UCHL1 Regulates Radiation Lung Injury via Sphingosine Kinase-1
Abstract
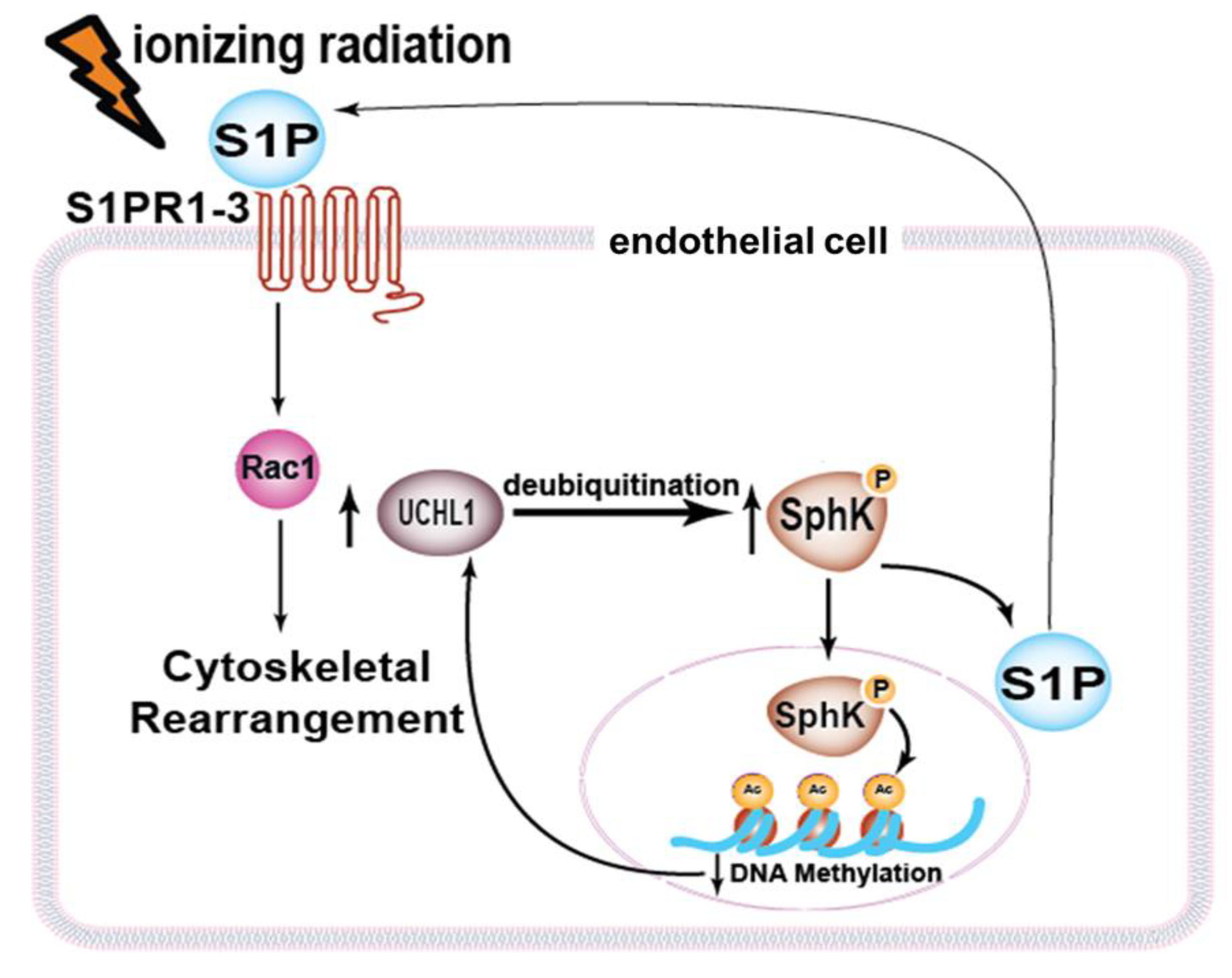
:1. Introduction
2. Materials and Methods
2.1. Antibodies and Reagents
2.2. Endothelial Cell Culture
2.3. Endothelial Cell (EC) siRNA Transfection and Treatment with a UCHL1 Inhibitor
2.4. UCHL1 Overexpression
2.5. Irradiation of EC
2.6. Immunoblotting and Immunoprecipitation
2.7. Transendothelial Electrical Resistance (TER) Measurement
2.8. Murine RILI Model
2.9. Statistics
3. Results
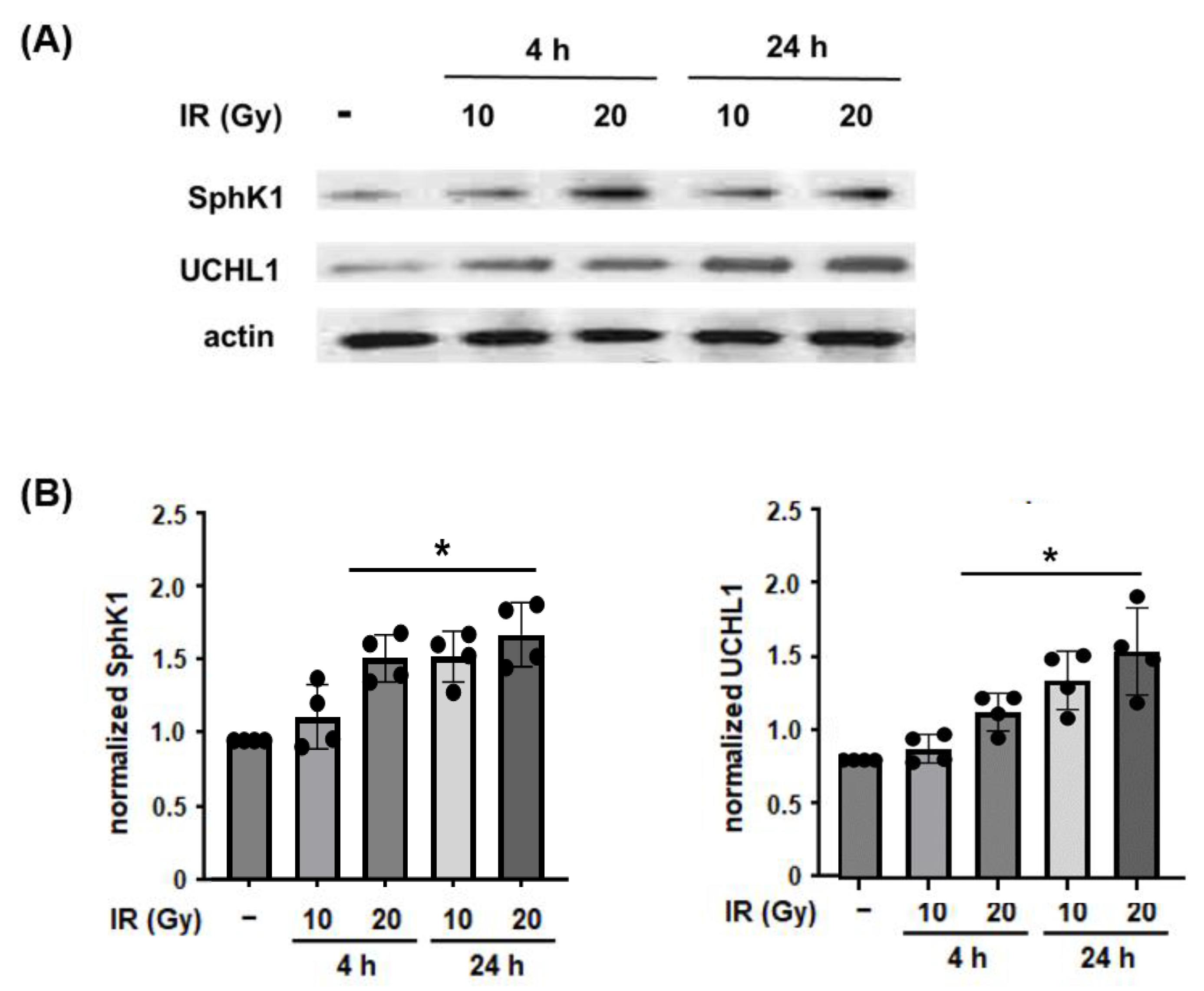
3.1. Effect of Radiation on Lung EC and SphK1 Expression
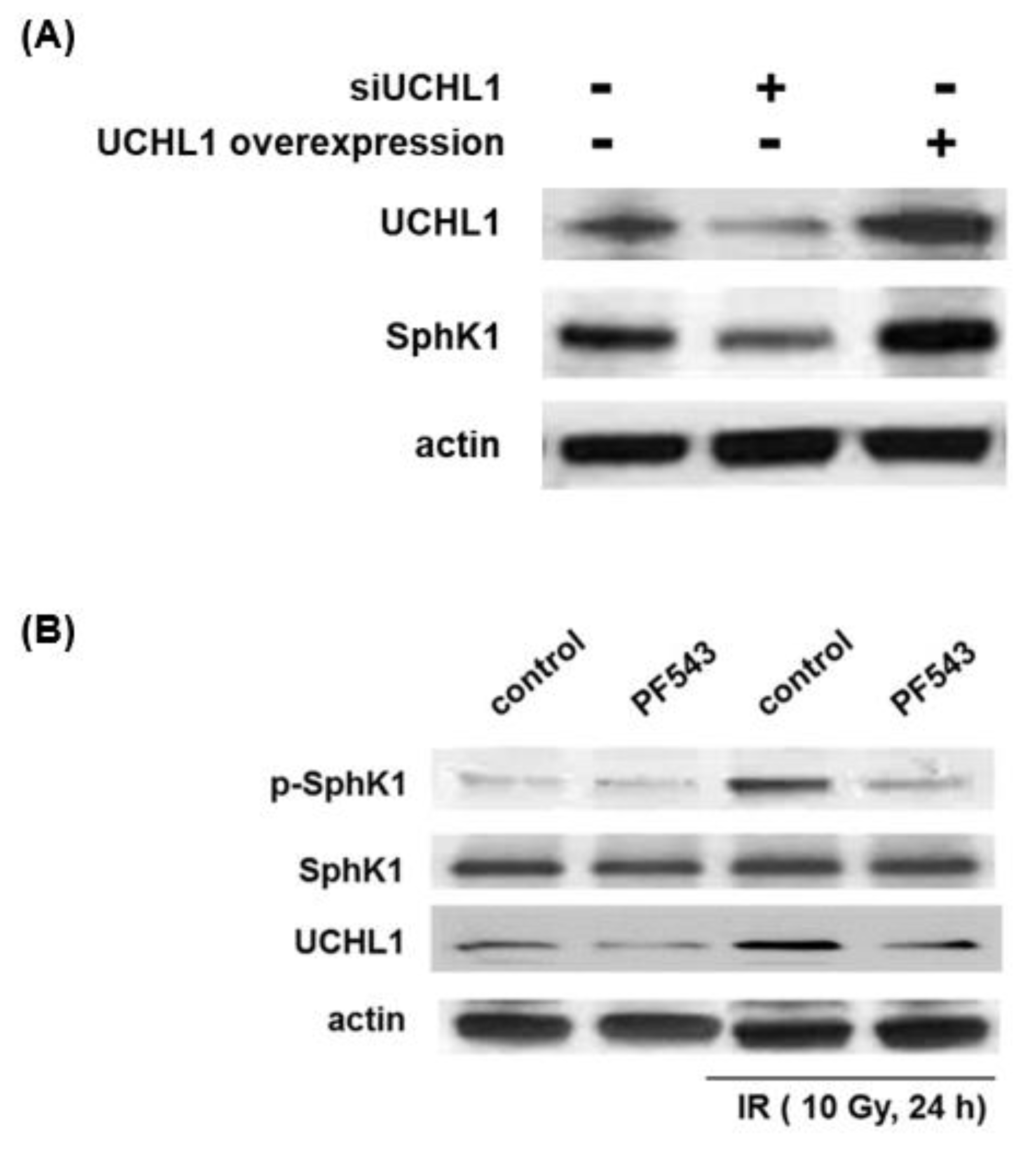
3.2. Role of UCHL1 in Radiation-Induced SphK1 Ubiquitination and Expression Levels
3.3. UCHL1 and SphK1 Interdependence
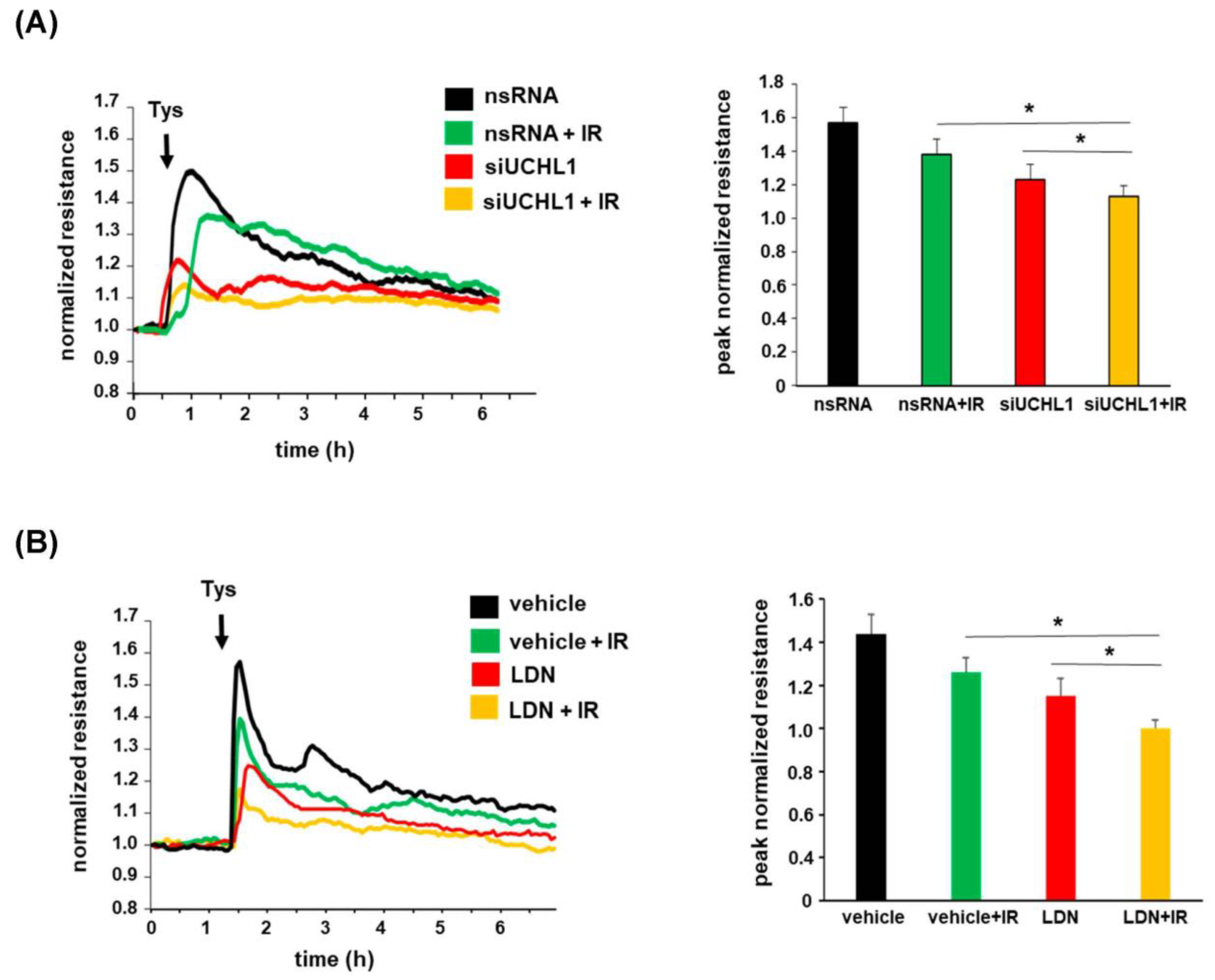
3.4. Role of UCHL1 in EC Barrier Function after Radiation
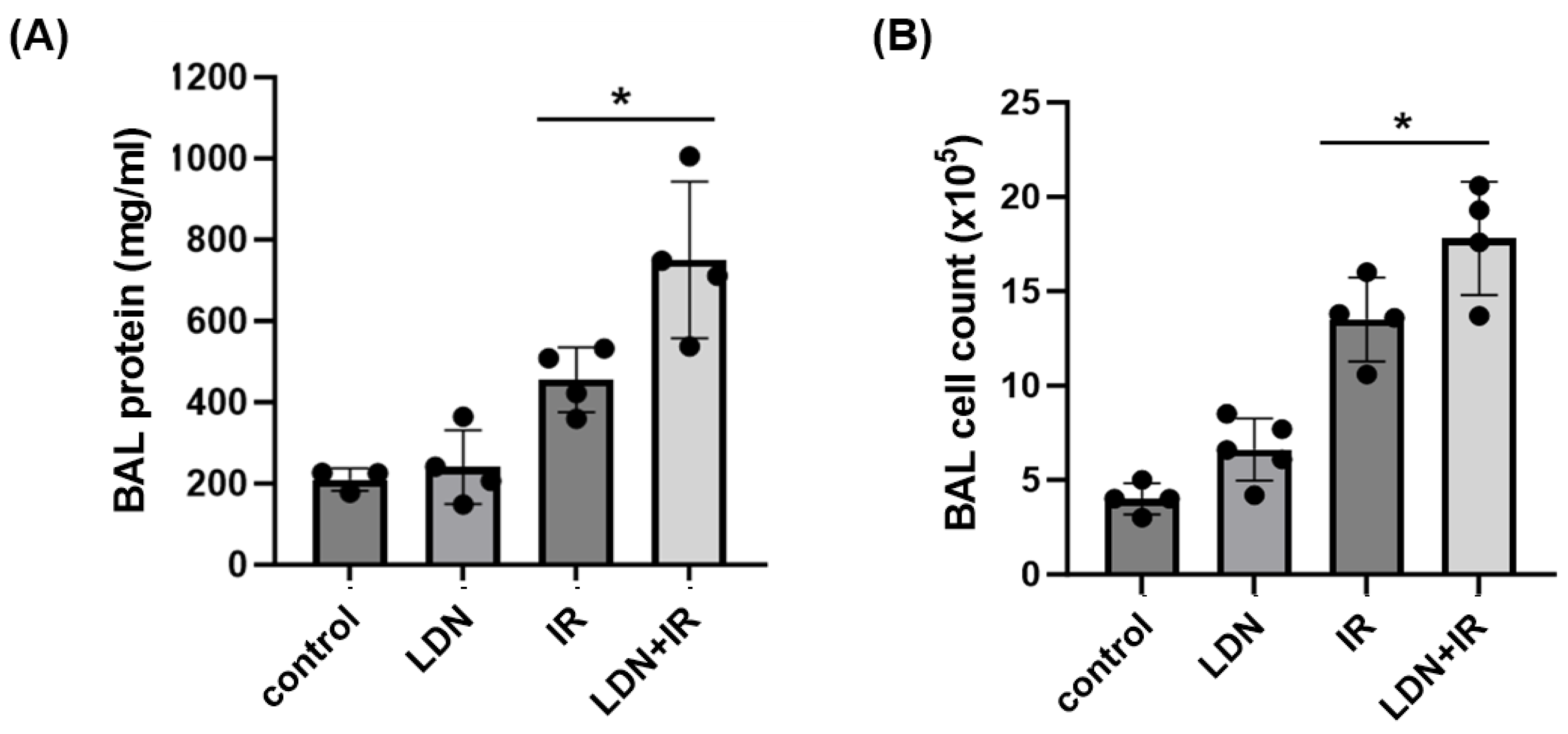
3.5. Role of UCHL1 In Vivo In Murine RILI
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Borst, G.R.; De Jaeger, K.; Belderbos, J.S.; Burgers, S.A.; Lebesque, J.V. Pulmonary function changes after radiotherapy in non-small-cell lung cancer patients with long-term disease-free survival. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 639–644. [Google Scholar] [CrossRef]
- Dang, J.; Li, G.; Zang, S.; Zhang, S.; Yao, L. Risk and predictors for early radiation pneumonitis in patients with stage III non-small cell lung cancer treated with concurrent or sequential chemoradiotherapy. Radiat. Oncol. 2014, 9, 172. [Google Scholar] [CrossRef]
