Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment
Abstract
1. Introduction
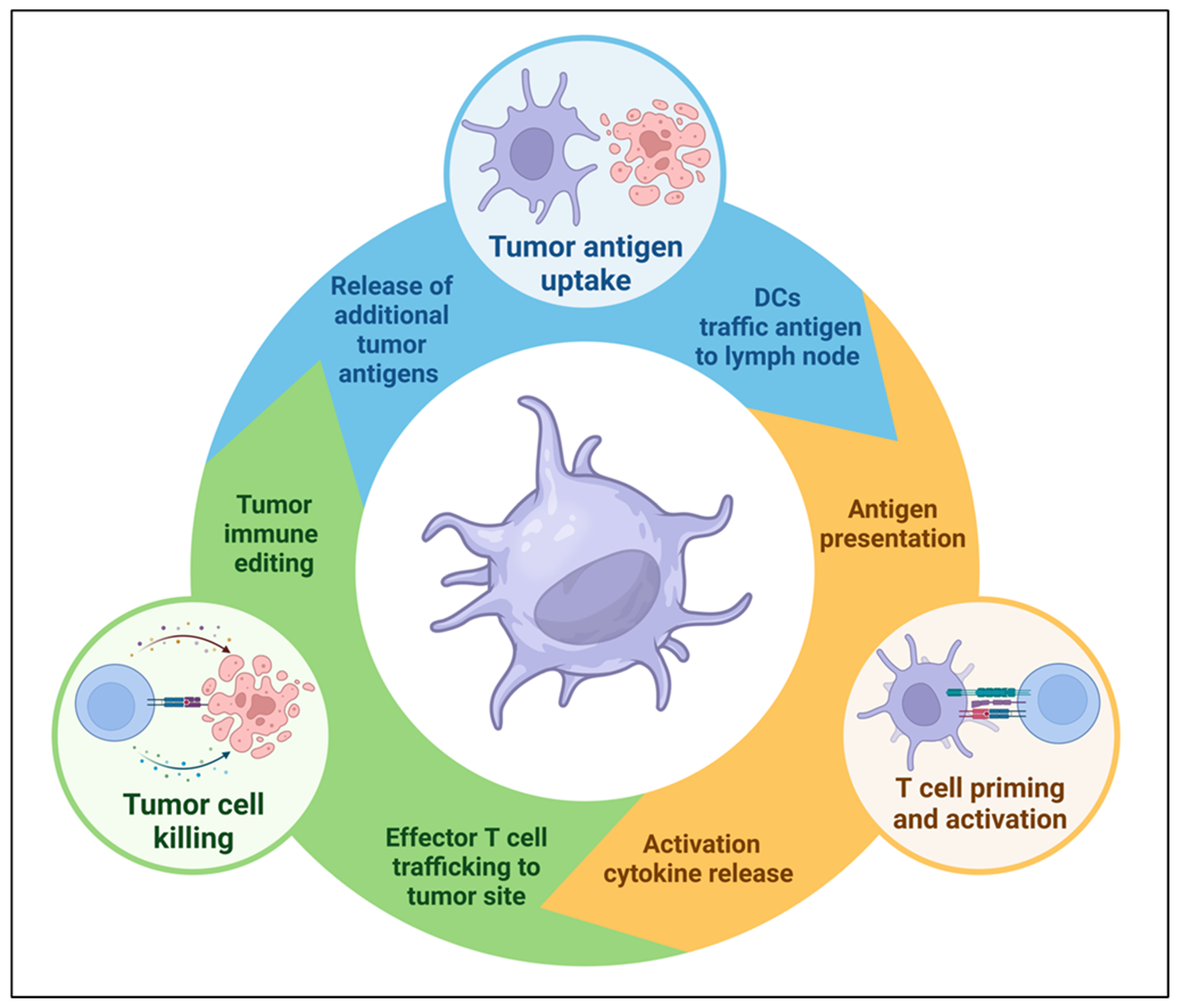
2. DC Diversity and Function
DC Subtypes and Functional Differences
3. Immune Cells in the NSCLC TME and Their Relationship with DCs
3.1. T Cells
3.2. B Cells and Tertiary Lymphoid Structures
3.3. Macrophages
3.4. Neutrophils and Myeloid-Derived Suppressor Cells
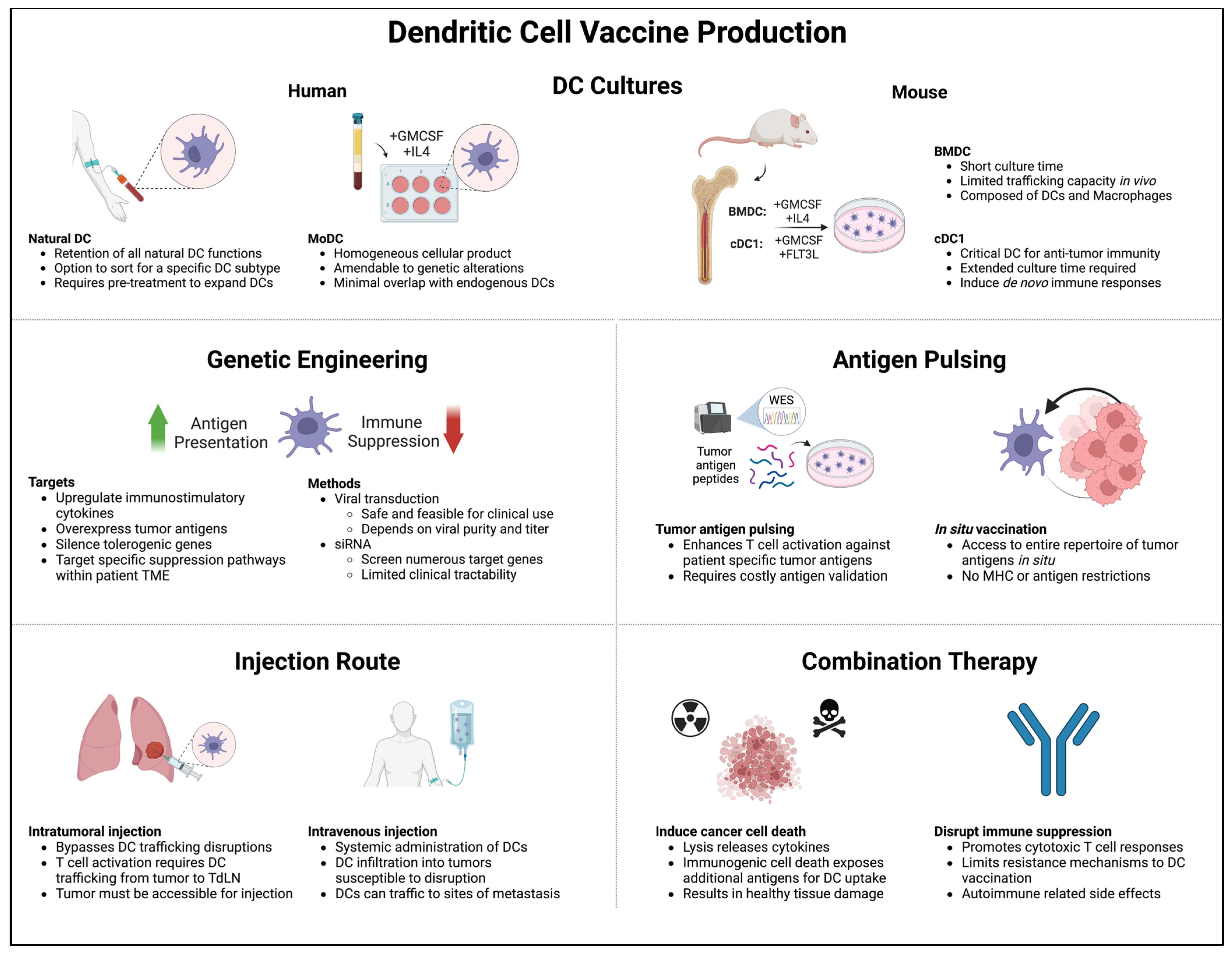
4. DCs as Cancer Vaccines
4.1. Murine BMDCs
4.2. MoDCs
4.3. Stem Cell-Derived DCs
4.4. cDC1s
4.5. Naturally Circulating DCs
4.6. Translation to the Clinic
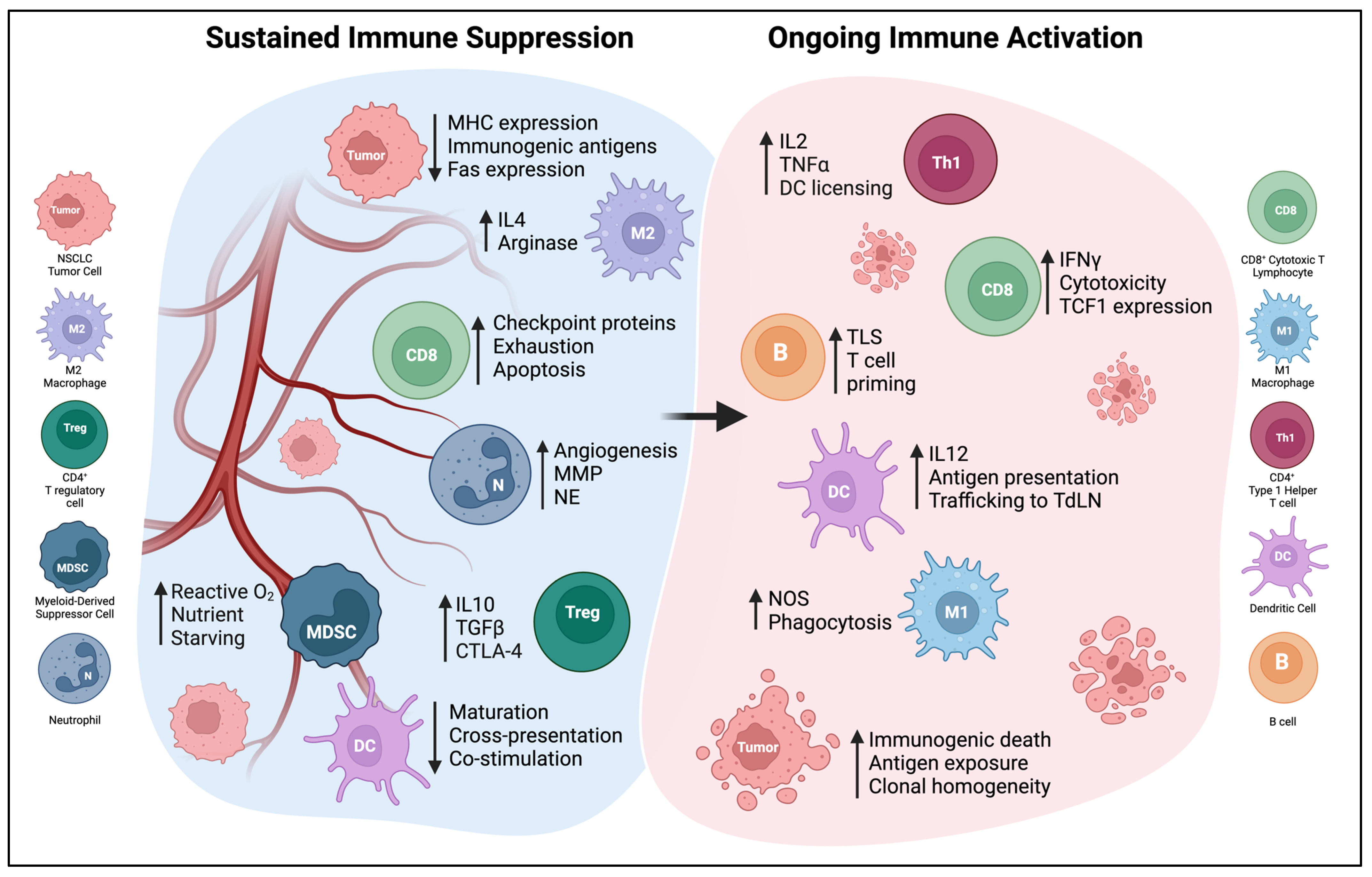
5. Microenvironmental and Systemic Changes Induced by DC Vaccine Therapy
5.1. Changes in Cytokine Profiles
| Vaccine Description | Clinical Study Design | Immune Monitoring | Ref. |
|---|---|---|---|
| ID MoDCs pulsed with tumor cell line lysate | Phase I in stage I-IIIB NSCLC after definitive therapy | Increased T cell IFNγ response to tumor lysate in 6/16 and 9/14 patients across two reports | [201,204] |
| LN injection of MoDCs pulsed with pleural effusion tumor lysate | Phase I in advanced refractory NSCLC | Increased T cell IFNγ response to tumor lysate in 3/8 patients | [200] |
| ID MoDCs pulsed with tumor lysate | Phase I in advanced refractory NSCLC | Increased T cell IFNγ response to tumor lysate in 5/9 patients | [202] |
| IV MoDCs and CIKs (<3 vs. ≥3 cycles) | Non-randomized study in resected NSCLC | Lower Treg frequency and IL10/TGFβ levels with ≥3 cycles | [212] |
| IV MoDCs pulsed with MUC1 and survivin | Phase I in resected NSCLC | Decreased Tregs; lower levels of TNFɑ and IL6 in 2/15 patients | [205] |
| IT MoDCs transduced with CCL21 | Phase I in advanced refractory NSCLC | Increased T cell IFNγ response to TAAs in 6/16 patients; induced tumor T cell infiltration in 7/13 | [185] |
| ID MoDCs pulsed with MAGE3 and survivin | Single-arm study in stage I-IIIB NSCLC after definitive therapy | Increased IFNγ production by peripheral T cells | [203] |
| IV/ID MoDCs transfected with TAAs | Phase I in GBM and NSCLC with brain metastases | Induced T cell responses to TAAs in 7/7 patients tested | [213] |
| ID MoDCs transduced with WT p53 | Phase I/II in untreated SCLC as maintenance after chemotherapy | Improved T cell response to p53 in 18/43 patients; fewer responses in those with elevated MDSCs | [186] |
| ID MoDCs transduced with WT p53 +/− ATRA | Phase I in untreated SCLC as maintenance after chemotherapy | Increased T cell IFNγ response to p53 in 3/15 patients; 5/12 in ATRA combination arm | [214] |
| ID MoDCs pulsed with MAGE-1 peptide | Single-arm study in metastatic melanoma | Induced TIL cytolytic activity against autologous tumor cells in 2/2 patients | [151] |
| IV MoDCs pulsed with neoantigen peptides | Phase I in melanoma after progression on ICB | Developed new T cell responses to neoantigens in 3/3 patients and a more diverse TCR repertoire | [215] |
| SC MoDCs pulsed with tumor antigens vs. irradiated tumor cells | Randomized phase II in metastatic melanoma | DCs associated with increase in Th1/Th17 serum cytokines | [208] |
| ID MoDCs pulsed with melanoma cell lysates | Phase I-II in advanced colorectal cancer | Patients with SD had higher plasma levels of GM-CSF, TNFɑ, IFNγ, IL2, and IL5 | [206] |
5.2. Changes in Myeloid Populations
5.3. Induction of Tumor-Specific T Cell Responses
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M. Classification and Pathology of Lung Cancer. Surg. Oncol. Clin. 2016, 25, 447–468. [Google Scholar] [CrossRef]
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; Reports of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. [Google Scholar]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of Mutational Processes in Human Cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Tai, Y.; Wang, Q.; Korner, H.; Zhang, L.; Wei, W. Molecular Mechanisms of T Cells Activation by Dendritic Cells in Autoimmune Diseases. Front. Pharmacol. 2018, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Amigorena, S. The Cell Biology of Antigen Presentation in Dendritic Cells. Curr. Opin. Immunol. 2001, 13, 45–51. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.-C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; de Chaisemartin, L.; et al. Long-Term Survival for Patients with Non-Small-Cell Lung Cancer with Intratumoral Lymphoid Structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B Cells in Tertiary Lymphoid Structures Is Associated with a Protective Immunity in Patients with Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Dai, F.; Liu, L.; Che, G.; Yu, N.; Pu, Q.; Zhang, S.; Ma, J.; Ma, L.; You, Z. The Number and Microlocalization of Tumor-Associated Immune Cells Are Associated with Patient’s Survival Time in Non-Small Cell Lung Cancer. BMC Cancer 2010, 10, 220. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.-A.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10. [Google Scholar] [CrossRef] [PubMed]
- Villar, J.; Segura, E. The More, the Merrier: DC3s Join the Human Dendritic Cell Family. Immunity 2020, 53, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Cytlak, U.; Resteu, A.; Pagan, S.; Green, K.; Milne, P.; Maisuria, S.; McDonald, D.; Hulme, G.; Filby, A.; Carpenter, B.; et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity 2020, 53, 353–370.e8. [Google Scholar] [CrossRef] [PubMed]
- Roberts, E.W.; Broz, M.L.; Binnewies, M.; Headley, M.B.; Nelson, A.E.; Wolf, D.M.; Kaisho, T.; Bogunovic, D.; Bhardwaj, N.; Krummel, M.F. Critical Role for CD103(+)/CD141(+) Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; e Sousa, C.R. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792. [Google Scholar] [CrossRef]
- Murphy, T.L.; Murphy, K.M. Dendritic Cells in Cancer Immunology. Cell Mol. Immunol. 2022, 19, 3–13. [Google Scholar] [CrossRef]
- Merad, M.; Sathe, P.; Helft, J.; Miller, J.; Mortha, A. The Dendritic Cell Lineage: Ontogeny and Function of Dendritic Cells and Their Subsets in the Steady State and the Inflamed Setting. Annu. Rev. Immunol. 2013, 31, 563–604. [Google Scholar] [CrossRef]
- Ferris, S.T.; Durai, V.; Wu, R.; Theisen, D.J.; Ward, J.P.; Bern, M.D.; Davidson, J.T.; Bagadia, P.; Liu, T.; Briseño, C.G.; et al. cDC1 Prime and Are Licensed by CD4+ T Cells to Induce Anti-Tumour Immunity. Nature 2020, 584, 624–629. [Google Scholar] [CrossRef]
- Saito, Y.; Komori, S.; Kotani, T.; Murata, Y.; Matozaki, T. The Role of Type-2 Conventional Dendritic Cells in the Regulation of Tumor Immunity. Cancers 2022, 14, 1976. [Google Scholar] [CrossRef]
- Ye, Y.; Gaugler, B.; Mohty, M.; Malard, F. Plasmacytoid Dendritic Cell Biology and Its Role in Immune-Mediated Diseases. Clin. Transl. Immunol. 2020, 9, e1139. [Google Scholar] [CrossRef]
- Koucký, V.; Bouček, J.; Fialová, A. Immunology of Plasmacytoid Dendritic Cells in Solid Tumors: A Brief Review. Cancers 2019, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; Terlizzi, M.; Di Crescenzo, V.G.; Popolo, A.; Pecoraro, M.; Perillo, G.; Galderisi, A.; Pinto, A. Human Lung Cancer-Derived Immunosuppressive Plasmacytoid Dendritic Cells Release IL-1α in an AIM2 Inflammasome-Dependent Manner. Am. J. Pathol. 2015, 185, 3115–3124. [Google Scholar] [CrossRef] [PubMed]
