Generation of Human Regulatory Dendritic Cells from Cryopreserved Healthy Donor Cells and Hematopoietic Stem Cell Transplant Recipients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mononuclear Cell Sources
2.2. Cell Preparation
2.3. Cryopreservation of Monocytes
2.4. DCreg and Conventional Dendritic Cell (cDC) Cultures
2.5. Flow Cytometric Reagents
2.6. Flow Cytometric Staining and Analysis
2.7. Culture Cyotokine Assessment
2.8. Treg Induction Assay
2.9. Live Cell Imaging
2.10. Statistical Analysis
3. Results
3.1. Compared to cDC, DCreg Express Lower Levels of CD11c and HLA-DR
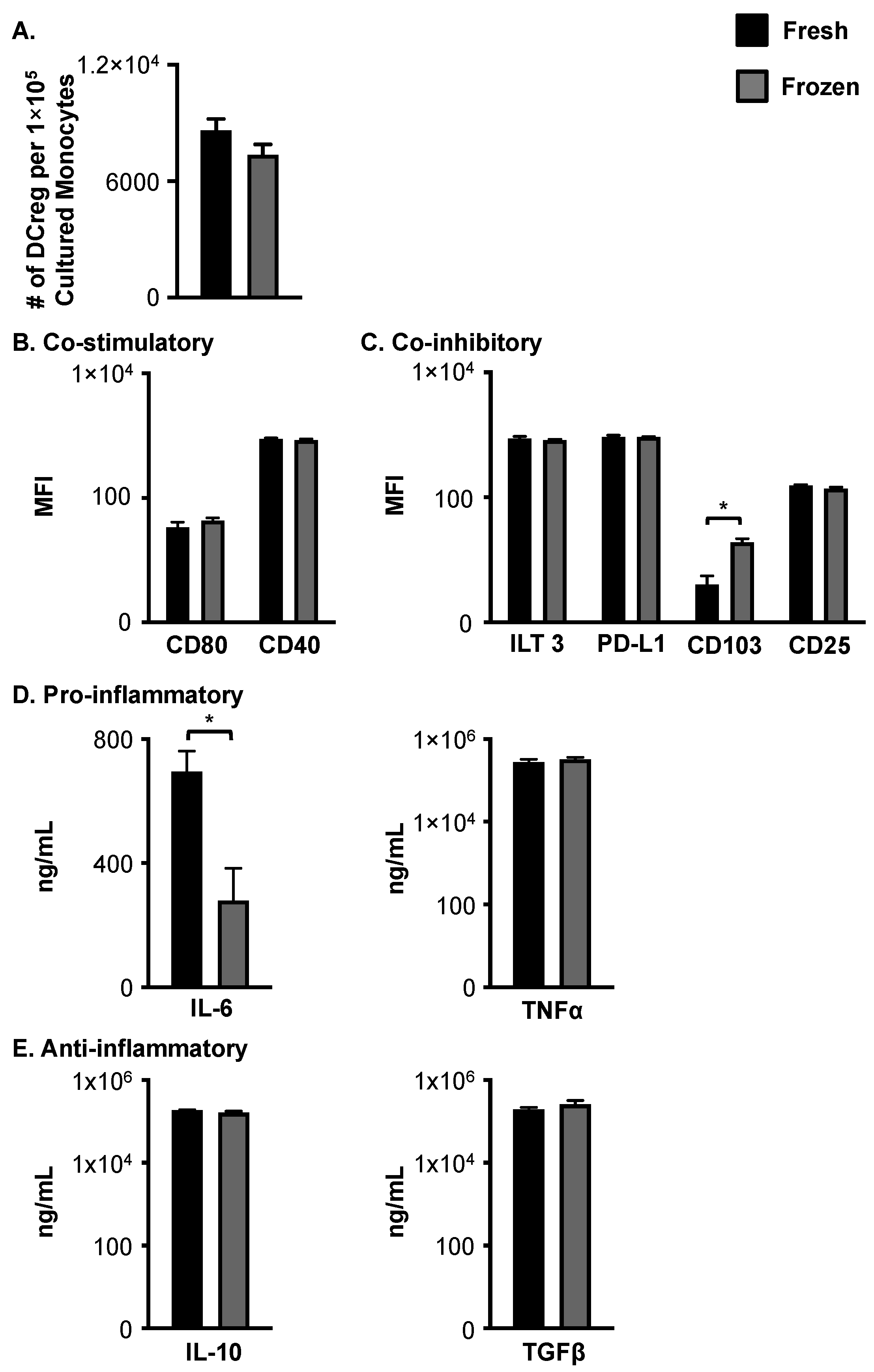
3.2. Cryopreservation of Young, Healthy Monocytes Prior to DCreg Generation Does Not Negatively Impact Cell Number, Phenotype, or Cytokine Production
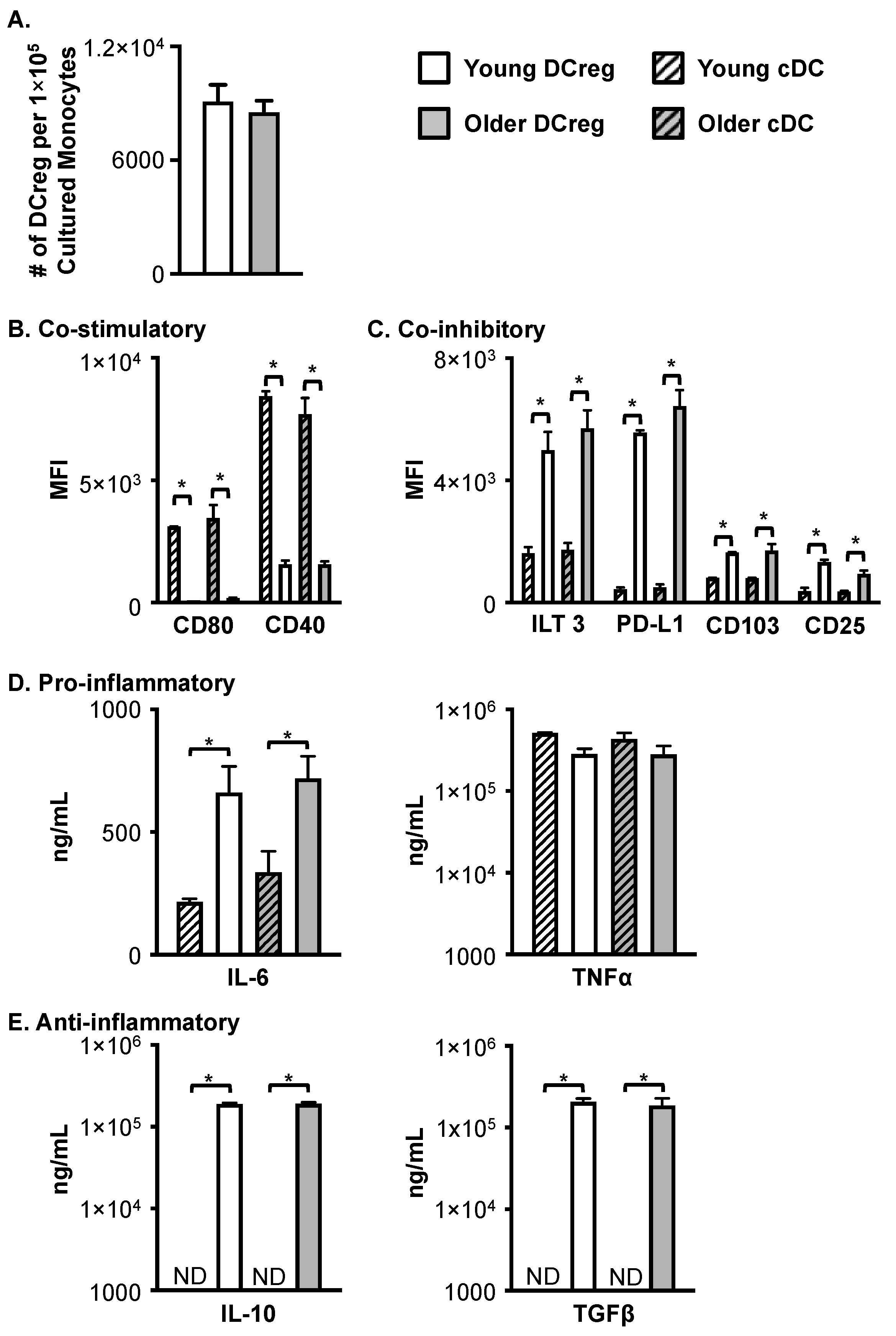
3.3. DCregs Generated from Older, Healthy Donors Have Comparable Numbers, Phenotype, and Function to DCregs Generated from Young, Healthy Donors
3.4. Monocytes from Patients with Various Hematological Diseases Generate Phenotypical and Functional DCregs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrara, J.L.M.; Cooke, K.R.; Teshima, T. The Pathophysiology of Acute Graft-versus-Host Disease. Int. J. Hematol. 2003, 78, 181–187. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Deeg, H.J. Graft-versus-host disease. N. Engl. J. Med. 1991, 324, 667–674. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Slater, S. Acure graft-versus-host disease. In Blood and Marrow Transplant Handbook; Mazairz, R.T., Slater, S., Eds.; Springer Science+Business, LLC: New York, NY, USA, 2011; pp. 167–187. [Google Scholar]
- Faraci, M.; Caviglia, I.; Biral, E.; Morreale, G.; Giardino, S.; Garbarino, L.; Castagnola, E.; Dini, G.; Lanino, E. Acute graft-versus-host disease in pediatric allogeneic hematopoietic stem cell transplantation. Single-center experience during 10 yr. Pediatr. Transplant. 2012, 16, 887–893. [Google Scholar] [CrossRef]
- Gatza, E.; Reddy, P.; Choi, S.W. Prevention and Treatment of Acute Graft-versus-Host Disease in Children, Adolescents, and Young Adults. Biol. Blood Marrow Transplant. 2020, 26, e101–e112. [Google Scholar] [CrossRef]
- Johnston, L. Acute graft-versus-host disease: Differing risk with differing graft sources and conditioning intensity. Best Pr Res. Clin. Haematol. 2008, 21, 177–192. [Google Scholar] [CrossRef]