- Rodrigues, G.; Lock, M.; D’Souza, D.; Yu, E.; Van Dyk, J. Prediction of radiation pneumonitis by dose—Volume histogram parameters in lung cancer—A systematic review. Radiother. Oncol. 2004, 71, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.A.; Senan, S.; Tsujino, K.; Barriger, R.B.; Rengan, R.; Moreno, M.; Bradley, J.D.; Kim, T.H.; Ramella, S.; Marks, L.B.; et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Leprieur, E.G.; Fernandez, D.; Chatellier, G.; Klotz, S.; Giraud, P.; Durdux, C. Acute radiation pneumonitis after conformational radiotherapy for nonsmall cell lung cancer: Clinical, dosimetric, and associated-treatment risk factors. J. Cancer Res. Ther. 2013, 9, 447–451. [Google Scholar] [CrossRef]
- Fujimoto, D.; Kato, R.; Morimoto, T.; Shimizu, R.; Sato, Y.; Kogo, M.; Ito, J.; Teraoka, S.; Nagata, K.; Nakagawa, A.; et al. Characteristics and Prognostic Impact of pneumonitis during systemic anti-cancer therapy in patients with advanced non-small-cell lung cancer. PLoS ONE 2016, 11, e0168465. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Huang, Y.; Jacobson, J.R.; Berdyshev, E.; Gerhold, L.M.; Wang, T.; Moreno-Vinasco, L.; Lang, G.; Zhao, Y.; Chen, C.T.; et al. Simvastatin attenuates radiation-induced murine lung injury and dysregulated lung gene expression. Am. J. Respir. Cell Mol. Biol. 2011, 44, 415–422. [Google Scholar] [CrossRef]
- Mathew, B.; Jacobson, J.R.; Berdyshev, E.; Huang, Y.; Sun, X.; Zhao, Y.; Gerhold, L.M.; Siegler, J.; Evenoski, C.; Wang, T.; et al. Role of sphingolipids in murine radiation-induced lung injury: Protection by sphingosine 1-phosphate analogs. FASEB J. 2011, 25, 3388–3400. [Google Scholar] [CrossRef]