- Tang-Huau, T.-L.; Segura, E. Human in Vivo-Differentiated Monocyte-Derived Dendritic Cells. Semin. Cell Dev. Biol. 2019, 86, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-B.; Huang, X.; Li, F.-R. Impaired Dendritic Cell Functions in Lung Cancer: A Review of Recent Advances and Future Perspectives. Cancer Commun. 2019, 39, 43. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Leader, A.M.; Chen, S.T.; Tung, N.; Chang, C.; LeBerichel, J.; Chudnovskiy, A.; Maskey, S.; Walker, L.; Finnigan, J.P.; et al. A Conserved Dendritic-Cell Regulatory Program Limits Antitumour Immunity. Nature 2020, 580, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Mpakali, A.; Stratikos, E. The Role of Antigen Processing and Presentation in Cancer and the Efficacy of Immune Checkpoint Inhibitor Immunotherapy. Cancers 2021, 13, 134. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Q.; Yang, Z.; Zhang, S.; Xu, J.; Wang, Z.; Bai, H.; Duan, J.; Zheng, B.; Li, W.; et al. Single-Cell Transcriptomic Profiling Reveals the Tumor Heterogeneity of Small-Cell Lung Cancer. Signal Transduct. Target. Ther. 2022, 7, 346. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2021, 124, 359–367. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef]
- Lerner, E.C.; Woroniecka, K.I.; D’Anniballe, V.M.; Wilkinson, D.S.; Mohan, A.A.; Lorrey, S.J.; Waibl-Polania, J.; Wachsmuth, L.P.; Miggelbrink, A.M.; Jackson, J.D.; et al. CD8+ T Cells Maintain Killing of MHC-I-Negative Tumor Cells through the NKG2D-NKG2DL Axis. Nat. Cancer 2023, 4, 1258–1272. [Google Scholar] [CrossRef]
- Ruffini, E.; Asioli, S.; Filosso, P.L.; Lyberis, P.; Bruna, M.C.; Macrì, L.; Daniele, L.; Oliaro, A. Clinical Significance of Tumor-Infiltrating Lymphocytes in Lung Neoplasms. Ann. Thorac. Surg. 2009, 87, 365–371; discussion 371–372. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K.; Miyamoto, M.; Cho, Y.; Suzuoki, M.; Oshikiri, T.; Nakakubo, Y.; Itoh, T.; Ohbuchi, T.; Kondo, S.; Katoh, H. Concurrent Infiltration by CD8+ T Cells and CD4+ T Cells Is a Favourable Prognostic Factor in Non-Small-Cell Lung Carcinoma. Br. J. Cancer 2006, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Al-Shibli, K.I.; Donnem, T.; Al-Saad, S.; Persson, M.; Bremnes, R.M.; Busund, L.-T. Prognostic Effect of Epithelial and Stromal Lymphocyte Infiltration in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2008, 14, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Kawai, O.; Ishii, G.; Kubota, K.; Murata, Y.; Naito, Y.; Mizuno, T.; Aokage, K.; Saijo, N.; Nishiwaki, Y.; Gemma, A.; et al. Predominant Infiltration of Macrophages and CD8(+) T Cells in Cancer Nests Is a Significant Predictor of Survival in Stage IV Nonsmall Cell Lung Cancer. Cancer 2008, 113, 1387–1395. [Google Scholar] [CrossRef]
- Goc, J.; Germain, C.; Vo-Bourgais, T.K.D.; Lupo, A.; Klein, C.; Knockaert, S.; de Chaisemartin, L.; Ouakrim, H.; Becht, E.; Alifano, M.; et al. Dendritic Cells in Tumor-Associated Tertiary Lymphoid Structures Signal a Th1 Cytotoxic Immune Contexture and License the Positive Prognostic Value of Infiltrating CD8+ T Cells. Cancer Res. 2014, 74, 705–715. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Anestopoulos, I.; Panayiotidis, M.I.; Mitrakas, A.; Pappa, A.; Koukourakis, M.I. Prognostic Relevance of the Relative Presence of CD4, CD8 and CD20 Expressing Tumor Infiltrating Lymphocytes in Operable Non-Small Cell Lung Cancer Patients. Anticancer. Res. 2021, 41, 3989–3995. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Brown, J.; Carvajal-Hausdorf, D.; McLaughlin, J.; Velcheti, V.; Syrigos, K.N.; Herbst, R.S.; Rimm, D.L. Objective Measurement and Clinical Significance of TILs in Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2015, 107, dju435. [Google Scholar] [CrossRef]
- Djenidi, F.; Adam, J.; Goubar, A.; Durgeau, A.; Meurice, G.; de Montpréville, V.; Validire, P.; Besse, B.; Mami-Chouaib, F. CD8+CD103+ Tumor-Infiltrating Lymphocytes Are Tumor-Specific Tissue-Resident Memory T Cells and a Prognostic Factor for Survival in Lung Cancer Patients. J. Immunol. 2015, 194, 3475–3486. [Google Scholar] [CrossRef]
- Horne, Z.D.; Jack, R.; Gray, Z.T.; Siegfried, J.M.; Wilson, D.O.; Yousem, S.A.; Nason, K.S.; Landreneau, R.J.; Luketich, J.D.; Schuchert, M.J. Increased Levels of Tumor-Infiltrating Lymphocytes Are Associated with Improved Recurrence-Free Survival in Stage 1A Non-Small-Cell Lung Cancer. J. Surg. Res. 2011, 171, 1–5. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef]
- Kennedy, R.; Celis, E. Multiple Roles for CD4+ T Cells in Anti-Tumor Immune Responses. Immunol. Rev. 2008, 222, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Laheurte, C.; Dosset, M.; Vernerey, D.; Boullerot, L.; Gaugler, B.; Gravelin, E.; Kaulek, V.; Jacquin, M.; Cuche, L.; Eberst, G.; et al. Distinct Prognostic Value of Circulating Anti-Telomerase CD4+ Th1 Immunity and Exhausted PD-1+/TIM-3+ T Cells in Lung Cancer. Br. J. Cancer 2019, 121, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.H.; Bretscher, P.A. Different Immune Correlates Associated with Tumor Progression and Regression: Implications for Prevention and Treatment of Cancer. Cancer Immunol. Immunother. 2008, 57, 1125–1136. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kuo, F.; Capistrano, K.J.; Kang, D.; Nixon, B.G.; Shi, W.; Chou, C.; Do, M.H.; Stamatiades, E.G.; Gao, S.; et al. TGF-β Suppresses Type 2 Immunity to Cancer. Nature 2020, 587, 115–120. [Google Scholar] [CrossRef]
- Lorvik, K.B.; Hammarström, C.; Fauskanger, M.; Haabeth, O.A.W.; Zangani, M.; Haraldsen, G.; Bogen, B.; Corthay, A. Adoptive Transfer of Tumor-Specific Th2 Cells Eradicates Tumors by Triggering an In Situ Inflammatory Immune Response. Cancer Res. 2016, 76, 6864–6876. [Google Scholar] [CrossRef]
- Wolf, M.T.; Ganguly, S.; Wang, T.L.; Anderson, C.W.; Sadtler, K.; Narain, R.; Cherry, C.; Parrillo, A.J.; Park, B.V.; Wang, G.; et al. A Biologic Scaffold-Associated Type 2 Immune Microenvironment Inhibits Tumor Formation and Synergizes with Checkpoint Immunotherapy. Sci. Transl. Med. 2019, 11, eaat7973. [Google Scholar] [CrossRef]
- Shimizu, K.; Nakata, M.; Hirami, Y.; Yukawa, T.; Maeda, A.; Tanemoto, K. Tumor-Infiltrating Foxp3+ Regulatory T Cells Are Correlated with Cyclooxygenase-2 Expression and Are Associated with Recurrence in Resected Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 585–590. [Google Scholar] [CrossRef]
- Petersen, R.P.; Campa, M.J.; Sperlazza, J.; Conlon, D.; Joshi, M.-B.; Harpole, D.H.; Patz, E.F. Tumor Infiltrating Foxp3+ Regulatory T-Cells Are Associated with Recurrence in Pathologic Stage I NSCLC Patients. Cancer 2006, 107, 2866–2872. [Google Scholar] [CrossRef]
- Tao, H.; Mimura, Y.; Aoe, K.; Kobayashi, S.; Yamamoto, H.; Matsuda, E.; Okabe, K.; Matsumoto, T.; Sugi, K.; Ueoka, H. Prognostic Potential of FOXP3 Expression in Non-Small Cell Lung Cancer Cells Combined with Tumor-Infiltrating Regulatory T Cells. Lung Cancer 2012, 75, 95–101. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, D.S.; Rexhepaj, E.; Gately, K.; Coate, L.; Delaney, D.; O’Donnell, D.M.; Kay, E.; O’Connell, F.; Gallagher, W.M.; O’Byrne, K.J. Tumour Islet Foxp3+ T-Cell Infiltration Predicts Poor Outcome in Nonsmall Cell Lung Cancer. Eur. Respir. J. 2015, 46, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Lu, L.; Barbi, J.; Pan, F. The Regulation of Immune Tolerance by FOXP3. Nat. Rev. Immunol. 2017, 17, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Baratelli, F.; Lin, Y.; Zhu, L.; Yang, S.-C.; Heuzé-Vourc’h, N.; Zeng, G.; Reckamp, K.; Dohadwala, M.; Sharma, S.; Dubinett, S.M. Prostaglandin E2 Induces FOXP3 Gene Expression and T Regulatory Cell Function in Human CD4+ T Cells. J. Immunol. 2005, 175, 1483–1490. [Google Scholar] [CrossRef]
