Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence
Abstract
1. Introduction
2. Materials and Methods
2.1. Islet Collection and Culture
2.2. Islet Transplantation
2.3. Hyperspectral Fluorescence Microscopy
2.4. Image Preparation and Analysis
2.5. Modelling and Unmixing
2.6. Statistical Analysis
3. Results
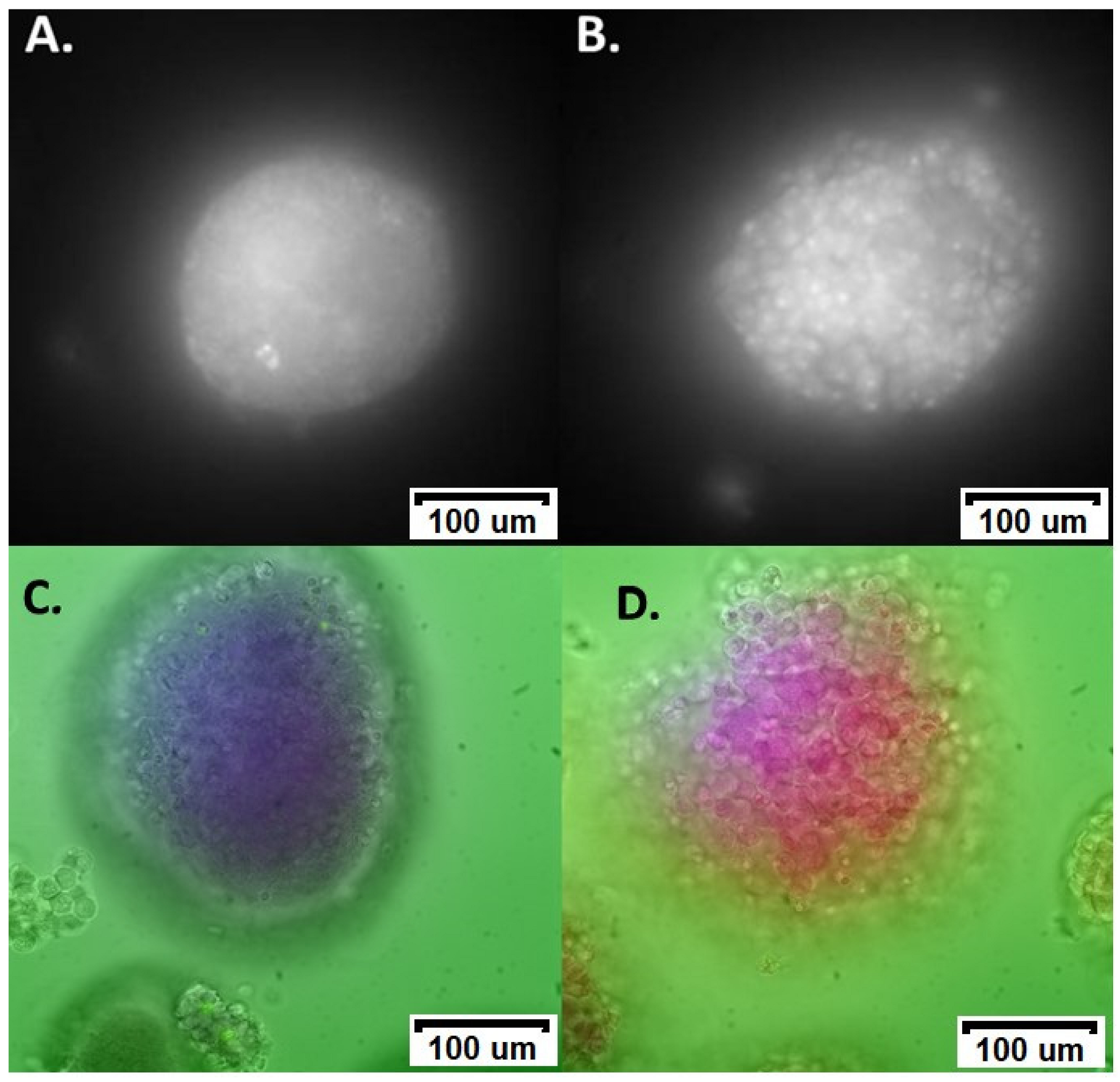
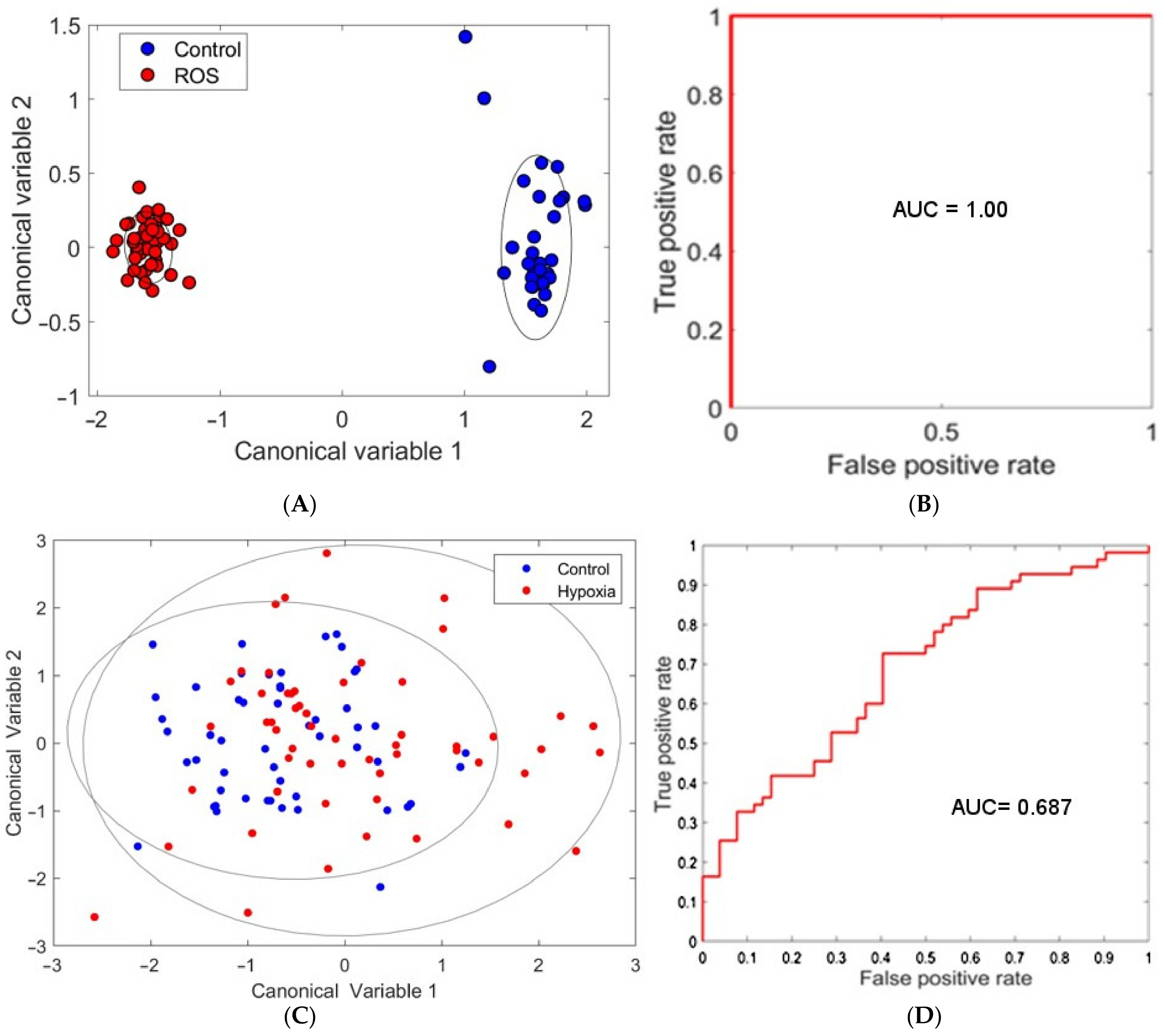
3.1. ROS Damage
3.2. Hypoxia
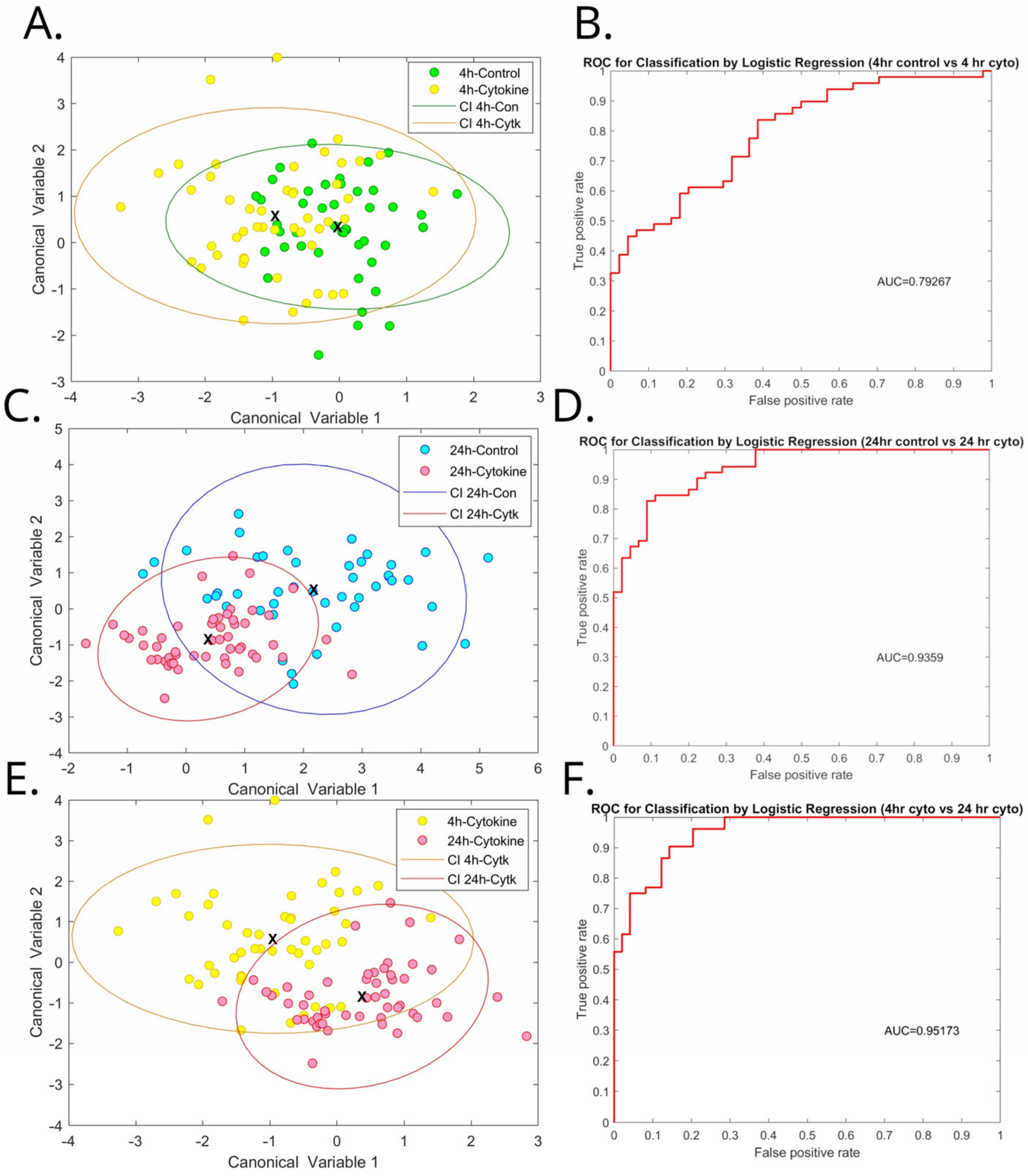
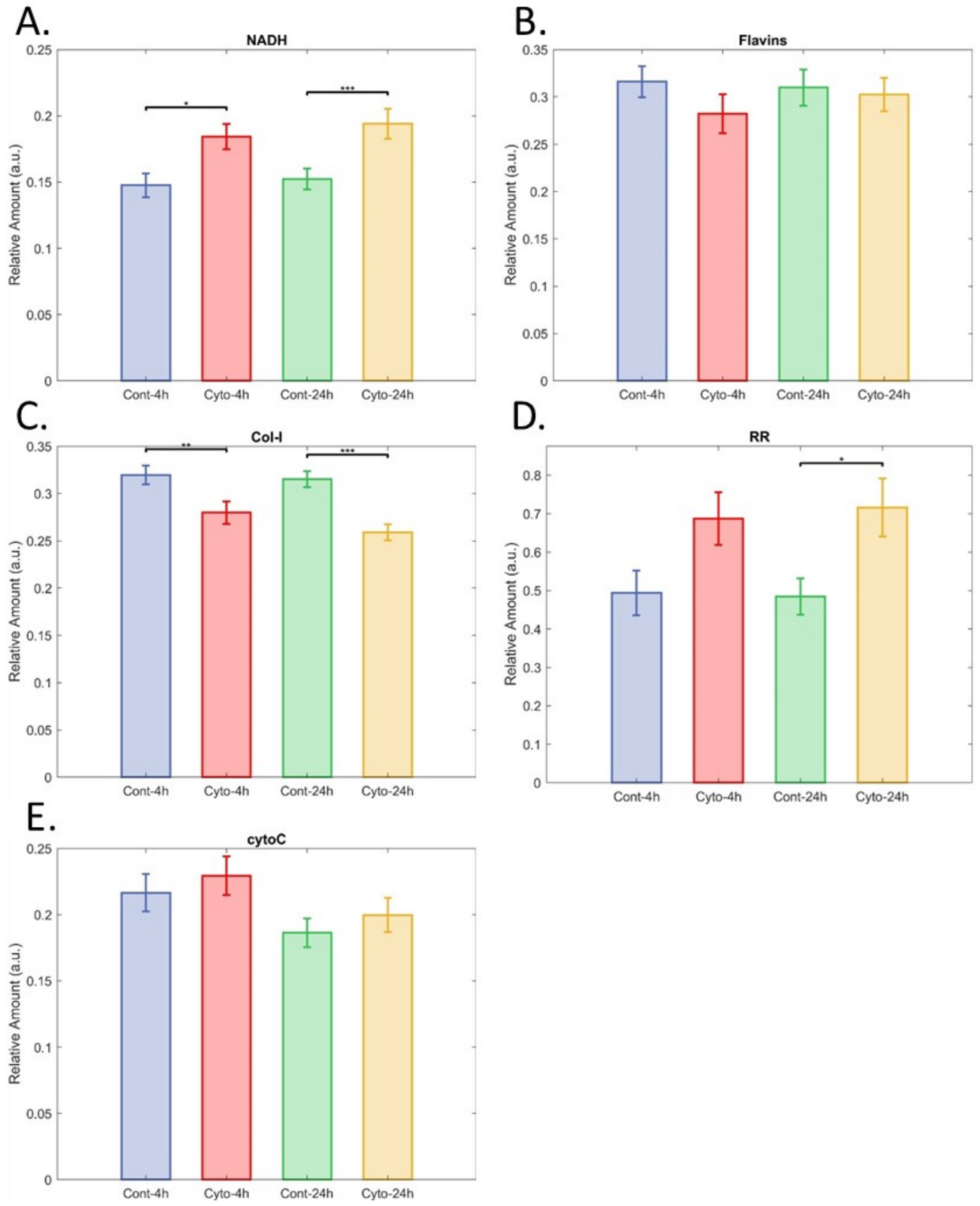
3.3. Inflammatory Cytokines
3.4. Warm Ischaemia
3.5. Encapsulation
3.6. Prediction of Transplant Success
4. Discussion
4.1. Islet Disaggregation
4.2. Biomarkers
4.3. Translation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Connell, P.J.; Holmes-Walker, D.J.; Goodman, D.; Hawthorne, W.J.; Loudovaris, T.; Gunton, J.E.; Thomas, H.E.; Grey, S.T.; Drogemuller, C.J.; Ward, G.M.; et al. Multicenter Australian Trial of Islet Transplantation: Improving Accessibility and Outcomes. Am. J. Transplant. 2013, 13, 1850–1858. [Google Scholar] [CrossRef]
- Anazawa, T.; Okajima, H.; Masui, T.; Uemoto, S. Current state and future evolution of pancreatic islet transplantation. Ann. Gastroenterol. Surg. 2019, 3, 34–42. [Google Scholar] [CrossRef]
- Barton, F.B.; Rickels, M.R.; Alejandro, R.; Hering, B.J.; Wease, S.; Naziruddin, B.; Oberholzer, J.; Odorico, J.S.; Garfinkel, M.R.; Levy, M.; et al. Improvement in Outcomes of Clinical Islet Transplantation: 1999–2010. Diabetes Care 2012, 35, 1436–1445. [Google Scholar] [CrossRef]
- Papas, K.K.; Suszynski, T.M.; Colton, C.K. Islet assessment for transplantation. Curr. Opin. Organ Transplant. 2009, 14, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Ricordi, C.; Lakey, J.; Hering, B. Challenges toward standardization of islet isolation technology. Transplant. Proc. 2001, 33, 1709. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, F.; Ricordi, C. Prediction of Clinical Outcome in Islet Allotransplantation. Diabetes Care 2007, 30, 410–417. [Google Scholar] [CrossRef][Green Version]