- Mathew, B.; Takekoshi, D.; Sammani, S.; Epshtein, Y.; Sharma, R.; Smith, B.D.; Mitra, S.; Desai, A.A.; Weichselbaum, R.R.; Garcia, J.G.; et al. Role of GADD45a in murine models of radiation- and bleomycin-induced lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1420–L1429. [Google Scholar] [CrossRef]
- Meyer, N.J.; Huang, Y.; Singleton, P.A.; Sammani, S.; Moitra, J.; Evenoski, C.L.; Husain, A.N.; Mitra, S.; Moreno-Vinasco, L.; Jacobson, J.R.; et al. GADD45a is a novel candidate gene in inflammatory lung injury via influences on Akt signaling. FASEB J. 2009, 23, 1325–1337. [Google Scholar] [CrossRef]
- Mitra, S.; Sammani, S.; Wang, T.; Boone, D.L.; Meyer, N.J.; Dudek, S.M.; Moreno-Vinasco, L.; Garcia, J.G.; Jacobson, J.R. Role of growth arrest and DNA damage-inducible alpha in Akt phosphorylation and ubiquitination after mechanical stress-induced vascular injury. Am. J. Respir. Crit. Care Med. 2011, 184, 1030–1040. [Google Scholar] [CrossRef]
- Loveridge, C.; Tonelli, F.; Leclercq, T.; Lim, K.G.; Long, J.S.; Berdyshev, E.; Tate, R.J.; Natarajan, V.; Pitson, S.M.; Pyne, N.J.; et al. The sphingosine kinase 1 inhibitor 2-(p-hydroxyanilino)-4-(p-chlorophenyl)thiazole induces proteasomal degradation of sphingosine kinase 1 in mammalian cells. J. Biol. Chem. 2010, 285, 38841–38852. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Sun, C.; Valentine, W.J.; Shuyu, E.; Liu, J.; Tigyi, G.; Bittman, R. Chiral vinylphosphonate and phosphonate analogues of the immunosuppressive agent FTY720. J. Org. Chem. 2009, 74, 3192–3195. