Protein Kinase D3 (PKD3) Requires Hsp90 for Stability and Promotion of Prostate Cancer Cell Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Migration
2.4. Gene Transfection
2.5. Cell Lysis
2.6. Western Blot
2.7. Proximity Ligation (PLA) Assay
2.8. Co-Immunoprecipitation (Co-IP)
2.9. Statistical Analysis
3. Results
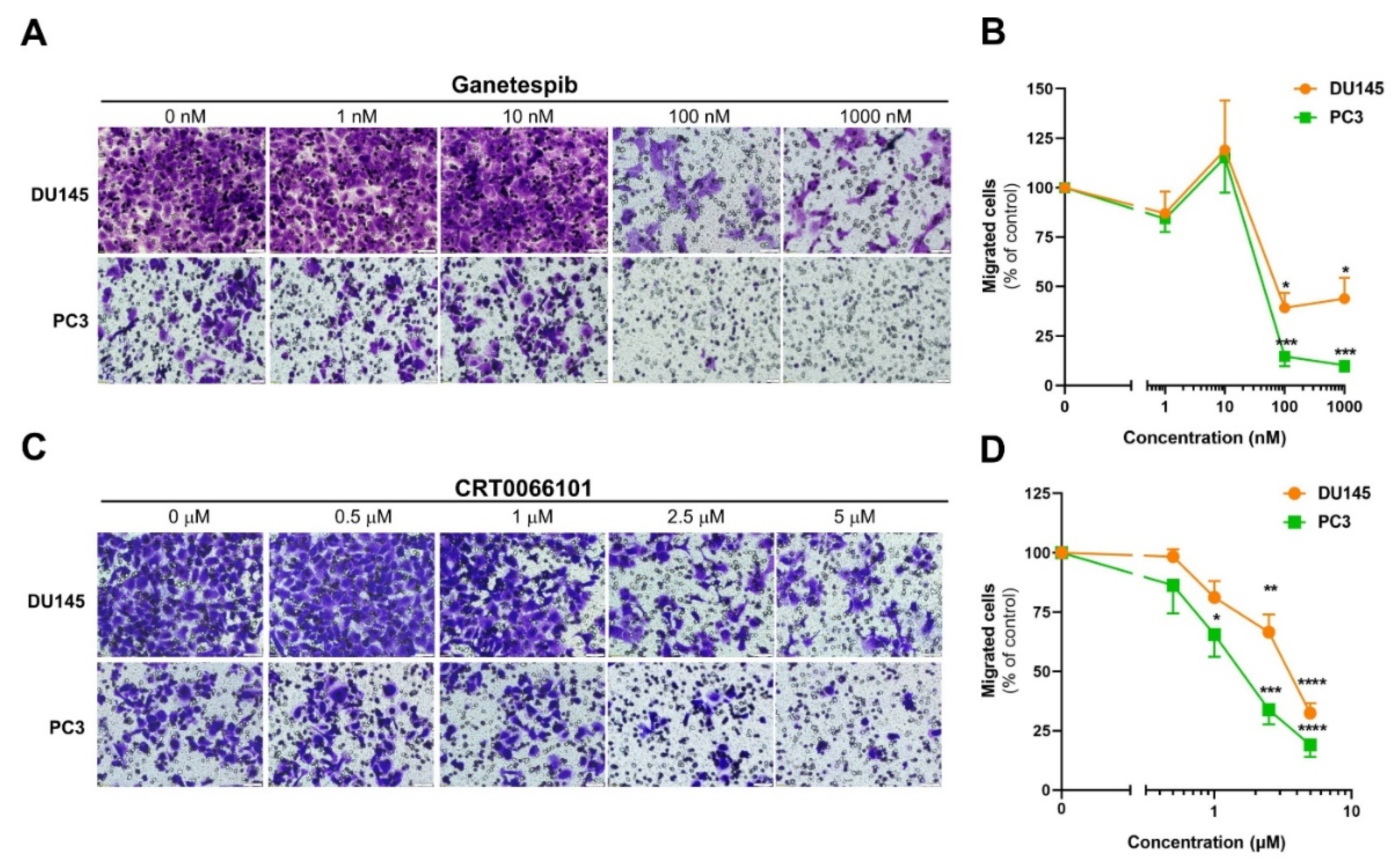
3.1. Inhibition of Hsp90 or PKD Arrests Androgen-Independent Prostate Cancer Cell Migration
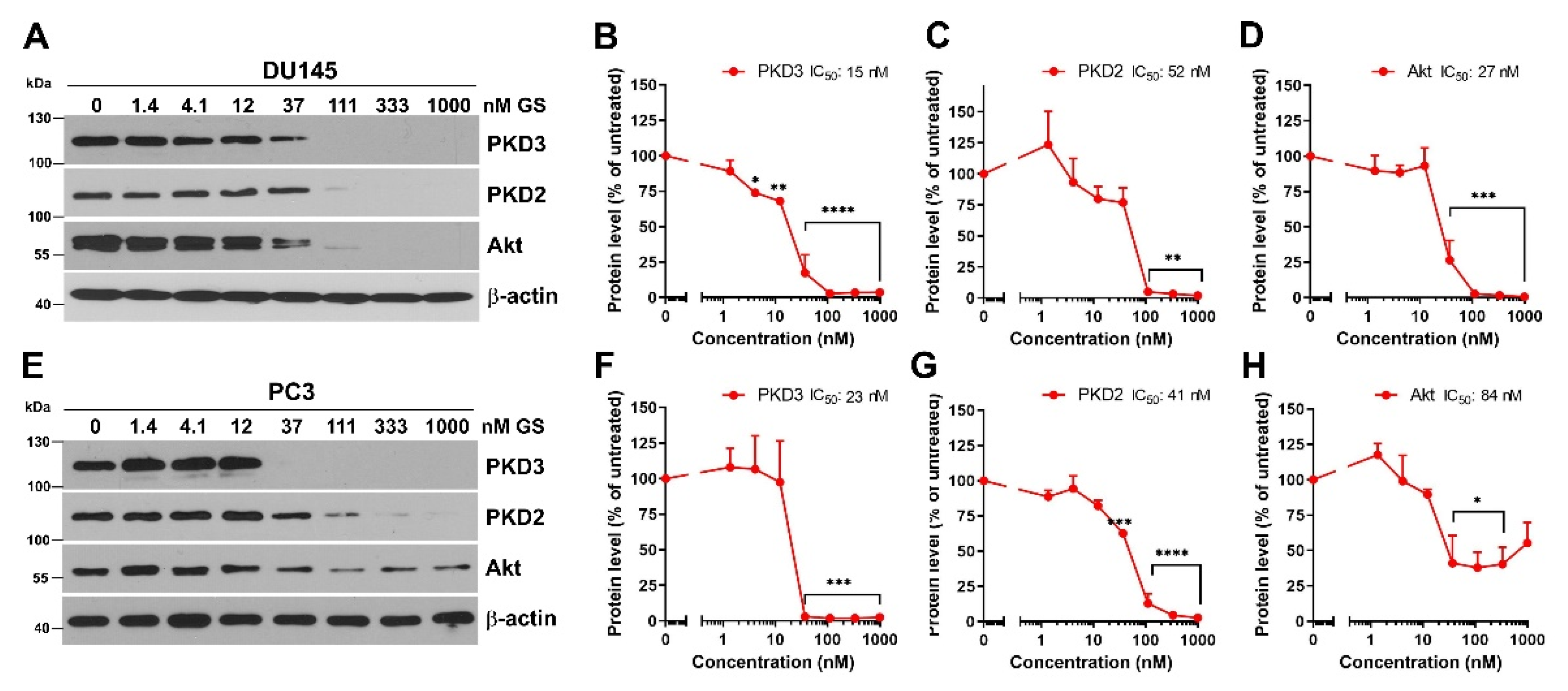
3.2. Hsp90 Inhibition Decreases PKD3 Protein Level in Prostate Cancer Cells
3.3. A Physical Interaction with Hsp90 Ensures PKD3 Conformational Stability
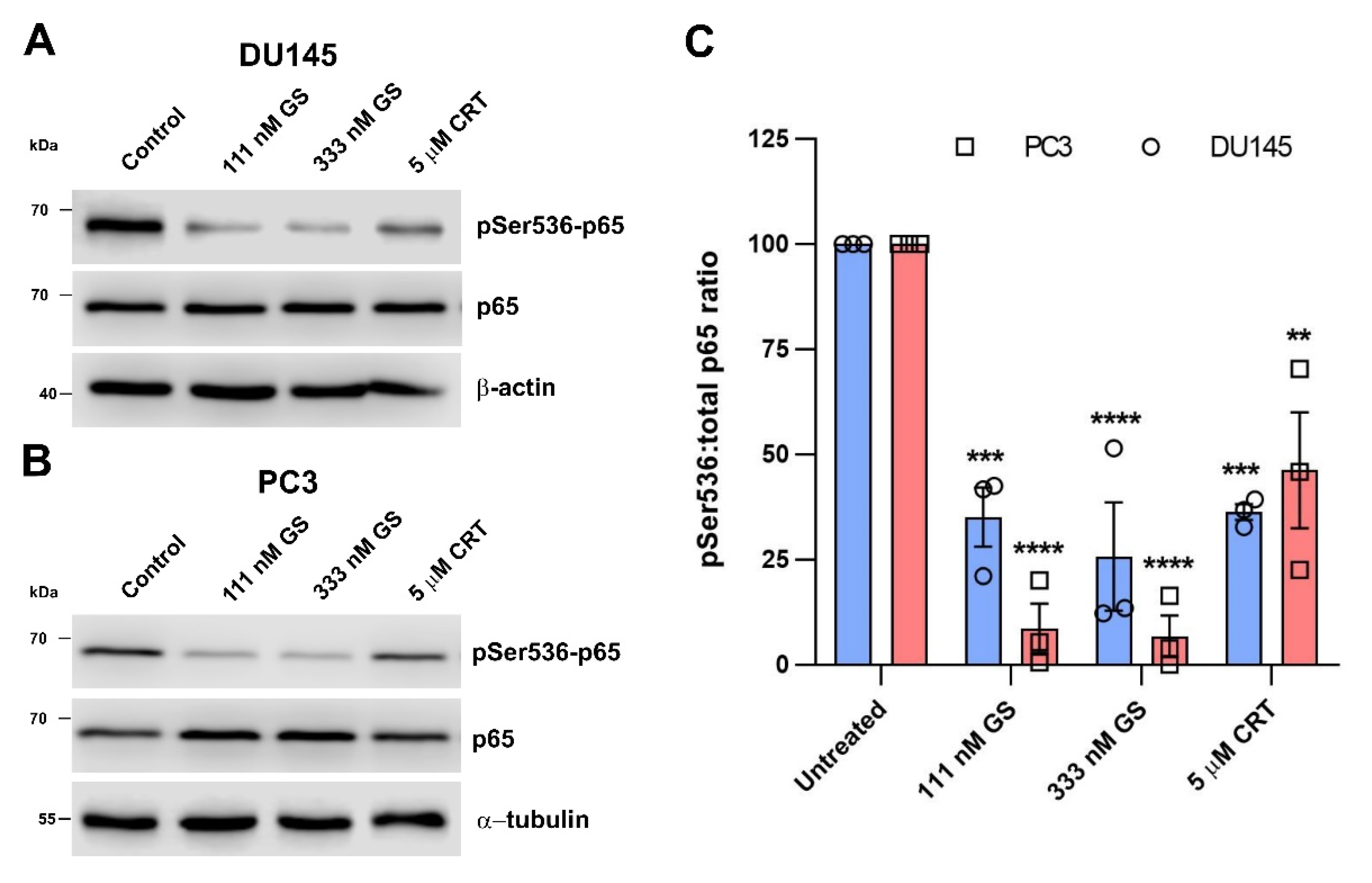
3.4. The Hsp90–PKD3 Interaction Is Required for PKD3-Dependent Phosphorylation of p65
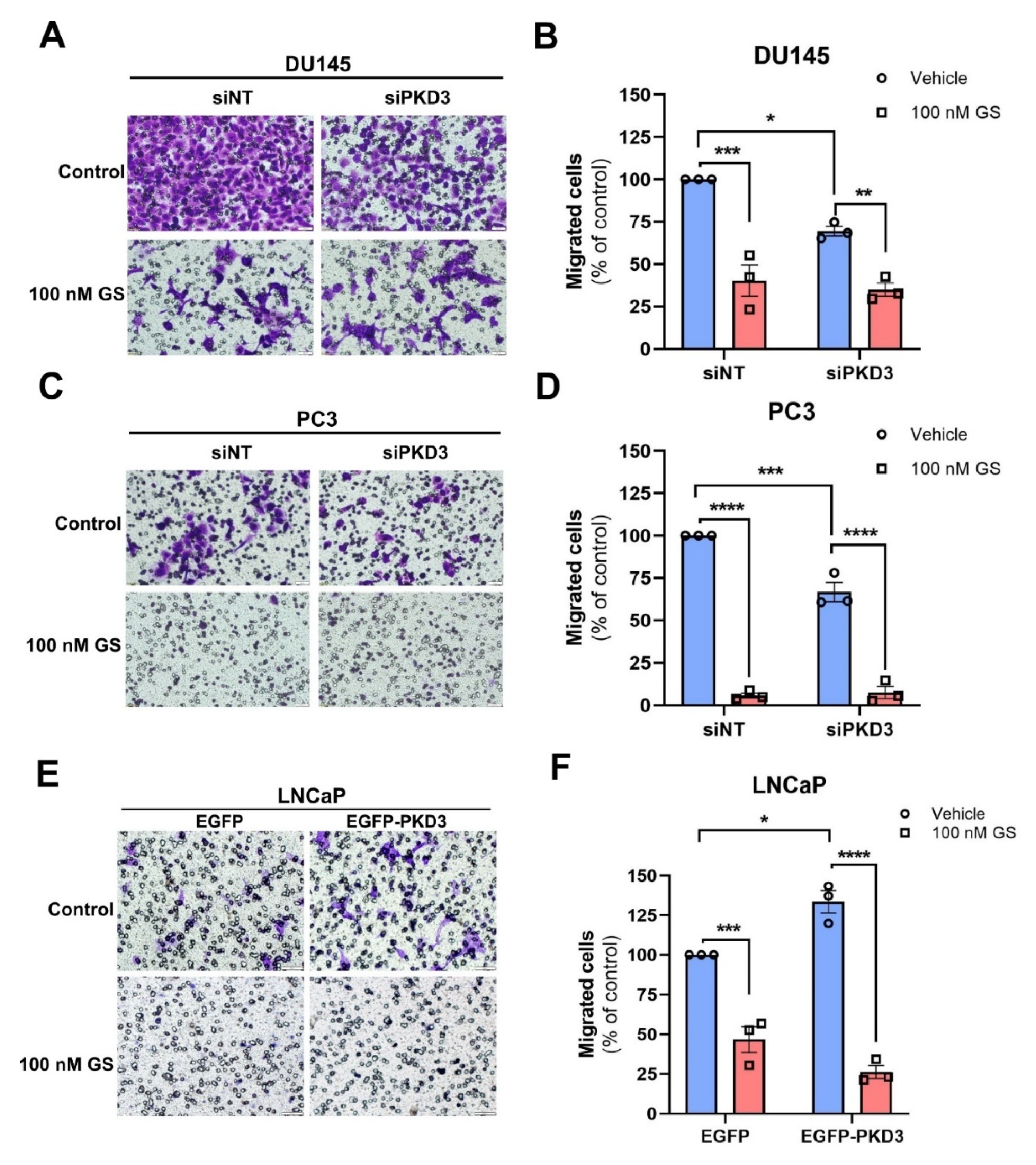
3.5. Hsp90 Chaperones PKD3-Mediated Prostate Cancer Cell Migration
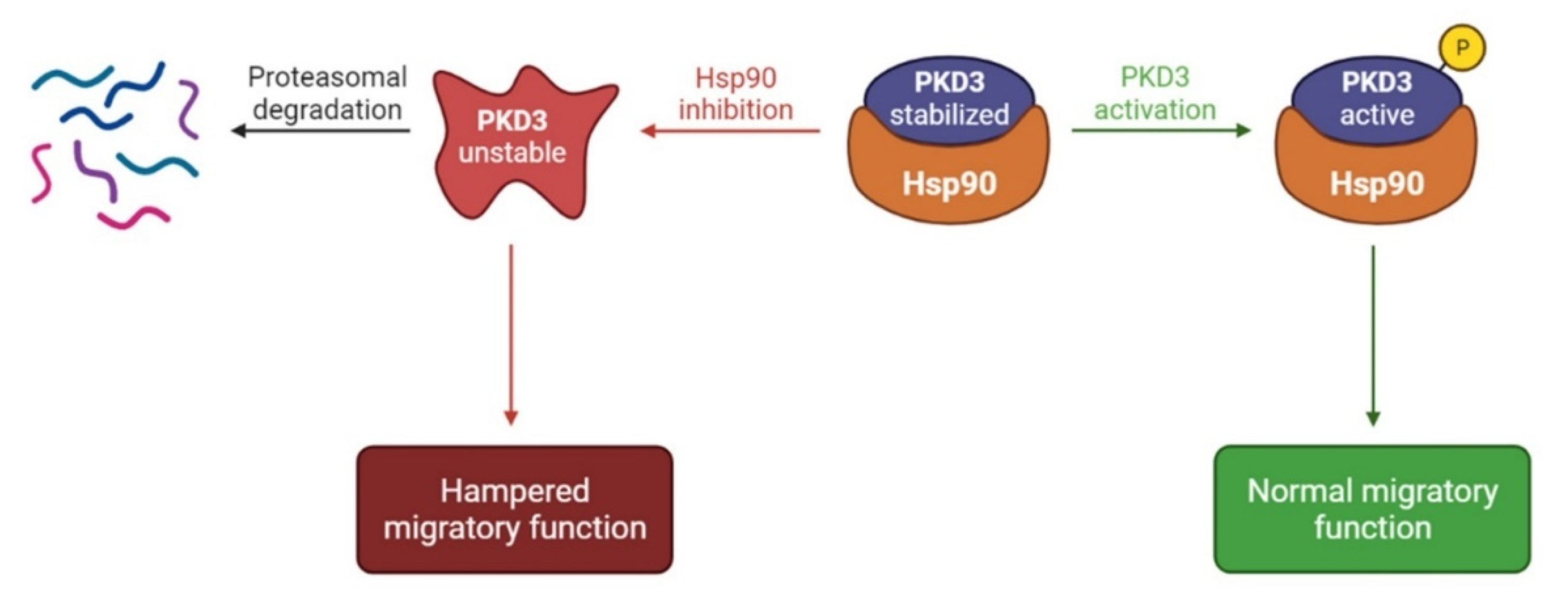
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi, N.; Gulley, J.L.; Dahut, W.L. Androgen deprivation therapy for prostate cancer. JAMA 2005, 294, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Dahut, W.L.; Steinberg, S.M.; Figg, W.D.; Tarassoff, C.; Arlen, P.; Gulley, J.L. A retrospective study of the time to clinical endpoints for advanced prostate cancer. BJU Int. 2005, 96, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.P.; Melo, C.M.; Saggioro, F.P.; Reis, R.B.D.; Squire, J.A. Epithelial-Mesenchymal Transition Signaling and Prostate Cancer Stem Cells: Emerging Biomarkers and Opportunities for Precision Therapeutics. Genes 2021, 12, 1900. [Google Scholar] [CrossRef]
- Valverde, A.M.; Sinnett-Smith, J.; Van Lint, J.; Rozengurt, E. Molecular cloning and characterization of protein kinase D: A target for diacylglycerol and phorbol esters with a distinctive catalytic domain. Proc. Natl. Acad. Sci. USA 1994, 91, 8572–8576. [Google Scholar] [CrossRef] [PubMed]