- Street, C.N.; Lakey, J.R.; Shapiro, A.J.; Imes, S.; Rajotte, R.V.; Ryan, E.A.; Lyon, J.G.; Kin, T.; Avila, J.; Street, C.N.; et al. Islet graft assessment in the Edmonton Protocol: Implications for predicting long-term clinical outcome. Diabetes 2004, 53, 3107–3114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salgado, M.; Gonzalez, N.; Medrano, L.; Rawson, J.; Omori, K.; Qi, M.; Al-Abdullah, I.; Kandeel, F.; Mullen, Y.; Komatsu, H. Semi-Automated Assessment of Human Islet Viability Predicts Transplantation Outcomes in a Diabetic Mouse Model. Cell Transplant. 2020, 29, 963689720919444. [Google Scholar] [CrossRef]
- Goto, M.; Holgersson, J.; Kumagai-Braesch, M.; Korsgren, O. The ADP/ATP Ratio: A Novel Predictive Assay for Quality Assessment of Isolated Pancreatic Islets. Am. J. Transplant. 2006, 6, 2483–2487. [Google Scholar] [CrossRef]
- Kitzmann, J.; O’Gorman, D.; Kin, T.; Gruessner, A.; Senior, P.; Imes, S.; Gruessner, R.; Shapiro, A.; Papas, K. Islet Oxygen Consumption Rate Dose Predicts Insulin Independence for First Clinical Islet Allotransplants. Transplant. Proc. 2014, 46, 1985–1988. [Google Scholar] [CrossRef]
- Cantley, J.; Walters, S.N.; Jung, M.-H.; Weinberg, A.; Cowley, M.J.; Whitworth, P.T.; Kaplan, W.; Hawthorne, W.J.; O’Connell, P.J.; Weir, G.; et al. A Preexistent Hypoxic Gene Signature Predicts Impaired Islet Graft Function and Glucose Homeostasis. Cell Transplant. 2013, 22, 2147–2159. [Google Scholar] [CrossRef]
- Cowley, M.J.; Weinberg, A.; Zammit, N.W.; Walters, S.N.; Hawthorne, W.J.; Loudovaris, T.; Thomas, H.; Kay, T.; Gunton, J.E.; Alexander, S.I.; et al. Human Islets Express a Marked Proinflammatory Molecular Signature Prior to Transplantation. Cell Transplant. 2012, 21, 2063–2078. [Google Scholar] [CrossRef] [PubMed]