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.G.; Liu, F.; Verin, A.D.; Birukova, A.; Dechert, M.A.; Gerthoffer, W.T.; Bamberg, J.R.; English, D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Investig. 2001, 108, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.; Haynes, S.R.; Gorospe, M. Clean western blot signals from immunopreciptated samples. Mol. Cel. Probes 2005, 19, 385–388. [Google Scholar] [CrossRef]
- McVerry, B.J.; Peng, X.; Hassoun, P.M.; Sammani, S.; Simon, B.A.; Garcia, J.G. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. Am. J. Respir. Crit. Care Med. 2004, 170, 987–993. [Google Scholar] [CrossRef]
- Peng, X.; Hassoun, P.M.; Sammani, S.; McVerry, B.J.; Burne, M.J.; Rabb, H.; Pearse, D.; Tuder, R.M.; Garcia, J.G. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Tauseef, M.; Kini, V.; Knezevic, N.; Brannan, M.; Ramchandaran, R.; Fyrst, H.; Saba, J.; Vogel, S.M.; Malik, A.B.; Mehta, D. Activation of sphingosine kinase-1 reverses the increase in lung vascular permeability through sphingosine-1-phosphate receptor signaling in endothelial cells. Circ. Res. 2008, 103, 1164–1172. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Xia, P.; Wang, L.; Gamble, J.R.; Vadas, M.A. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J. Biol. Chem. 1999, 274, 34499–34505. [Google Scholar] [CrossRef]
- Venkataraman, K.; Lee, Y.M.; Michaud, J.; Thangada, S.; Ai, Y.; Bonkovsky, H.L.; Parikh, N.S.; Habrukowich, C.; Hla, T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res. 2008, 102, 669–676. [Google Scholar] [CrossRef]