- Sturany, S.; Van Lint, J.; Muller, F.; Wilda, M.; Hameister, H.; Hocker, M.; Brey, A.; Gern, U.; Vandenheede, J.; Gress, T.; et al. Molecular cloning and characterization of the human protein kinase D2. A novel member of the protein kinase D family of serine threonine kinases. J. Biol. Chem. 2001, 276, 3310–3318. [Google Scholar] [CrossRef]
- Hayashi, A.; Seki, N.; Hattori, A.; Kozuma, S.; Saito, T. PKCnu, a new member of the protein kinase C family, composes a fourth subfamily with PKCmu. Biochim. Biophys. Acta 1999, 1450, 99–106. [Google Scholar] [CrossRef]
- Sanchez-Ruiloba, L.; Cabrera-Poch, N.; Rodriguez-Martinez, M.; Lopez-Menendez, C.; Jean-Mairet, R.M.; Higuero, A.M.; Iglesias, T. Protein kinase D intracellular localization and activity control kinase D-interacting substrate of 220-kDa traffic through a postsynaptic density-95/discs large/zonula occludens-1-binding motif. J. Biol. Chem. 2006, 281, 18888–18900. [Google Scholar] [CrossRef]
- Zhang, X.; Connelly, J.; Chao, Y.; Wang, Q.J. Multifaceted Functions of Protein Kinase D in Pathological Processes and Human Diseases. Biomolecules 2021, 11, 483. [Google Scholar] [CrossRef]
- Doppler, H.; Bastea, L.I.; Borges, S.; Spratley, S.J.; Pearce, S.E.; Storz, P. Protein kinase d isoforms differentially modulate cofilin-driven directed cell migration. PLoS ONE 2014, 9, e98090. [Google Scholar] [CrossRef]
- Borges, S.; Perez, E.A.; Thompson, E.A.; Radisky, D.C.; Geiger, X.J.; Storz, P. Effective Targeting of Estrogen Receptor-Negative Breast Cancers with the Protein Kinase D Inhibitor CRT0066101. Mol. Cancer Ther. 2015, 14, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, F.; Singh, S.V.; Wang, Q.J. Protein kinase D3 (PKD3) contributes to prostate cancer cell growth and survival through a PKCepsilon/PKD3 pathway downstream of Akt and ERK 1/2. Cancer Res. 2008, 68, 3844–3853. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hua, L.; Fan, H.; He, Y.; Xu, W.; Zhang, L.; Yang, J.; Deng, F.; Zeng, F. Interplay of PKD3 with SREBP1 Promotes Cell Growth via Upregulating Lipogenesis in Prostate Cancer Cells. J. Cancer 2019, 10, 6395–6404. [Google Scholar] [CrossRef]