- Abdelli, S.; Ansite, J.; Roduit, R.; Borsello, T.; Matsumoto, I.; Sawada, T.; Allaman-Pillet, N.; Henry, H.; Beckmann, J.S.; Hering, B.J.; et al. Intracellular Stress Signaling Pathways Activated During Human Islet Preparation and Following Acute Cytokine Exposure. Diabetes 2004, 53, 2815–2823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartolomé, F.; Abramov, A.Y. Measurement of Mitochondrial NADH and FAD Autofluorescence in Live Cells. Methods Mol. Biol. 2015, 1264, 263–270. [Google Scholar] [CrossRef]
- Heaster, T.M.; Walsh, A.J.; Zhao, Y.; Hiebert, S.W.; Skala, M.C. Autofluorescence imaging identifies tumor cell-cycle status on a single-cell level. J. Biophotonics 2018, 11, e201600276. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, J.H.; McMahon, C.M.; Lem, S.; Millon, S.R.; Brown, J.Q.; Seewaldt, V.L.; Ramanujam, N. Optical Redox Ratio Differentiates Breast Cancer Cell Lines Based on Estrogen Receptor Status. Cancer Res 2010, 70, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Meleshina, A.V.; Dudenkova, V.V.; Bystrova, A.S.; Kuznetsova, D.S.; Shirmanova, M.V.; Zagaynova, E.V. Two-photon FLIM of NAD(P)H and FAD in mesenchymal stem cells undergoing either osteogenic or chondrogenic differentiation. Stem Cell Res. Ther. 2017, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, N. Fluorescence Spectroscopy of Neoplastic and Non-Neoplastic Tissues. Neoplasia 2000, 2, 89–117. [Google Scholar] [CrossRef]
- Campbell, J.M.; Habibalahi, A.; Mahbub, S.; Gosnell, M.; Anwer, A.G.; Paton, S.; Gronthos, S.; Goldys, E. Non-destructive, label free identification of cell cycle phase in cancer cells by multispectral microscopy of autofluorescence. BMC Cancer 2019, 19, 1242. [Google Scholar] [CrossRef]
- Mahbub, S.B.; Guller, A.; Campbell, J.M.; Anwer, A.G.; Gosnell, M.E.; Vesey, G.; Goldys, E.M. Non-Invasive Monitoring of Functional State of Articular Cartilage Tissue with Label-Free Unsupervised Hyperspectral Imaging. Sci. Rep. 2019, 9, 4398. [Google Scholar] [CrossRef]