- Singleton, P.A.; Dudek, S.M.; Chiang, E.T.; Garcia, J.G. Regulation of sphingosine 1-phosphate-induced endothelial cytoskeletal rearrangement and barrier enhancement by S1P1 receptor, PI3 kinase, Tiam1/Rac1, and alpha-actinin. FASEB J. 2005, 19, 1646–1656. [Google Scholar] [CrossRef]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef]
- Ham, S.J.; Lee, D.; Xu, W.J.; Cho, E.; Choi, S.; Min, S.; Park, S.; Chung, J. Loss of UCHL1 rescues the defects related to Parkinson’s disease by suppressing glycolysis. Sci. Adv. 2021, 7, eabg4574. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.S.L.; Tan, Y.J.; Lu, Z.; Ng, E.Y.; Ng, S.Y.E.; Chia, N.S.Y.; Setiawan, F.; Xu, Z.; Keong, N.C.H.; Tay, K.Y.; et al. Plasma ubiquitin C-terminal hydrolase L1 levels reflect disease stage and motor severity in Parkinson’s disease. Aging (Albany NY) 2020, 12, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. Ubiquitin carboxyl-terminal hydrolase L-1 in brain: Focus on its oxidative/nitrosative modification and role in brains of subjects with Alzheimer disease and mild cognitive impairment. Free Radic. Biol. Med. 2021, 177, 278–286. [Google Scholar] [CrossRef]
- Zhong, J.; Zhao, M.; Ma, Y.; Luo, Q.; Liu, J.; Wang, J.; Yuan, X.; Sang, J.; Huang, C. UCHL1 acts as a colorectal cancer oncogene via activation of the beta-catenin/TCF pathway through its deubiquitinating activity. Int. J. Mol. Med. 2012, 30, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Foreman, O.; Perkins, S.L.; Witzig, T.E.; Miles, R.R.; van Deursen, J.; Galardy, P.J. The de-ubiquitinase UCH-L1 is an oncogene that drives the development of lymphoma in vivo by deregulating PHLPP1 and Akt signaling. Leukemia 2010, 24, 1641–1655. [Google Scholar] [CrossRef]
