1 |
| Robo | Roundabout |
| RYK | related to receptor tyrosine kinase |
| SC | Schlemm’s canal |
| S1P | sphingosine 1-phosphate |
| S1PR1 | sphingosine 1-phosphate receptor 1 |
| Sema | semaphorin |
| siRNA | small interfering RNA |
| sVEGFR | soluble vascular endothelial growth factor receptor |
| Tie | angiopoietin receptor |
| TIR | Toll/IL-1 receptor |
| TGFBIp | transforming growth factor-β-induced protein |
| TNF-α | tumor necrosis factor α |
| TSP-1 | thrombospondin 1 |
| UNC5B | Unc5 netrin receptor B |
| VEGF | vascular endothelial growth factor |
| VEGF-A | vascular endothelial growth factor A |
| VEGF-C | vascular endothelial growth factor C |
| VEGF-D | vascular endothelial growth factor D |
| VEGFR | vascular endothelial growth factor receptor |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
| VEGFR-3 | vascular endothelial growth factor receptor 3 |
| Wnt | wingless and integrated |
References
- Del Monte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Meek, K.M.; Knupp, C. Corneal structure and transparency. Prog. Retin. Eye Res. 2015, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Azar, D.T. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis). Trans. Am. Ophthalmol. Soc. 2006, 104, 264–302. [Google Scholar] [PubMed]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.-H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [PubMed]
- Ecoiffier, T.; Yuen, N.; Chen, L. Differential distribution of blood and lymphatic vessels in the murine cornea. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Rummelt, C.; Jünemann, A.; Vorwerk, C.; Neuhuber, W.; E Kruse, F.; Schroedl, F. Absence of blood and lymphatic vessels in the developing human cornea. Cornea 2006, 25, 722–726. [Google Scholar] [CrossRef]
- Hori, J.; Yamaguchi, T.; Keino, H.; Hamrah, P.; Maruyama, K. Immune privilege in corneal transplantation. Prog. Retin. Eye Res. 2019, 72, 100758. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Tse, J.; Tilahun, F.; Qiu, M.; Chen, L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Investig. Ophthalmol. Vis. Sci. 2011, 52, 334–338. [Google Scholar] [CrossRef]
- Lee, H.-K.; Lee, S.-M.; Lee, D.-I. Corneal Lymphangiogenesis: Current Pathophysiological Understandings and Its Functional Role in Ocular Surface Disease. Int. J. Mol. Sci. 2021, 22, 11628. [Google Scholar] [CrossRef]
- Masood, F.; Bhattaram, R.; Rosenblatt, M.I.; Kazlauskas, A.; Chang, J.-H.; Azar, D.T. Lymphatic Vessel Regression and Its Therapeutic Applications: Learning from Principles of Blood Vessel Regression. Front. Physiol. 2022, 13, 846936. [Google Scholar] [CrossRef]
- Yang, J.F.; Walia, A.; Huang, Y.-H.; Han, K.-Y.; Rosenblatt, M.I.; Azar, D.T.; Chang, J.-H. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv. Ophthalmol. 2016, 61, 272–296. [Google Scholar] [CrossRef] [PubMed]
- Chennakesavalu, M.; Somala, S.R.R.; Dommaraju, S.R.; Peesapati, M.P.; Guo, K.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Corneal lymphangiogenesis as a potential target in dry eye disease—A systematic review. Surv. Ophthalmol. 2021, 66, 960–976. [Google Scholar] [CrossRef]
- Clahsen, T.; Büttner, C.; Hatami, N.; Reis, A.; Cursiefen, C. Role of Endogenous Regulators of Hem- and Lymphangiogenesis in Corneal Transplantation. J. Clin. Med. 2020, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Hos, D.; Bukowiecki, A.; Horstmann, J.; Bock, F.; Bucher, F.; Heindl, L.M.; Siebelmann, S.; Steven, P.; Dana, R.; Eming, S.A.; et al. Transient Ingrowth of Lymphatic Vessels into the Physiologically Avascular Cornea Regulates Corneal Edema and Transparency. Sci. Rep. 2017, 7, 7227. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narimatsu, A.; Hattori, T.; Koike, N.; Tajima, K.; Nakagawa, H.; Yamakawa, N.; Usui, Y.; Kumakura, S.; Matsumoto, T.; Goto, H. Corneal lymphangiogenesis ameliorates corneal inflammation and edema in late stage of bacterial keratitis. Sci. Rep. 2019, 9, 2984. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, M.; Doh, S.J.; Santosa, S.M.; Montana, M.; Qin, E.C.; Kong, H.; Han, K.-Y.; Yu, C.; Rosenblatt, M.I.; Kazlauskas, A.; et al. Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies—A review. Med. Res. Rev. 2018, 38, 1769–1798. [Google Scholar] [CrossRef]
- Wang, C.; Chu, M. Advances in Drugs Targeting Lymphangiogenesis for Preventing Tumor Progression and Metastasis. Front. Oncol. 2021, 11, 783309. [Google Scholar] [CrossRef]
- Vaahtomeri, K.; Karaman, S.; Makinen, T.; Alitalo, K. Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev. 2017, 31, 1615–1634. [Google Scholar] [CrossRef]
- Doh, S.J.; Yamakawa, M.; Santosa, S.M.; Montana, M.; Guo, K.; Sauer, J.R.; Curran, N.; Han, K.-Y.; Yu, C.; Ema, M.; et al. Fluorescent reporter transgenic mice for in vivo live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2018, 21, 677–698. [Google Scholar] [CrossRef]
- Jafree, D.J.; Long, D.A.; Scambler, P.J.; Ruhrberg, C. Mechanisms and cell lineages in lymphatic vascular development. Angiogenesis 2021, 24, 271–288. [Google Scholar] [CrossRef]
- Han, K.-Y.; Chang, J.-H.; Dugas-Ford, J.; Alexander, J.S.; Azar, D.T. Involvement of lysosomal degradation in VEGF-C-induced down-regulation of VEGFR-3. FEBS Lett. 2014, 588, 4357–4363. [Google Scholar] [CrossRef] [PubMed]
- Bock, F.; Onderka, J.; Dietrich, T.; Bachmann, B.; Kruse, F.E.; Paschke, M.; Zahn, G.; Cursiefen, C. Bevacizumab as a potent inhibitor of inflammatory corneal angiogenesis and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Jitariu, A.A.; Cimpean, A.M.; Kundnani, N.R.; Raica, M. Platelet-derived growth factors induced lymphangiogenesis: Evidence, unanswered questions and upcoming challenges. Arch. Med. Sci. 2015, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Yuen, D.; Grimaldo, S.; Sessa, R.; Ecoiffier, T.; Truong, T.; Huang, E.; Bernas, M.; Daley, S.; Witte, M.; Chen, L. Role of angiopoietin-2 in corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3320–3327. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- He, Y.; Rajantie, I.; Ilmonen, M.; Makinen, T.; Karkkainen, M.J.; Haiko, P.; Salven, P.; Alitalo, K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004, 64, 3737–3740. [Google Scholar] [CrossRef]
- Connor, A.L.; Kelley, P.M.; Tempero, R.M. Lymphatic endothelial lineage assemblage during corneal lymphangiogenesis. Lab. Investig. 2016, 96, 270–282. [Google Scholar] [CrossRef]
- Geng, X.; Yanagida, K.; Akwii, R.G.; Choi, D.; Chen, L.; Ho, Y.; Cha, B.; Mahamud, R.; de Ruiz, K.B.; Ichise, H.; et al. S1PR1 regulates the quiescence of lymphatic vessels by inhibiting laminar shear stress-dependent VEGF-C signaling. JCI Insight 2020, 5, e137652. [Google Scholar] [CrossRef]