- Mahbub, S.B.; Nguyen, L.T.; Habibalahi, A.; Campbell, J.M.; Anwer, A.G.; Qadri, U.M.; Gill, A.; Chou, A.; Wong, M.G.; Gosnell, M.; et al. Non-invasive assessment of exfoliated kidney cells extracted from urine using multispectral autofluorescence features. Sci. Rep. 2021, 11, 10655. [Google Scholar]
- Habibalahi, A.; Moghari, M.D.; Campbell, J.M.; Anwer, A.G.; Mahbub, S.B.; Gosnell, M.; Saad, S.; Pollock, C.; Goldys, E.M. Non-invasive real-time imaging of reactive oxygen species (ROS) using auto-fluorescence multispectral imaging technique: A novel tool for redox biology. Redox Biol. 2020, 34, 101561. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Mahbub, S.; Habibalahi, A.; Paton, S.; Gronthos, S.; Goldys, E. Ageing human bone marrow mesenchymal stem cells have depleted NAD(P)H and distinct multispectral autofluorescence. GeroScience 2020, 43, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Grey, S.T.; Longo, C.; Shukri, T.; Patel, V.I.; Csizmadia, E.; Daniel, S.; Arvelo, M.B.; Tchipashvili, V.; Ferran, C. Genetic engineering of a suboptimal islet graft with A20 preserves beta cell mass and function. J. Immunol. 2003, 170, 6250–6256. [Google Scholar] [CrossRef]
- Goffart, S.; Tikkanen, P.; Michell, C.; Wilson, T.; Pohjoismäki, J.L.O. The Type and Source of Reactive Oxygen Species Influences the Outcome of Oxidative Stress in Cultured Cells. Cells 2021, 10, 1075. [Google Scholar] [CrossRef]
- Tesi, M.; Bugliani, M.; Ferri, G.; Suleiman, M.; De Luca, C.; Bosi, E.; Masini, M.; De Tata, V.; Gysemans, C.; Cardarelli, F.; et al. Pro-Inflammatory Cytokines Induce Insulin and Glucagon Double Positive Human Islet Cells that are Resistant to Apoptosis. Biomolecules 2021, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Cetkovic-Cvrlje, M.; Eizirik, D.L. TNF-α and IFN-γ potentiate the deleterious effects of IL-1 β on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine 1994, 6, 399–406. [Google Scholar] [CrossRef]
