Establishing Hedgehog Gradients during Neural Development
Abstract
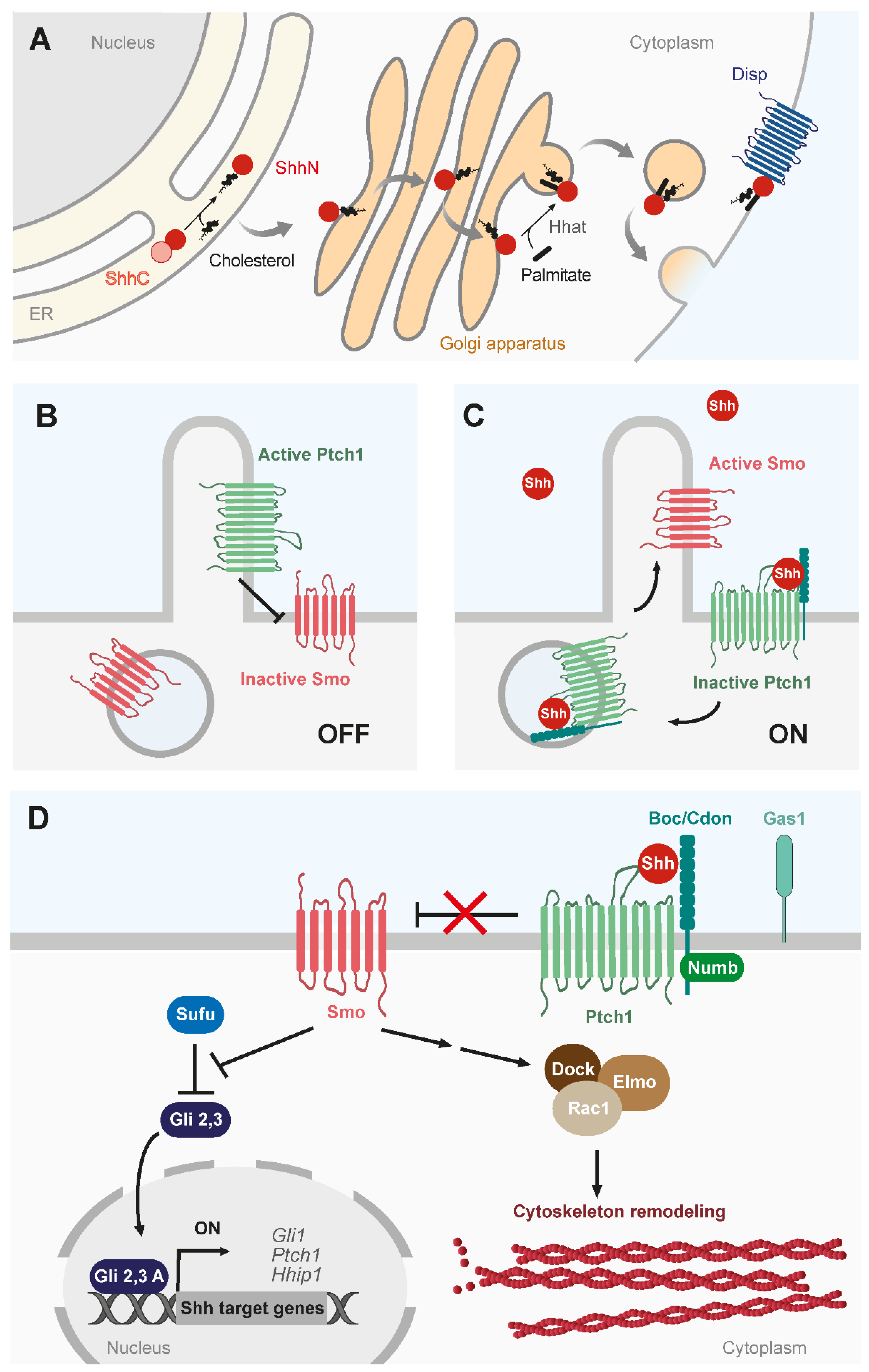
1. Introduction
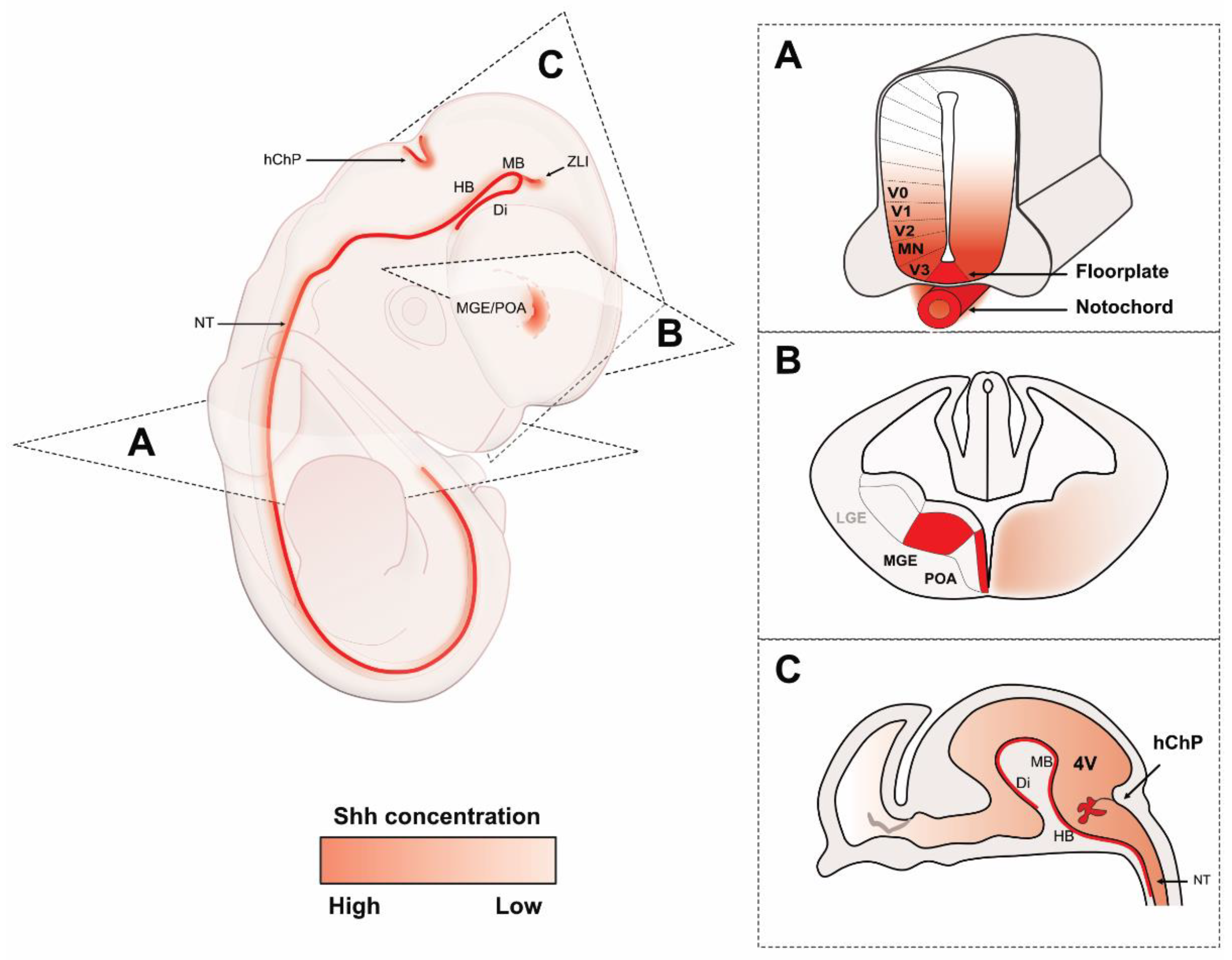
2. Shh Sources in the Vertebrate Neural Axis
2.1. Neural Tube
2.2. Anterior Shh Sources in the Neural Axis
2.3. Embryonic Cerebrospinal Fluid
2.4. Shh Sources in the Adult Brain
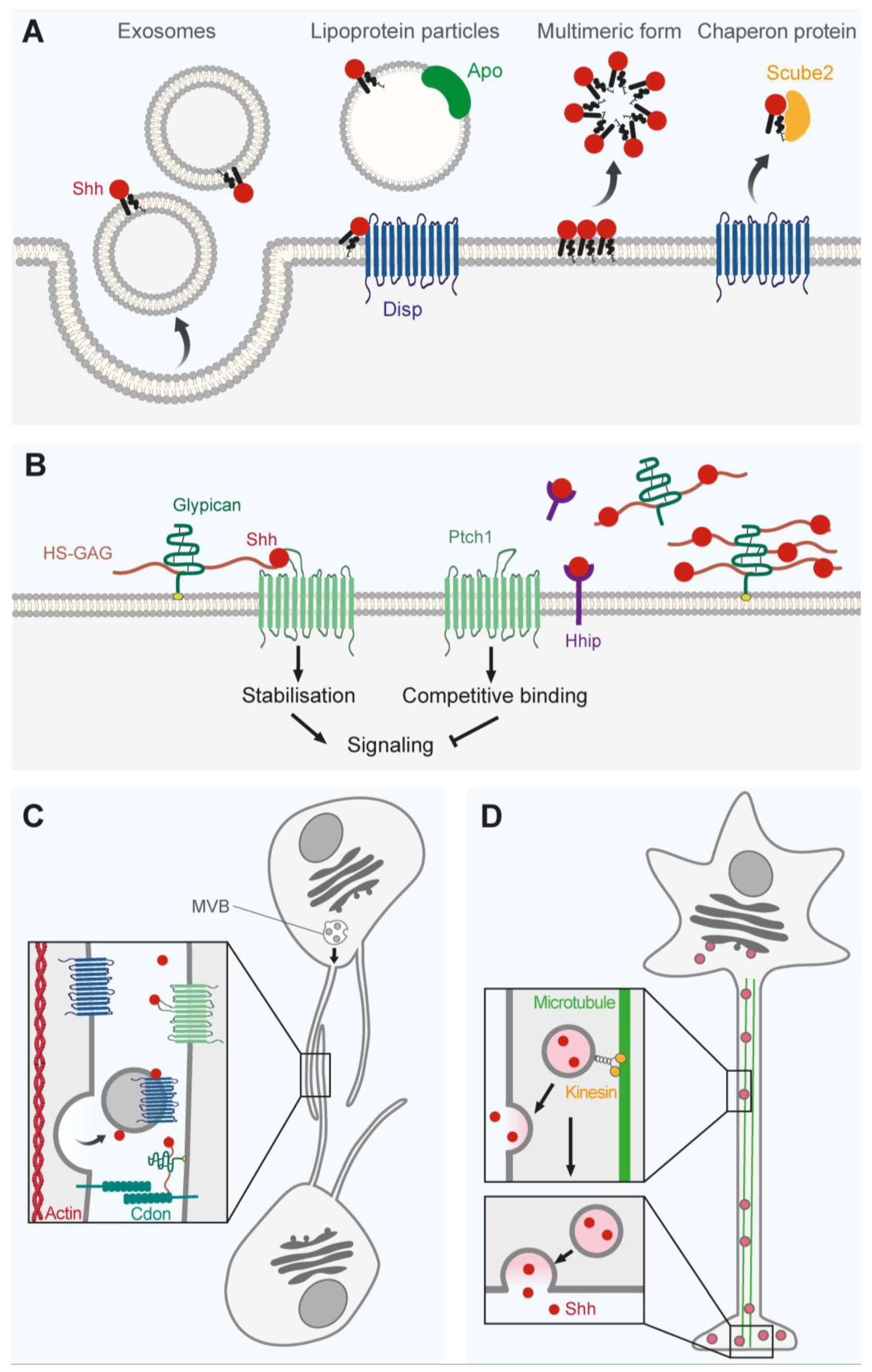
3. Shh Transport
3.1. Extracellular Vesicles and Multimers
3.2. Membrane-Bound Regulators
3.3. Cytonemes
3.4. Axonal Transport and Neuronal Communication
4. Shh Distribution Defects in Neurological Disorders
4.1. Neurodevelopmental Disorders
4.2. Cancer
4.3. Brain Injury and Inflammation
5. Challenges and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Turing, A. The Chemical Basis of Morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1952, 237, 37–72. [Google Scholar]
- Wolpert, L. The French Flag Problem: A Contribution to the Discussion on Pattern Development and Regulation. In The Origin of Life; Waddington, C.H., Ed.; Routledge: London, UK, 1968; pp. 125–133. [Google Scholar]
- Wolpert, L. Positional Information and the Spatial Pattern of Cellular Differentiation. J. Theor. Biol. 1969, 25, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Stapornwongkul, K.S.; Vincent, J.P. Generation of Extracellular Morphogen Gradients: The Case for Diffusion. Nat. Rev. Genet. 2021, 22, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Dessaud, E.; McMahon, A.P.; Briscoe, J. Pattern Formation in the Vertebrate Neural Tube: A Sonic Hedgehog Morphogen-Regulated Transcriptional Network. Development 2008, 135, 2489–2503. [Google Scholar] [CrossRef] [PubMed]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations Affecting Segment Number and Polarity in Drosophila. Nature 1980, 287, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Sagner, A.; Briscoe, J. Establishing Neuronal Diversity in the Spinal Cord: A Time and a Place. Development 2019, 146, dev.182154. [Google Scholar] [CrossRef]
- Ferent, J.; Traiffort, E. Hedgehog: Multiple Paths for Multiple Roles in Shaping the Brain and Spinal Cord. Neuroscientist 2015, 21, 356–371. [Google Scholar] [CrossRef]
- Porter, J.A.; Young, K.E.; Beachy, P.A. Cholesterol Modification of Hedgehog Signaling Proteins in Animal Development. Science 1996, 274, 255–259. [Google Scholar] [CrossRef]
- Pepinsky, R.B.; Zeng, C.; Went, D.; Rayhorn, P.; Baker, D.P.; Williams, K.P.; Bixler, S.A.; Ambrose, C.M.; Garber, E.A.; Miatkowski, K.; et al. Identification of a Palmitic Acid-Modified Form of Human Sonic Hedgehog. J. Biol. Chem. 1998, 273, 14037–14045. [Google Scholar] [CrossRef]
- Wang, Q.; Asarnow, D.E.; Ding, K.; Mann, R.K.; Hatakeyama, J.; Zhang, Y.; Ma, Y.; Cheng, Y.; Beachy, P.A. Dispatched Uses Na + Flux to Power Release of Lipid-Modified Hedgehog. Nature 2021, 599, 320–324. [Google Scholar] [CrossRef]
- Cannac, F.; Qi, C.; Falschlunger, J.; Hausmann, G.; Basler, K.; Korkhov, V.M. Cryo-EM Structure of the Hedgehog Release Protein Dispatched. Sci. Adv. 2020, 6, 2–10. [Google Scholar] [CrossRef]
- Briscoe, J.; Ericson, J. Specification of Neuronal Fates in the Ventral Neural Tube. Curr. Opin. Neurobiol. 2001, 11, 43–49. [Google Scholar] [CrossRef]
- Ingham, P.W. Hedgehog Signaling, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 149, ISBN 9780128170977. [Google Scholar]
- Okada, A.; Charron, F.; Morin, S.; Shin, D.S.; Wong, K.; Fabre, P.J.; Tessier-Lavigne, M.; McConnell, S.K. Boc Is a Receptor for Sonic Hedgehog in the Guidance of Commissural Axons. Nature 2006, 444, 369–373. [Google Scholar] [CrossRef]
- Tenzen, T.; Allen, B.L.; Cole, F.; Kang, J.S.; Krauss, R.S.; McMahon, A.P. The Cell Surface Membrane Proteins Cdo and Boc Are Components and Targets of the Hedgehog Signaling Pathway and Feedback Network in Mice. Dev. Cell 2006, 10, 647–656. [Google Scholar] [CrossRef]
- Casali, A.; Struhl, G. Reading the Hedgehog Morphogen Gradient by Measuring the Ratio of Bound to Unbound Patched Protein. Nature 2004, 431, 76–80. [Google Scholar] [CrossRef]
- Corbit, K.C.; Aanstad, P.; Singla, V.; Norman, A.R.; Stainier, D.Y.R.; Reiter, J.F. Vertebrate Smoothened Functions at the Primary Cilium. Nature 2005, 437, 1018–1021. [Google Scholar] [CrossRef]
- Santos, N.; Reiter, J.F. A Central Region of Gli2 Regulates Its Localization to the Primary Cilium and Transcriptional Activity. J. Cell Sci. 2014, 127, 1500–1510. [Google Scholar] [CrossRef]
- Thibert, C.; Teillet, M.A.; Lapointe, F.; Mazelin, L.; Le Douarin, N.M.; Mehlen, P. Inhibition of Neuroepithelial Patched-Induced Apoptosis by Sonic Hedgehog. Science 2003, 301, 843–846. [Google Scholar] [CrossRef]
- Belgacem, Y.H.; Borodinsky, L.N. Sonic Hedgehog Signaling Is Decoded by Calcium Spike Activity in the Developing Spinal Cord. Proc. Natl. Acad. Sci. USA 2011, 108, 4482–4487. [Google Scholar] [CrossRef]
- Bijlsma, M.F.; Damhofer, H.; Roelink, H. Hedgehog-Stimulated Chemotaxis Is Mediated by Smoothened Located Outside the Primary Cilium. Sci. Signal. 2012, 5, ra60. [Google Scholar] [CrossRef]
- Alvarez-Medina, R.; Cayuso, J.; Okubo, T.; Takada, S.; Martí, E. Wnt Canonical Pathway Restricts Graded Shh/Gli Patterning Activity through the Regulation of Gli3 Expression. Development 2008, 135, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Avilés, E.C.; Wilson, N.H.; Stoeckli, E.T. Sonic Hedgehog and Wnt: Antagonists in Morphogenesis but Collaborators in Axon Guidance. Front. Cell. Neurosci. 2013, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Borday, C.; Cabochette, P.; Parain, K.; Mazurier, N.; Janssens, S.; Tran, H.T.; Sekkali, B.; Bronchain, O.; Vleminckx, K.; Locker, M.; et al. Antagonistic Cross-Regulation between Wnt and Hedgehog Signalling Pathways Controls Post-Embryonic Retinal Proliferation. Development 2012, 139, 3499–3509. [Google Scholar] [CrossRef]
- Lei, Q.; Jeong, Y.; Misra, K.; Li, S.; Zelman, A.K.; Epstein, D.J.; Matise, M.P. Wnt Signaling Inhibitors Regulate the Transcriptional Response to Morphogenetic Shh-Gli Signaling in the Neural Tube. Dev. Cell 2006, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Manzari-Tavakoli, A.; Babajani, A.; Farjoo, M.H.; Hajinasrollah, M.; Bahrami, S.; Niknejad, H. The Cross-Talks among Bone Morphogenetic Protein (BMP) Signaling and Other Prominent Pathways Involved in Neural Differentiation. Front. Mol. Neurosci. 2022, 15, 827275. [Google Scholar] [CrossRef]
- Morales, A.V.; Espeso-Gil, S.; Ocaña, I.; Nieto-Lopez, F.; Calleja, E.; Bovolenta, P.; Lewandoski, M.; Diez del Corral, R. FGF Signaling Enhances a Sonic Hedgehog Negative Feedback Loop at the Initiation of Spinal Cord Ventral Patterning. Dev. Neurobiol. 2016, 76, 956–971. [Google Scholar] [CrossRef]
- Ulloa, F.; Martí, E. Wnt Won the War: Antagonistic Role of Wnt over Shh Controls Dorso-Ventral Patterning of the Vertebrate Neural Tube. Dev. Dyn. 2010, 239, 69–76. [Google Scholar] [CrossRef]