- Sharma, S.; Yang, S.-C.; Zhu, L.; Reckamp, K.; Gardner, B.; Baratelli, F.; Huang, M.; Batra, R.K.; Dubinett, S.M. Tumor Cyclooxygenase-2/Prostaglandin E2–Dependent Promotion of FOXP3 Expression and CD4+CD25+ T Regulatory Cell Activities in Lung Cancer. Cancer Res. 2005, 65, 5211–5220. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Ganesan, A.-P.; Johansson, M.; Ruffell, B.; Yagui-Beltrán, A.; Lau, J.; Jablons, D.M.; Coussens, L.M. Tumor-Infiltrating Regulatory T Cells Inhibit Endogenous Cytotoxic T Cell Responses to Lung Adenocarcinoma. J. Immunol. 2013, 191, 2009–2017. [Google Scholar] [CrossRef]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-Cell Exhaustion: Characteristics, Causes and Conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef]
- Wherry, E.J. T Cell Exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Cheng, Y.; Shao, Z.; Chen, L.; Zheng, Q.; Zhang, Q.; Ding, W.; Zhang, M.; Yu, Q.; Gao, D. Role, Function and Regulation of the Thymocyte Selection-Associated High Mobility Group Box Protein in CD8+ T Cell Exhaustion. Immunol. Lett. 2021, 229, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, S.; Park, S.Y.; Kim, G.; Park, S.M.; Cho, J.-W.; Kim, D.H.; Park, Y.M.; Koh, Y.W.; Kim, H.R.; et al. Single-Cell Transcriptome Analysis Reveals TOX as a Promoting Factor for T Cell Exhaustion and a Predictor for Anti-PD-1 Responses in Human Cancer. Genome Med. 2020, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Devel Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Schaeuble, K.; Chennupati, V.; Fuertes Marraco, S.A.; Calderon-Copete, S.; Pais Ferreira, D.; Carmona, S.J.; Scarpellino, L.; Gfeller, D.; Pradervand, S.; et al. Intratumoral Tcf1+PD-1+CD8+ T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 2019, 50, 195–211.e10. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of Exhausted CD8+ T Cells Differentially Mediate Tumor Control and Respond to Checkpoint Blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Feng, K.; Gao, R.; Xue, Z.; Zhang, S.; Zhang, Y.; Corse, E.; Hu, Y.; Han, W.; et al. Temporal Single-Cell Tracing Reveals Clonal Revival and Expansion of Precursor Exhausted T Cells during Anti-PD-1 Therapy in Lung Cancer. Nat. Cancer 2022, 3, 108–121. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next Generation of Immune Checkpoint Inhibitors and Beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef]
- Smith-Garvin, J.E.; Koretzky, G.A.; Jordan, M.S. T Cell Activation. Annu. Rev. Immunol. 2009, 27, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Marciscano, A.E.; Anandasabapathy, N. The Role of Dendritic Cells in Cancer and Anti-Tumor Immunity. Semin. Immunol. 2021, 52, 101481. [Google Scholar] [CrossRef] [PubMed]
- Padovan, E.; Spagnoli, G.C.; Ferrantini, M.; Heberer, M. IFN-Alpha2a Induces IP-10/CXCL10 and MIG/CXCL9 Production in Monocyte-Derived Dendritic Cells and Enhances Their Capacity to Attract and Stimulate CD8+ Effector T Cells. J. Leukoc. Biol. 2002, 71, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Woo, S.-R.; Burnett, B.; Fu, Y.-X.; Gajewski, T.F. Type I Interferon Response and Innate Immune Sensing of Cancer. Trends Immunol. 2013, 34, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-Redundant Requirement for CXCR3 Signalling during Tumoricidal T-Cell Trafficking across Tumour Vascular Checkpoints. Nat. Commun. 2015, 6, 7458. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4. [Google Scholar] [CrossRef]
- Litchfield, K.; Reading, J.L.; Puttick, C.; Thakkar, K.; Abbosh, C.; Bentham, R.; Watkins, T.B.K.; Rosenthal, R.; Biswas, D.; Rowan, A.; et al. Meta-Analysis of Tumor- and T Cell-Intrinsic Mechanisms of Sensitization to Checkpoint Inhibition. Cell 2021, 184, 596–614.e14. [Google Scholar] [CrossRef]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on Dendritic Cells Attenuates T Cell Activation and Regulates Response to Immune Checkpoint Blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R. Tertiary Lymphoid Organs in Cancer Tissues. Front. Immunol. 2016, 7, 244. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Thommen, D.S. Tertiary Lymphoid Structures in Cancer. Science 2022, 375, eabf9419. [Google Scholar] [CrossRef]
- Pelletier, M.P.; Edwardes, M.D.; Michel, R.P.; Halwani, F.; Morin, J.E. Prognostic Markers in Resectable Non-Small Cell Lung Cancer: A Multivariate Analysis. Can. J. Surg. 2001, 44, 180–188. [Google Scholar] [PubMed]
- Feng, H.; Yang, F.; Qiao, L.; Zhou, K.; Wang, J.; Zhang, J.; Tian, T.; Du, Y.; Shangguan, H. Prognostic Significance of Gene Signature of Tertiary Lymphoid Structures in Patients With Lung Adenocarcinoma. Front. Oncol. 2021, 11, 693234. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral Plasma Cells Predict Outcomes to PD-L1 Blockade in Non-Small Cell Lung Cancer. Cancer Cell 2022, 40, 289–300.e4. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.-C.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Sautès-Fridman, C. Tertiary Lymphoid Structures, Drivers of the Anti-Tumor Responses in Human Cancers. Immunol. Rev. 2016, 271, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Siliņa, K.; Soltermann, A.; Attar, F.M.; Casanova, R.; Uckeley, Z.M.; Thut, H.; Wandres, M.; Isajevs, S.; Cheng, P.; Curioni-Fontecedro, A.; et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018, 78, 1308–1320. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.B.; Wagner, M.; Hornung, V.; Giese, T.; Schnurr, M.; Endres, S.; Hartmann, G. Plasmacytoid Dendritic Cells Control TLR7 Sensitivity of Naive B Cells via Type I IFN. J. Immunol. 2005, 174, 4043–4050. [Google Scholar] [CrossRef]
- Jego, G.; Palucka, A.K.; Blanck, J.-P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid Dendritic Cells Induce Plasma Cell Differentiation through Type I Interferon and Interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef]
- Dubois, B.; Vanbervliet, B.; Fayette, J.; Massacrier, C.; Van Kooten, C.; Brière, F.; Banchereau, J.; Caux, C. Dendritic Cells Enhance Growth and Differentiation of CD40-Activated B Lymphocytes. J. Exp. Med. 1997, 185, 941–951. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via Arginase or Nitric Oxide Synthase: Two Competing Arginine Pathways in Macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Ma, R.-Y.; Black, A.; Qian, B.-Z. Macrophage Diversity in Cancer Revisited in the Era of Single-Cell Omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef]
- Gautier, E.L.; Yvan-Charvet, L. Understanding Macrophage Diversity at the Ontogenic and Transcriptomic Levels. Immunol. Rev. 2014, 262, 85–95. [Google Scholar] [CrossRef]