- Brandhorst, D.; Brandhorst, H.; Acreman, S.; Johnson, P.R.V. The ischaemic preconditioning paradox and its implications for islet isolation from heart-beating and non heart-beating donors. Sci. Rep. 2022, 12, 19321. [Google Scholar] [CrossRef]
- Pham-Hua, D.; Padgett, L.E.; Xue, B.; Anderson, B.; Zeiger, M.; Barra, J.M.; Bethea, M.; Hunter, C.S.; Kozlovskaya, V.; Kharlampieva, E.; et al. Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking. Biomaterials 2017, 128, 19–32. [Google Scholar] [CrossRef]
- Walters, S.; Webster, K.E.; Sutherland, A.; Gardam, S.; Groom, J.; Liuwantara, D.; Mariño, E.; Thaxton, J.; Weinberg, A.; Mackay, F.; et al. Increased CD4+ Foxp3+ T Cells in BAFF-Transgenic Mice Suppress T Cell Effector Responses. J. Immunol. 2009, 182, 793–801. [Google Scholar] [CrossRef]
- Hesse, U.J.; Schmitz-Rode, M.; Voss, S. Islet counting by staining with dithizone in various concentrations. Transplant. Proc. 1992, 24, 2989. [Google Scholar]
- Mahbub, S.B.; Plöschner, M.; Gosnell, M.E.; Anwer, A.G.; Goldys, E.M. Statistically strong label-free quantitative identification of native fluorophores in a biological sample. Sci. Rep. 2017, 7, 15792. [Google Scholar] [CrossRef]
- Habibalahi, A.; Bala, C.; Allende, A.; Anwer, A.G.; Goldys, E.M. Novel automated non invasive detection of ocular surface squamous neoplasia using multispectral autofluorescence imaging. Ocul. Surf. 2019, 17, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Gosnell, M.E.; Anwer, A.G.; Mahbub, S.B.; Perinchery, S.M.; Inglis, D.W.; Adhikary, P.P.; Jazayeri, J.A.; Cahill, M.A.; Saad, S.; Pollock, C.A.; et al. Quantitative non-invasive cell characterisation and discrimination based on multispectral autofluorescence features. Sci. Rep. 2016, 6, 23453. [Google Scholar] [CrossRef]