- Zeng, A.; Wang, S.-R.; He, Y.-X.; Yan, Y.; Zhang, Y. Progress in understanding of the stalk and tip cells formation involvement in angiogenesis mechanisms. Tissue Cell 2021, 73, 101626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tammela, T.; Yamamoto, M.; Anisimov, A.; Holopainen, T.; Kaijalainen, S.; Karpanen, T.; Lehti, K.; Ylä-Herttuala, S.; Alitalo, K. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood 2011, 118, 1154–1162. [Google Scholar] [CrossRef]
- Tessier-Lavigne, M.; Goodman, C.S. The molecular biology of axon guidance. Science 1996, 274, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Leithner, A.; Eichner, A.; Müller, J.; Reversat, A.; Brown, M.; Schwarz, J.; Merrin, J.; De Gorter, D.J.J.; Schur, F.; Bayerl, J.; et al. Diversified actin protrusions promote environmental exploration but are dispensable for locomotion of leukocytes. Nat. Cell Biol. 2016, 18, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Benest, A.V.; Harper, S.J.; Herttuala, S.Y.; Alitalo, K.; Bates, D.O. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc. Res. 2008, 78, 315–323. [Google Scholar] [CrossRef]
- Gerhardt, H.; Golding, M.; Fruttiger, M.; Ruhrberg, C.; Lundkvist, A.; Abramsson, A.; Jeltsch, M.; Mitchell, C.; Alitalo, K.; Shima, D.; et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 2003, 161, 1163–1177. [Google Scholar] [CrossRef]
- Bautch, V.L. VEGF-directed blood vessel patterning: From cells to organism. Cold Spring Harb. Perspect. Med. 2012, 2, a006452. [Google Scholar] [CrossRef]
- Adams, R.H.; Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2010, 2, a001875. [Google Scholar] [CrossRef]
- Chen, W.; Xia, P.; Wang, H.; Tu, J.; Liang, X.; Zhang, X.; Li, L. The endothelial tip-stalk cell selection and shuffling during angiogenesis. J. Cell Commun. Signal. 2019, 13, 291–301. [Google Scholar] [CrossRef]
- Baluk, P.; Tammela, T.; Ator, E.; Lyubynska, N.; Achen, M.G.; Hicklin, D.J.; Jeltsch, M.; Petrova, T.V.; Pytowski, B.; Stacker, S.A.; et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Investig. 2005, 115, 247–257. [Google Scholar] [CrossRef]
- Detry, B.; Bruyère, F.; Erpicum, C.; Paupert, J.; Lamaye, F.; Maillard, C.; Lenoir, B.; Foidart, J.-M.; Thiry, M.; Noël, A. Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. 2011, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Wirzenius, M.; Tammela, T.; Uutela, M.; He, Y.; Odorisio, T.; Zambruno, G.; Nagy, J.A.; Dvorak, H.F.; Ylä-Herttuala, S.; Shibuya, M.; et al. Distinct vascular endothelial growth factor signals for lymphatic vessel enlargement and sprouting. J. Exp. Med. 2007, 204, 1431–1440. [Google Scholar] [CrossRef]
- Suchting, S.; Freitas, C.; le Noble, F.; Benedito, R.; Bréant, C.; Duarte, A.; Eichmann, A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc. Natl. Acad. Sci. USA 2007, 104, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Culver, A.; Culver, F.; Liu, T.; Dietz, W.H.; Thomson, B.R.; Hadjantonakis, A.-K.; Quaggin, S.E.; Kume, T. Murine Notch1 is required for lymphatic vascular morphogenesis during development. Dev. Dyn. 2014, 243, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Amano, S.; Usui, T.; Kaji, Y.; Oshika, T.; Ishii, Y. Expression of vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 in corneal lymphangiogenesis. Exp. Eye Res. 2001, 72, 71–78. [Google Scholar] [CrossRef]
- Yuen, N.; Pytowski, B.; Chen, L. Combined blockade of VEGFR-2 and VEGFR-3 inhibits inflammatory lymphangiogenesis in early and middle stages. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2593–2597. [Google Scholar] [CrossRef][Green Version]
- Cursiefen, C.; Ikeda, S.; Nishina, P.M.; Smith, R.S.; Ikeda, A.; Jackson, D.; Mo, J.-S.; Chen, L.; Dana, M.R.; Pytowski, B.; et al. Spontaneous corneal hem- and lymphangiogenesis in mice with destrin-mutation depend on VEGFR3 signaling. Am. J. Pathol. 2005, 166, 1367–1377. [Google Scholar] [CrossRef]
- Wang, G.; Muhl, L.; Padberg, Y.; Dupont, L.; Peterson-Maduro, J.; Stehling, M.; le Noble, F.; Colige, A.; Betsholtz, C.; Schulte-Merker, S.; et al. Specific fibroblast subpopulations and neuronal structures provide local sources of Vegfc-processing components during zebrafish lymphangiogenesis. Nat. Commun. 2020, 11, 2724. [Google Scholar] [CrossRef]
- Shin, J.W.; Min, M.; Larrieu-Lahargue, F.; Canron, X.; Kunstfeld, R.; Nguyen, L.; Henderson, J.; Bikfalvi, A.; Detmar, M.; Hong, Y.-K. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: A role for FGF signaling in lymphangiogenesis. Mol. Biol. Cell 2006, 17, 576–584. [Google Scholar] [CrossRef]
- Cao, R.; Ji, H.; Feng, N.; Zhang, Y.; Yang, X.; Andersson, P.; Sun, Y.; Tritsaris, K.; Hansen, A.J.; Dissing, S.; et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 15894–15899. [Google Scholar] [CrossRef]
- Tzeng, H.-E.; Chang, A.-C.; Tsai, C.-H.; Wang, S.-W.; Tang, C.-H. Basic fibroblast growth factor promotes VEGF-C-dependent lymphangiogenesis via inhibition of miR-381 in human chondrosarcoma cells. Oncotarget 2016, 7, 38566–38578. [Google Scholar] [CrossRef]
- Hamrah, P.; Chen, L.; Zhang, Q.; Dana, M.R. Novel expression of vascular endothelial growth factor receptor (VEGFR)-3 and VEGF-C on corneal dendritic cells. Am. J. Pathol. 2003, 163, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Caunt, M.; Mak, J.; Liang, W.-C.; Stawicki, S.; Pan, Q.; Tong, R.K.; Kowalski, J.; Ho, C.; Reslan, H.B.; Ross, J.; et al. Blocking neuropilin-2 function inhibits tumor cell metastasis. Cancer Cell 2008, 13, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, L.; Mak, J.; Pardanaud, L.; Caunt, M.; Kasman, I.; Larrivée, B.; Del Toro, R.; Suchting, S.; Medvinsky, A.; et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J. Cell Biol. 2010, 188, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Sun, J.-F.; Wang, X.-Y.; Du, L.-L.; Liu, P. Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol. Vis. 2010, 16, 2354–2361. [Google Scholar]
- Egea, J.; Klein, R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Jiang, Z.; Liu, X.; Meng, A. Eph/ephrin signaling maintains the boundary of dorsal forerunner cell cluster during morphogenesis of the zebrafish embryonic left-right organizer. Development 2016, 143, 2603–2615. [Google Scholar] [CrossRef]
- Zhang, G.; Brady, J.R.; Liang, W.-C.; Wu, Y.; Henkemeyer, M.; Yan, M. EphB4 forward signalling regulates lymphatic valve development. Nat. Commun. 2015, 6, 6625. [Google Scholar] [CrossRef]
- Abéngozar, M.A.; De Frutos, S.; Ferreiro, S.; Soriano, J.; Perez-Martinez, M.; Olmeda, D.; Marenchino, M.; Cañamero, M.; Ortega, S.; Megias, D.; et al. Blocking ephrinB2 with highly specific antibodies inhibits angiogenesis, lymphangiogenesis, and tumor growth. Blood 2012, 119, 4565–4576. [Google Scholar] [CrossRef]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Lüthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef]