- Liu, S.; Gonzalez-Prieto, R.; Zhang, M.; Geurink, P.P.; Kooij, R.; Iyengar, P.V.; van Dinther, M.; Bos, E.; Zhang, X.; Le Devedec, S.E.; et al. Deubiquitinase activity profiling identifies UCHL1 as a candidate oncoprotein that promotes TGFbeta-induced breast cancer metastasis. Clin. Cancer Res. 2020, 26, 1460–1473. [Google Scholar] [CrossRef]
- Xiang, T.; Li, L.; Yin, X.; Yuan, C.; Tan, C.; Su, X.; Xiong, L.; Putti, T.C.; Oberst, M.; Kelly, K.; et al. The ubiquitin peptidase UCHL1 induces G0/G1 cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer. PLoS ONE 2012, 7, e29783. [Google Scholar] [CrossRef]
- Jin, C.; Yu, W.; Lou, X.; Zhou, F.; Han, X.; Zhao, N.; Lin, B. UCHL1 Is a Putative Tumor Suppressor in Ovarian Cancer Cells and Contributes to Cisplatin Resistance. J. Cancer 2013, 4, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lei, Y.; He, S.W.; Li, Y.Q.; Wang, Y.Q.; Hong, X.H.; Liang, Y.L.; Li, J.Y.; Chen, Y.; Luo, W.J.; et al. Hypermethylation of UCHL1 promotes metastasis of nasopharyngeal carcinoma by suppressing degradation of cortactin (CTTN). Cells 2020, 9, 559. [Google Scholar] [CrossRef]
- Ummanni, R.; Jost, E.; Braig, M.; Lohmann, F.; Mundt, F.; Barett, C.; Schlomm, T.; Sauter, G.; Senff, T.; Bokemeyer, C.; et al. Ubiquitin carboxyl-terminal hydrolase 1 (UCHL1) is a potential tumour suppressor in prostate cancer and is frequently silenced by promoter methylation. Mol. Cancer 2011, 10, 129. [Google Scholar] [CrossRef]