- LaValle, C.R.; Zhang, L.; Xu, S.; Eiseman, J.L.; Wang, Q.J. Inducible silencing of protein kinase D3 inhibits secretion of tumor-promoting factors in prostate cancer. Mol. Cancer Ther. 2012, 11, 1389–1399. [Google Scholar] [CrossRef]
- Zou, Z.; Zeng, F.; Xu, W.; Wang, C.; Ke, Z.; Wang, Q.J.; Deng, F. PKD2 and PKD3 promote prostate cancer cell invasion by modulating NF-kappaB- and HDAC1-mediated expression and activation of uPA. J. Cell Sci. 2012, 125, 4800–4811. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohaszka, Z.; Nardai, G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef] [PubMed]
- Biebl, M.M.; Buchner, J. Structure, Function, and Regulation of the Hsp90 Machinery. Cold Spring Harb. Perspect. Biol. 2019, 11, a034017. [Google Scholar] [CrossRef]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Bio. 2010, 11, 515–528. [Google Scholar] [CrossRef]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative Analysis of Hsp90-Client Interactions Reveals Principles of Substrate Recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef]
- Azoitei, N.; Diepold, K.; Brunner, C.; Rouhi, A.; Genze, F.; Becher, A.; Kestler, H.; van Lint, J.; Chiosis, G.; Koren, J., 3rd; et al. HSP90 supports tumor growth and angiogenesis through PRKD2 protein stabilization. Cancer Res. 2014, 74, 7125–7136. [Google Scholar] [CrossRef]
- Solit, D.B.; Zheng, F.F.; Drobnjak, M.; Munster, P.N.; Higgins, B.; Verbel, D.; Heller, G.; Tong, W.; Cordon-Cardo, C.; Agus, D.B.; et al. 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin. Cancer Res. 2002, 8, 986–993. [Google Scholar] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.M.; Whitesell, L. HSP90: Enabler of Cancer Adaptation. Annu. Rev. Annu. Rev. Cancer Biol. 2019, 97, 275. [Google Scholar] [CrossRef]
- Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell 1997, 90, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Du, Z.; Sun, L.; Foley, K.P.; Proia, D.A.; Blackman, R.K.; Zhou, D.; Inoue, T.; Tatsuta, N.; Sang, J.; et al. Ganetespib, a unique triazolone-containing Hsp90 inhibitor, exhibits potent antitumor activity and a superior safety profile for cancer therapy. Mol. Cancer Ther. 2012, 11, 475–484. [Google Scholar] [CrossRef]
- Proia, D.A.; Bates, R.C. Ganetespib and HSP90: Translating preclinical hypotheses into clinical promise. Cancer Res. 2014, 74, 1294–1300. [Google Scholar] [CrossRef]
- Fu, Z.; Jia, B. Advances in the role of heat shock protein 90 in prostate cancer. Andrologia 2022, 54, e14376. [Google Scholar] [CrossRef]
- Alqawi, O.; Moghaddas, M.; Singh, G. Effects of geldanamycin on HIF-1alpha mediated angiogenesis and invasion in prostate cancer cells. Prostate Cancer Prostatic Dis. 2006, 9, 126–135. [Google Scholar] [CrossRef]
- Peng, R.; Li, Z.; Lin, Z.; Wang, Y.; Wang, W.; Hu, B.; Wang, X.; Zhang, J.; Wang, Y.; Zhou, R.; et al. The HSP90 inhibitor 17-PAG effectively inhibits the proliferation and migration of androgen-independent prostate cancer cells. Am. J. Cancer Res. 2015, 5, 3198–3209. [Google Scholar]
- Armstrong, H.K.; Gillis, J.L.; Johnson, I.R.D.; Nassar, Z.D.; Moldovan, M.; Levrier, C.; Sadowski, M.C.; Chin, M.Y.; Tomlinson Guns, E.S.; Tarulli, G.; et al. Dysregulated fibronectin trafficking by Hsp90 inhibition restricts prostate cancer cell invasion. Sci. Rep. 2018, 8, 2090. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Csermely, P.; Soti, C. Hsp90 chaperones PPARgamma and regulates differentiation and survival of 3T3-L1 adipocytes. Cell Death Differ. 