- Sessa, R.; Yuen, D.; Wan, S.; Rosner, M.; Padmanaban, P.; Ge, S.; Smith, A.; Fletcher, R.; Baudhuin-Kessel, A.; Yamaguchi, T.P.; et al. Monocyte-derived Wnt5a regulates inflammatory lymphangiogenesis. Cell Res. 2016, 26, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Lutze, G.; Haarmann, A.; Demanou Toukam, J.A.; Buttler, K.; Wilting, J.; Becker, J. Non-canonical WNT-signaling controls differentiation of lymphatics and extension lymphangiogenesis via RAC and JNK signaling. Sci. Rep. 2019, 9, 4739. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.; Geng, X.; Mahamud, M.R.; Fu, J.; Mukherjee, A.; Kim, Y.; Jho, E.-H.; Kim, T.H.; Kahn, M.; Xia, L.; et al. Mechanotransduction activates canonical Wnt/beta-catenin signaling to promote lymphatic vascular patterning and the development of lymphatic and lymphovenous valves. Genes Dev. 2016, 30, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Larrieu-Lahargue, F.; Welm, A.L.; Thomas, K.R.; Li, D.Y. Netrin-4 induces lymphangiogenesis in vivo. Blood 2010, 115, 5418–5426. [Google Scholar] [CrossRef]
- Larrieu-Lahargue, F.; Thomas, K.R.; Li, D.Y. Netrin ligands and receptors: Lessons from neurons to the endothelium. Trends Cardiovasc. Med. 2012, 22, 44–47. [Google Scholar] [CrossRef]
- Yang, X.-M.; Han, H.-X.; Sui, F.; Dai, Y.-M.; Chen, M.; Geng, J.-G. Slit-Robo signaling mediates lymphangiogenesis and promotes tumor lymphatic metastasis. Biochem. Biophys. Res. Commun. 2010, 396, 571–577. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Kuzontkoski, P.M.; Jiang, S.; Zhu, W.; Li, D.Y.; Groopman, J.E. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun. Signal. 2014, 12, 25. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 Axis in Tumor Immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef]
- Döring, Y.; Pawig, L.; Weber, C.; Noels, H. The CXCL12/CXCR4 chemokine ligand/receptor axis in cardiovascular disease. Front. Physiol. 2014, 5, 212. [Google Scholar]
- Zhuo, W.; Jia, L.; Song, N.; Lu, X.-A.; Ding, Y.; Wang, X.; Song, X.; Fu, Y.; Luo, Y. The CXCL12-CXCR4 chemokine pathway: A novel axis regulates lymphangiogenesis. Clin. Cancer Res. 2012, 18, 5387–5398. [Google Scholar] [CrossRef]
- Jung, B.; Obinata, H.; Galvani, S.; Mendelson, K.; Ding, B.-S.; Skoura, A.; Kinzel, B.; Brinkmann, V.; Rafii, S.; Evans, T.; et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 2012, 23, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Huang, Y.-L.; Shyu, M.-K.; Chen, S.-U.; Lin, C.-H.; Ju, T.-K.; Lu, J.; Lee, H. Sphingosine-1-phosphate induces VEGF-C expression through a MMP-2/FGF-1/FGFR-1-dependent pathway in endothelial cells in vitro. Acta Pharmacol. Sin. 2013, 34, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Surya, V.N.; Michalaki, E.; Huang, E.Y.; Fuller, G.G.; Dunn, A.R. Sphingosine 1-phosphate receptor 1 regulates the directional migration of lymphatic endothelial cells in response to fluid shear stress. J. R. Soc. Interface 2016, 13, 20160823. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Y.; Huang, Y. Topical application of sphingosine 1-phosphate receptor 1 prolongs corneal graft survival in mice. Mol. Med. Rep. 2015, 11, 3800–3807. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Xiao, Y.; Han, G.; Jia, L.; Wang, L.; Lei, T.; Huang, Y. Prolonging survival of corneal transplantation by selective sphingosine-1-phosphate receptor 1 agonist. PLoS ONE 2014, 9, e105693. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Baluk, P.; Xu, Y.; Grigorova, I.; Bankovich, A.; Pappu, R.; Coughlin, S.R.; McDonald, D.M.; Schwab, S.; Cyster, J.G. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med. 2010, 207, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, H.; Liu, Y.; He, Y.; Yang, C.; Du, Y.; Wu, M.; Zhang, G.; Gao, F. The cooperative role of S1P3 with LYVE-1 in LMW-HA-induced lymphangiogenesis. Exp. Cell Res. 2015, 336, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Puri, S.; Mutoji, K.N.; Coulson-Thomas, Y.M.; Hascall, V.C.; Jackson, D.G.; Gesteira, T.F.; Coulson-Thomas, V.J. Hyaluronan Derived from the Limbus is a Key Regulator of Corneal Lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1050–1062. [Google Scholar] [CrossRef]
- Bazigou, E.; Xie, S.; Chen, C.; Weston, A.; Miura, N.; Sorokin, L.; Adams, R.; Muro, A.F.; Sheppard, D.; Makinen, T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev. Cell 2009, 17, 175–186. [Google Scholar] [CrossRef]
- Oommen, S.; Gupta, S.K.; Vlahakis, N.E. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: Identification of a specific {alpha}9{beta}1 binding site. J. Biol. Chem. 2011, 286, 1083–1092. [Google Scholar] [CrossRef]
- Karpanen, T.; Padberg, Y.; van de Pavert, S.A.; Dierkes, C.; Morooka, N.; Peterson-Maduro, J.; van de Hoek, G.; Adrian, M.; Mochizuki, N.; Sekiguchi, K.; et al. An Evolutionarily Conserved Role for Polydom/Svep1 during Lymphatic Vessel Formation. Circ. Res. 2017, 120, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Garmy-Susini, B.; Avraamides, C.J.; Schmid, M.C.; Foubert, P.; Ellies, L.G.; Barnes, L.; Feral, C.; Papayannopoulou, T.; Lowy, A.; Blair, S.L.; et al. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010, 70, 3042–3051. [Google Scholar] [CrossRef] [PubMed]
- Larrieu-Lahargue, F.; Welm, A.L.; Thomas, K.R.; Li, D.Y. Netrin-4 activates endothelial integrin {alpha}6{beta}1. Circ. Res. 2011, 109, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, Y.; Lee, Y.G.; Akatsu, Y.; Taguchi, L.; Suzuki, H.I.; Cunha, S.I.; Maruyama, K.; Suzuki, Y.; Yamazaki, T.; Katsura, A.; et al. Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc. Natl. Acad. Sci. USA 2013, 110, 18940–18945. [Google Scholar] [CrossRef]
- Hu, H.; Wang, S.; He, Y.; Shen, S.; Yao, B.; Xu, D.; Liu, X.; Zhang, Y. The role of bone morphogenetic protein 4 in corneal injury repair. Exp. Eye Res. 2021, 212, 108769. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.E.; Choi, D.-K.; Jang, J.Y.; Jung, J.-J.; Kiyonari, H.; Shioi, G.; Chang, W.; Suda, T.; Mochizuki, N.; et al. Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci. Transl. Med. 2013, 5, 203ra127. [Google Scholar] [CrossRef]
- Qu, X.; Tompkins, K.; Batts, L.E.; Puri, M.; Baldwin, H.S. Abnormal embryonic lymphatic vessel development in Tie1 hypomorphic mice. Development 2010, 137, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.W.; Thurston, G.; Hackett, S.F.; Renard, R.; Wang, Q.; McClain, J.; Martin, C.; Witte, C.; Witte, M.H.; Jackson, D.; et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 2002, 3, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, X.; Lu, Y.; Gong, L. TGFBIp mediates lymphatic sprouting in corneal lymphangiogenesis. J. Cell. Mol. Med. 2019, 23, 7602–7616. [Google Scholar] [CrossRef]
- Sakurai, A.; Doci, C.; Gutkind, J.S. Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res. 2012, 22, 23–32. [Google Scholar] [CrossRef]