- Wu, M.; Hernandez, M.; Shen, S.; Sabo, J.K.; Kelkar, D.; Wang, J.; O’Leary, R.; Phillips, G.R.; Cate, H.S.; Casaccia, P. Differential Modulation of the Oligodendrocyte Transcriptome by Sonic Hedgehog and Bone Morphogenetic Protein 4 via Opposing Effects on Histone Acetylation. J. Neurosci. 2012, 32, 6651–6664. [Google Scholar] [CrossRef]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic Hedgehog, a Member of a Family of Putative Signaling Molecules, Is Implicated in the Regulation of CNS Polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef]
- Roelink, H.; Augsburger, A.; Heemskerk, J.; Korzh, V.; Norlin, S.; i Altaba, A.R.; Tanabe, Y.; Placzek, M.; Edlund, T.; Jessell, T.M.; et al. Floor Plate and Motor Neuron Induction by Vhh-1, a Vertebrate Homolog of Hedgehog Expressed by the Notochord. Cell 1994, 76, 761–775. [Google Scholar] [CrossRef]
- Ribes, V.; Balaskas, N.; Sasai, N.; Cruz, C.; Dessaud, E.; Cayuso, J.; Tozer, S.; Yang, L.L.; Novitch, B.; Marti, E.; et al. Distinct Sonic Hedgehog Signaling Dynamics Specify Floor Plate and Ventral Neuronal Progenitors in the Vertebrate Neural Tube. Genes Dev. 2010, 24, 1186–1200. [Google Scholar] [CrossRef]
- Arkell, R.; Beddington, R.S.P. BMP-7 Influences Pattern and Growth of the Developing Hindbrain of Mouse Embryos. Development 1997, 124, 1–12. [Google Scholar] [CrossRef]
- Patten, I.; Placzek, M. Opponent Activities of Shh and BMP Signaling during Floor Plate Induction in Vivo. Curr. Biol. 2002, 12, 47–52. [Google Scholar] [CrossRef]
- Ericson, J.; Rashbass, P.; Schedl, A.; Brenner-Morton, S.; Kawakami, A.; Van Heyningen, V.; Jessell, T.M.; Briscoe, J. Pax6 Controls Progenitor Cell Identity and Neuronal Fate in Response to Graded Shh Signaling. Cell 1997, 90, 169–180. [Google Scholar] [CrossRef]
- Roelink, H.; Porter, J.A.; Chiang, C.; Tanabe, Y.; Chang, D.T.; Beachy, P.A.; Jessell, T.M. Floor Plate and Motor Neuron Induction by Different Concentrations of the Amino-Terminal Cleavage Product of Sonic Hedgehog Autoproteolysis. Cell 1995, 81, 445–455. [Google Scholar] [CrossRef]
- Wijgerde, M.; McMahon, J.A.; Rule, M.; McMahon, A.P. A Direct Requirement for Hedgehog Signaling for Normal Specification of All Ventral Progenitor Domains in the Presumptive Mammalian Spinal Cord. Genes Dev. 2002, 16, 2849–2864. [Google Scholar] [CrossRef]
- Dessaud, E.; Ribes, V.; Balaskas, N.; Yang, L.L.; Pierani, A.; Kicheva, A.; Novitch, B.G.; Briscoe, J.; Sasai, N. Dynamic Assignment and Maintenance of Positional Identity in the Ventral Neural Tube by the Morphogen Sonic Hedgehog. PLoS Biol. 2010, 8, e1000382. [Google Scholar] [CrossRef]
- Li, P.; Markson, J.S.; Wang, S.; Chen, S.; Vachharajani, V.; Elowitz, M.B. Morphogen Gradient Reconstitution Reveals Hedgehog Pathway Design Principles. Science 2018, 360, 543–548. [Google Scholar] [CrossRef]
- Exelby, K.; Herrera-Delgado, E.; Perez, L.G.; Perez-Carrasco, R.; Sagner, A.; Metzis, V.; Sollich, P.; Briscoe, J. Precision of Tissue Patterning Is Controlled by Dynamical Properties of Gene Regulatory Networks. Development 2021, 148, dev197566. [Google Scholar] [CrossRef]
- Uygur, A.; Young, J.; Huycke, T.R.; Koska, M.; Briscoe, J.; Tabin, C.J. Scaling Pattern to Variations in Size during Development of the Vertebrate Neural Tube. Dev. Cell 2016, 37, 127–135. [Google Scholar] [CrossRef]
- Zagorski, M.; Tabata, Y.; Brandenberg, N.; Lutolf, M.P.; Tkačik, G.; Bollenbach, T.; Briscoe, J.; Kicheva, A. Decoding of Position in the Developing Neural Tube from Antiparallel Morphogen Gradients. Science 2017, 356, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Vetter, R.; Iber, D. Precision of Morphogen Gradients in Neural Tube Development. Nat. Commun. 2022, 13, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, C.E.; Jeong, J.; Guo, C.; Allen, B.L.; McMahon, A.P. Notochord-Derived Shh Concentrates in Close Association with the Apically Positioned Basal Body in Neural Target Cells and Forms a Dynamic Gradient during Neural Patterning. Development 2008, 135, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Gritli-Linde, A.; Lewis, P.; McMahon, A.P.; Linde, A. The Whereabouts of a Morphogen: Direct Evidence for Short- and Graded Long-Range Activity of Hedgehog Signaling Peptides. Dev. Biol. 2001, 236, 364–386. [Google Scholar] [CrossRef] [PubMed]
- Sloan, T.F.W.; Qasaimeh, M.A.; Juncker, D.; Yam, P.T.; Charron, F. Integration of Shallow Gradients of Shh and Netrin-1 Guides Commissural Axons. PLoS Biol. 2015, 13, e1002119. [Google Scholar] [CrossRef] [PubMed]
- Kahane, N.; Kalcheim, C. Neural Tube Development Depends on Notochord-Derived Sonic Hedgehog Released into the Sclerotome. Development 2020, 147, dev183996. [Google Scholar] [CrossRef]
- Charron, F.; Stein, E.; Jeong, J.; McMahon, A.P.; Tessier-Lavigne, M. The Morphogen Sonic Hedgehog Is an Axonal Chemoattractant That Collaborates with Netrin-1 in Midline Axon Guidance. Cell 2003, 113, 11–23. [Google Scholar] [CrossRef]
- Ferent, J.; Giguère, F.; Jolicoeur, C.; Morin, S.; Michaud, J.F.; Makihara, S.; Yam, P.T.; Cayouette, M.; Charron, F. Boc Acts via Numb as a Shh-Dependent Endocytic Platform for Ptch1 Internalization and Shh-Mediated Axon Guidance. Neuron 2019, 102, 1157–1171.e5. [Google Scholar] [CrossRef]
- Yam, P.T.; Langlois, S.D.; Morin, S.; Charron, F. Sonic Hedgehog Guides Axons through a Noncanonical, Src-Family-Kinase-Dependent Signaling Pathway. Neuron 2009, 62, 349–362. [Google Scholar] [CrossRef]
- Ferent, J.; Constable, S.; Gigante, E.D.; Yam, P.T.; Mariani, L.E.; Legué, E.; Liem, K.F.; Caspary, T.; Charron, F. The Ciliary Protein Arl13b Functions Outside of the Primary Cilium in Shh-Mediated Axon Guidance. Cell Rep. 2019, 29, 3356–3366.e3. [Google Scholar] [CrossRef]
- Makihara, S.; Morin, S.; Ferent, J.; Côté, J.-F.; Yam, P.T.; Charron, F. Polarized Dock Activity Drives Shh-Mediated Axon Guidance. Dev. Cell 2018, 46, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Yam, P.T.; Kent, C.B.; Morin, S.; Farmer, W.T.; Alchini, R.; Lepelletier, L.; Colman, D.R.; Tessier-Lavigne, M.; Fournier, A.E.; Charron, F. 14-3-3 Proteins Regulate a Cell-Intrinsic Switch from Sonic Hedgehog-Mediated Commissural Axon Attraction to Repulsion after Midline Crossing. Neuron 2012, 76, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Dominici, C.; Moreno-Bravo, J.A.; Puiggros, S.R.; Rappeneau, Q.; Rama, N.; Vieugue, P.; Bernet, A.; Mehlen, P.; Chédotal, A. Floor-Plate-Derived Netrin-1 Is Dispensable for Commissural Axon Guidance. Nature 2017, 545, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Varadarajan, S.G.; Kong, J.H.; Phan, K.D.; Kao, T.J.; Panaitof, S.C.; Cardin, J.; Eltzschig, H.; Kania, A.; Novitch, B.G.; Butler, S.J. Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 2017, 94, 790–799.e3. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Bravo, J.A.; Puiggros, S.R.; Blockus, H.; Dominici, C.; Zelina, P.; Mehlen, P.; Chédotal, A. Commissural Neurons Transgress the Cns/Pns Boundary in Absence of Ventricular Zone-Derived Netrin 1. Development 2018, 145, dev159400. [Google Scholar] [CrossRef]