- El Aziz, M.A.; Selim, I.M.; Xiong, S. Automatic Detection of Galaxy Type from Datasets of Galaxies Image Based on Image Retrieval Approach. Sci. Rep. 2017, 7, 4463. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Balloux, F. Discriminant analysis of principal components: A new method for the analysis of genetically structured populations. BMC Genet. 2010, 11, 1471–2156. [Google Scholar] [CrossRef] [PubMed]
- Naganathan, G.K.; Grimes, L.M.; Subbiah, J.; Calkins, C.R.; Samal, A.; Meyer, G.E. Visible/near-infrared hyperspectral imaging for beef tenderness prediction. Comput. Electron. Agric. 2008, 64, 225–233. [Google Scholar] [CrossRef]
- Vapnik, V. The Nature of Statistical Learning Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ Repletion Rescues Female Fertility during Reproductive Aging. Cell Rep. 2020, 30, 1670–1681.e7. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, S.B. Unsupervised Hyperspectral Unmixing Analysis for Label-free Quantitative Identification of Native Fluorophores in a Biological Sample by a Robust Dependent Component Analysis (RoDECA). Ph.D. Thesis, Department of Physics and Astronomy, Faculty of Science and Engineering, Macquarie University, Macquarie Park, NSW, Australia, 2017; p. 295. [Google Scholar]
- Campbell, P.M.; Senior, P.A.; Salam, A.; LaBranche, K.; Bigam, D.L.; Kneteman, N.M.; Imes, S.; Halpin, A.; Ryan, E.A.; Shapiro, A.M.J. High Risk of Sensitization After Failed Islet Transplantation. Am. J. Transplant. 2007, 7, 2311–2317. [Google Scholar] [CrossRef]
- Cardani, R.; Pileggi, A.; Ricordi, C.; Gomez, C.; Baidal, D.A.; Ponte, G.G.; Mineo, D.; Faradji, R.N.; Froud, T.; Ciancio, G.; et al. Allosensitization of Islet Allograft Recipients. Transplantation 2007, 84, 1413–1427. [Google Scholar] [CrossRef]