- Tran, T.S.; Kolodkin, A.L.; Bharadwaj, R. Semaphorin regulation of cellular morphology. Annu. Rev. Cell Dev. Biol. 2007, 23, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y. Semaphorin signaling in vertebrate neural circuit assembly. Front. Mol. Neurosci. 2012, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Toledano, S.; Nir-Zvi, I.; Engelman, R.; Kessler, O.; Neufeld, G. Class-3 Semaphorins and Their Receptors: Potent Multifunctional Modulators of Tumor Progression. Int. J. Mol. Sci. 2019, 20, 556. [Google Scholar] [CrossRef]
- Bouvrée, K.; Brunet, I.; Del Toro, R.; Gordon, E.; Prahst, C.; Cristofaro, B.; Mathivet, T.; Xu, Y.; Soueid, J.; Fortuna, V.; et al. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circ. Res. 2012, 111, 437–445. [Google Scholar] [CrossRef]
- Jurisic, G.; Hajjami, H.M.-E.; Karaman, S.; Ochsenbein, A.; Alitalo, A.; Siddiqui, S.; Pereira, C.O.; Petrova, T.; Detmar, M. An unexpected role of semaphorin3a-neuropilin-1 signaling in lymphatic vessel maturation and valve formation. Circ. Res. 2012, 111, 426–436. [Google Scholar] [CrossRef]
- Degenhardt, K.; Singh, M.K.; Aghajanian, H.; Massera, D.; Wang, Q.; Li, J.; Li, L.; Choi, C.; Yzaguirre, A.D.; Francey, L.J.; et al. Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Nat. Med. 2013, 19, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, H.; Choi, C.; Ho, V.C.; Gupta, M.; Singh, M.K.; Epstein, J.A. Semaphorin 3d and semaphorin 3e direct endothelial motility through distinct molecular signaling pathways. J. Biol. Chem. 2014, 289, 17971–17979. [Google Scholar] [CrossRef]
- Doçi, C.L.; Mikelis, C.M.; Lionakis, M.S.; Molinolo, A.A.; Gutkind, J.S. Genetic Identification of SEMA3F as an Antilymphangiogenic Metastasis Suppressor Gene in Head and Neck Squamous Carcinoma. Cancer Res. 2015, 75, 2937–2948. [Google Scholar] [CrossRef]
- Bielenberg, D.R.; Hida, Y.; Shimizu, A.; Kaipainen, A.; Kreuter, M.; Kim, C.C.; Klagsbrun, M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J. Clin. Investig. 2004, 114, 1260–1271. [Google Scholar] [CrossRef]
- Favier, B.; Alam, A.; Barron, P.; Bonnin, J.; Laboudie, P.; Fons, P.; Mandron, M.; Herault, J.-P.; Neufeld, G.; Savi, P.; et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood 2006, 108, 1243–1250. [Google Scholar] [CrossRef]
- Liu, X.; Uemura, A.; Fukushima, Y.; Yoshida, Y.; Hirashima, M. Semaphorin 3G Provides a Repulsive Guidance Cue to Lymphatic Endothelial Cells via Neuropilin-2/PlexinD1. Cell Rep. 2016, 17, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Cueni, L.N.; Detmar, M. New insights into the molecular control of the lymphatic vascular system and its role in disease. J. Investig. Dermatol. 2006, 126, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. VEGF-VEGFR System as a Target for Suppressing Inflammation and other Diseases. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Kaipainen, A.; Korhonen, J.; Mustonen, T.; van Hinsbergh, V.W.; Fang, G.H.; Dumont, D.; Breitman, M.; Alitalo, K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 1995, 92, 3566–3570. [Google Scholar] [CrossRef]
- Zheng, W.; Aspelund, A.; Alitalo, K. Lymphangiogenic factors, mechanisms, and applications. J. Clin. Investig. 2014, 124, 878–887. [Google Scholar] [CrossRef]
- Hirakawa, S.; Kodama, S.; Kunstfeld, R.; Kajiya, K.; Brown, L.F.; Detmar, M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005, 201, 1089–1099. [Google Scholar] [CrossRef]
- Nakao, S.; Zandi, S.; Hata, Y.; Kawahara, S.; Arita, R.; Schering, A.; Sun, D.; Melhorn, M.I.; Ito, Y.; Lara-Castillo, N.; et al. Blood vessel endothelial VEGFR-2 delays lymphangiogenesis: An endogenous trapping mechanism links lymph- and angiogenesis. Blood 2011, 117, 1081–1090. [Google Scholar] [CrossRef]
- Kubo, H.; Cao, R.; Bräkenhielm, E.; Mäkinen, T.; Cao, Y.; Alitalo, K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 2002, 99, 8868–8873. [Google Scholar] [CrossRef]
- Wuest, T.R.; Carr, D.J. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J. Exp. Med. 2010, 207, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chauhan, S.K.; El Annan, J.; Nallasamy, N.; Zhang, Q.; Dana, R. Evidence of corneal lymphangiogenesis in dry eye disease: A potential link to adaptive immunity? Arch. Ophthalmol. 2010, 128, 819–824. [Google Scholar] [CrossRef]
- Platonova, N.; Miquel, G.; Regenfuss, B.; Taouji, S.; Cursiefen, C.; Chevet, E.; Bikfalvi, A. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood 2013, 121, 1229–1237. [Google Scholar] [CrossRef]
- Hajrasouliha, A.R.; Sadrai, Z.; Chauhan, S.K.; Dana, R. b-FGF induces corneal blood and lymphatic vessel growth in a spatially distinct pattern. Cornea 2012, 31, 804–809. [Google Scholar] [CrossRef]
- Tanabe, K.; Wada, J.; Sato, Y. Targeting angiogenesis and lymphangiogenesis in kidney disease. Nat. Rev. Nephrol. 2020, 16, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Sun, J.; Du, L.; Du, H.; Wang, L.; Mai, J.; Zhang, F.; Liu, P. Neuropilin-2 contributes to LPS-induced corneal inflammatory lymphangiogenesis. Exp. Eye Res. 2016, 143, 110–119. [Google Scholar] [CrossRef]
- Niethamer, T.K.; Bush, J.O. Getting direction(s): The Eph/ephrin signaling system in cell positioning. Dev. Biol. 2019, 447, 42–57. [Google Scholar] [CrossRef]
- Eichmann, A.; Makinen, T.; Alitalo, K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005, 19, 1013–1021. [Google Scholar] [CrossRef]
- Germain, S.; Eichmann, A. VEGF and ephrin-B2: A bloody duo. Nat. Med. 2010, 16, 752–754. [Google Scholar] [CrossRef]
- Mäkinen, T.; Adams, R.H.; Bailey, J.; Lu, Q.; Ziemiecki, A.; Alitalo, K.; Klein, R.; Wilkinson, G.A. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005, 19, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Katsuta, H.; Fukushima, Y.; Maruyama, K.; Hirashima, M.; Nishida, K.; Nishikawa, S.-I.; Uemura, A. EphrinB2-EphB4 signals regulate formation and maintenance of funnel-shaped valves in corneal lymphatic capillaries. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4102–4108. [Google Scholar] [CrossRef]
- Dejana, E. The role of wnt signaling in physiological and pathological angiogenesis. Circ. Res. 2010, 107, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Navankasattusas, S.; Whitehead, K.J.; Suli, A.; Sorensen, L.K.; Lim, A.H.; Zhao, J.; Park, K.W.; Wythe, J.; Thomas, K.R.; Chien, C.-B.; et al. The netrin receptor UNC5B promotes angiogenesis in specific vascular beds. Development 2008, 135, 659–667. [Google Scholar] [CrossRef]
- Yaniv, K.; Isogai, S.; Castranova, D.; Dye, L.; Hitomi, J.; Weinstein, B.M. Live imaging of lymphatic development in the zebrafish. Nat. Med. 2006, 12, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.-K.; Klein, S.; Kociok, N.; Riechardt, A.; Gundlach, E.; Reichhart, N.; Strauß, O.; Joussen, A. Netrin-4 Mediates Corneal Hemangiogenesis but Not Lymphangiogenesis in the Mouse-Model of Suture-Induced Neovascularization. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1387–1396. [Google Scholar] [CrossRef]