2013, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S. Proximity Ligation Assay (PLA). Curr. Protoc. Immunol. 2018, 123, e58. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.D.; Solit, D.B.; Chiosis, G.; Giri, B.; Tsichlis, P.; Rosen, N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J. Biol. Chem. 2002, 277, 39858–39866. [Google Scholar] [CrossRef] [PubMed]
- Giubellino, A.; Sourbier, C.; Lee, M.J.; Scroggins, B.; Bullova, P.; Landau, M.; Ying, W.; Neckers, L.; Trepel, J.B.; Pacak, K. Targeting heat shock protein 90 for the treatment of malignant pheochromocytoma. PLoS ONE 2013, 8, e56083. [Google Scholar] [CrossRef][Green Version]
- Eiseler, T.; Doppler, H.; Yan, I.K.; Kitatani, K.; Mizuno, K.; Storz, P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat. Cell Biol. 2009, 11, 545–556. [Google Scholar] [CrossRef]
- Siebert, C.; Ciato, D.; Murakami, M.; Frei-Stuber, L.; Perez-Rivas, L.G.; Monteserin-Garcia, J.L.; Nolting, S.; Maurer, J.; Feuchtinger, A.; Walch, A.K.; et al. Heat Shock Protein 90 as a Prognostic Marker and Therapeutic Target for Adrenocortical Carcinoma. Front. Endocrinol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Kumar, P.; Siripini, S.; Sreedhar, A.S. The matrix metalloproteinase 7 (MMP7) links Hsp90 chaperone with acquired drug resistance and tumor metastasis. Cancer Rep. 2020, 5, e1261. [Google Scholar] [CrossRef]
- Rozengurt, E.; Rey, O.; Waldron, R.T. Protein kinase D signaling. J. Biol. Chem. 2005, 280, 13205–13208. [Google Scholar] [CrossRef]
- Baker, J.; Falconer, A.M.D.; Wilkinson, D.J.; Europe-Finner, G.N.; Litherland, G.J.; Rowan, A.D. Protein kinase D3 modulates MMP1 and MMP13 expression in human chondrocytes. PLoS ONE 2018, 13, e0195864. [Google Scholar] [CrossRef]