- Wu, Z.; Makihara, S.; Yam, P.T.; Teo, S.; Renier, N.; Balekoglu, N.; Moreno-Bravo, J.A.; Olsen, O.; Chédotal, A.; Charron, F.; et al. Long-Range Guidance of Spinal Commissural Axons by Netrin1 and Sonic Hedgehog from Midline Floor Plate Cells. Neuron 2019, 101, 635–647.e4. [Google Scholar] [CrossRef]
- Aoto, K.; Shikata, Y.; Imai, H.; Matsumaru, D.; Tokunaga, T.; Shioda, S.; Yamada, G.; Motoyama, J. Mouse Shh Is Required for Prechordal Plate Maintenance during Brain and Craniofacial Morphogenesis. Dev. Biol. 2009, 327, 106–120. [Google Scholar] [CrossRef]
- Amano, T. Gene Regulatory Landscape of the Sonic Hedgehog Locus in Embryonic Development. Dev. Growth Differ. 2020, 62, 334–342. [Google Scholar] [CrossRef]
- Shimamura, K.; Rubenstein, J.L.R. Inductive Interactions Direct Early Regionalization of the Mouse Forebrain. Development 1997, 124, 2709–2718. [Google Scholar] [CrossRef]
- Pera, E.M.; Kessel, M. Patterning of the Chick Forebrain Anlage by the Prechordal Plate. Development 1997, 124, 4153–4162. [Google Scholar] [CrossRef]
- Dale, J.K.; Vesque, C.; Lints, T.J.; Sampath, T.K.; Furley, A.; Dodd, J.; Placzek, M. Cooperation of BMP7 and SHH in the Induction of Forebrain Ventral Midline Cells by Prechordal Mesoderm. Cell 1997, 90, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Sagai, T.; Amano, T.; Maeno, A.; Ajima, R.; Shiroishi, T. SHH Signaling Mediated by a Prechordal and Brain Enhancer Controls Forebrain Organization. Proc. Natl. Acad. Sci. USA 2019, 116, 23636–23642. [Google Scholar] [CrossRef] [PubMed]
- Szabó, N.E.; Zhao, T.; Zhou, X.; Alvarez-Bolado, G. The Role of Sonic Hedgehog of Neural Origin in Thalamic Differentiation in the Mouse. J. Neurosci. 2009, 29, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; McMahon, A.P. A Sonic Hedgehog-Dependent Signaling Relay Regulates Growth of Diencephalic and Mesencephalic Primordia in the Early Mouse Embryo. Development 2002, 129, 4807–4819. [Google Scholar] [CrossRef] [PubMed]
- Szabó, N.E.; Zhao, T.; Çankaya, M.; Theil, T.; Zhou, X.; Alvarez-Bolado, G. Role of Neuroepithelial Sonic Hedgehog in Hypothalamic Patterning. J. Neurosci. 2009, 29, 6989–7002. [Google Scholar] [CrossRef]
- Kitamura, K.; Miura, H.; Yanazawa, M.; Miyashita, T.; Kato, K. Expression Patterns of Brx1 (Rieg Gene), Sonic Hedgehog, Nkx2.2, Dlx1 and Arx during Zona Limitans Intrathalamica and Embryonic Ventral Lateral Geniculate Nuclear Formation. Mech. Dev. 1997, 67, 83–96. [Google Scholar] [CrossRef]
- Kiecker, C.; Lumsden, A. Hedgehog Signaling from the ZLI Regulates Diencephalic Regional Identity. Nat. Neurosci. 2004, 7, 1242–1249. [Google Scholar] [CrossRef]
- Andreu-Cervera, A.; Catala, M.; Schneider-Maunoury, S. Cilia, Ciliopathies and Hedgehog-Related Forebrain Developmental Disorders. Neurobiol. Dis. 2021, 150, 105236. [Google Scholar] [CrossRef]
- Andreu-Cervera, A.; Anselme, I.; Karam, A.; Laclef, C.; Catala, M.; Schneider-Maunoury, S. The Ciliopathy Gene Ftm/Rpgrip1l Controls Mouse Forebrain Patterning via Region-Specific Modulation of Hedgehog/Gli Signaling. J. Neurosci. 2019, 39, 2398–2415. [Google Scholar] [CrossRef]
- Ericson, J.; Muhr, J.; Placzek, M.; Lints, T.; Jessel, T.M.; Edlund, T. Sonic Hedgehog Induces the Differentiation of Ventral Forebrain Neurons: A Common Signal for Ventral Patterning within the Neural Tube. Cell 1995, 81, 747–756. [Google Scholar] [CrossRef]
- Gunhaga, L.; Jessell, T.M.; Edlund, T. Sonic Hedgehog Signaling at Gastrula Stages Specifies Ventral Telencephalic Cells in the Chick Embryo. Development 2000, 127, 3283–3293. [Google Scholar] [CrossRef] [PubMed]
- Kohtz, J.D.; Baker, D.P.; Corte, G.; Fishell, G. Regionalization within the Mammalian Telencephalon Is Mediated by Changes in Responsiveness to Sonic Hedgehog. Development 1998, 125, 5079–5089. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Litingtung, Y.; Lee, E.; Youngt, K.E.; Cordent, J.L.; Westphal, H.; Beachyt, P.A. Mice Lacking Sonic Hedgehog Gene Function. Nature 1996, 383, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Gofflot, F.; Kolf-Clauw, M.; Clotman, F.; Roux, C.; Picard, J.J. Absence of Ventral Cell Populations in the Developing Brain in a Rat Model of the Smith-Lemli-Opitz Syndrome. Am. J. Med. Genet. 1999, 87, 207–216. [Google Scholar] [CrossRef]
- Sussel, L.; Marin, O.; Kimura, S.; Rubenstein, J.L.R. Loss of Nkx2.1 Homeobox Gene Function Results in a Ventral to Dorsal Molecular Respecification within the Basal Telencephalon: Evidence for a Transformation of the Pallidum into the Striatum. Development 1999, 126, 3359–3370. [Google Scholar] [CrossRef]
- Gulacsi, A.; Anderson, S.A. Shh Maintains Nkx2.1 in the MGE by a Gli3-Independent Mechanism. Cereb. Cortex 2006, 16, 89–95. [Google Scholar] [CrossRef]
- Flandin, P.; Zhao, Y.; Vogt, D.; Jeong, J.; Long, J.; Potter, G.; Westphal, H.; Rubenstein, J.L.R. Lhx6 and Lhx8 Coordinately Induce Neuronal Expression of Shh That Controls the Generation of Interneuron Progenitors. Neuron 2011, 70, 939–950. [Google Scholar] [CrossRef]
- Gelman, D.M.; Martini, F.J.; Nóbrega-Pereira, S.; Pierani, A.; Kessaris, N.; Marín, O. The Embryonic Preoptic Area Is a Novel Source of Cortical GABAergic Interneurons. J. Neurosci. 2009, 29, 9380–9389. [Google Scholar] [CrossRef]
- Winkler, C.C.; Yabut, O.R.; Fregoso, S.P.; Gomez, H.G.; Dwyer, B.E.; Pleasure, S.J.; Franco, S.J. The Dorsal Wave of Neocortical Oligodendrogenesis Begins Embryonically and Requires Multiple Sources of Sonic Hedgehog. J. Neurosci. 2018, 38, 5237–5250. [Google Scholar] [CrossRef]
- Moreau, M.X.; Saillour, Y.; Cwetsch, A.W.; Pierani, A.; Causeret, F. Single-Cell Transcriptomics of the Early Developing Mouse Cerebral Cortex Disentangle the Spatial and Temporal Components of Neuronal Fate Acquisition. Development 2021, 148, dev197962. [Google Scholar] [CrossRef]
- Baudoin, J.P.; Viou, L.; Launay, P.S.; Luccardini, C.; Espeso Gil, S.; Kiyasova, V.; Irinopoulou, T.; Alvarez, C.; Rio, J.P.; Boudier, T.; et al. Tangentially Migrating Neurons Assemble a Primary Cilium That Promotes Their Reorientation to the Cortical Plate. Neuron 2012, 76, 1108–1122. [Google Scholar] [CrossRef]
- Brady, M.V.; Vaccarino, F.M. Role of Shh in Patterning Human Pluripotent Cells towards Ventral Forebrain Fates. Cells 2021, 10, 914. [Google Scholar] [CrossRef]
- Xiang, Y.; Tanaka, Y.; Patterson, B.; Kang, Y.J.; Govindaiah, G.; Roselaar, N.; Cakir, B.; Kim, K.Y.; Lombroso, A.P.; Hwang, S.M.; et al. Fusion of Regionally Specified HPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 2017, 21, 383–398.e7. [Google Scholar] [CrossRef]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of Functionally Integrated Human Forebrain Spheroids. Nature 2017, 545, 54–59. [Google Scholar] [CrossRef]
- Ozone, C.; Suga, H.; Eiraku, M.; Kadoshima, T.; Yonemura, S.; Takata, N.; Oiso, Y.; Tsuji, T.; Sasai, Y. Functional Anterior Pituitary Generated in Self-Organizing Culture of Human Embryonic Stem Cells. Nat. Commun. 2016, 7, 10351. [Google Scholar] [CrossRef]