- Han, X.; Zhang, M.-C. Potential anti-angiogenic role of Slit2 in corneal neovascularization. Exp. Eye Res. 2010, 90, 742–749. [Google Scholar] [CrossRef]
- Onder, L.; Ludewig, B. A Fresh View on Lymph Node Organogenesis. Trends Immunol. 2018, 39, 775–787. [Google Scholar] [CrossRef]
- Niimi, K.; Kohara, M.; Sedoh, E.; Fukumoto, M.; Shibata, S.; Sawano, T.; Tashiro, F.; Miyazaki, S.; Kubota, Y.; Miyazaki, J.-I.; et al. FOXO1 regulates developmental lymphangiogenesis by upregulating CXCR4 in the mouse-tail dermis. Development 2020, 147, dev181545. [Google Scholar] [CrossRef]
- Lee, K.M.; Danuser, R.; Stein, J.V.; Graham, D.; Nibbs, R.J.; Graham, G.J. The chemokine receptors ACKR2 and CCR2 reciprocally regulate lymphatic vessel density. EMBO J. 2014, 33, 2564–2580. [Google Scholar] [CrossRef]
- Klein, K.R.; Karpinich, N.O.; Espenschied, S.T.; Willcockson, H.H.; Dunworth, W.P.; Hoopes, S.L.; Kushner, E.J.; Bautch, V.L.; Caron, K.M. Decoy receptor CXCR7 modulates adrenomedullin-mediated cardiac and lymphatic vascular development. Dev. Cell 2014, 30, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Tobia, C.; Chiodelli, P.; Barbieri, A.; Buraschi, S.; Ferrari, E.; Mitola, S.; Borsani, G.; Guerra, J.; Presta, M. Atypical Chemokine Receptor 3 Generates Guidance Cues for CXCL12-Mediated Endothelial Cell Migration. Front. Immunol. 2019, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Forrester, J.V.; Graham, G.J.; Kuffova, L. The atypical chemokine receptor-2 does not alter corneal graft survival but regulates early stage of corneal graft-induced lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1875–1882. [Google Scholar] [CrossRef]
- Du, L.L.; Liu, P. CXCL12/CXCR4 axis regulates neovascularization and lymphangiogenesis in sutured corneas in mice. Mol. Med. Rep. 2016, 13, 4987–4994. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Liu, X.; Wu, J.; Li, J.; Dong, C.; Wu, X.; Xiao, X.; Yu, F.-S.X. CXCL10 suppression of hem- and lymph-angiogenesis in inflamed corneas through MMP13. Angiogenesis 2017, 20, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Nagahashi, M.; Terracina, K.P.; Takabe, K. Emerging Role of Sphingosine-1-phosphate in Inflammation, Cancer and Lymphangiogenesis. Biomolecules 2013, 3, 408–434. [Google Scholar] [CrossRef]
- Porter, H.; Qi, H.; Prabhu, N.; Grambergs, R.; McRae, J.; Hopiavuori, B.; Mandal, N. Characterizing Sphingosine Kinases and Sphingosine 1-Phosphate Receptors in the Mammalian Eye and Retina. Int. J. Mol. Sci. 2018, 19, 3885. [Google Scholar] [CrossRef]
- Wu, M.; Du, Y.; Liu, Y.; He, Y.; Yang, C.; Wang, W.; Gao, F. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS ONE 2014, 9, e92857. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, A.S.; Nguyen-Thanh, T.; Kang, K.P.; Lee, S.; Jang, K.Y.; Kim, M.K.; Kim, S.H.; Park, S.K.; Kim, W. Hyaluronan-induced VEGF-C promotes fibrosis-induced lymphangiogenesis via Toll-like receptor 4-dependent signal pathway. Biochem. Biophys. Res. Commun. 2015, 466, 339–345. [Google Scholar] [CrossRef]
- Chen, J.; Alexander, J.S.; Orr, A.W. Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis. Int. J. Cell Biol. 2012, 2012, 853703. [Google Scholar] [CrossRef]
- Ito, K.; Morimoto, J.; Kihara, A.; Matsui, Y.; Kurotaki, D.; Kanayama, M.; Simmons, S.; Ishii, M.; Sheppard, D.; Takaoka, A.; et al. Integrin alpha9 on lymphatic endothelial cells regulates lymphocyte egress. Proc. Natl. Acad. Sci. USA 2014, 111, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Morooka, N.; Futaki, S.; Sato-Nishiuchi, R.; Nishino, M.; Totani, Y.; Shimono, C.; Nakano, I.; Nakajima, H.; Mochizuki, N.; Sekiguchi, K. Polydom Is an Extracellular Matrix Protein Involved in Lymphatic Vessel Remodeling. Circ. Res. 2017, 120, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Altiok, E.; Ecoiffier, T.; Sessa, R.; Yuen, N.; Grimaldo, S.; Tran, C.; Li, D.; Rosner, M.; Lee, N.; Uede, T.; et al. Integrin Alpha-9 Mediates Lymphatic Valve Formation in Corneal Lymphangiogenesis. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6313–6319. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz-Krzywiec, P.; Martín-Pérez, R.; Ehling, M.; García-Caballero, M.; Pinioti, S.; Pretto, S.; Kroes, R.; Aldeni, C.; Di Matteo, M.; Prenen, H.; et al. Podoplanin-Expressing Macrophages Promote Lymphangiogenesis and Lymphoinvasion in Breast Cancer. Cell Metab. 2019, 30, 917–936.e10. [Google Scholar] [CrossRef]
- Chen, W.-S.; Cao, Z.; Sugaya, S.; Lopez, M.J.; Sendra, V.G.; Laver, N.; Leffler, H.; Nilsson, U.; Fu, J.; Song, J.; et al. Pathological lymphangiogenesis is modulated by galectin-8-dependent crosstalk between podoplanin and integrin-associated VEGFR-3. Nat. Commun. 2016, 7, 11302. [Google Scholar] [CrossRef]
- Grimaldo, S.; Yuen, N.; Ecoiffier, T.; Chen, L. Very late antigen-1 mediates corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4808–4812. [Google Scholar] [CrossRef]
- Dietrich, T.; Onderka, J.; Bock, F.; Kruse, F.E.; Vossmeyer, D.; Stragies, R.; Zahn, G.; Cursiefen, C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am. J. Pathol. 2007, 171, 361–372. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Xiang, L.X.; Shao, J.Z. Bone morphogenetic protein. Biochem. Biophys. Res. Commun. 2007, 362, 550–553. [Google Scholar] [CrossRef]
- Levet, S.; Ciais, D.; Merdzhanova, G.; Mallet, C.; Zimmers, T.A.; Lee, S.-J.; Navarro, F.; Texier, I.; Feige, J.-J.; Bailly, S.; et al. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood 2013, 122, 598–607. [Google Scholar] [CrossRef]
- Subileau, M.; Merdzhanova, G.; Ciais, D.; Collin-Faure, V.; Feige, J.-J.; Bailly, S.; Vittet, D. Bone Morphogenetic Protein 9 Regulates Early Lymphatic-Specified Endothelial Cell Expansion during Mouse Embryonic Stem Cell Differentiation. Stem Cell Rep. 2019, 12, 98–111. [Google Scholar] [CrossRef]
- Masood, F.; Chang, J.-H.; Akbar, A.; Song, A.; Hu, W.-Y.; Azar, D.T.; Rosenblatt, M.I. Therapeutic Strategies for Restoring Perturbed Corneal Epithelial Homeostasis in Limbal Stem Cell Deficiency: Current Trends and Future Directions. Cells 2022, 11, 3247. [Google Scholar] [CrossRef] [PubMed]
- Suri, C.; Jones, P.F.; Patan, S.; Bartunkova, S.; Maisonpierre, P.C.; Davis, S.; Sato, T.N.; Yancopoulos, G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 1996, 87, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Bernier-Latmani, J.; Petrova, T.V. All TIEd up: Mechanisms of Schlemm’s canal maintenance. J. Clin. Investig. 2017, 127, 3594–3597. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.; Park, D.-Y.; Bae, H.; Park, D.Y.; Kim, D.; Lee, C.-K.; Song, S.; Chung, T.-Y.; Lim, D.H.; Kubota, Y.; et al. Impaired angiopoietin/Tie2 signaling compromises Schlemm’s canal integrity and induces glaucoma. J. Clin. Investig. 2017, 127, 3877–3896. [Google Scholar] [CrossRef]