- Rey, O.; Yuan, J.; Young, S.H.; Rozengurt, E. Protein kinase C nu/protein kinase D3 nuclear localization, catalytic activation, and intracellular redistribution in response to G protein-coupled receptor agonists. J. Biol. Chem. 2003, 278, 23773–23785. [Google Scholar] [CrossRef] [PubMed]
- Rodina, A.; Wang, T.; Yan, P.; Gomes, E.D.; Dunphy, M.P.; Pillarsetty, N.; Koren, J.; Gerecitano, J.F.; Taldone, T.; Zong, H.; et al. The epichaperome is an integrated chaperome network that facilitates tumour survival. Nature 2016, 538, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Moulick, K.; Ahn, J.H.; Zong, H.; Rodina, A.; Cerchietti, L.; Gomes DaGama, E.M.; Caldas-Lopes, E.; Beebe, K.; Perna, F.; Hatzi, K.; et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat. Chem. Biol. 2011, 7, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Huck, B.; Kemkemer, R.; Franz-Wachtel, M.; Macek, B.; Hausser, A.; Olayioye, M.A. GIT1 phosphorylation on serine 46 by PKD3 regulates paxillin trafficking and cellular protrusive activity. J. Biol. Chem. 2012, 287, 34604–34613. [Google Scholar] [CrossRef]
- Chakraborty, A.; Edkins, A.L. HSP90 as a regulator of extracellular matrix dynamics. Biochem. Soc. Trans. 2021, 49, 2611–2625. [Google Scholar] [CrossRef]
- Baker-Williams, A.J.; Hashmi, F.; Budzynski, M.A.; Woodford, M.R.; Gleicher, S.; Himanen, S.V.; Makedon, A.M.; Friedman, D.; Cortes, S.; Namek, S.; et al. Co-chaperones TIMP2 and AHA1 Competitively Regulate Extracellular HSP90:Client MMP2 Activity and Matrix Proteolysis. Cell Rep. 2019, 28, 1894–1906.e1896. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Zeng, F.; Li, S.; Guo, W.; Chen, L.; Li, G.; Zhang, Z.; Wang, Q.J.; Deng, F. Protein kinase Ds promote tumor angiogenesis through mast cell recruitment and expression of angiogenic factors in prostate cancer microenvironment. J. Exp. Clin. Cancer Res. 2019, 38, 114. [Google Scholar] [CrossRef]