- Welsh, T.J.; Green, R.H.; Richardson, D.; Waller, D.A.; O’Byrne, K.J.; Bradding, P. Macrophage and Mast-Cell Invasion of Tumor Cell Islets Confers a Marked Survival Advantage in Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 8959–8967. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-W.; Min, H.S.; Lee, K.-H.; Kim, Y.J.; Oh, D.-Y.; Jeon, Y.K.; Lee, S.-H.; Im, S.-A.; Chung, D.H.; Kim, Y.T.; et al. High Tumour Islet Macrophage Infiltration Correlates with Improved Patient Survival but Not with EGFR Mutations, Gene Copy Number or Protein Expression in Resected Non-Small Cell Lung Cancer. Br. J. Cancer 2008, 98, 1118–1124. [Google Scholar] [CrossRef]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 Form of Tumor-Associated Macrophages in Non-Small Cell Lung Cancer Is Positively Associated with Survival Time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of M1 and M2 Macrophages in Tumor Islets and Stroma in Relation to Prognosis of Non-Small Cell Lung Cancer. BMC Immunol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Ohri, C.M.; Shikotra, A.; Green, R.H.; Waller, D.A.; Bradding, P. Macrophages within NSCLC Tumour Islets Are Predominantly of a Cytotoxic M1 Phenotype Associated with Extended Survival. Eur. Respir. J. 2009, 33, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yao, G.; Zhang, Y.; Gao, J.; Yang, B.; Rao, Z.; Gao, J. M2-Polarized Tumor-Associated Macrophages Are Associated with Poor Prognoses Resulting from Accelerated Lymphangiogenesis in Lung Adenocarcinoma. Clinics 2011, 66, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Takanami, I.; Takeuchi, K.; Kodaira, S. Tumor-Associated Macrophage Infiltration in Pulmonary Adenocarcinoma: Association with Angiogenesis and Poor Prognosis. Oncology 1999, 57, 138–142. [Google Scholar] [CrossRef]
- Sumitomo, R.; Hirai, T.; Fujita, M.; Murakami, H.; Otake, Y.; Huang, C.-L. M2 Tumor-Associated Macrophages Promote Tumor Progression in Non-Small-Cell Lung Cancer. Exp. Ther. Med. 2019, 18, 4490–4498. [Google Scholar] [CrossRef]
- Guo, Z.; Song, J.; Hao, J.; Zhao, H.; Du, X.; Li, E.; Kuang, Y.; Yang, F.; Wang, W.; Deng, J.; et al. M2 Macrophages Promote NSCLC Metastasis by Upregulating CRYAB. Cell Death Dis. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.F.; Atkins, R.C. Defective Cytostatic Activity of Pulmonary Alveolar Macrophages in Primary Lung Cancer. Chest 1990, 98, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Siziopikou, K.P.; Harris, J.E.; Casey, L.; Nawas, Y.; Braun, D.P. Impaired Tumoricidal Function of Alveolar Macrophages from Patients with Non-Small Cell Lung Cancer. Cancer 1991, 68, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Kataki, A.; Scheid, P.; Piet, M.; Marie, B.; Martinet, N.; Martinet, Y.; Vignaud, J.-M. Tumor Infiltrating Lymphocytes and Macrophages Have a Potential Dual Role in Lung Cancer by Supporting Both Host-Defense and Tumor Progression. J. Lab. Clin. Med. 2002, 140, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, H.; Takeuchi, E.; Suzuki, Y.; Hanibuchi, M.; Haku, T.; Ohmoto, Y.; Sone, S. Production of Interleukin-10 by Alveolar Macrophages from Lung Cancer Patients. Respir. Med. 1999, 93, 666–671. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Dalla, E.; Leader, A.M.; LeBerichel, J.; Nikolic, J.; Morales, B.M.; Brown, M.; Chang, C.; Troncoso, L.; Chen, S.T.; et al. Tissue-Resident Macrophages Provide a pro-Tumorigenic Niche to Early NSCLC Cells. Nature 2021, 595, 578–584. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.T.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 Blocks CD8+ T Cell-Dependent Responses to Chemotherapy by Suppressing IL-12 Expression in Intratumoral Dendritic Cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Mittal, S.K.; Roche, P.A. Suppression of Antigen Presentation by IL-10. Curr. Opin. Immunol. 2015, 34, 22–27. [Google Scholar] [CrossRef]
- Bedoret, D.; Wallemacq, H.; Marichal, T.; Desmet, C.; Quesada Calvo, F.; Henry, E.; Closset, R.; Dewals, B.; Thielen, C.; Gustin, P.; et al. Lung Interstitial Macrophages Alter Dendritic Cell Functions to Prevent Airway Allergy in Mice. J. Clin. Invest. 2009, 119, 3723–3738. [Google Scholar] [CrossRef]
- Sharma, S.K.; Chintala, N.K.; Vadrevu, S.K.; Patel, J.; Karbowniczek, M.; Markiewski, M.M. Pulmonary Alveolar Macrophages Contribute to the Premetastatic Niche by Suppressing Antitumor T Cell Responses in the Lungs. J. Immunol. 2015, 194, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Oliver, J.; Bilyk, N.; McMenamin, C.; McMenamin, P.G.; Kraal, G.; Thepen, T. Downregulation of the Antigen Presenting Cell Function(s) of Pulmonary Dendritic Cells in Vivo by Resident Alveolar Macrophages. J. Exp. Med. 1993, 177, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Isobe, K.I.; Hasegawa, Y.; Nakashima, I.; Shimokata, K. Immunosuppressive Activity Induced by Nitric Oxide in Culture Supernatant of Activated Rat Alveolar Macrophages. Immunology 1992, 76, 72–78. [Google Scholar] [PubMed]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil Diversity and Plasticity in Tumour Progression and Therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-Associated Neutrophils as a New Prognostic Factor in Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef]
- Kargl, J.; Zhu, X.; Zhang, H.; Yang, G.H.Y.; Friesen, T.J.; Shipley, M.; Maeda, D.Y.; Zebala, J.A.; McKay-Fleisch, J.; Meredith, G.; et al. Neutrophil Content Predicts Lymphocyte Depletion and Anti-PD1 Treatment Failure in NSCLC. JCI Insight 2019, 4, e130850. [Google Scholar] [CrossRef]
- Akinci Ozyurek, B.; Sahin Ozdemirel, T.; Buyukyaylaci Ozden, S.; Erdogan, Y.; Kaplan, B.; Kaplan, T. Prognostic Value of the Neutrophil to Lymphocyte Ratio (NLR) in Lung Cancer Cases. Asian Pac. J. Cancer Prev. 2017, 18, 1417–1421. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, I.; Salataj, E.; Said Abu Egal, E.; Beswick, E.J. G-CSF in Tumors: Aggressiveness, Tumor Microenvironment and Immune Cell Regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef] [PubMed]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-Tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Tumor Angiogenesis: MMP-Mediated Induction of Intravasation- and Metastasis-Sustaining Neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Zajac, E.; Juncker-Jensen, A.; Kupriyanova, T.A.; Welter, L.; Quigley, J.P. Tissue-Infiltrating Neutrophils Constitute the Major in Vivo Source of Angiogenesis-Inducing MMP-9 in the Tumor Microenvironment. Neoplasia 2014, 16, 771–788. [Google Scholar] [CrossRef]
- Hattar, K.; Franz, K.; Ludwig, M.; Sibelius, U.; Wilhelm, J.; Lohmeyer, J.; Savai, R.; Subtil, F.S.B.; Dahlem, G.; Eul, B.; et al. Interactions between Neutrophils and Non-Small Cell Lung Cancer Cells: Enhancement of Tumor Proliferation and Inflammatory Mediator Synthesis. Cancer Immunol. Immunother. 2014, 63, 1297–1306. [Google Scholar] [CrossRef]
- Gong, L.; Cumpian, A.M.; Caetano, M.S.; Ochoa, C.E.; De la Garza, M.M.; Lapid, D.J.; Mirabolfathinejad, S.G.; Dickey, B.F.; Zhou, Q.; Moghaddam, S.J. Promoting Effect of Neutrophils on Lung Tumorigenesis Is Mediated by CXCR2 and Neutrophil Elastase. Mol. Cancer 2013, 12, 154. [Google Scholar] [CrossRef]
- Shaul, M.E.; Fridlender, Z.G. The Dual Role of Neutrophils in Cancer. Semin. Immunol. 2021, 57, 101582. [Google Scholar] [CrossRef]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A Neutrophil Response Linked to Tumor Control in Immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef]
- Hagerling, C.; Gonzalez, H.; Salari, K.; Wang, C.-Y.; Lin, C.; Robles, I.; van Gogh, M.; Dejmek, A.; Jirström, K.; Werb, Z. Immune Effector Monocyte-Neutrophil Cooperation Induced by the Primary Tumor Prevents Metastatic Progression of Breast Cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 21704–21714. [Google Scholar] [CrossRef]