- Pasquini, M.C. Impact of graft-versus-host disease on survival. Best Pr Res. Clin. Haematol. 2008, 21, 193–204. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Wang, Z.; Horowitz, M.M.; Gale, R.P. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transpl. 2010, 24, 87–105. [Google Scholar]
- Holtan, S.G.; Yu, J.; Choe, H.K.; Paranagama, D.; Tang, J.; Naim, A.; Galvin, J.; Joachim Deeg, H. Disease progression, treatments, hospitalization, and clinical outcomes in acute GVHD: A multicenter chart review. Bone Marrow Transplant. 2022, 57, 1581–1585. [Google Scholar] [CrossRef]
- El Jurdi, N.; Rayes, A.; MacMillan, M.L.; Holtan, S.G.; DeFor, T.E.; Witte, J.; Arora, M.; Young, J.-A.; Weisdorf, D.J. Steroid-dependent acute GVHD after allogeneic hematopoietic cell transplantation: Risk factors and clinical outcomes. Blood Adv. 2021, 5, 1352–1359. [Google Scholar] [CrossRef]
- Ordemann, R.; Hutchinson, R.; Friedman, J.; Burakoff, S.J.; Reddy, P.; Duffner, U.; Braun, T.M.; Liu, C.; Teshima, T.; Ferrara, J.L. Enhanced allostimulatory activity of host antigen-presenting cells in old mice intensifies acute graft-versus-host disease. J. Clin. Investig. 2002, 109, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.R.; Mielke, S.; Savani, B.N.; Montero, A.; Wisch, L.; Childs, R.; Hensel, N.; Schindler, J.; Ghetie, V.; Leitman, S.F.; et al. Selective depletion of alloreactive donor lymphocytes: A novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood 2005, 106, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.J.; Artz, A.S. Allogeneic hematopoietic cell transplantation for older patients. Hematology 2021, 2021, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yamashita, N.; Baba, M.; Matsuyama, T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood 2003, 101, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Scroggins, S.M.; Olivier, A.K.; Meyerholz, D.K.; Schlueter, A.J. Characterization of Regulatory Dendritic Cells That Mitigate Acute Graft-versus-Host Disease in Older Mice Following Allogeneic Bone Marrow Transplantation. PLoS ONE 2013, 8, e75158. [Google Scholar] [CrossRef]
- Chorny, A.; Gonzalez-Rey, E.; Fernandez-Martin, A.; Ganea, D.; Delgado, M. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood 2006, 107, 3787–3794. [Google Scholar] [CrossRef]