- Yan, Z.-X.; Jiang, Z.-H.; Liu, N.-F. Angiopoietin-2 promotes inflammatory lymphangiogenesis and its effect can be blocked by the specific inhibitor L1-10. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H215–H223. [Google Scholar] [CrossRef][Green Version]
- Jiao, B.; Liu, S.; Tan, X.; Lu, P.; Wang, D.; Xu, H. Class-3 semaphorins: Potent multifunctional modulators for angiogenesis-associated diseases. Biomed. Pharmacother. 2021, 137, 111329. [Google Scholar] [CrossRef]
- Uchida, Y.; James, J.M.; Suto, F.; Mukouyama, Y.-S. Class 3 semaphorins negatively regulate dermal lymphatic network formation. Biol. Open 2015, 4, 1194–1205. [Google Scholar] [CrossRef]
- Black, S.; Nelson, A.C.; Gurule, N.; Futscher, B.W.; Lyons, T.R. Semaphorin 7a exerts pleiotropic effects to promote breast tumor progression. Oncogene 2016, 35, 5170–5178. [Google Scholar] [CrossRef]
- Reuer, T.; Schneider, A.-C.; Cakir, B.; Bühler, A.D.; Walz, J.M.; Lapp, T.; Lange, C.; Agostini, H.; Schlunck, G.; Cursiefen, C.; et al. Semaphorin 3F Modulates Corneal Lymphangiogenesis and Promotes Corneal Graft Survival. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5277–5284. [Google Scholar] [CrossRef]
- Andersson, E.R.; Sandberg, R.; Lendahl, U. Notch signaling: Simplicity in design, versatility in function. Development 2011, 138, 3593–3612. [Google Scholar] [CrossRef]
- Murtomaki, A.; Uh, M.K.; Choi, Y.K.; Kitajewski, C.; Borisenko, V.; Kitajewski, J.; Shawber, C.J. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 2013, 140, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Muley, A.; Uh, M.K.; Simone, G.S.-D.; Swaminathan, B.; James, J.M.; Murtomaki, A.; Youn, S.W.; McCarron, J.D.; Kitajewski, C.; Buethe, M.G.; et al. Unique functions for Notch4 in murine embryonic lymphangiogenesis. Angiogenesis 2022, 25, 205–224. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, X.; Luo, W.; Ge, H.; Sun, D.; Liu, P. Notch Signaling Pathway Is Involved in bFGF-Induced Corneal Lymphangiogenesis and Hemangiogenesis. J. Ophthalmol. 2019, 2019, 9613923. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, R.-F.; Li, F.-F.; Liang, Y.-L.; Wang, C.; Qin, Y.-W.; Huang, S.; Zhao, X.-X.; Jing, Q. MicroRNA-126a Directs Lymphangiogenesis through Interacting with Chemokine and Flt4 Signaling in Zebrafish. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2381–2393. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.; Choi, J.-S.; Rho, C.R.; Joo, C.-K.; Lee, S.K. MicroRNA miR-466 inhibits Lymphangiogenesis by targeting prospero-related homeobox 1 in the alkali burn corneal injury model. J. Biomed. Sci. 2015, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Seo, M.; Choi, J.-S.; Joo, C.-K.; Lee, S.K. MiR-199a/b-5p Inhibits Lymphangiogenesis by Targeting Discoidin Domain Receptor 1 in Corneal Injury. Mol. Cells 2018, 41, 93–102. [Google Scholar] [PubMed]
- Du, H.-T.; Du, L.-L.; Tang, X.-L.; Ge, H.-Y.; Liu, P. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 1573–1579. [Google Scholar] [CrossRef]
- Wong, H.L.X.; Jin, G.; Cao, R.; Zhang, S.; Cao, Y.; Zhou, Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 2016, 7, 10824. [Google Scholar] [CrossRef]
- Künnapuu, J.; Bokharaie, H.; Jeltsch, M. Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs. Biology 2021, 10, 167. [Google Scholar] [CrossRef]
- Dupont, L.; Joannes, L.; Morfoisse, F.; Blacher, S.; Monseur, C.; Deroanne, C.F.; Noël, A.; Colige, A.C. ADAMTS2 and ADAMTS14 can substitute for ADAMTS3 in adults for pro-VEGFC activation and lymphatic homeostasis. JCI Insight 2022, 7, e151509. [Google Scholar] [CrossRef]
- Jeltsch, M.; Jha, S.K.; Tvorogov, D.; Anisimov, A.; Leppänen, V.-M.; Holopainen, T.; Kivelä, R.; Ortega, S.; Kärpanen, T.; Alitalo, K. CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 2014, 129, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Lee, J.L.; Kang, H.G.; Gu, N.; Byun, H.; Yeo, A.; Noh, H.; Kim, S.; Choi, E.Y.; Song, J.S.; et al. Corneal lymphangiogenesis facilitates ocular surface inflammation and cell trafficking in dry eye disease. Ocul. Surf. 2018, 16, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Ny, A.; Koch, M.; Vandevelde, W.; Schneider, M.; Fischer, C.; Diez-Juan, A.; Neven, E.; Geudens, I.; Maity, S.; Moons, L.; et al. Role of VEGF-D and VEGFR-3 in developmental lymphangiogenesis, a chemicogenetic study in Xenopus tadpoles. Blood 2008, 112, 1740–1749. [Google Scholar] [CrossRef]
- Lee, S.J.; Im, S.-T.; Wu, J.; Cho, C.S.; Jo, D.H.; Chen, Y.; Dana, R.; Kim, J.H.; Lee, S.-M. Corneal lymphangiogenesis in dry eye disease is regulated by substance P/neurokinin-1 receptor system through controlling expression of vascular endothelial growth factor receptor 3. Ocul. Surf. 2021, 22, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bignami, F.; Giacomini, C.; Lorusso, A.; Aramini, A.; Rama, P.; Ferrari, G. NK1 receptor antagonists as a new treatment for corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6783–6794. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.K.; Jin, Y.; Goyal, S.; Lee, H.S.; Fuchsluger, T.A.; Lee, H.K.; Dana, R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood 2011, 118, 4630–4634. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Maruyama, K.; Bock, F.; Saban, D.; Sadrai, Z.; Lawler, J.; Dana, R.; Masli, S. Thrombospondin 1 inhibits inflammatory lymphangiogenesis by CD36 ligation on monocytes. J. Exp. Med. 2011, 208, 1083–1092. [Google Scholar] [CrossRef]
- Schöllhorn, L.; Bock, F.; Cursiefen, C. Thrombospondin-1 as a Regulator of Corneal Inflammation and Lymphangiogenesis: Effects on Dry Eye Disease and Corneal Graft Immunology. J. Ocul. Pharmacol. Ther. 2015, 31, 376–385. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Dana, M.R.; Streilein, J.W. Corneal lymphangiogenesis: Evidence, mechanisms, and implications for corneal transplant immunology. Cornea 2003, 22, 273–281. [Google Scholar] [CrossRef]
- Hou, Y.; Bock, F.; Hos, D.; Cursiefen, C. Lymphatic Trafficking in the Eye: Modulation of Lymphatic Trafficking to Promote Corneal Transplant Survival. Cells 2021, 10, 1661. [Google Scholar] [CrossRef]
- Bachmann, B.; Taylor, R.S.; Cursiefen, C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: An evidence-based meta-analysis. Ophthalmology 2010, 117, 1300–1305.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Montana, M.; Santosa, S.M.; Isjwara, I.D.; Huang, Y.-H.; Han, K.-Y.; O’Neil, C.; Wang, A.; Cortina, M.S.; de la Cruz, J.; et al. Angiogenesis and lymphangiogenesis in corneal transplantation—A review. Surv. Ophthalmol. 2018, 63, 453–479. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting edge: Lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Cao, J.; Chen, L.; Liu, Y.; Maruyama, K.; Jackson, D.; Kruse, F.; Wiegand, S.; Dana, R.; Streilein, W. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2666–2673. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Saban, D.R.; Okanobo, A.; Nallasamy, N.; Sadrai, Z.; Chauhan, S.K.; Hajrasouliha, A.R.; Dana, R. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2411–2417. [Google Scholar] [CrossRef][Green Version]