- Hirschhorn, D.; Budhu, S.; Kraehenbuehl, L.; Gigoux, M.; Schröder, D.; Chow, A.; Ricca, J.M.; Gasmi, B.; De Henau, O.; Mangarin, L.M.B.; et al. T Cell Immunotherapies Engage Neutrophils to Eliminate Tumor Antigen Escape Variants. Cell 2023, 186, 1432–1447.e17. [Google Scholar] [CrossRef]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-Type Oxidized LDL Receptor-1 Distinguishes Population of Human Polymorphonuclear Myeloid-Derived Suppressor Cells in Cancer Patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef]
- Youn, J.-I.; Park, S.-M.; Park, S.; Kim, G.; Lee, H.-J.; Son, J.; Hong, M.H.; Ghaderpour, A.; Baik, B.; Islam, J.; et al. Peripheral Natural Killer Cells and Myeloid-Derived Suppressor Cells Correlate with Anti-PD-1 Responses in Non-Small Cell Lung Cancer. Sci. Rep. 2020, 10, 9050. [Google Scholar] [CrossRef]
- Li, R.; Salehi-Rad, R.; Crosson, W.; Momcilovic, M.; Lim, R.J.; Ong, S.L.; Huang, Z.L.; Zhang, T.; Abascal, J.; Dumitras, C.; et al. Inhibition of Granulocytic Myeloid-Derived Suppressor Cells Overcomes Resistance to Immune Checkpoint Inhibition in LKB1-Deficient Non-Small Cell Lung Cancer. Cancer Res. 2021, 81, 3295–3308. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef]
- Hoechst, B.; Gamrekelashvili, J.; Manns, M.P.; Greten, T.F.; Korangy, F. Plasticity of Human Th17 Cells and iTregs Is Orchestrated by Different Subsets of Myeloid Cells. Blood 2011, 117, 6532–6541. [Google Scholar] [CrossRef]
- Schmielau, J.; Finn, O.J. Activated Granulocytes and Granulocyte-Derived Hydrogen Peroxide Are the Underlying Mechanism of Suppression of t-Cell Function in Advanced Cancer Patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar] [PubMed]
- Mazzoni, A.; Bronte, V.; Visintin, A.; Spitzer, J.H.; Apolloni, E.; Serafini, P.; Zanovello, P.; Segal, D.M. Myeloid Suppressor Lines Inhibit T Cell Responses by an NO-Dependent Mechanism. J. Immunol. 2002, 168, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Huang, H.; Zhu, Y.; Yu, G.; Gao, X.; Xu, Y.; Liu, C.; Hou, J.; Zhang, X. A Novel Subset of B7-H3+CD14+HLA-DR-/Low Myeloid-Derived Suppressor Cells Are Associated with Progression of Human NSCLC. Oncoimmunology 2015, 4, e977164. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Zhang, B.; Wang, B.; Zhang, F.; Fan, K.-X.; Guo, Y.-J. Increased CD14(+)HLA-DR (-/Low) Myeloid-Derived Suppressor Cells Correlate with Extrathoracic Metastasis and Poor Response to Chemotherapy in Non-Small Cell Lung Cancer Patients. Cancer Immunol. Immunother. 2013, 62, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Mu, S.; Wang, Y.; Wang, H.; Cai, L.; Li, W.; Hu, Y. Prognostic Role of Myeloid-Derived Suppressor Cells in Cancers: A Systematic Review and Meta-Analysis. BMC Cancer 2018, 18, 1220. [Google Scholar] [CrossRef] [PubMed]
- Van Gisbergen, K.P.J.M.; Sanchez-Hernandez, M.; Geijtenbeek, T.B.H.; van Kooyk, Y. Neutrophils Mediate Immune Modulation of Dendritic Cells through Glycosylation-Dependent Interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005, 201, 1281–1292. [Google Scholar] [CrossRef]
- Megiovanni, A.M.; Sanchez, F.; Robledo-Sarmiento, M.; Morel, C.; Gluckman, J.C.; Boudaly, S. Polymorphonuclear Neutrophils Deliver Activation Signals and Antigenic Molecules to Dendritic Cells: A New Link between Leukocytes Upstream of T Lymphocytes. J. Leukoc. Biol. 2006, 79, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Riise, R.E.; Bernson, E.; Aurelius, J.; Martner, A.; Pesce, S.; Della Chiesa, M.; Marcenaro, E.; Bylund, J.; Hellstrand, K.; Moretta, L.; et al. TLR-Stimulated Neutrophils Instruct NK Cells To Trigger Dendritic Cell Maturation and Promote Adaptive T Cell Responses. J. Immunol. 2015, 195, 1121–1128. [Google Scholar] [CrossRef]
- Ugolini, A.; Tyurin, V.A.; Tyurina, Y.Y.; Tcyganov, E.N.; Donthireddy, L.; Kagan, V.E.; Gabrilovich, D.I.; Veglia, F. Polymorphonuclear Myeloid-Derived Suppressor Cells Limit Antigen Cross-Presentation by Dendritic Cells in Cancer. JCI Insight 2020, 5, e138581. [Google Scholar] [CrossRef]
- Markowitz, J.; Wang, J.; Vangundy, Z.; You, J.; Yildiz, V.; Yu, L.; Foote, I.P.; Branson, O.E.; Stiff, A.R.; Brooks, T.R.; et al. Nitric Oxide Mediated Inhibition of Antigen Presentation from DCs to CD4+ T Cells in Cancer and Measurement of STAT1 Nitration. Sci. Rep. 2017, 7, 15424. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.L.; Lesinski, G.B.; Jaime-Ramirez, A.C.; Benninger, K.; Khan, M.; Kuppusamy, P.; Guenterberg, K.; Kondadasula, S.V.; Chaudhury, A.R.; La Perle, K.M.; et al. Myeloid-Derived Suppressor Cell Inhibition of the IFN Response in Tumor-Bearing Mice. Cancer Res. 2011, 71, 5101–5110. [Google Scholar] [CrossRef]
- Mundy-Bosse, B.L.; Young, G.S.; Bauer, T.; Binkley, E.; Bloomston, M.; Bill, M.A.; Bekaii-Saab, T.; Carson, W.E.; Lesinski, G.B. Distinct Myeloid Suppressor Cell Subsets Correlate with Plasma IL-6 and IL-10 and Reduced Interferon-Alpha Signaling in CD4+ T Cells from Patients with GI Malignancy. Cancer Immunol. Immunother. 2011, 60, 1269–1279. [Google Scholar] [CrossRef]
- Mukherji, B.; Chakraborty, N.G.; Yamasaki, S.; Okino, T.; Yamase, H.; Sporn, J.R.; Kurtzman, S.K.; Ergin, M.T.; Ozols, J.; Meehan, J. Induction of Antigen-Specific Cytolytic T Cells in Situ in Human Melanoma by Immunization with Synthetic Peptide-Pulsed Autologous Antigen Presenting Cells. Proc. Natl. Acad. Sci. USA 1995, 92, 8078–8082. [Google Scholar] [CrossRef]
- Mayordomo, J.I.; Zorina, T.; Storkus, W.J.; Zitvogel, L.; Celluzzi, C.; Falo, L.D.; Melief, C.J.; Ildstad, S.T.; Kast, W.M.; Deleo, A.B. Bone Marrow-Derived Dendritic Cells Pulsed with Synthetic Tumour Peptides Elicit Protective and Therapeutic Antitumour Immunity. Nat. Med. 1995, 1, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, N.; Yamazaki, K.; Tamura, Y.; Imai, A.; Kikuchi, E.; Yokouchi, H.; Hommura, F.; Oizumi, S.; Nishimura, M. Immunotherapy with Dendritic Cells Pulsed with Tumor-Derived Gp96 against Murine Lung Cancer Is Effective through Immune Response of CD8+ Cytotoxic T Lymphocytes and Natural Killer Cells. Cancer Immunol. Immunother. 2008, 57, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, K.; Ohshio, Y.; Fujita, T.; Hanaoka, J.; Kontani, K. Simultaneous Activation of T Helper Function Can Augment the Potency of Dendritic Cell-Based Cancer Immunotherapy. J. Cancer Res. Clin. Oncol. 2013, 139, 861–870. [Google Scholar] [CrossRef]
- Pan, J.; Zeng, W.; Jia, J.; Shi, Y.; Wang, D.; Dong, J.; Fang, Z.; He, J.; Yang, X.; Zhang, R.; et al. A Novel Therapeutic Tumor Vaccine Targeting MUC1 in Combination with PD-L1 Elicits Specific Anti-Tumor Immunity in Mice. Vaccines 2022, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuan, F.; Shu, Y.; Tian, Y.; Zhou, B.; Yi, L.; Zhang, X.; Ding, Z.; Xu, H.; Yang, L. Personalized Neoantigen-Pulsed Dendritic Cell Vaccines Show Superior Immunogenicity to Neoantigen-Adjuvant Vaccines in Mouse Tumor Models. Cancer Immunol. Immunother. 2020, 69, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Tüting, T.; DeLeo, A.B.; Lotze, M.T.; Storkus, W.J. Genetically Modified Bone Marrow-Derived Dendritic Cells Expressing Tumor-Associated Viral or “Self” Antigens Induce Antitumor Immunity in Vivo. Eur. J. Immunol. 1997, 27, 2702–2707. [Google Scholar] [CrossRef]
- Markov, O.V.; Mironova, N.L.; Sennikov, S.V.; Vlassov, V.V.; Zenkova, M.A. Prophylactic Dendritic Cell-Based Vaccines Efficiently Inhibit Metastases in Murine Metastatic Melanoma. PLoS ONE 2015, 10, e0136911. [Google Scholar] [CrossRef][Green Version]
- Yang, S.-C.; Hillinger, S.; Riedl, K.; Zhang, L.; Zhu, L.; Huang, M.; Atianzar, K.; Kuo, B.Y.; Gardner, B.; Batra, R.K.; et al. Intratumoral Administration of Dendritic Cells Overexpressing CCL21 Generates Systemic Antitumor Responses and Confers Tumor Immunity. Clin. Cancer Res. 2004, 10, 2891–2901. [Google Scholar] [CrossRef]