- Blacker, T.S.; Duchen, M.R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 2016, 100, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, O.I.; Quinn, K.P.; Hassinen, I.E.; Machida, K.; Münzel, T.; Daiber, A.; Zhang, M.; Ying, W.; Lorusso, J.S.; Sviderskiy, O.A.; et al. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal. 2019, 30, 875–889. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Gao, D.; Pararasa, C. Redox regulation in metabolic programming and inflammation. Redox Biol. 2017, 12, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.E.; Lamont, I.L. Biochemistry Changes That Occur after Death: Potential Markers for Determining Post-Mortem Interval. PLoS ONE 2013, 8, e82011. [Google Scholar] [CrossRef]
- Papayan, G.; Petrishchev, N.; Galagudza, M. Autofluorescence spectroscopy for NADH and flavoproteins redox state monitoring in the isolated rat heart subjected to ischemia-reperfusion. Photodiagnosis Photodyn. Ther. 2014, 11, 400–408. [Google Scholar] [CrossRef]
- Levitt, J.M.; Baldwin, A.; Papadakis, A.; Puri, S.; Xylas, J.; Münger, K.; Georgakoudi, I. Intrinsic fluorescence and redox changes associated with apoptosis of primary human epithelial cells. J. Biomed. Opt. 2006, 11, 064012. [Google Scholar] [CrossRef]
- Cantley, J.; Grey, S.T.; Maxwell, P.H.; Withers, D.J. The hypoxia response pathway and β-cell function. Diabetes Obes. Metab. 2010, 12, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Liu, J.-W.; Tang, D.G.; Canela, N.; Rodriguez-Vilarrupla, A.; Estanyol, J.M.; Díaz, C.; Pujol, M.J.; Agell, N.; Bachs, O. Early Mitochondrial Activation and Cytochrome c Up-regulation during Apoptosis. Perspect. Surg. 2002, 277, 50842–50854. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Finke, E.H.; Olack, B.J.; Scharp, D.W. Automated method for isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Epidemiology of Gender Differences in Diabetes and Obesity. Adv. Exp. Med. Biol. 2017, 1043, 3–8. [Google Scholar] [CrossRef]