- Huang, W.K.; Wong, S.Z.H.; Pather, S.R.; Nguyen, P.T.T.; Zhang, F.; Zhang, D.Y.; Zhang, Z.; Lu, L.; Fang, W.; Chen, L.; et al. Generation of Hypothalamic Arcuate Organoids from Human Induced Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 1657–1670.e10. [Google Scholar] [CrossRef]
- Cederquist, G.Y.; Asciolla, J.J.; Tchieu, J.; Walsh, R.M.; Cornacchia, D.; Resh, M.D.; Studer, L. Specification of Positional Identity in Forebrain Organoids. Nat. Biotechnol. 2019, 37, 436–444. [Google Scholar] [CrossRef]
- Bitgood, M.J.; McMahon, A.P. Hedgehog and Bmp Genes Are Coexpressed at Many Diverse Sites of Cell-Cell Interaction in the Mouse Embryo. Dev. Biol. 1995, 172, 126–138. [Google Scholar] [CrossRef]
- Huang, X.; Ketova, T.; Fleming, J.T.; Wang, H.; Dey, S.K.; Litingtung, Y.; Chiang, C. Sonic Hedgehog Signaling Regulates a Novel Epithelial Progenitor Domain of the Hindbrain Choroid Plexus. Development 2009, 136, 2535–2543. [Google Scholar] [CrossRef]
- Dani, N.; Herbst, R.H.; McCabe, C.; Green, G.S.; Kaiser, K.; Head, J.P.; Cui, J.; Shipley, F.B.; Jang, A.; Dionne, D.; et al. A Cellular and Spatial Map of the Choroid Plexus across Brain Ventricles and Ages. Cell 2021, 184, 3056–3074.e21. [Google Scholar] [CrossRef]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and Functions of the Choroid Plexus-Cerebrospinal Fluid System. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Johnson, M.B.; Broadbelt, K.G.; Watanabe, M.; Kang, Y.J.; Chau, K.F.; Springel, M.W.; Malesz, A.; Sousa, A.M.M.; Pletikos, M.; et al. Spatially Heterogeneous Choroid Plexus Transcriptomes Encode Positional Identity and Contribute to Regional CSF Production. J. Neurosci. 2015, 35, 4903–4916. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.F.; Springel, M.W.; Broadbelt, K.G.; Park, H.y.; Topal, S.; Lun, M.P.; Mullan, H.; Maynard, T.; Steen, H.; LaMantia, A.S.; et al. Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes Following Neural Tube Closure. Dev. Cell 2015, 35, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, J.; Ketova, T.; Fleming, J.T.; Grover, V.K.; Cooper, M.K.; Litingtung, Y.; Chiang, C. Transventricular Delivery of Sonic Hedgehog Is Essential to Cerebellar Ventricular Zone Development. Proc. Natl. Acad. Sci. USA 2010, 107, 8422–8427. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Grausam, K.B.; Wang, J.; Lun, M.P.; Ohli, J.; Lidov, H.G.W.; Calicchio, M.L.; Zeng, E.; Salisbury, J.L.; Wechsler-Reya, R.J.; et al. Sonic Hedgehog Promotes Proliferation of Notch-Dependent Monociliated Choroid Plexus Tumour Cells. Nat. Cell Biol. 2016, 18, 418–430. [Google Scholar] [CrossRef]
- Nielsen, C.M.; Dymecki, S.M. Sonic Hedgehog Is Required for Vascular Outgrowth in the Hindbrain Choroid Plexus. Dev. Biol. 2010, 340, 430–437. [Google Scholar] [CrossRef]
- Dahmane, N.; Ruiz, I.; Altaba, A. Sonic Hedgehog Regulates the Growth and Patterning of the Cerebellum. Development 1999, 126, 3089–3100. [Google Scholar] [CrossRef]
- De Luca, A.; Parmigiani, E.; Tosatto, G.; Martire, S.; Hoshino, M.; Buffo, A.; Leto, K.; Rossi, F. Exogenous Sonic Hedgehog Modulates the Pool of GABAergic Interneurons During Cerebellar Development. Cerebellum 2015, 14, 72–85. [Google Scholar] [CrossRef]
- Corrales, J.M.D.; Rocco, G.L.; Blaess, S.; Guo, Q.; Joyner, A.L. Spatial Pattern of Sonic Hedgehog Signaling through Gli Genes during Cerebellum Development. Development 2004, 131, 5581–5590. [Google Scholar] [CrossRef]
- Wallace, V.A. Purkinje-Cell-Derived Sonic Hedgehog Regulates Granule Neuron Precursor Cell Proliferation in the Developing Mouse Cerebellum. Curr. Biol. 1999, 9, 445–448. [Google Scholar] [CrossRef]
- Shqirat, M.; Kinoshita, A.; Kageyama, R.; Ohtsuka, T. Sonic Hedgehog Expands Neural Stem Cells in the Neocortical Region Leading to an Expanded and Wrinkled Neocortical Surface. Genes Cells 2021, 26, 399–410. [Google Scholar] [CrossRef]
- Yabut, O.R.; Pleasure, S.J. Sonic Hedgehog Signaling Rises to the Surface: Emerging Roles in Neocortical Development. Brain Plast. 2018, 3, 119–128. [Google Scholar] [CrossRef]
- Komada, M.; Saitsu, H.; Kinboshi, M.; Miura, T.; Shiota, K.; Ishibashi, M. Hedgehog Signaling Is Involved in Development of the Neocortex. Development 2008, 135, 2717–2727. [Google Scholar] [CrossRef]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic Hedgehog Is Required for Progenitor Cell Maintenance in Telencephalic Stem Cell Niches. Neuron 2003, 40, 189–190, Erratum in: Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef]
- Charytoniuk, D.; Porcel, B.; Rodriíguez Gomez, J.; Faure, H.; Ruat, M.; Traiffort, E. Sonic Hedgehog Signalling in the Developing and Adult Brain. J. Physiol. Paris 2002, 96, 9–16. [Google Scholar] [CrossRef]
- Traiffort, E.; Charytoniuk, D.A.; Faure, H.; Ruat, M. Regional Distribution of Sonic Hedgehog, Patched, and Smoothened MRNA in the Adult Rat Brain. J. Neurochem. 1998, 70, 1327–1330. [Google Scholar] [CrossRef]
- Wechsler-Reya, R.J.; Scott, M.P. Control of Neuronal Precursor Proliferation in the Cerebellum by Sonic Hedgehog. Neuron 1999, 22, 103–114. [Google Scholar] [CrossRef]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog Signaling and Primary Cilia Are Required for the Formation of Adult Neural Stem Cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef]
- Lai, K.; Kaspar, B.K.; Gage, F.H.; Schaffer, D.V. Sonic Hedgehog Regulates Adult Neural Progenitor Proliferation in Vitro and in Vivo. Nat. Neurosci. 2003, 6, 21–27. [Google Scholar] [CrossRef]
- Angot, E.; Loulier, K.; Nguyen-Ba-Charvet, K.T.; Gadeau, A.-P.; Ruat, M.; Traiffort, E. Chemoattractive Activity of Sonic Hedgehog in the Adult Subventricular Zone Modulates the Number of Neural Precursors Reaching the Olfactory Bulb. Stem Cells 2008, 26, 2311–2320. [Google Scholar] [CrossRef]
- Ferent, J.; Zimmer, C.; Durbec, P.; Ruat, M.; Traiffort, E. Sonic Hedgehog Signaling Is a Positive Oligodendrocyte Regulator during Demyelination. J. Neurosci. 2013, 33, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Ihrie, R.A.; Shah, J.K.; Harwell, C.C.; Levine, J.H.; Guinto, C.D.; Lezameta, M.; Kriegstein, A.R.; Alvarez-Buylla, A. Persistent Sonic Hedgehog Signaling in Adult Brain Determines Neural Stem Cell Positional Identity. Neuron 2011, 71, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Harwell, C.C.; Parker, P.R.L.; Gee, S.M.; Okada, A.; McConnell, S.K.; Kreitzer, A.C.; Kriegstein, A.R. Sonic Hedgehog Expression in Corticofugal Projection Neurons Directs Cortical Microcircuit Formation. Neuron 2012, 73, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kuan, A.T.; Wang, W.; Herbert, Z.T.; Mosto, O.; Olukoya, O.; Adam, M.; Vu, S.; Kim, M.; Tran, D.; et al. Astrocyte-Neuron Crosstalk through Hedgehog Signaling Mediates Cortical Synapse Development. Cell Rep. 2022, 38, 110416. [Google Scholar] [CrossRef] [PubMed]