- Okada, N.; Mori, N.; Koretomo, R.; Okada, Y.; Nakayama, T.; Yoshie, O.; Mizuguchi, H.; Hayakawa, T.; Nakagawa, S.; Mayumi, T.; et al. Augmentation of the Migratory Ability of DC-Based Vaccine into Regional Lymph Nodes by Efficient CCR7 Gene Transduction. Gene Ther. 2005, 12, 129–139. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, L.; Tao, X.; Li, X.; Su, Y.; Hou, X.; Shi, H. Antitumor Effects of Murine Bone Marrow-Derived Dendritic Cells Infected with Xenogeneic Livin Alpha Recombinant Adenoviral Vectors against Lewis Lung Carcinoma. Lung Cancer 2010, 68, 338–345. [Google Scholar] [CrossRef]
- Ma, J.; Wang, S.; Ma, B.; Mao, C.; Tong, J.; Yang, M.; Wu, C.; Jiao, Z.; Lu, L.; Xu, H. Dendritic Cells Engineered to Express GITRL Enhance Therapeutic Immunity in Murine Lewis Lung Carcinoma. Cancer Lett. 2011, 301, 142–150. [Google Scholar] [CrossRef]
- Sun, Q.F.; Zhao, X.N.; Peng, C.L.; Hao, Y.T.; Zhao, Y.P.; Jiang, N.; Xue, H.; Guo, J.Z.; Yun, C.H.; Cong, B.; et al. Immunotherapy for Lewis Lung Carcinoma Utilizing Dendritic Cells Infected with CK19 Gene Recombinant Adenoviral Vectors. Oncol. Rep. 2015, 34, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Chen, X.; Zhou, W.; Fan, G.; Zhao, P.; Ren, S.; Zhou, C.; Zhang, J. Immunotherapy with Dendritic Cells Modified with Tumor-Associated Antigen Gene Demonstrates Enhanced Antitumor Effect Against Lung Cancer. Transl. Oncol. 2017, 10, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Makino, K.; Kajihara, R.; Yokoi, T.; Araki, R.; Abe, M.; Minderman, H.; Chang, A.E.; Odunsi, K.; Ito, F. In Situ Delivery of iPSC-Derived Dendritic Cells with Local Radiotherapy Generates Systemic Antitumor Immunity and Potentiates PD-L1 Blockade in Preclinical Poorly Immunogenic Tumor Models. J. Immunother. Cancer 2021, 9, e002432. [Google Scholar] [CrossRef] [PubMed]
- Wculek, S.K.; Amores-Iniesta, J.; Conde-Garrosa, R.; Khouili, S.C.; Melero, I.; Sancho, D. Effective Cancer Immunotherapy by Natural Mouse Conventional Type-1 Dendritic Cells Bearing Dead Tumor Antigen. J. Immunother. Cancer 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Slone, N.; Chrisikos, T.T.; Kyrysyuk, O.; Babcock, R.L.; Medik, Y.B.; Li, H.S.; Kleinerman, E.S.; Watowich, S.S. Vaccine Efficacy against Primary and Metastatic Cancer with in Vitro-Generated CD103+ Conventional Dendritic Cells. J. Immunother. Cancer 2020, 8, e000474. [Google Scholar] [CrossRef] [PubMed]
- Ferris, S.T.; Ohara, R.A.; Ou, F.; Wu, R.; Huang, X.; Kim, S.; Chen, J.; Liu, T.-T.; Schreiber, R.D.; Murphy, T.L.; et al. cDC1 Vaccines Drive Tumor Rejection by Direct Presentation Independently of Host cDC1. Cancer Immunol. Res. 2022, 10, 920–931. [Google Scholar] [CrossRef]
- Lou, Y.; Liu, C.; Kim, G.J.; Liu, Y.-J.; Hwu, P.; Wang, G. Plasmacytoid Dendritic Cells Synergize with Myeloid Dendritic Cells in the Induction of Antigen-Specific Antitumor Immune Responses. J. Immunol. 2007, 178, 1534–1541. [Google Scholar] [CrossRef]
- Inaba, K.; Inaba, M.; Romani, N.; Aya, H.; Deguchi, M.; Ikehara, S.; Muramatsu, S.; Steinman, R.M. Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med. 1992, 176, 1693–1702. [Google Scholar] [CrossRef]
- Grauer, O.; Wohlleben, G.; Seubert, S.; Weishaupt, A.; Kämpgen, E.; Gold, R. Analysis of Maturation States of Rat Bone Marrow-Derived Dendritic Cells Using an Improved Culture Technique. Histochem. Cell Biol. 2002, 117, 351–362. [Google Scholar] [CrossRef]
- Helft, J.; Böttcher, J.; Chakravarty, P.; Zelenay, S.; Huotari, J.; Schraml, B.U.; Goubau, D.; Reis e Sousa, C. GM-CSF Mouse Bone Marrow Cultures Comprise a Heterogeneous Population of CD11c(+)MHCII(+) Macrophages and Dendritic Cells. Immunity 2015, 42, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, P.; Liu, H.; Fang, C.; Guo, H.; Chen, X.; Tan, M.; Zhang, Y.; Min, W. Silencing IDO2 in Dendritic Cells: A Novel Strategy to Strengthen Cancer Immunotherapy in a Murine Lung Cancer Model. Int. J. Oncol. 2020, 57, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.; Ingels, J.; Van Lint, S.; Vandekerckhove, B.; Vermaelen, K. Dendritic Cell-Based Immunotherapy in Lung Cancer. Front. Immunol. 2020, 11, 620374. [Google Scholar] [CrossRef]
- Ng, P.M.L.; Kaliaperumal, N.; Lee, C.Y.; Chin, W.J.; Tan, H.C.; Au, V.B.; Goh, A.X.-H.; Tan, Q.W.; Yeo, D.S.G.; Connolly, J.E.; et al. Enhancing Antigen Cross-Presentation in Human Monocyte-Derived Dendritic Cells by Recruiting the Intracellular Fc Receptor TRIM21. J. Immunol. 2019, 202, 2307–2319. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Hernández, M.; Leylek, R.; Wagar, L.E.; Engleman, E.G.; Keler, T.; Marinkovich, M.P.; Davis, M.M.; Nolan, G.P.; Idoyaga, J. High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization. Immunity 2017, 47, 1037–1050.e6. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Kühn, U.; Müller, G.; Steinbrink, K.; Paragnik, L.; Schmitt, E.; Knop, J.; Enk, A.H. Pro-Inflammatory Cytokines and Prostaglandins Induce Maturation of Potent Immunostimulatory Dendritic Cells under Fetal Calf Serum-Free Conditions. Eur. J. Immunol. 1997, 27, 3135–3142. [Google Scholar] [CrossRef]
- Van Elsas, A.; van der Burg, S.H.; van der Minne, C.E.; Borghi, M.; Mourer, J.S.; Melief, C.J.; Schrier, P.I. Peptide-Pulsed Dendritic Cells Induce Tumoricidal Cytotoxic T Lymphocytes from Healthy Donors against Stably HLA-A*0201-Binding Peptides from the Melan-A/MART-1 Self Antigen. Eur. J. Immunol. 1996, 26, 1683–1689. [Google Scholar] [CrossRef]
- Von Euw, E.M.; Barrio, M.M.; Furman, D.; Bianchini, M.; Levy, E.M.; Yee, C.; Li, Y.; Wainstok, R.; Mordoh, J. Monocyte-Derived Dendritic Cells Loaded with a Mixture of Apoptotic/Necrotic Melanoma Cells Efficiently Cross-Present Gp100 and MART-1 Antigens to Specific CD8(+) T Lymphocytes. J. Transl. Med. 2007, 5, 19. [Google Scholar] [CrossRef]
- Laureano, R.S.; Sprooten, J.; Vanmeerbeerk, I.; Borras, D.M.; Govaerts, J.; Naulaerts, S.; Berneman, Z.N.; Beuselinck, B.; Bol, K.F.; Borst, J.; et al. Trial Watch: Dendritic Cell (DC)-Based Immunotherapy for Cancer. Oncoimmunology 2022, 11, 2096363. [Google Scholar] [CrossRef]
- Baratelli, F.; Takedatsu, H.; Hazra, S.; Peebles, K.; Luo, J.; Kurimoto, P.S.; Zeng, G.; Batra, R.K.; Sharma, S.; Dubinett, S.M.; et al. Pre-Clinical Characterization of GMP Grade CCL21-Gene Modified Dendritic Cells for Application in a Phase I Trial in Non-Small Cell Lung Cancer. J. Transl. Med. 2008, 6, 38. [Google Scholar] [CrossRef]
- Cyster, J.G. Chemokines and the Homing of Dendritic Cells to the T Cell Areas of Lymphoid Organs. J. Exp. Med. 1999, 189, 447–450. [Google Scholar] [CrossRef]
- Pfeiffer, I.A.; Hoyer, S.; Gerer, K.F.; Voll, R.E.; Knippertz, I.; Gückel, E.; Schuler, G.; Schaft, N.; Dörrie, J. Triggering of NF-κB in Cytokine-Matured Human DCs Generates Superior DCs for T-Cell Priming in Cancer Immunotherapy. Eur. J. Immunol. 2014, 44, 3413–3428. [Google Scholar] [CrossRef] [PubMed]
- Bosch, N.C.; Voll, R.E.; Voskens, C.J.; Gross, S.; Seliger, B.; Schuler, G.; Schaft, N.; Dörrie, J. NF-κB Activation Triggers NK-Cell Stimulation by Monocyte-Derived Dendritic Cells. Ther. Adv. Med. Oncol. 2019, 11, 1758835919891622. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, M.-H.; Garon, E.; Goldman, J.W.; Salehi-Rad, R.; Baratelli, F.E.; Schaue, D.; Wang, G.; Rosen, F.; Yanagawa, J.; et al. Phase I Trial of Intratumoral Injection of CCL21 Gene-Modified Dendritic Cells in Lung Cancer Elicits Tumor-Specific Immune Responses and CD8+ T-Cell Infiltration. Clin. Cancer Res. 2017, 23, 4556–4568. [Google Scholar] [CrossRef]