- Tan, T.C.Y.; Mahbub, S.B.; Campbell, J.M.; Habibalahi, A.; Campugan, C.A.; Rose, R.D.; Chow, D.J.X.; Mustafa, S.; Goldys, E.M.; Dunning, K.R. Non-invasive, label-free optical analysis to detect aneuploidy within the inner cell mass of the preimplantation embryo. Hum. Reprod. 2021, 37, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Cardona, K.; Korbutt, G.S.; Milas, Z.; Lyon, J.; Cano, J.; Jiang, W.; Bello-Laborn, H.; Hacquoil, B.; Strobert, E.; Gangappa, S.; et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med. 2006, 12, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Odorico, J.; Markmann, J.; Melton, D.; Greenstein, J.; Hwa, A.; Nostro, C.; Rezania, A.; Oberholzer, J.; Pipeleers, D.; Yang, L.; et al. Report of the Key Opinion Leaders Meeting on Stem Cell-derived Beta Cells. Transplantation 2018, 102, 1223–1229. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, J.M.; Walters, S.N.; Habibalahi, A.; Mahbub, S.B.; Anwer, A.G.; Handley, S.; Grey, S.T.; Goldys, E.M. Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence. Cells 2023, 12, 2302. https://doi.org/10.3390/cells12182302
Campbell JM, Walters SN, Habibalahi A, Mahbub SB, Anwer AG, Handley S, Grey ST, Goldys EM. Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence. Cells. 2023; 12(18):2302. https://doi.org/10.3390/cells12182302
Chicago/Turabian StyleCampbell, Jared M., Stacey N. Walters, Abbas Habibalahi, Saabah B. Mahbub, Ayad G. Anwer, Shannon Handley, Shane T. Grey, and Ewa M. Goldys. 2023. "Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence" Cells 12, no. 18: 2302. https://doi.org/10.3390/cells12182302
APA StyleCampbell, J. M., Walters, S. N., Habibalahi, A., Mahbub, S. B., Anwer, A. G., Handley, S., Grey, S. T., & Goldys, E. M. (2023). Pancreatic Islet Viability Assessment Using Hyperspectral Imaging of Autofluorescence. Cells, 12(18), 2302. https://doi.org/10.3390/cells12182302