- Tirou, L.; Russo, M.; Faure, H.; Pellegrino, G.; Sharif, A.; Ruat, M. C9C5 Positive Mature Oligodendrocytes Are a Source of Sonic Hedgehog in the Mouse Brain. PLoS ONE 2020, 15, e0229362. [Google Scholar] [CrossRef]
- Creanga, A.; Glenn, T.D.; Mann, R.K.; Saunders, A.M.; Talbot, W.S.; Beachy, P.A. Scube/You Activity Mediates Release of Dually Lipid-Modified Hedgehog Signal in Soluble Form. Genes Dev. 2012, 26, 1312–1325. [Google Scholar] [CrossRef]
- Huang, P.; Wierbowski, B.M.; Lian, T.; Chan, C.; García-Linares, S.; Jiang, J.; Salic, A. Structural Basis for Catalyzed Assembly of the Sonic Hedgehog–Patched1 Signaling Complex. Dev. Cell 2022, 57, 670–685.e8. [Google Scholar] [CrossRef]
- Petrov, K.; Wierbowski, B.M.; Liu, J.; Salic, A. Distinct Cation Gradients Power Cholesterol Transport at Different Key Points in the Hedgehog Signaling Pathway. Dev. Cell 2020, 55, 314–327.e7. [Google Scholar] [CrossRef]
- Wierbowski, B.M.; Petrov, K.; Aravena, L.; Gu, G.; Xu, Y.; Salic, A. Hedgehog Pathway Activation Requires Coreceptor-Catalyzed, Lipid-Dependent Relay of the Sonic Hedgehog Ligand. Dev. Cell 2020, 55, 450–467.e8. [Google Scholar] [CrossRef]
- Gradilla, A.C.; González, E.; Seijo, I.; Andrés, G.; Bischoff, M.; González-Mendez, L.; Sánchez, V.; Callejo, A.; Ibáñez, C.; Guerra, M.; et al. Exosomes as Hedgehog Carriers in Cytoneme-Mediated Transport and Secretion. Nat. Commun. 2014, 5, 5649. [Google Scholar] [CrossRef]
- Panáková, D.; Sprong, H.; Marois, E.; Thiele, C.; Eaton, S. Lipoprotein Particles Are Required for Hedgehog and Wingless Signalling. Nature 2005, 435, 58–65. [Google Scholar] [CrossRef]
- Plochberger, B.; Sych, T.; Weber, F.; Novacek, J.; Axmann, M.; Stangl, H.; Sezgin, E. Lipoprotein Particles Interact with Membranes and Transfer Their Cargo without Receptors. Biochemistry 2020, 59, 4421–4428. [Google Scholar] [CrossRef]
- Palm, W.; Swierczynska, M.M.; Kumari, V.; Ehrhart-Bornstein, M.; Bornstein, S.R.; Eaton, S. Secretion and Signaling Activities of Lipoprotein-Associated Hedgehog and Non-Sterol-Modified Hedgehog in Flies and Mammals. PLOS Biol. 2013, 11, e1001505. [Google Scholar] [CrossRef]
- Coulter, M.E.; Dorobantu, C.M.; Lodewijk, G.A.; Delalande, F.; Cianferani, S.; Ganesh, V.S.; Smith, R.S.; Lim, E.T.; Xu, C.S.; Pang, S.; et al. The ESCRT-III Protein CHMP1A Mediates Secretion of Sonic Hedgehog on a Distinctive Subtype of Extracellular Vesicles. Cell Rep. 2018, 24, 973–986. [Google Scholar] [CrossRef]
- Parchure, A.; Vyas, N.; Ferguson, C.; Parton, R.G.; Mayor, S. Oligomerization and Endocytosis of Hedgehog Is Necessary for Its Efficient Exovesicular Secretion. Mol. Biol. Cell 2015, 26, 4700. [Google Scholar] [CrossRef]
- Vyas, N.; Walvekar, A.; Tate, D.; Lakshmanan, V.; Bansal, D.; Lo Cicero, A.; Raposo, G.; Palakodeti, D.; Dhawan, J. Vertebrate Hedgehog Is Secreted on Two Types of Extracellular Vesicles with Different Signaling Properties. Sci. Rep. 2014, 4, 7357. [Google Scholar] [CrossRef]
- Juan, T.; Fürthauer, M. Biogenesis and Function of ESCRT-Dependent Extracellular Vesicles. Semin. Cell Dev. Biol. 2018, 74, 66–77. [Google Scholar] [CrossRef]
- Zeng, X.; Goetz, J.A.; Suber, L.M.; Scott, W.J.; Schreiner, C.M.; Robbins, D.J. A Freely Diffusible Form of Sonic Hedgehog Mediates Long-Range Signalling. Nature 2001, 411, 716–720. [Google Scholar] [CrossRef]
- Chen, M.H.; Li, Y.J.; Kawakami, T.; Xu, S.M.; Chuang, P.T. Palmitoylation Is Required for the Production of a Soluble Multimeric Hedgehog Protein Complex and Long-Range Signaling in Vertebrates. Genes Dev. 2004, 18, 641–659. [Google Scholar] [CrossRef]
- Vyas, N.; Goswami, D.; Manonmani, A.; Sharma, P.; Ranganath, H.A.; VijayRaghavan, K.; Shashidhara, L.S.; Sowdhamini, R.; Mayor, S. Nanoscale Organization of Hedgehog Is Essential for Long-Range Signaling. Cell 2008, 133, 1214–1227. [Google Scholar] [CrossRef]
- Chuang, P.T.; McMahon, A.P. Vertebrate Hedgehog Signalling Modulated by Induction of a Hedgehog-Binding Protein. Nature 1999, 397, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Loulier, K.; Ruat, M.; Traiffort, E. Analysis of Hedgehog Interacting Protein in the Brain and Its Expression in Nitric Oxide Synthase-Positive Cells. Neuroreport 2005, 16, 1959–1962. [Google Scholar] [CrossRef] [PubMed]
- Holtz, A.M.; Peterson, K.A.; Nishi, Y.; Morin, S.; Song, J.Y.; Charron, F.; Mcmahon, A.P.; Allen, B.L. Essential Role for Ligand-Dependent Feedback Antagonism of Vertebrate Hedgehog Signaling by PTCH1, PTCH2 AND HHIP1 during Neural Patterning. Development 2013, 140, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; McMahon, A.P. Growth and Pattern of the Mammalian Neural Tube Are Governed by Partially Overlapping Feedback Activities of the Hedgehog Antagonists Patched 1 and Hhip1. Development 2005, 132, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Bishop, B.; Aricescu, A.R.; Harlos, K.; O’Callaghan, C.A.; Jones, E.Y.; Siebold, C. Structural Insights into Hedgehog Ligand Sequestration by the Human Hedgehog-Interacting Protein HHIP. Nat. Struct. Mol. Biol. 2009, 16, 698–703. [Google Scholar] [CrossRef]
- Bosanac, I.; Maun, H.R.; Scales, S.J.; Wen, X.; Lingel, A.; Bazan, J.F.; De Sauvage, F.J.; Hymowitz, S.G.; Lazarus, R.A. The Structure of SHH in Complex with HHIP Reveals a Recognition Role for the Shh Pseudo Active Site in Signaling. Nat. Struct. Mol. Biol. 2009, 16, 691–697. [Google Scholar] [CrossRef]
- Holtz, A.M.; Griffiths, S.C.; Davis, S.J.; Bishop, B.; Siebold, C.; Allen, B.L. Secreted HHIP1 Interacts with Heparan Sulfate and Regulates Hedgehog Ligand Localization and Function. J. Cell Biol. 2015, 209, 739–758. [Google Scholar] [CrossRef]
- Bourikas, D.; Pekarik, V.; Baeriswyl, T.; Grunditz, Å.; Sadhu, R.; Nardó, M.; Stoeckli, E.T. Sonic Hedgehod Guides Commissural Axons along the Longitudinal Axis of the Spinal Cord. Nat. Neurosci. 2005, 8, 297–304. [Google Scholar] [CrossRef]
- Filmus, J.; Capurro, M. The Role of Glypicans in Hedgehog Signaling. Matrix Biol. 2014, 35, 248–252. [Google Scholar] [CrossRef]
- Matsuo, I.; Kimura-Yoshida, C. Extracellular Distribution of Diffusible Growth Factors Controlled by Heparan Sulfate Proteoglycans during Mammalian Embryogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130545. [Google Scholar] [CrossRef]
- Yan, D.; Lin, X. Shaping Morphogen Gradients by Proteoglycans. Cold Spring Harb. Perspect. Biol. 2009, 1, a002493. [Google Scholar] [CrossRef] [PubMed]
- Häcker, U.; Nybakken, K.; Perrimon, N. Heparan Sulphate Proteoglycans: The Sweet Side of Development. Nat. Rev. Mol. Cell Biol. 2005, 6, 530–541. [Google Scholar] [CrossRef]
- Bellaiche, Y.; The, I.; Perrimon, N. Tout-velu is a Drosophila Homologue of the Putative Tumour Suppresor EXT-1 and is Needed for Hh Diffusion. Nature 1998, 394, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Bornemann, D.J.; Duncan, J.E.; Staatz, W.; Selleck, S.; Warrior, R. Abrogation of Heparan Sulface Synthesis in Drosophila Disrupts the Wingless, Hedgehog and Decapentaplegic Signaling Pathways. Development 2004, 131, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Roelink, H. Loss of the Heparan Sulfate Proteoglycan Glypican5 Facilitates Long-Range Sonic Hedgehog Signaling. Stem Cells 2019, 37, 899–909. [Google Scholar] [CrossRef]