- Dohlman, T.H.; Omoto, M.; Hua, J.; Stevenson, W.; Lee, S.M.; Chauhan, S.K.; Dana, R. VEGF-trap aflibercept significantly improves long-term graft survival in high-risk corneal transplantation. Transplantation 2015, 99, 678–686. [Google Scholar] [CrossRef]
- Albuquerque, R.J.; Hayashi, T.; Gil Cho, W.; Kleinman, M.E.; Dridi, S.; Takeda, A.; Baffi, J.Z.; Yamada, K.; Kaneko, H.; Green, M.G.; et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009, 15, 1023–1030. [Google Scholar] [CrossRef]
- Singh, N.; Tiem, M.; Watkins, R.; Cho, Y.K.; Wang, Y.; Olsen, T.; Uehara, H.; Mamalis, C.; Luo, L.; Oakey, Z.; et al. Soluble vascular endothelial growth factor receptor 3 is essential for corneal alymphaticity. Blood 2013, 121, 4242–4249. [Google Scholar] [CrossRef]
- Kang, G.J.; Truong, T.; Huang, E.; Su, V.; Ge, S.; Chen, L. Integrin Alpha 9 Blockade Suppresses Lymphatic Valve Formation and Promotes Transplant Survival. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5935–5939. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Li, G.; Sessa, R.; Kang, G.J.; Shi, M.; Ge, S.; Gong, A.J.; Wen, Y.; Chintharlapalli, S.; Chen, L. Angiopoietin-2 Blockade Promotes Survival of Corneal Transplants. Investig. Ophthalmol. Vis. Sci. 2017, 58, 79–86. [Google Scholar] [CrossRef]
- Maruyama, Y.; Maruyama, K.; Kato, Y.; Kajiya, K.; Moritoh, S.; Yamamoto, K.; Matsumoto, Y.; Sawane, M.; Kerjaschki, N.; Nakazawa, T.; et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4813–4822. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef] [PubMed]
- Mulik, S.; Sharma, S.; Suryawanshi, A.; Veiga-Parga, T.; Reddy, P.B.J.; Rajasagi, N.K.; Rouse, B.T. Activation of endothelial roundabout receptor 4 reduces the severity of virus-induced keratitis. J. Immunol. 2011, 186, 7195–7204. [Google Scholar] [CrossRef]
- Giménez, F.; Suryawanshi, A.; Rouse, B.T. Pathogenesis of herpes stromal keratitis—A focus on corneal neovascularization. Prog. Retin. Eye Res. 2013, 33, 1–9. [Google Scholar] [CrossRef]
- Park, P.J.; Chang, M.; Garg, N.; Zhu, J.; Chang, J.-H.; Shukla, D. Corneal lymphangiogenesis in herpetic stromal keratitis. Surv. Ophthalmol. 2015, 60, 60–71. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Sadrai, Z.; Saban, D.R.; Zhang, Q.; Dana, R. Corneal penetration of topical and subconjunctival bevacizumab. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8718–8723. [Google Scholar] [CrossRef]
- Foulsham, W.; Dohlman, T.H.; Mittal, S.K.; Taketani, Y.; Singh, R.B.; Masli, S.; Dana, R. Thrombospondin-1 in ocular surface health and disease. Ocul. Surf. 2019, 17, 374–383. [Google Scholar] [CrossRef]
- Hos, D.; Regenfuss, B.; Bock, F.; Onderka, J.; Cursiefen, C. Blockade of insulin receptor substrate-1 inhibits corneal lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5778–5785. [Google Scholar] [CrossRef]
- Cursiefen, C.; Viaud, E.; Bock, F.; Geudelin, B.; Ferry, A.; Kadlecová, P.; Lévy, M.; Al Mahmood, S.; Colin, S.; Thorin, E.; et al. Aganirsen antisense oligonucleotide eye drops inhibit keratitis-induced corneal neovascularization and reduce need for transplantation: The I-CAN study. Ophthalmology 2014, 121, 1683–1692. [Google Scholar] [CrossRef]
- Singh, P.; Tyagi, M.; Kumar, Y.; Gupta, K.K.; Sharma, P.D. Ocular chemical injuries and their management. Oman J. Ophthalmol. 2013, 6, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Qi, C.; Ling, S.; Li, W.; Liang, L. Lymphatic vessels correlate closely with inflammation index in alkali burned cornea. Curr. Eye Res. 2010, 35, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheng, J.; Yu, B.-J.; Zhou, L.; Xu, H.-F.; Yang, L.-L. LRG1 promotes corneal angiogenesis and lymphangiogenesis in a corneal alkali burn mouse model. Int. J. Ophthalmol. 2020, 13, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shi, S.; Xia, F.; Luo, B.; Ha, Y.; Luisi, J.; Gupta, P.K.; Merkley, K.H.; Motamedi, M.; Liu, H.; et al. CXCR3 deletion aggravates corneal neovascularization in a corneal alkali-burn model. Exp. Eye Res. 2022, 225, 109265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Q.; Hanus, J.; Anderson, C.; Zhang, H.; Dellinger, M.; Brekken, R.; Wang, S. Inhibition of multiple pathogenic pathways by histone deacetylase inhibitor SAHA in a corneal alkali-burn injury model. Mol. Pharm. 2013, 10, 307–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, W.; Gao, X.; Wang, S.; Han, K.; Ema, M.; Adams, S.; Adams, R.H.; Rosenblatt, M.I.; Chang, J.-H.; Azar, D.T. Prox1-GFP/Flt1-DsRed transgenic mice: An animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis 2017, 20, 581–598. [Google Scholar] [CrossRef]
- Zhang, W.; Schönberg, A.; Bassett, F.; Hadrian, K.; Hos, D.; Becker, M.; Bock, F.; Cursiefen, C. Different Murine High-Risk Corneal Transplant Settings Vary Significantly in Their (Lymph)angiogenic and Inflammatory Cell Signatures. Investig. Ophthalmol. Vis. Sci. 2022, 63, 18. [Google Scholar] [CrossRef]
- Giacomini, C.; Ferrari, G.; Bignami, F.; Rama, P. Alkali burn versus suture-induced corneal neovascularization in C57BL/6 mice: An overview of two common animal models of corneal neovascularization. Exp. Eye Res. 2014, 121, 1–4. [Google Scholar] [CrossRef]
- Zhu, J.; Dugas-Ford, J.; Chang, M.; Purta, P.; Han, K.-Y.; Hong, Y.-K.; Dickinson, M.; Rosenblatt, M.; Chang, J.-H.; Azar, D. Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. FEBS J. 2015, 282, 1458–1467. [Google Scholar] [CrossRef]
- Ling, S.; Qi, C.; Li, W.; Xu, J.; Kuang, W. Crucial role of corneal lymphangiogenesis for allograft rejection in alkali-burned cornea bed. Clin. Exp. Ophthalmol. 2009, 37, 874–883. [Google Scholar] [CrossRef]
- Liu, Y.; Shu, Y.; Yin, L.; Xie, T.; Zou, J.; Zhan, P.; Wang, Y.; Wei, T.; Zhu, L.; Yang, X.; et al. Protective roles of the TIR/BB-loop mimetic AS-1 in alkali-induced corneal neovascularization by inhibiting ERK phosphorylation. Exp. Eye. Res. 2021, 207, 108568. [Google Scholar] [CrossRef] [PubMed]
- Shokirova, H.; Inomata, T.; Saitoh, T.; Zhu, J.; Fujio, K.; Okumura, Y.; Yanagawa, A.; Fujimoto, K.; Sung, J.; Eguchi, A.; et al. Topical administration of the kappa opioid receptor agonist nalfurafine suppresses corneal neovascularization and inflammation. Sci. Rep. 2021, 11, 8647. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, Y.; Li, Z.; Li, H.; Chen, Y.; Chen, X.; Su, W.; Liang, D. Subconjunctival injections of dimethyl fumarate inhibit lymphangiogenesis and allograft rejection in the rat cornea. Int. Immunopharmacol. 2021, 96, 107580. [Google Scholar] [CrossRef] [PubMed]