- Chiappori, A.A.; Soliman, H.; Janssen, W.E.; Antonia, S.J.; Gabrilovich, D.I. INGN-225: A Dendritic Cell-Based P53 Vaccine (Ad.P53-DC) in Small Cell Lung Cancer: Observed Association between Immune Response and Enhanced Chemotherapy Effect. Expert. Opin. Biol. Ther. 2010, 10, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Senju, S.; Matsunaga, Y.; Fukushima, S.; Hirata, S.; Motomura, Y.; Fukuma, D.; Matsuyoshi, H.; Nishimura, Y. Immunotherapy with Pluripotent Stem Cell-Derived Dendritic Cells. Semin. Immunopathol. 2011, 33, 603–612. [Google Scholar] [CrossRef]
- Senju, S.; Hirata, S.; Matsuyoshi, H.; Masuda, M.; Uemura, Y.; Araki, K.; Yamamura, K.; Nishimura, Y. Generation and Genetic Modification of Dendritic Cells Derived from Mouse Embryonic Stem Cells. Blood 2003, 101, 3501–3508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mayer, C.T.; Ghorbani, P.; Nandan, A.; Dudek, M.; Arnold-Schrauf, C.; Hesse, C.; Berod, L.; Stüve, P.; Puttur, F.; Merad, M.; et al. Selective and Efficient Generation of Functional Batf3-Dependent CD103+ Dendritic Cells from Mouse Bone Marrow. Blood 2014, 124, 3081–3091. [Google Scholar] [CrossRef]
- Satpathy, A.T.; Briseño, C.G.; Lee, J.S.; Ng, D.; Manieri, N.A.; Kc, W.; Wu, X.; Thomas, S.R.; Lee, W.-L.; Turkoz, M.; et al. Notch2-Dependent Classical Dendritic Cells Orchestrate Intestinal Immunity to Attaching-and-Effacing Bacterial Pathogens. Nat. Immunol. 2013, 14, 937–948. [Google Scholar] [CrossRef]
- Kirkling, M.E.; Cytlak, U.; Lau, C.M.; Lewis, K.L.; Resteu, A.; Khodadadi-Jamayran, A.; Siebel, C.W.; Salmon, H.; Merad, M.; Tsirigos, A.; et al. Notch Signaling Facilitates In Vitro Generation of Cross-Presenting Classical Dendritic Cells. Cell Rep. 2018, 23, 3658–3672.e6. [Google Scholar] [CrossRef]
- Lutz, M.B.; Ali, S.; Audiger, C.; Autenrieth, S.E.; Berod, L.; Bigley, V.; Cyran, L.; Dalod, M.; Dörrie, J.; Dudziak, D.; et al. Guidelines for Mouse and Human DC Generation. Eur. J. Immunol. 2022, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Van Eck van der Sluijs, J.; van Ens, D.; Thordardottir, S.; Vodegel, D.; Hermens, I.; van der Waart, A.B.; Falkenburg, J.H.F.; Kester, M.G.D.; de Rink, I.; Heemskerk, M.H.M.; et al. Clinically Applicable CD34+-Derived Blood Dendritic Cell Subsets Exhibit Key Subset-Specific Features and Potently Boost Anti-Tumor T and NK Cell Responses. Cancer Immunol. Immunother. 2021, 70, 3167–3181. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, F.; Schreibelt, G.; Sköld, A.E.; Figdor, C.G.; De Vries, I.J.M. Paradigm Shift in Dendritic Cell-Based Immunotherapy: From in Vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets. Front. Immunol. 2014, 5, 165. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, M.; Bol, K.F.; Boudewijns, S.; Gorris, M.A.J.; de Wilt, J.H.W.; Croockewit, S.A.J.; van Rossum, M.M.; de Goede, A.L.; Petry, K.; Koornstra, R.H.T.; et al. Immunological Responses to Adjuvant Vaccination with Combined CD1c+ Myeloid and Plasmacytoid Dendritic Cells in Stage III Melanoma Patients. Oncoimmunology 2022, 11, 2015113. [Google Scholar] [CrossRef] [PubMed]
- Schreibelt, G.; Bol, K.F.; Westdorp, H.; Wimmers, F.; Aarntzen, E.H.J.G.; Duiveman-de Boer, T.; van de Rakt, M.W.M.M.; Scharenborg, N.M.; de Boer, A.J.; Pots, J.M.; et al. Effective Clinical Responses in Metastatic Melanoma Patients after Vaccination with Primary Myeloid Dendritic Cells. Clin. Cancer Res. 2016, 22, 2155–2166. [Google Scholar] [CrossRef]
- Anassi, E.; Ndefo, U.A. Sipuleucel-T (Provenge) Injection: The First Immunotherapy Agent (Vaccine) for Hormone-Refractory Prostate Cancer. Pharm. Ther. 2011, 36, 197–202. [Google Scholar]
- Ridgway, D. The First 1000 Dendritic Cell Vaccinees. Cancer Invest 2003, 21, 873–886. [Google Scholar] [CrossRef]
- Kamath, A.T.; Pooley, J.; O’Keeffe, M.A.; Vremec, D.; Zhan, Y.; Lew, A.M.; D’Amico, A.; Wu, L.; Tough, D.F.; Shortman, K. The Development, Maturation, and Turnover Rate of Mouse Spleen Dendritic Cell Populations. J. Immunol. 2000, 165, 6762–6770. [Google Scholar] [CrossRef]
- Chang, G.-C.; Lan, H.-C.; Juang, S.-H.; Wu, Y.-C.; Lee, H.-C.; Hung, Y.-M.; Yang, H.-Y.; Whang-Peng, J.; Liu, K.-J. A Pilot Clinical Trial of Vaccination with Dendritic Cells Pulsed with Autologous Tumor Cells Derived from Malignant Pleural Effusion in Patients with Late-Stage Lung Carcinoma. Cancer 2005, 103, 763–771. [Google Scholar] [CrossRef]
- Hirschowitz, E.A.; Foody, T.; Hidalgo, G.E.; Yannelli, J.R. Immunization of NSCLC Patients with Antigen-Pulsed Immature Autologous Dendritic Cells. Lung Cancer 2007, 57, 365–372. [Google Scholar] [CrossRef]
- Um, S.-J.; Choi, Y.J.; Shin, H.-J.; Son, C.H.; Park, Y.-S.; Roh, M.S.; Kim, Y.S.; Kim, Y.D.; Lee, S.-K.; Jung, M.H.; et al. Phase I Study of Autologous Dendritic Cell Tumor Vaccine in Patients with Non-Small Cell Lung Cancer. Lung Cancer 2010, 70, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; He, S. MAGE3 and Survivin Activated Dendritic Cell Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Oncol. Lett. 2018, 15, 8777–8783. [Google Scholar] [CrossRef]
- Hirschowitz, E.A.; Foody, T.; Kryscio, R.; Dickson, L.; Sturgill, J.; Yannelli, J. Autologous Dendritic Cell Vaccines for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2004, 22, 2808–2815. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Li, R.; Song, H.; Geng, T.; Yang, J.; Tan, Q.; Song, L.; Wang, Y.; Xue, Y.; Li, Z.; et al. Phase I Clinical Trial of a Novel Autologous Modified-DC Vaccine in Patients with Resected NSCLC. BMC Cancer 2017, 17, 884. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.K.; Claesson, M.H.; Nielsen, H.J.; Rosenberg, J. Changes in Cytokine and Biomarker Blood Levels in Patients with Colorectal Cancer during Dendritic Cell-Based Vaccination. Acta Oncol. 2009, 48, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.A.; Wesley, J.D.; Chadwick, E.; Perdue, N.; Dela Rosa, C.P.; Frohlich, M.W.; Stewart, F.P.; Urdal, D.L. Characterization of Antigen-Specific T-Cell Activation and Cytokine Expression Induced by Sipuleucel-T. J. Clin. Oncol. 2011, 29, 155. [Google Scholar] [CrossRef]
- Nistor, G.I.; Dillman, R.O. Cytokine Network Analysis of Immune Responses before and after Autologous Dendritic Cell and Tumor Cell Vaccine Immunotherapies in a Randomized Trial. J. Transl. Med. 2020, 18, 176. [Google Scholar] [CrossRef]
- Trabanelli, S.; Očadlíková, D.; Ciciarello, M.; Salvestrini, V.; Lecciso, M.; Jandus, C.; Metz, R.; Evangelisti, C.; Laury-Kleintop, L.; Romero, P.; et al. The SOCS3-Independent Expression of IDO2 Supports the Homeostatic Generation of T Regulatory Cells by Human Dendritic Cells. J. Immunol. 2014, 192, 1231–1240. [Google Scholar] [CrossRef]
- Lan, X.; Chen, Y.; Wang, Z.; Yuan, C.; Wang, G.; Lu, G.; Mao, S.; Jin, X.; Xia, Q. Immunotherapy of DC-CIK Cells Enhances the Efficacy of Chemotherapy for Solid Cancer: A Meta-Analysis of Randomized Controlled Trials in Chinese Patients. J. Zhejiang Univ. Sci. B 2015, 16, 743–756. [Google Scholar] [CrossRef]
- Meng, Y.; Yu, Z.; Wu, Y.; Du, T.; Chen, S.; Meng, F.; Su, N.; Ma, Y.; Li, X.; Sun, S.; et al. Cell-Based Immunotherapy with Cytokine-Induced Killer (CIK) Cells: From Preparation and Testing to Clinical Application. Hum. Vaccin. Immunother. 2017, 13, 1379–1387. [Google Scholar] [CrossRef]