- Aguirre-Tamaral, A.; Cambón, M.; Poyato, D.; Soler, J.; Guerrero, I. Predictive Model for Cytoneme Guidance in Hedgehog Signaling Based on Ihog- Glypicans Interaction. Nat. Commun. 2022, 13, 5647. [Google Scholar] [CrossRef]
- Capurro, M.; Izumikawa, T.; Suarez, P.; Shi, W.; Cydzik, M.; Kaneiwa, T.; Gariepy, J.; Bonafe, L.; Filmus, J. Glypican-6 Promotes the Growth of Developing Long Bones by Stimulating Hedgehog Signaling. J. Cell Biol. 2017, 216, 2911–2926. [Google Scholar] [CrossRef]
- Capurro, M.I.; Xu, P.; Shi, W.; Li, F.; Jia, A.; Filmus, J. Glypican-3 Inhibits Hedgehog Signaling during Development by Competing with Patched for Hedgehog Binding. Dev. Cell 2008, 14, 700–711. [Google Scholar] [CrossRef]
- Li, F.; Shi, W.; Capurro, M.; Filmus, J. Glypican-5 Stimulates Rhabdomyosarcoma Cell Proliferation by Activating Hedgehog Signaling. J. Cell Biol. 2011, 192, 691–704. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wierbowski, B.M.; Salic, A. Hedgehog Pathway Modulation by Glypican 3-Conjugated Heparan Sulfate. J. Cell Sci. 2022, 135, jcs259297. [Google Scholar] [CrossRef]
- Wilson, N.H.; Stoeckli, E.T. Sonic Hedgehog Regulates Its Own Receptor on Postcrossing Commissural Axons in a Glypican1-Dependent Manner. Neuron 2013, 79, 478–491. [Google Scholar] [CrossRef]
- Farshi, P.; Ohlig, S.; Pickhinke, U.; Höing, S.; Jochmann, K.; Lawrence, R.; Dreier, R.; Dierker, T.; Grobe, K. Dual Roles of the Cardin-Weintraub Motif in Multimeric Sonic Hedgehog. J. Biol. Chem. 2011, 286, 23608–23619. [Google Scholar] [CrossRef]
- Traister, A.; Shi, W.; Filmus, J. Mammalian Notum Induces the Release of Glypicans and Other GPI-Anchored Proteins from the Cell Surface. Biochem. J. 2008, 410, 503–511. [Google Scholar] [CrossRef]
- Capurro, M.; Shi, W.; Izumikawa, T.; Kitagawa, H.; Filmus, J. Processing by Convertases Is Required for Glypican-3-Induced Inhibition of Hedgehog Signaling. J. Biol. Chem. 2015, 290, 7576–7585. [Google Scholar] [CrossRef]
- Witt, R.M.; Hecht, M.L.; Pazyra-Murphy, M.F.; Cohen, S.M.; Noti, C.; Van Kuppevelt, T.H.; Fuller, M.; Chan, J.A.; Hopwood, J.J.; Seeberger, P.H.; et al. Heparan Sulfate Proteoglycans Containing a Glypican 5 Core and 2-O-Sulfo-Iduronic Acid Function as Sonic Hedgehog Co-Receptors to Promote Proliferation. J. Biol. Chem. 2013, 288, 26275–26288. [Google Scholar] [CrossRef]
- Danesin, C.; Agius, E.; Escalas, N.; Ai, X.; Emerson, C.; Cochard, P.; Soula, C. Ventral Neural Progenitors Switch toward an Oligodendroglial Fate in Response to Increased Sonic Hedgehog (Shh) Activity: Involvement of Sulfatase 1 in Modulating Shh Signaling in the Ventral Spinal Cord. J. Neurosci. 2006, 26, 5037–5048. [Google Scholar] [CrossRef]
- Ramsbottom, S.A.; Maguire, R.J.; Fellgett, S.W.; Pownall, M.E. Sulf1 Influences the Shh Morphogen Gradient during the Dorsal Ventral Patterning of the Neural Tube in Xenopus Tropicalis. Dev. Biol. 2014, 391, 207–218. [Google Scholar] [CrossRef]
- Grobe, K.; Inatani, M.; Pallerla, S.R.; Castagnola, J.; Yamaguchi, Y.; Esko, J.D. Cerebral Hypoplasia and Craniofacial Defects in Mice Lacking Heparan Sulfate Ndst1 Gene Function. Development 2005, 132, 3777–3786. [Google Scholar] [CrossRef]
- Inatani, M.; Irie, F.; Plump, A.S.; Tessier-Lavigne, M.; Yamaguchi, Y. Mammalian Brain Morphogenesis and Midline Axon Guidance Require Heparan Sulfate. Science 2003, 302, 1044–1046. [Google Scholar] [CrossRef]
- Jen, Y.H.L.; Musacchio, M.; Lander, A.D. Glypican-1 Controls Brain Size through Regulation of Fibroblast Growth Factor Signaling in Early Neurogenesis. Neural Dev. 2009, 4, 33. [Google Scholar] [CrossRef]
- Gallet, A. Hedgehog Morphogen: From Secretion to Reception. Trends Cell Biol. 2011, 21, 238–246. [Google Scholar] [CrossRef]
- Bischoff, M.; Gradilla, A.C.; Seijo, I.; Andrés, G.; Rodríguez-Navas, C.; González-Méndez, L.; Guerrero, I. Cytonemes Are Required for the Establishment of a Normal Hedgehog Morphogen Gradient in Drosophila Epithelia. Nat. Cell Biol. 2013, 15, 1269–1281. [Google Scholar] [CrossRef]
- Ramírez-Weber, F.A.; Kornberg, T.B. Cytonemes: Cellular Processes That Project to the Principal Signaling Center in Drosophila Imaginal Discs. Cell 1999, 97, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Daly, C.A.; Hall, E.T.; Ogden, S.K. Regulatory Mechanisms of Cytoneme-Based Morphogen Transport. Cell. Mol. Life Sci. 2022, 79, 119. [Google Scholar] [CrossRef] [PubMed]
- González-Méndez, L.; Seijo-Barandiarán, I.; Guerrero, I. Cytoneme-Mediated Cell-Cell Contacts for Hedgehog Reception. eLife 2017, 6, e24045. [Google Scholar] [CrossRef]
- Hall, E.T.; Dillard, M.E.; Stewart, D.P.; Zhang, Y.; Wagner, B.; Levine, R.M.; Pruett-Miller, S.M.; Sykes, A.; Temirov, J.; Cheney, R.E.; et al. Cytoneme Delivery of Sonic Hedgehog from Ligand-Producing Cells Requires Myosin 10 and a Dispatched-Boc/Cdon Co-Receptor Complex. eLife 2021, 10, e61432. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Llagostera, E.; Barna, M. Specialized Filopodia Direct Long-Range Transport of SHH during Vertebrate Tissue Patterning. Nature 2013, 497, 628–632. [Google Scholar] [CrossRef]
- Gradilla, A.C.; Guerrero, I. Hedgehog on Track: Long-Distant Signal Transport and Transfer through Direct Cell-to-Cell Contact, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 150, ISBN 9780128201558. [Google Scholar]
- Bodeen, W.J.; Marada, S.; Truong, A.; Ogden, S.K. A Fixation Method to Preserve Cultured Cell Cytonemes Facilitates Mechanistic Interrogation of Morphogen Transport. Development 2017, 144, 3612–3624. [Google Scholar] [CrossRef]
- Takei, Y.; Ozawa, Y.; Sato, M.; Watanabe, A.; Tabata, T. Three Drosophila EXT Genes Shape Morphogen Gradients through Synthesis of Heparan Sulfate Proteoglycans. Development 2004, 131, 73–82. [Google Scholar] [CrossRef]
- Hall, E.T.; Daly, C.A.; Zhang, Y.; Dillard, M.E.; Ogden, S.K. Fixation of Embryonic Mouse Tissue for Cytoneme Analysis. J. Vis. Exp. 2022, 184, e64100. [Google Scholar] [CrossRef]
- Heimsath, E.G.; Yim, Y.I.; Mustapha, M.; Hammer, J.A.; Cheney, R.E. Myosin-X Knockout Is Semi-Lethal and Demonstrates That Myosin-X Functions in Neural Tube Closure, Pigmentation, Hyaloid Vasculature Regression, and Filopodia Formation. Sci. Rep. 2017, 7, 17354. [Google Scholar] [CrossRef]
- Arellano, J.I.; Morozov, Y.M.; Micali, N.; Rakic, P. Radial Glial Cells: New Views on Old Questions. Neurochem. Res. 2021, 46, 2512–2524. [Google Scholar] [CrossRef]
- Gallo, G. Mechanisms Underlying the Initiation and Dynamics of Neuronal Filopodia. From Neurite Formation to Synaptogenesis; Elsevier: Amsterdam, The Netherlands, 2013; Volume 301, ISBN 9780124077041. [Google Scholar]