- Sato, K.; Yamashita, N.; Yamashita, N.; Baba, M.; Matsuyama, T. Regulatory Dendritic Cells Protect Mice from Murine Acute Graft-versus-Host Disease and Leukemia Relapse. Immunity 2003, 18, 367–379. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Z.; Jiang, X.; Yao, Y.; Gao, Q.; Ding, Y.; Cao, X. Regulatory dendritic cells program generation of interleukin-4–producing alternative memory CD4 T cells with suppressive activity. Blood 2011, 117, 1218–1227. [Google Scholar] [CrossRef]
- Napoletano, C.; Pinto, D.; Bellati, F.; Taurino, F.; Rahimi, H.; Tomao, F.; Panici, P.B.; Rughetti, A.; Frati, L.; Nuti, M. A Comparative Analysis of Serum and Serum-free Media for Generation of Clinical Grade DCs. J. Immunother. 2007, 30, 567–576. [Google Scholar] [CrossRef]
- Wan, X.; Bao, L.; Ma, G.; Long, T.; Li, H.; Zhang, Y.; Jiang, H. Tolerogenic dendritic cells alleviate collagen-induced arthritis by forming microchimerism and affecting the expression of immune checkpoint molecules. Eur. J. Immunol. 2022, 52, 1980–1992. [Google Scholar] [CrossRef]
- Raïch-Regué, D.; Glancy, M.; Thomson, A.W. Regulatory dendritic cell therapy: From rodents to clinical application. Immunol. Lett. 2013, 161, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Amarnath, S.; Costanzo, C.M.; Mariotti, J.; Ullman, J.L.; Telford, W.G.; Kapoor, V.; Riley, J.L.; Levine, B.L.; June, C.H.; Fong, T.; et al. Regulatory T Cells and Human Myeloid Dendritic Cells Promote Tolerance via Programmed Death Ligand-1. PLoS Biol. 2010, 8, e1000302. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhu, H.; Lei, P.; Yagita, H.; Liu, J.; Wen, X.; Zhou, W.; Gong, F.; Shen, G.; Fang, M. Programmed Death-1 Signaling Is Essential for the Skin Allograft Protection by Alternatively Activated Dendritic Cell Infusion in Mice. Transplantation 2009, 88, 864–873. [Google Scholar] [CrossRef]
- del Rio, M.-L.; Bernhardt, G.; Rodriguez-Barbosa, J.-I.; Förster, R. Development and functional specialization of CD103+dendritic cells. Immunol. Rev. 2010, 234, 268–281. [Google Scholar] [CrossRef]
- Driesen, J.; Popov, A.; Schultze, J.L. CD25 as an immune regulatory molecule expressed on myeloid dendritic cells. Immunobiology 2008, 213, 849–858. [Google Scholar] [CrossRef]