- Huck, B.; Duss, S.; Hausser, A.; Olayioye, M.A. Elevated protein kinase D3 (PKD3) expression supports proliferation of triple-negative breast cancer cells and contributes to mTORC1-S6K1 pathway activation. J. Biol. Chem. 2014, 289, 3138–3147. [Google Scholar] [CrossRef]
- Liu, Y.; Song, H.; Yu, S.; Huang, K.H.; Ma, X.; Zhou, Y.; Yu, S.; Zhang, J.; Chen, L. Protein Kinase D3 promotes the cell proliferation by activating the ERK1/c-MYC axis in breast cancer. J. Cell Mol. Med. 2020, 24, 2135–2144. [Google Scholar] [CrossRef]
- Hao, Q.; McKenzie, R.; Gan, H.; Tang, H. Protein kinases D2 and D3 are novel growth regulators in HCC1806 triple-negative breast cancer cells. Anticancer Res. 2013, 33, 393–399. [Google Scholar]
- Cui, B.; Chen, J.; Luo, M.; Wang, L.; Chen, H.; Kang, Y.; Wang, J.; Zhou, X.; Feng, Y.; Zhang, P. Protein kinase D3 regulates the expression of the immunosuppressive protein, PDL1, through STAT1/STAT3 signaling. Int. J. Oncol. 2020, 56, 909–920. [Google Scholar] [CrossRef]
- Mayer, A.E.; Loffler, M.C.; Loza Valdes, A.E.; Schmitz, W.; El-Merahbi, R.; Viera, J.T.; Erk, M.; Zhang, T.; Braun, U.; Heikenwalder, M.; et al. The kinase PKD3 provides negative feedback on cholesterol and triglyceride synthesis by suppressing insulin signaling. Sci. Signal. 2019, 12, eaav9150. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Cai, X.; Sun, H.; Jiao, J.; Bai, T.; Zhou, X.W.; Chen, X.; Gill, D.L.; Tang, X.D. Protein kinase D3 is a pivotal activator of pathological cardiac hypertrophy by selectively increasing the expression of hypertrophic transcription factors. J. Biol. Chem. 2011, 286, 40782–40791. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, H.; Yin, M.; Pei, X.; Hausser, A.; Ishikawa, E.; Yamasaki, S.; Jin, Z.G. Deletion of Protein Kinase D3 Promotes Liver Fibrosis in Mice. Hepatology 2020, 72, 1717–1734. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, K.; Pfizenmaier, K.; Lutz, S.; Hausser, A. Expression patterns of protein kinase D 3 during mouse development. BMC Dev. Biol. 2008, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A.; Ishikawa, T. Cell cycle transition under stress conditions controlled by vertebrate heat shock factors. EMBO J. 2001, 20, 2885–2895. [Google Scholar] [CrossRef] [PubMed]