- Mitra, S.; Epshtein, Y.; Sammani, S.; Quijada, H.; Chen, W.; Bandela, M.; Desai, A.A.; Garcia, J.G.N.; Jacobson, J.R. UCHL1, a deubiquitinating enzyme, regulates lung endothelial cell permeability in vitro and in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L497–L507. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Zeng, L.; Yeom, C.J.; Zhu, Y.; Morinibu, A.; Shinomiya, K.; Kobayashi, M.; Hirota, K.; Itasaka, S.; Yoshimura, M.; et al. UCHL1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on HIF-1alpha. Nat. Commun. 2015, 6, 6153. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.J.; Cao, Y.; Li, C.Y.; Dewhirst, M.W. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell 2004, 5, 429–441. [Google Scholar] [CrossRef]
- Nagata, A.; Itoh, F.; Sasho, A.; Sugita, K.; Suzuki, R.; Hinata, H.; Shimoda, Y.; Suzuki, E.; Maemoto, Y.; Inagawa, T.; et al. The evolutionary conserved deubiqutinase UBH1/UCH-L1 augments DAF7/TGF-β signaling, inhibits dauer larva formation, and enhances lung tumorigenesis. J. Biol. Chem. 2020, 295, 9105–9120. [Google Scholar] [CrossRef]
- Rube, C.E.; Uthe, D.; Schmid, K.W.; Richter, K.D.; Wessel, J.; Schuck, A.; Willich, N.; Rube, C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042. [Google Scholar] [CrossRef]
- Yi, E.S.; Bedoya, A.; Lee, H.; Chin, E.; Saunders, W.; Kim, S.J.; Danielpour, D.; Remick, D.G.; Yin, S.; Ulich, T.R. Radiation-induced lung injury in vivo: Expression of transforming growth factor-beta precedes fibrosis. Inflammation 1996, 20, 339–352. [Google Scholar] [CrossRef]