- Guilliams, M.; Crozat, K.; Henri, S.; Tamoutounour, S.; Grenot, P.; Devilard, E.; de Bovis, B.; Alexopoulou, L.; Dalod, M.; Malissen, B. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood 2010, 115, 1958–1968. [Google Scholar] [CrossRef]
- Lan, Y.Y.; Wang, Z.; Raimondi, G.; Wu, W.; Colvin, B.L.; De Creus, A.; Thomson, A.W. “Alternatively Activated” Dendritic Cells Preferentially Secrete IL-10, Expand Foxp3+CD4+ T Cells, and Induce Long-Term Organ Allograft Survival in Combination with CTLA4-Ig. J. Immunol. 2006, 177, 5868–5877. [Google Scholar] [CrossRef]
- Li, H.; Demetris, A.J.; McNiff, J.; Matte-Martone, C.; Tan, H.S.; Rothstein, D.M.; Lakkis, F.G.; Shlomchik, W.D. Profound Depletion of Host Conventional Dendritic Cells, Plasmacytoid Dendritic Cells, and B Cells Does Not Prevent Graft-versus-Host Disease Induction. J. Immunol. 2012, 188, 3804–3811. [Google Scholar] [CrossRef]
- Matta, B.M.; Castellaneta, A.; Thomson, A.W. Tolerogenic plasmacytoid DC. Eur. J. Immunol. 2010, 40, 2667–2676. [Google Scholar] [CrossRef]
- Scott, C.L.; Aumeunier, A.M.; Mowat, A.M. Intestinal CD103+ dendritic cells: Master regulators of tolerance? Trends Immunol. 2011, 32, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Li, X.; Yao, S.; Wang, L.; Chen, Y.; Zhao, D.; Johnston, H.F.; Young, J.S.; Liu, H.; Todorov, I.; et al. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients. J. Immunol. 2011, 186, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Josefowicz, S.Z.; Rudensky, A. Control of Regulatory T Cell Lineage Commitment and Maintenance. Immunity 2009, 30, 616–625. [Google Scholar] [CrossRef]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and Functions of the IL-10 Family of Cytokines in Inflammation and Disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Chorny, A.; Gonzalez-Rey, E.; Fernandez-Martin, A.; Pozo, D.; Ganea, D.; Delgado, M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc. Natl. Acad. Sci. USA 2005, 102, 13562–13567. [Google Scholar] [CrossRef]
- Fujita, S.; Seino, K.-I.; Sato, K.; Sato, Y.; Eizumi, K.; Yamashita, N.; Taniguchi, M.; Sato, K. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood 2006, 107, 3656–3664. [Google Scholar] [CrossRef] [PubMed]