- Nardai, G.; Csermely, P.; Soti, C. Chaperone function and chaperone overload in the aged. A preliminary analysis. Exp. Gerontol. 2002, 37, 1257–1262. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Kurop, M.K.; Huyen, C.M.; Kelly, J.H.; Blagg, B.S.J. The heat shock response and small molecule regulators. Eur. J. Med. Chem. 2021, 226, 113846. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, A.; Nguyen, M.T.; Pénzes, K.; Bátai, B.; Gyulavári, P.; Gurbi, B.; Murányi, J.; Csermely, P.; Csala, M.; Vántus, T.; et al. Protein Kinase D3 (PKD3) Requires Hsp90 for Stability and Promotion of Prostate Cancer Cell Migration. Cells 2023, 12, 212. https://doi.org/10.3390/cells12020212
Varga A, Nguyen MT, Pénzes K, Bátai B, Gyulavári P, Gurbi B, Murányi J, Csermely P, Csala M, Vántus T, et al. Protein Kinase D3 (PKD3) Requires Hsp90 for Stability and Promotion of Prostate Cancer Cell Migration. Cells. 2023; 12(2):212. https://doi.org/10.3390/cells12020212
Chicago/Turabian StyleVarga, Attila, Minh Tu Nguyen, Kinga Pénzes, Bence Bátai, Pál Gyulavári, Bianka Gurbi, József Murányi, Péter Csermely, Miklós Csala, Tibor Vántus, and et al. 2023. "Protein Kinase D3 (PKD3) Requires Hsp90 for Stability and Promotion of Prostate Cancer Cell Migration" Cells 12, no. 2: 212. https://doi.org/10.3390/cells12020212
APA StyleVarga, A., Nguyen, M. T., Pénzes, K., Bátai, B., Gyulavári, P., Gurbi, B., Murányi, J., Csermely, P., Csala, M., Vántus, T., & Sőti, C. (2023). Protein Kinase D3 (PKD3) Requires Hsp90 for Stability and Promotion of Prostate Cancer Cell Migration. Cells, 12(2), 212. https://doi.org/10.3390/cells12020212