- Takami, Y.; Nakagami, H.; Morishita, R.; Katsuya, T.; Cui, T.X.; Ichikawa, T.; Saito, Y.; Hayashi, H.; Kikuchi, Y.; Nishikawa, T.; et al. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-kappaB activation. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2184–2190. [Google Scholar] [CrossRef]
- Rube, C.E.; Wilfert, F.; Uthe, D.; Schmid, K.W.; Knoop, R.; Willich, N.; Schuck, A.; Rube, C. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother. Oncol. 2002, 64, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Tonse, R.; Ramamoorthy, V.; Rubens, M.; Saxena, A.; McGranaghan, P.; Veledar, E.; Hall, M.D.; Chuong, M.D.; Ahluwalia, M.S.; Mehta, M.P.; et al. Hospitalization rates from radiotherapy complications in the United States. Sci. Rep. 2022, 12, 4371. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Epshtein, Y.; Mathew, B.; Chen, W.; Jacobson, J.R. UCHL1 Regulates Radiation Lung Injury via Sphingosine Kinase-1. Cells 2023, 12, 2405. https://doi.org/10.3390/cells12192405
Epshtein Y, Mathew B, Chen W, Jacobson JR. UCHL1 Regulates Radiation Lung Injury via Sphingosine Kinase-1. Cells. 2023; 12(19):2405. https://doi.org/10.3390/cells12192405
Chicago/Turabian StyleEpshtein, Yulia, Biji Mathew, Weiguo Chen, and Jeffrey R. Jacobson. 2023. "UCHL1 Regulates Radiation Lung Injury via Sphingosine Kinase-1" Cells 12, no. 19: 2405. https://doi.org/10.3390/cells12192405
APA StyleEpshtein, Y., Mathew, B., Chen, W., & Jacobson, J. R. (2023). UCHL1 Regulates Radiation Lung Injury via Sphingosine Kinase-1. Cells, 12(19), 2405. https://doi.org/10.3390/cells12192405