- Salabarria, A.-C.; Koch, M.; Schönberg, A.; Zinser, E.; Hos, D.; Hamdorf, M.; Imhof, T.; Braun, G.; Cursiefen, C.; Bock, F. Topical VEGF-C/D Inhibition Prevents Lymphatic Vessel Ingrowth into Cornea but Does Not Improve Corneal Graft Survival. J. Clin. Med. 2020, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; Shin, E.Y.; Uehara, H.; Ambati, B. Antiangiogenesis Effect of Albendazole on the Cornea. J. Ocul. Pharmacol. Ther. 2019, 35, 254–261. [Google Scholar] [CrossRef]
- Hossein Pourgholami, M.; Yan Cai, Z.; Lu, Y.; Wang, L.; Lawson Morris, D. Albendazole: A potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin. Cancer Res. 2006, 12, 1928–1935. [Google Scholar] [CrossRef]
- Cho, Y.K.; Shin, E.Y.; Uehara, H.; Ambati, B. The Effect of 0.5% Timolol Maleate on Corneal(Lymph)Angiogenesis in a Murine Suture Model. J. Ocul. Pharmacol. Ther. 2018, 34, 403–409. [Google Scholar] [CrossRef]
- Dunn, F.G.; Frohlich, E.D. Pharmacokinetics, mechanisms of action, indications, and adverse effects of timolol maleate, a nonselective beta-adrenoreceptor blocking agent. Pharmacotherapy 1981, 1, 188–200. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Bower, N.I.; Koltowska, K.; Pichol-Thievend, C.; Virshup, I.; Paterson, S.; Lagendijk, A.K.; Wang, W.; Lindsey, B.W.; Bent, S.J.; Baek, S.; et al. Mural lymphatic endothelial cells regulate meningeal angiogenesis in the zebrafish. Nat. Neurosci. 2017, 20, 774–783. [Google Scholar] [CrossRef]
- van Lessen, M.; Shibata-Germanos, S.; van Impel, A.; Hawkins, T.A.; Rihel, J.; Schulte-Merker, S. Intracellular uptake of macromolecules by brain lymphatic endothelial cells during zebrafish embryonic development. Elife 2017, 6, e25932. [Google Scholar] [CrossRef] [PubMed]
- Bussmann, J.; Bos, F.L.; Urasaki, A.; Kawakami, K.; Duckers, H.J.; Schulte-Merker, S. Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk. Development 2010, 137, 2653–2657. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.A. Rejection and recipient age. Transpl. Immunol. 2002, 10, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yuen, D.; Leu, R.; Sadovnikova, A.; Chen, L. Increased lymphangiogenesis and hemangiogenesis in infant cornea. Lymphat. Res. Biol. 2011, 9, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Cursiefen, C.; Maruyama, K.; Jackson, D.G.; Streilein, J.W.; Kruse, F.E. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea 2006, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Chung, E.-S.; Saban, D.R.; Chauhan, S.K.; Dana, R. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
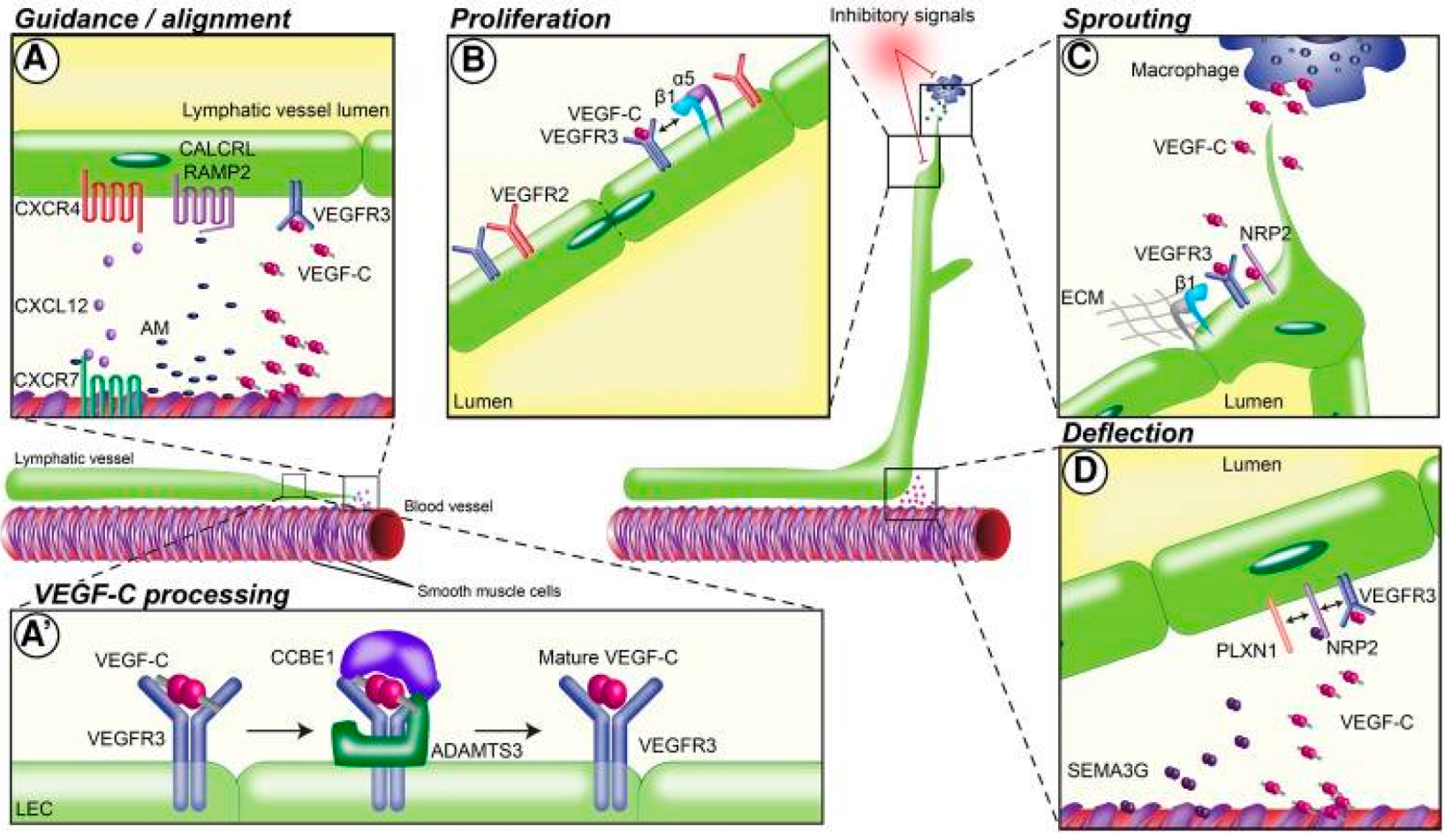
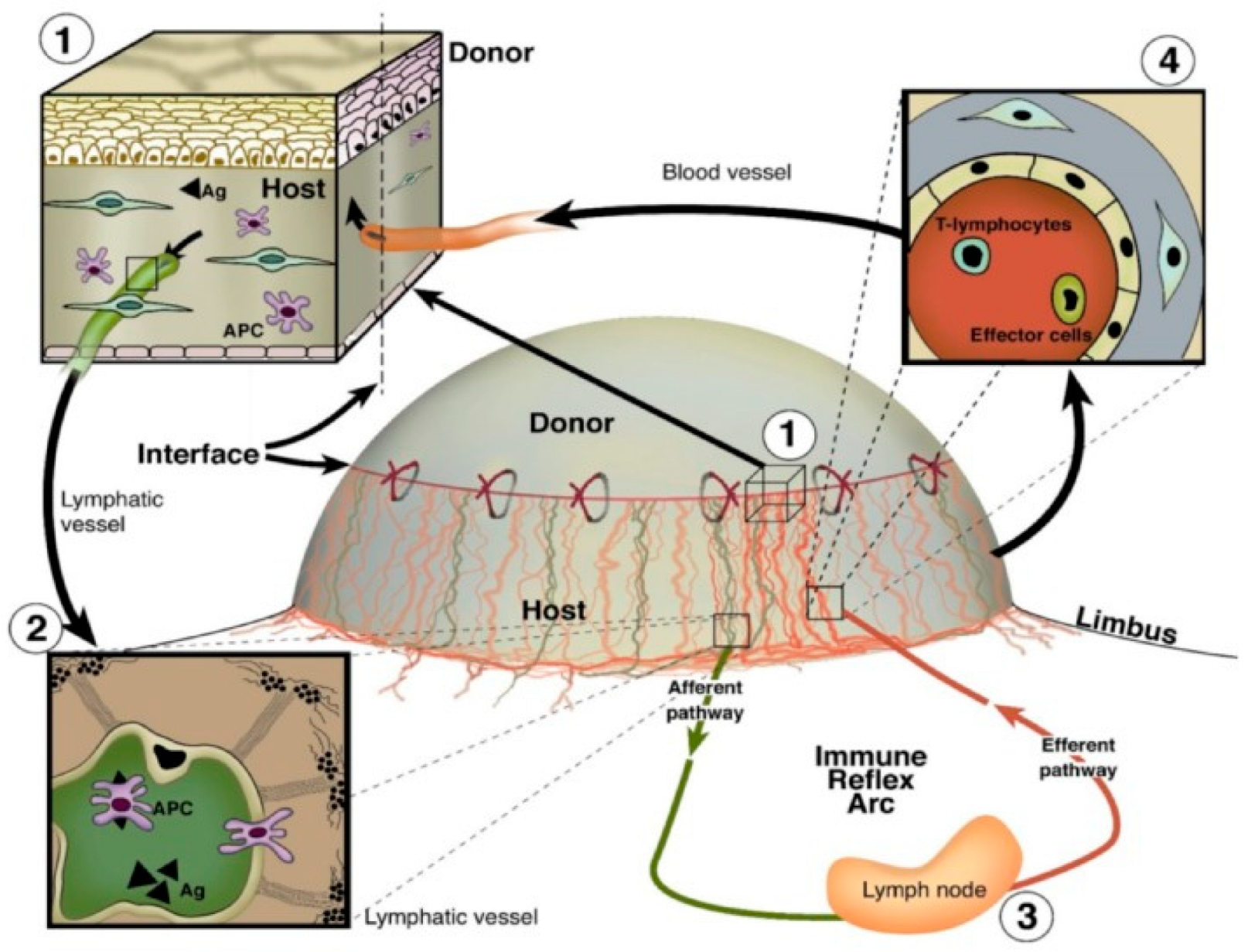
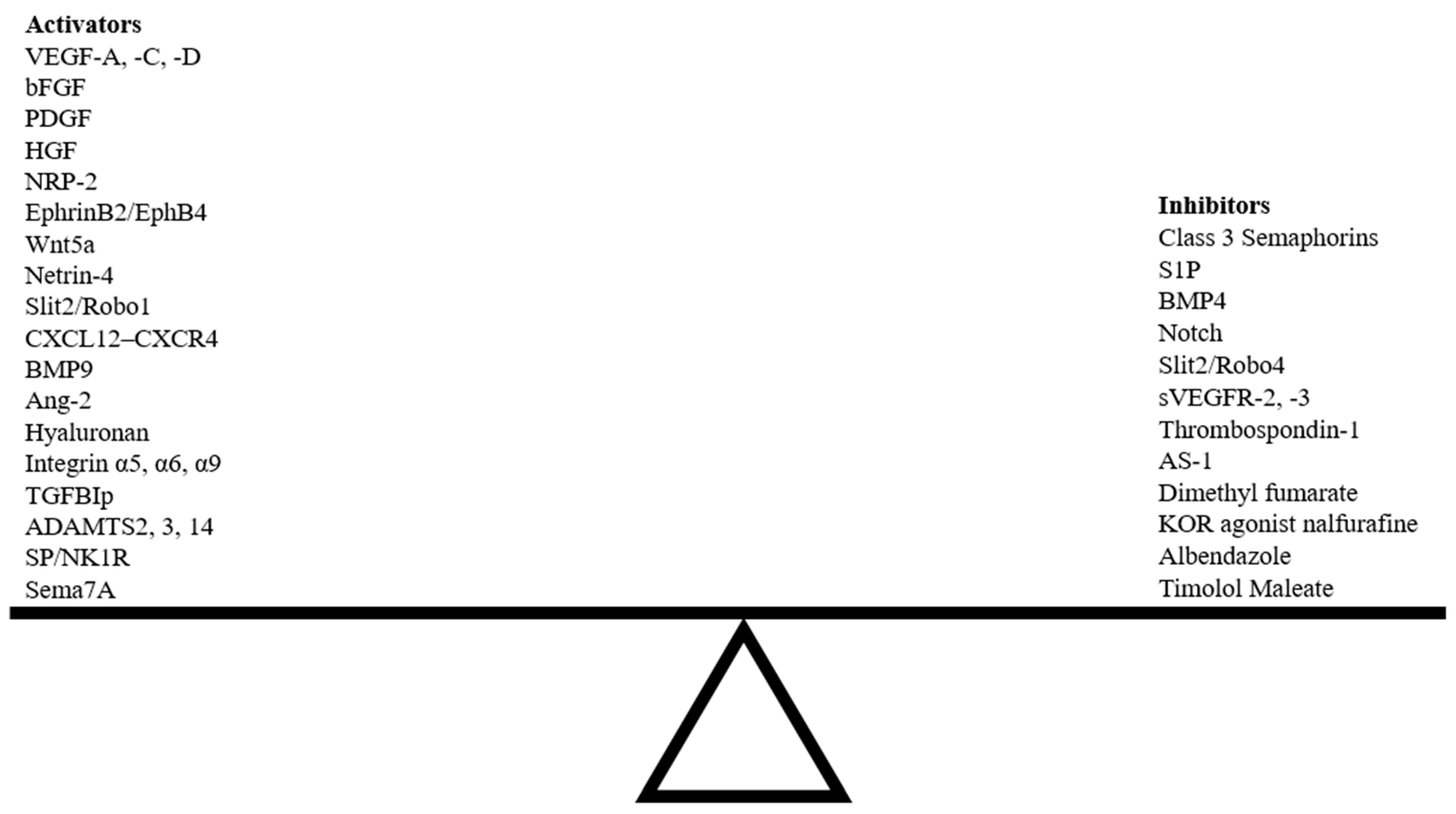
| Molecule | Signaling Pathway Targeted | Mechanism of Action | Findings | Reference(s) |
|---|---|---|---|---|
| AS1 | IL-1β/IL1R1/ MyD88/NF-κB | A synthetic Toll/IL-1 receptor (TIR)/BB-loop mimetic that prohibits interaction between IL-1RI and MyD88 | AS1 treatment decreases corneal lymphatic vessel ingrowth, VEGF-A/-C and LYVE-1 expression, and proinflammatory cytokine levels. AS1 alleviates corneal opacity, edema, and inflammatory cell infiltration post-alkali burn injury. | [211] |
| Nalfurafine | Kappa opioid receptor (KOR) signaling | KOR agonism | Nalfurafine treatment suppresses corneal lymphangiogenesis and VEGF-A/-C expression. | [212] |
| Dimethyl fumarate (DMF) | Cytokine-mediated macrophage signaling (TNF-α, IL-6, IL-1β, and VEGF-C) | Inhibition of NF-κB pathway activation in macrophages | Subconjunctival DMF injections decrease CD11b+ macrophage infiltration into the cornea and reduce mRNA expression of various proinflammatory cytokines (IL-1β, IL-6, TNF-α, MCP-1) following mouse corneal transplantation. As a result, DMF treatment inhibits macrophage-induced corneal lymphangiogenesis and decreases corneal graft rejection. | [213] |
| Topical VEGF-C/D Trap | VEGF-C/D/ VEGFR-3 | VEGF Trap (sVEGFR3 + Fc portion) binds to VEGF-C/-D and blocks activity | VEGF-C/-D Trap reduced lymphangiogenesis in a suture-induced corneal injury mouse model while increasing the frequency of CD11b+ macrophages. No benefit was observed for corneal graft survival. | [214] |
| Albendazole | VEGF/VEGFR + TNF-α | Inhibition of VEGF transcription | Treatment with albendazole inhibits corneal lymphangiogenesis and downregulates VEGF-A/-C and TNF-α expression in a suture-induced corneal injury mouse model. Combination treatment with bevacizumab offers an additive effect on lymphangiogenesis reduction. | [215,216] |
| Timolol maleate | β adrenergic receptors | Nonselective blockage of β adrenergic receptors | Treatment with timolol maleate inhibits corneal lymphangiogenesis, VEGF-A/-C and VEGFR-2/-3 expression, and inflammatory cell infiltration in a suture-induced corneal injury mouse model. | [217,218] |
| Guidance Cue | Reference/ID | Agent Name | Mechanism of Action | Indication/Disease | Trial Phase |
|---|---|---|---|---|---|
| VEGF | NCT01072357 | Bevacizumab | Anti-VEGF-A mAb | Corneal neovascularization Corneal graft failure | Phase 1/2 completed |
| NCT01868360 | Aflibercept | VEGF Trap (recombinant fusion protein) | Corneal neovascularization | Terminated | |
| NCT02342392 | Ranibizumab | VEGFR-1,-2,-3 inhibitor | Pterygium | Phase 2/3 completed | |
| Hyaluronan | NCT00599716 | Vismed | Contains sodium hyaluronate | Dry eye disease | Phase 3 completed |
| NCT01387620 | Hyaluronic acid | Source of hyaluronan | Corneal edema | Phase 4 completed | |
| NCT05313425 | Ectohylo eye drops | Contains sodium hyaluronate | Corneal haze Photorefractive keratectomy | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnam, M.; Dommaraju, S.R.; Masood, F.; Herbst, P.; Chang, J.-H.; Hu, W.-Y.; Rosenblatt, M.I.; Azar, D.T. Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea. Cells 2023, 12, 319. https://doi.org/10.3390/cells12020319
Patnam M, Dommaraju SR, Masood F, Herbst P, Chang J-H, Hu W-Y, Rosenblatt MI, Azar DT. Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea. Cells. 2023; 12(2):319. https://doi.org/10.3390/cells12020319
Chicago/Turabian StylePatnam, Mehul, Sunil R. Dommaraju, Faisal Masood, Paula Herbst, Jin-Hong Chang, Wen-Yang Hu, Mark I. Rosenblatt, and Dimitri T. Azar. 2023. "Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea" Cells 12, no. 2: 319. https://doi.org/10.3390/cells12020319
APA StylePatnam, M., Dommaraju, S. R., Masood, F., Herbst, P., Chang, J.-H., Hu, W.-Y., Rosenblatt, M. I., & Azar, D. T. (2023). Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea. Cells, 12(2), 319. https://doi.org/10.3390/cells12020319