- Nelson, B.R.; Hodge, R.D.; Bedogni, F.; Hevner, R.F. Dynamic Interactions between Intermediate Neurogenic Progenitors and Radial Glia in Embryonic Mouse Neocortex: Potential Role in Dll1-Notch Signaling. J. Neurosci. 2013, 33, 9122–9139. [Google Scholar] [CrossRef]
- Traiffort, E.; Moya, K.L.; Faure, H.; Hässig, R.; Ruat, M. High Expression and Anterograde Axonal Transport of Aminoterminal Sonic Hedgehog in the Adult Hamster Brain. Eur. J. Neurosci. 2001, 14, 839–850. [Google Scholar] [CrossRef]
- Sánchez-Camacho, C.; Bovolenta, P. Autonomous and Non-Autonomous Shh Signalling Mediate the in Vivo Growth and Guidance of Mouse Retinal Ganglion Cell Axons. Development 2008, 135, 3531–3541. [Google Scholar] [CrossRef]
- Fabre, P.J.; Shimogori, T.; Charron, F. Segregation of Ipsilateral Retinal Ganglion Cell Axons at the Optic Chiasm Requires the Shh Receptor Boc. J. Neurosci. 2010, 30, 266–275. [Google Scholar] [CrossRef]
- Peng, J.; Fabre, P.J.; Dolique, T.; Swikert, S.M.; Kermasson, L.; Shimogori, T.; Charron, F. Sonic Hedgehog Is a Remotely Produced Cue That Controls Axon Guidance Trans-Axonally at a Midline Choice Point. Neuron 2018, 97, 326–340.e4. [Google Scholar] [CrossRef]
- Beug, S.T.; Parks, R.J.; McBride, H.M.; Wallace, V.A. Processing-Dependent Trafficking of Sonic Hedgehog to the Regulated Secretory Pathway in Neurons. Mol. Cell. Neurosci. 2011, 46, 583–596. [Google Scholar] [CrossRef]
- Campbell, C.; Beug, S.; Nickerson, P.E.B.; Peng, J.; Mazerolle, C.; Bassett, E.A.; Ringuette, R.; Jama, F.A.; Morales, C.; Christ, A.; et al. Sortilin Regulates Sorting and Secretion of Sonic Hedgehog. J. Cell Sci. 2016, 129, 3832–3844. [Google Scholar] [CrossRef]
- Herrera, E.; Sitko, A.A.; Bovolenta, P. Shh-Ushing Midline Crossing through Remote Protein Transport. Neuron 2018, 97, 256–258. [Google Scholar] [CrossRef]
- Deven Somaiya, R.; Stebbins, K.; Gingrich, E.C.; Xie, H.; Campbell, J.N.; Garcia, D.R.; Fox, M.A. Sonic Hedgehog-Dependent Recruitment of GABAergic Interneurons into the Developing Visual Thalamus. eLife 2022, 11, 79833. [Google Scholar] [CrossRef] [PubMed]
- Pascual, O.; Traiffort, E.; Baker, D.P.; Galdes, A.; Ruat, M.; Champagnat, J. Sonic Hedgehog Signalling in Neurons of Adult Ventrolateral Nucleus Tractus Solitarius. Eur. J. Neurosci. 2005, 22, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Baufreton, J.; Owens, G.; Crossman, A.R.; Dudek, H.; Taupignon, A.; Brotchie, J.M.; Gang, B. Sonic Hedgehog Is a Neuromodulator in the Adult Subthalamic Nucleus. FASEB J. 2003, 17, 2337–2338. [Google Scholar] [CrossRef] [PubMed]
- Delmotte, Q.; Hamze, M.; Medina, I.; Buhler, E.; Zhang, J.; Belgacem, Y.H.; Porcher, C. Smoothened Receptor Signaling Regulates the Developmental Shift of GABA Polarity in Rat Somatosensory Cortex. J. Cell Sci. 2020, 133, jcs247700. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Reyes, L.E.; Verbitsky, M.; Blesa, J.; Jackson-Lewis, V.; Paredes, D.; Tillack, K.; Phani, S.; Kramer, E.R.; Przedborski, S.; Kottmann, A.H. Sonic Hedgehog Maintains Cellular and Neurochemical Homeostasis in the Adult Nigrostriatal Circuit. Neuron 2012, 75, 306–319. [Google Scholar] [CrossRef]
- Turcato, F.C.; Wegman, E.; Lu, T.; Ferguson, N.; Luo, Y. Dopaminergic Neurons Are Not a Major Sonic Hedgehog Ligand Source for Striatal Cholinergic or PV Interneurons. iScience 2022, 25, 105278. [Google Scholar] [CrossRef]
- Rivell, A.; Petralia, R.S.; Wang, Y.X.; Clawson, E.; Moehl, K.; Mattson, M.P.; Yao, P.J. Sonic Hedgehog Expression in the Postnatal Brain. Biol. Open 2019, 8, bio040592. [Google Scholar] [CrossRef]
- Eitan, E.; Petralia, R.S.; Wang, Y.X.; Indig, F.E.; Mattson, M.P.; Yao, P.J. Probing Extracellular Sonic Hedgehog in Neurons. Biol. Open 2016, 5, 1086–1092. [Google Scholar] [CrossRef]
- Mitchell, N.; Petralia, R.S.; Currier, D.G.; Wang, Y.X.; Kim, A.; Mattson, M.P.; Yao, P.J. Sonic Hedgehog Regulates Presynaptic Terminal Size, Ultrastructure and Function in Hippocampal Neurons. J. Cell Sci. 2012, 125, 4207–4213. [Google Scholar] [CrossRef]
- Dubourg, C.; Bendavid, C.; Pasquier, L.; Henry, C.; Odent, S.; David, V. Holoprosencephaly. Orphanet J. Rare Dis. 2007, 2, 8. [Google Scholar] [CrossRef]
- Nagase, T.; Nagase, M.; Osumi, N.; Fukuda, S.; Nakamura, S.; Ohsaki, K.; Harii, K.; Asato, H.; Yoshimura, K. Craniofacial Anomalies of the Cultured Mouse Embryo Induced by Inhibition of Sonic Hedgehog Signaling: An Animal Model of Holoprosencephaly. J. Craniofac. Surg. 2005, 16, 80–88. [Google Scholar] [CrossRef]
- Kim, A.; Le Douce, J.; Diab, F.; Ferovova, M.; Dubourg, C.; Odent, S.; Dupé, V.; David, V.; Diambra, L.; Watrin, E.; et al. Synonymous Variants in Holoprosencephaly Alter Codon Usage and Impact the Sonic Hedgehog Protein. Brain 2020, 143, 2027–2038. [Google Scholar] [CrossRef]
- Kim, A.; Savary, C.; Dubourg, C.; Carré, W.; Mouden, C.; Hamdi-Rozé, H.; Guyodo, H.; Le Douce, J.; Génin, E.; Campion, D.; et al. Integrated Clinical and Omics Approach to Rare Diseases: Novel Genes and Oligogenic Inheritance in Holoprosencephaly. Brain 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Costa, R.; Urbani, A.; Salvalaio, M.; Bellesso, S.; Cieri, D.; Zancan, I.; Filocamo, M.; Bonaldo, P.; Szabo, I.; Tomanin, R.; et al. Perturbations in Cell Signaling Elicit Early Cardiac Defects in Mucopolysaccharidosis Type II. Hum. Mol. Genet. 2017, 26, 1643–1655. [Google Scholar] [CrossRef]
- Fiorenza, M.T.; Moro, E.; Erickson, R.P. The Pathogenesis of Lysosomal Storage Disorders: Beyond the Engorgement of Lysosomes to Abnormal Development and Neuroinflammation. Hum. Mol. Genet. 2018, 27, R119–R129. [Google Scholar] [CrossRef]
- Canterini, S.; Dragotto, J.; Dardis, A.; Zampieri, S.; De Stefano, M.E.; Mangia, F.; Erickson, R.P.; Fiorenza, M.T. Shortened Primary Cilium Length and Dysregulated Sonic Hedgehog Signaling in Niemann-Pick C1 Disease. Hum. Mol. Genet. 2017, 26, 2277–2289. [Google Scholar] [CrossRef]
- Maerz, L.D.; Burkhalter, M.D.; Schilpp, C.; Wittekindt, O.H.; Frick, M.; Philipp, M. Pharmacological Cholesterol Depletion Disturbs Ciliogenesis and Ciliary Function in Developing Zebrafish. Commun. Biol. 2019, 2, 31. [Google Scholar] [CrossRef]
- Kinnebrew, M.; Iverson, E.J.; Patel, B.B.; Pusapati, G.V.; Kong, J.H.; Johnson, K.A.; Luchetti, G.; Eckert, K.M.; McDonald, J.G.; Covey, D.F.; et al. Cholesterol Accessibility at the Ciliary Membrane Controls Hedgehog Signaling. eLife 2019, 8, e50051. [Google Scholar] [CrossRef]
- Gallet, A.; Rodriguez, R.; Ruel, L.; Therond, P.P. Cholesterol Modification of Hedgehog Is Required for Trafficking and Movement, Revealing an Asymmetric Cellular Response to Hedgehog. Dev. Cell 2003, 4, 191–204. [Google Scholar] [CrossRef]
- Lewis, P.M.; Dunn, M.P.; McMahon, J.A.; Logan, M.; Martin, J.F.; St-Jacques, B.; McMahon, A.P. Cholesterol Modification of Sonic Hedgehog Is Required for Long-Range Signaling Activity and Effective Modulation of Signaling by Ptc1. Cell 2001, 105, 599–612. [Google Scholar] [CrossRef]