- Song, H.; Liu, S.; Zhao, Z.; Sun, W.; Wei, X.; Ma, X.; Zhao, P.; Gao, D. Increased Cycles of DC/CIK Immunotherapy Decreases Frequency of Tregs in Patients with Resected NSCLC. Int. Immunopharmacol. 2017, 52, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-T.; Nie, Y.; Sun, S.-N.; Lin, T.; Han, R.-J.; Jiang, J.; Li, Z.; Li, J.-Q.; Xiao, Y.-P.; Fan, Y.-Y.; et al. Tumor-Associated Antigen-Based Personalized Dendritic Cell Vaccine in Solid Tumor Patients. Cancer Immunol. Immunother. 2020, 69, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Iclozan, C.; Antonia, S.; Chiappori, A.; Chen, D.-T.; Gabrilovich, D. Therapeutic Regulation of Myeloid-Derived Suppressor Cells and Immune Response to Cancer Vaccine in Patients with Extensive Stage Small Cell Lung Cancer. Cancer Immunol. Immunother. 2013, 62, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer Immunotherapy. A Dendritic Cell Vaccine Increases the Breadth and Diversity of Melanoma Neoantigen-Specific T Cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Doughty, M.J. Rose Bengal Staining as an Assessment of Ocular Surface Damage and Recovery in Dry Eye Disease-a Review. Cont. Lens Anterior Eye 2013, 36, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Kunda, N.; Qiao, G.; Calata, J.F.; Pardiwala, K.; Prabhakar, B.S.; Maker, A.V. Colon Cancer Cell Treatment with Rose Bengal Generates a Protective Immune Response via Immunogenic Cell Death. Cell Death Dis. 2017, 8, e2584. [Google Scholar] [CrossRef]
- Zhang, L.; Du, J.; Song, Q.; Zhang, C.; Wu, X. A Novel In Situ Dendritic Cell Vaccine Triggered by Rose Bengal Enhances Adaptive Antitumour Immunity. J. Immunol. Res. 2022, 2022, 1178874. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Cheng, F.; Yu, B.; Nefedova, Y.; Sotomayor, E.; Lush, R.; Gabrilovich, D. All-Trans-Retinoic Acid Eliminates Immature Myeloid Cells from Tumor-Bearing Mice and Improves the Effect of Vaccination. Cancer Res. 2003, 63, 4441–4449. [Google Scholar]
- Macri, C.; Dumont, C.; Johnston, A.P.; Mintern, J.D. Targeting Dendritic Cells: A Promising Strategy to Improve Vaccine Effectiveness. Clin. Transl. Immunol. 2016, 5, e66. [Google Scholar] [CrossRef]
- Joffre, O.P.; Sancho, D.; Zelenay, S.; Keller, A.M.; Reis e Sousa, C. Efficient and Versatile Manipulation of the Peripheral CD4+ T-Cell Compartment by Antigen Targeting to DNGR-1/CLEC9A. Eur. J. Immunol. 2010, 40, 1255–1265. [Google Scholar] [CrossRef]
- Sancho, D.; Mourão-Sá, D.; Joffre, O.P.; Schulz, O.; Rogers, N.C.; Pennington, D.J.; Carlyle, J.R.; Reis e Sousa, C. Tumor Therapy in Mice via Antigen Targeting to a Novel, DC-Restricted C-Type Lectin. J. Clin. Investig. 2008, 118, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Verduzco, D.; Gatenby, R.A. Evolutionary Dynamics of Carcinogenesis and Why Targeted Therapy Does Not Work. Nat. Rev. Cancer 2012, 12, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of Resistance to EGFR-Targeted Drugs: Lung Cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Preclinical Efficacy | Mechanistic Findings | Ref. |
|---|---|---|---|
| IV BMDCs | Pulsing with TAA expressed by target tumor cell line improved efficacy | Splenocytes showed cytolytic activity against tumor cells after vaccine therapy | [152] |
| SC BMDCs | Pulsing with gp96 improved efficacy compared to non-pulsed DC or gp96 alone | Antitumor effect abrogated via the depletion of NK cells and CD8+/CD4+ T cells | [153] |
| SC BMDCs | Pulsing with both TAA- and MHC-II peptides proved more efficacious than using only one peptide pool | Induced stronger IFNγ response by CD8+ T to tumor antigens; Tregs decreased in spleen | [154] |
| ID BMDCs | Pulsing with MUC1-PD-L1 fusion protein improved antitumor efficacy | Induced splenic T cell activation and cytokine secretion; increased serum anti-PD-L1 antibody titers | [155] |
| IV BMDCs | Pulsing with neoantigen peptide improved efficacy compared to non-pulsed DCs | Increased tumor infiltration via IFNγ-producing CD8+ T cells | [156] |
| IV BMDCs | Transfection with E7 or p53 genes improved antitumor efficacy | Improved tumor-specific lysis and IFNγ production in splenocytes | [157] |
| IV BMDCs | Transfection with tumor total RNA proved more efficacious than pulsing with tumor lysate | Serum Th1 cytokines increased with therapeutic vaccination | [158] |
| IT BMDCs | Transduction with CCL21 improved antitumor efficacy | Efficacy diminished via IFNγ, CXCL9, or CXCL10 depletion; activity seen in contralateral tumors | [159] |
| ID BMDCs | Transduction with CCR7 promoted mature DC phenotype | CCR7-DCs showed greater migration to lymph nodes | [160] |
| SC BMDCs | Transduction with human livin α improved efficacy | Induced cytolytic activity against tumor cells in splenic T cells | [161] |
| IT BMDCs | Transduction with GITRL and pulsing with tumor cell lysates proved more efficacious than pulsing alone | Increased IFNγ-producing CD8+ T cells and deceased Tregs in the spleen | [162] |
| SC BMDCs | Transduction with CK19 improved antitumor efficacy | Spurred T cell proliferation in vitro; induced cytolytic activity against tumor cells in splenic T cells | [163] |
| IT and IV BMDCs | Transduction with OVA improved response against OVA-expressing tumors | T cell proliferation and cytolytic activity improved in DC co-culture | [164] |
| IT iPSC-DCs and RT | iPSC-DC vaccine was synergistic with RT in treating tumors | iPSC-DCs resembled cDC2s; RT induced DC trafficking to TdLN and increased DC/CD8+ T cell aggregates | [165] |
| ID cDC1s | cDC1 vaccine pulsed with tumor cell lysate was synergistic with anti-PD-1 in treating tumors | Enhanced activation of TdLN T cells; increased tumor T cell infiltration | [166] |
| IT cDC1s | cDC1 vaccine pulsed with OVA or tumor lysate proved more efficacious than BMDC vaccine | Increased tumor and TdLN infiltration by antigen-specific and IFNγ-producing T cells | [167] |
| IT cDC1s | cDC1 vaccine proved more efficacious than BMDCs in a cDC1-deficient model | cDC1s migrated to TdLN; increased splenic antigen-specific T cells; efficacy seen in contralateral tumors | [168] |
| SC pDCs and mDCs | A mix of pDCs and mDCs pulsed with a OVA peptide proved more efficacious than either vaccine alone | pDCs increased peripheral antigen-specific T cells; mixed vaccine requires mDC but not pDC MHC-I expression | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abascal, J.; Oh, M.S.; Liclican, E.L.; Dubinett, S.M.; Salehi-Rad, R.; Liu, B. Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells 2023, 12, 2404. https://doi.org/10.3390/cells12192404
Abascal J, Oh MS, Liclican EL, Dubinett SM, Salehi-Rad R, Liu B. Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells. 2023; 12(19):2404. https://doi.org/10.3390/cells12192404
Chicago/Turabian StyleAbascal, Jensen, Michael S. Oh, Elvira L. Liclican, Steven M. Dubinett, Ramin Salehi-Rad, and Bin Liu. 2023. "Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment" Cells 12, no. 19: 2404. https://doi.org/10.3390/cells12192404
APA StyleAbascal, J., Oh, M. S., Liclican, E. L., Dubinett, S. M., Salehi-Rad, R., & Liu, B. (2023). Dendritic Cell Vaccination in Non-Small Cell Lung Cancer: Remodeling the Tumor Immune Microenvironment. Cells, 12(19), 2404. https://doi.org/10.3390/cells12192404