- Torres-Aguilar, H.; Aguilar-Ruiz, S.R.; González-Pérez, G.; Munguía, R.; Bajaña, S.; Meraz-Ríos, M.A.; Sánchez-Torres, C. Tolerogenic Dendritic Cells Generated with Different Immunosuppressive Cytokines Induce Antigen-Specific Anergy and Regulatory Properties in Memory CD4+ T Cells. Pediatrics 2010, 184, 1765–1775. [Google Scholar] [CrossRef]
- Boks, M.A.; Zwaginga, J.J.; Van Ham, S.M.; Brinke, A.T. An Optimized CFSE-based T-cell Suppression Assay to Evaluate the Suppressive Capacity of Regulatory T-Cells Induced by Human Tolerogenic Dendritic Cells. Scand. J. Immunol. 2010, 72, 158–168. [Google Scholar] [CrossRef]
- Chorny, A.; Gonzalez-Rey, E.; Delgado, M. Regulation of Dendritic Cell Differentiation by Vasoactive Intestinal Peptide: Therapeutic Applications on Autoimmunity and Transplantation. Ann. N. Y. Acad. Sci. 2006, 1088, 187–194. [Google Scholar] [CrossRef]
- Fujita, S.; Sato, Y.; Sato, K.; Eizumi, K.; Fukaya, T.; Kubo, M.; Yamashita, N.; Sato, K. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+CD25+Foxp3+ regulatory T cells. Blood 2007, 110, 3793–3803. [Google Scholar] [CrossRef]
- Rossetti, M.; Gregori, S.; Roncarolo, M.G. Granulocyte-colony stimulating factor drives the in vitro differentiation of human dendritic cells that induce anergy in naïve T cells. Eur. J. Immunol. 2010, 40, 3097–3106. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scroggins, S.M.; Schlueter, A.J. Generation of Human Regulatory Dendritic Cells from Cryopreserved Healthy Donor Cells and Hematopoietic Stem Cell Transplant Recipients. Cells 2023, 12, 2372. https://doi.org/10.3390/cells12192372
Scroggins SM, Schlueter AJ. Generation of Human Regulatory Dendritic Cells from Cryopreserved Healthy Donor Cells and Hematopoietic Stem Cell Transplant Recipients. Cells. 2023; 12(19):2372. https://doi.org/10.3390/cells12192372
Chicago/Turabian StyleScroggins, Sabrina M., and Annette J. Schlueter. 2023. "Generation of Human Regulatory Dendritic Cells from Cryopreserved Healthy Donor Cells and Hematopoietic Stem Cell Transplant Recipients" Cells 12, no. 19: 2372. https://doi.org/10.3390/cells12192372
APA StyleScroggins, S. M., & Schlueter, A. J. (2023). Generation of Human Regulatory Dendritic Cells from Cryopreserved Healthy Donor Cells and Hematopoietic Stem Cell Transplant Recipients. Cells, 12(19), 2372. https://doi.org/10.3390/cells12192372