- Gorivodsky, M.; Mukhopadhyay, M.; Wilsch-Braeuninger, M.; Phillips, M.; Teufel, A.; Kim, C.; Malik, N.; Huttner, W.; Westphal, H. Intraflagellar Transport Protein 172 Is Essential for Primary Cilia Formation and Plays a Vital Role in Patterning the Mammalian Brain. Dev. Biol. 2009, 325, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Willaredt, M.A.; Hasenpusch-Theil, K.; Gardner, H.A.R.; Kitanovic, I.; Hirschfeld-Warneken, V.C.; Gojak, C.P.; Gorgas, K.; Bradford, C.L.; Spatz, J.; Wölfl, S.; et al. A Crucial Role for Primary Cilia in Cortical Morphogenesis. J. Neurosci. 2008, 28, 12887–12900. [Google Scholar] [CrossRef] [PubMed]
- Spassky, N.; Han, Y.G.; Aguilar, A.; Strehl, L.; Besse, L.; Laclef, C.; Romaguera Ros, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Primary Cilia Are Required for Cerebellar Development and Shh-Dependent Expansion of Progenitor Pool. Dev. Biol. 2008, 317, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Jones, D.T.W.; Jäger, N.; Northcott, P.A.; Pugh, T.J.; Hovestadt, V.; Piro, R.M.; Esparza, L.A.; Markant, S.L.; Remke, M.; et al. Genome Sequencing of SHH Medulloblastoma Predicts Genotype-Related Response to Smoothened Inhibition. Cancer Cell 2014, 25, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Bar, E.E.; Chaudhry, A.; Lin, A.; Fan, X.; Schreck, K.; Matsui, W.; Piccirillo, S.; Vescovi, A.L.; Dimeco, F.; Olvi, A.; et al. Cyclopamine-Mediated Hedgehog Pathway Inhibition Depletes Stem-like Cancer Cells in Glioblastoma. Stem Cells 2007, 25, 2524–2533. [Google Scholar] [CrossRef]
- Dahmane, N.; Sánchez, P.; Gitton, Y.; Palma, V.; Sun, T.; Beyna, M. The Sonic Hedgehog-Gli Pathway Regulates Dorsal Brain Growth and Tumorigenesis. Development 2001, 128, 5201–5212. [Google Scholar] [CrossRef]
- Boyd, N.H.; Tran, A.N.; Bernstock, J.D.; Etminan, T.; Jones, A.B.; Yancey Gillespie, G.; Friedman, G.K.; Hjelmeland, A.B. Glioma Stem Cells and Their Roles within the Hypoxic Tumor Microenvironment. Theranostics 2020, 11, 665–683. [Google Scholar] [CrossRef]
- Hung, H.C.; Liu, C.C.; Chuang, J.Y.; Su, C.L.; Gean, P.W. Inhibition of Sonic Hedgehog Signaling Suppresses Glioma Stem-like Cells Likely Through Inducing Autophagic Cell Death. Front. Oncol. 2020, 10, 1233. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Dodelet-Devillers, A.; Kebir, H.; Ifergan, I.; Fabre, P.J.; Terouz, S.; Sabbagh, M.; Wosik, K.; Bourbonnière, L.; Bernard, M.; et al. The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science 2011, 334, 1727–1731. [Google Scholar] [CrossRef]
- Amankulor, N.M.; Hambardzumyan, D.; Pyonteck, S.M.; Becher, O.J.; Joyce, J.A.; Holland, E.C. Sonic Hedgehog Pathway Activation Is Induced by Acute Brain Injury and Regulated by Injury-Related Inflammation. J. Neurosci. 2009, 29, 10299–10308. [Google Scholar] [CrossRef]
- Chechneva, O.V.; Mayrhofer, F.; Daugherty, D.J.; Krishnamurty, R.G.; Bannerman, P.; Pleasure, D.E.; Deng, W. A Smoothened Receptor Agonist Is Neuroprotective and Promotes Regeneration after Ischemic Brain Injury. Cell Death Dis. 2014, 5, e1481. [Google Scholar] [CrossRef]
- Jin, Y.; Raviv, N.; Barnett, A.; Bambakidis, N.C.; Filichia, E.; Luo, Y. The Shh Signaling Pathway Is Upregulated in Multiple Cell Types in Cortical Ischemia and Influences the Outcome of Stroke in an Animal Model. PLoS ONE 2015, 10, e0124657. [Google Scholar] [CrossRef]
- Pitter, K.L.; Tamagno, I.; Feng, X.; Ghosal, K.; Amankulor, N.; Holland, E.C.; Hambardzumyan, D. The SHH/Gli Pathway Is Reactivated in Reactive Glia and Drives Proliferation in Response to Neurodegeneration-Induced Lesions. Glia 2014, 62, 1595–1607. [Google Scholar] [CrossRef]
- Wang, Y.; Imitola, J.; Rasmussen, S.; O’Connor, K.C.; Khoury, S.J. Paradoxical Dysregulation of the Neural Stem Cell Pathway Sonic Hedgehog-Gli1 in Autoimmune Encephalomyelitis and Multiple Sclerosis. Ann. Neurol. 2008, 64, 417–427. [Google Scholar] [CrossRef]
- Xing, G.; Zhao, T.; Zhang, X.; Li, H.; Li, X.; Cui, P.; Li, M.; Li, D.; Zhang, N.; Jiang, W. Astrocytic Sonic Hedgehog Alleviates Intracerebral Hemorrhagic Brain Injury via Modulation of Blood-Brain Barrier Integrity. Front. Cell. Neurosci. 2020, 14, 575690. [Google Scholar] [CrossRef]
- Bambakidis, N.C. Improvement of Neurological Recovery and Stimulation of Neural Progenitor Cell Proliferation by Intrathecal Administration of Sonic Hedgehog Laboratory Investigation. J. Neurosurg. 2013, 118, 488, Erratum in: J. Neurosurg. 2012, 116, 1114–1120. [Google Scholar] [CrossRef]
- Sirko, S.; Behrendt, G.; Johansson, P.A.; Tripathi, P.; Costa, M.; Bek, S.; Heinrich, C.; Tiedt, S.; Colak, D.; Dichgans, M.; et al. Reactive Glia in the Injured Brain Acquire Stem Cell Properties in Response to Sonic Hedgehog Glia. Cell Stem Cell 2013, 12, 426–439. [Google Scholar] [CrossRef]
- Seifert, T.; Bauer, J.; Weissert, R.; Fazekas, F.; Storch, M.K. Differential Expression of Sonic Hedgehog Immunoreactivity during Lesion Evolution in Autoimmune Encephalomyelitis. J. Neuropathol. Exp. Neurol. 2005, 64, 404–411. [Google Scholar] [CrossRef]
- Sanchez, M.A.; Armstrong, R.C. Postnatal Sonic Hedgehog (Shh) Responsive Cells Give Rise to Oligodendrocyte Lineage Cells during Myelination and in Adulthood Contribute to Remyelination. Exp. Neurol. 2018, 299, 122–136. [Google Scholar] [CrossRef]
- Ballester, A.; Guijarro, A.; Bravo, B.; Hernández, J.; Murillas, R.; Gallego, M.I.; Ballester, S. Hedgehog Signalling Modulates Immune Response and Protects against Experimental Autoimmune Encephalomyelitis. Int. J. Mol. Sci. 2022, 23, 3171. [Google Scholar] [CrossRef]
- Laouarem, Y.; Kassoussi, A.; Zahaf, A.; Hutteau-Hamel, T.; Mellouk, A.; Bobé, P.; Mattern, C.; Schumacher, M.; Traiffort, E. Functional Cooperation of the Hedgehog and Androgen Signaling Pathways during Developmental and Repairing Myelination. Glia 2021, 69, 1369–1392. [Google Scholar] [CrossRef] [PubMed]
- Macchi, M.; Magalon, K.; Zimmer, C.; Peeva, E.; El Waly, B.; Brousse, B.; Jaekel, S.; Grobe, K.; Kiefer, F.; Williams, A.; et al. Mature Oligodendrocytes Bordering Lesions Limit Demyelination and Favor Myelin Repair via Heparan Sulfate Production. eLife 2020, 9, e51735. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douceau, S.; Deutsch Guerrero, T.; Ferent, J. Establishing Hedgehog Gradients during Neural Development. Cells 2023, 12, 225. https://doi.org/10.3390/cells12020225
Douceau S, Deutsch Guerrero T, Ferent J. Establishing Hedgehog Gradients during Neural Development. Cells. 2023; 12(2):225. https://doi.org/10.3390/cells12020225
Chicago/Turabian StyleDouceau, Sara, Tanya Deutsch Guerrero, and Julien Ferent. 2023. "Establishing Hedgehog Gradients during Neural Development" Cells 12, no. 2: 225. https://doi.org/10.3390/cells12020225
APA StyleDouceau, S., Deutsch Guerrero, T., & Ferent, J. (2023). Establishing Hedgehog Gradients during Neural Development. Cells, 12(2), 225. https://doi.org/10.3390/cells12020225





