In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling
Abstract
1. Introduction
2. Materials and Methods
3. Results
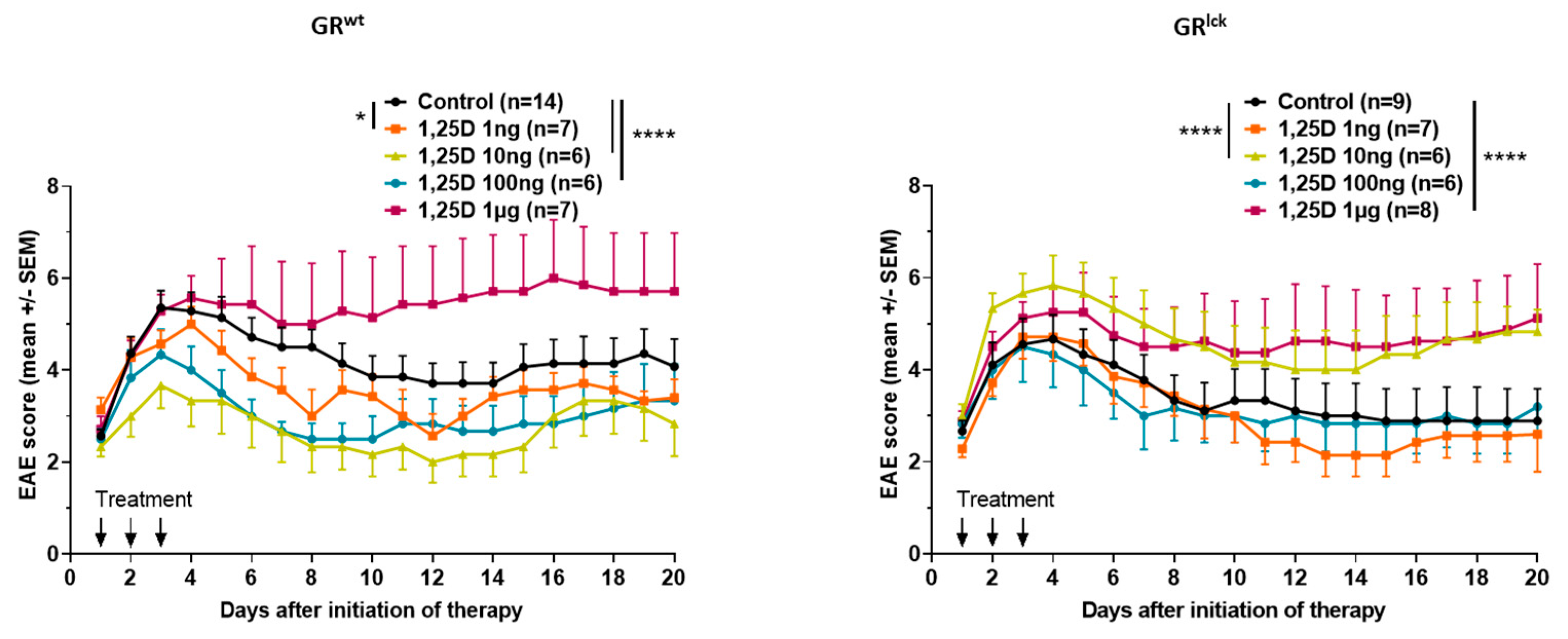
3.1. Calcitriol Improves MOG35–55 EAE Disease Course in WT but Not in GRlck Mice In Vivo
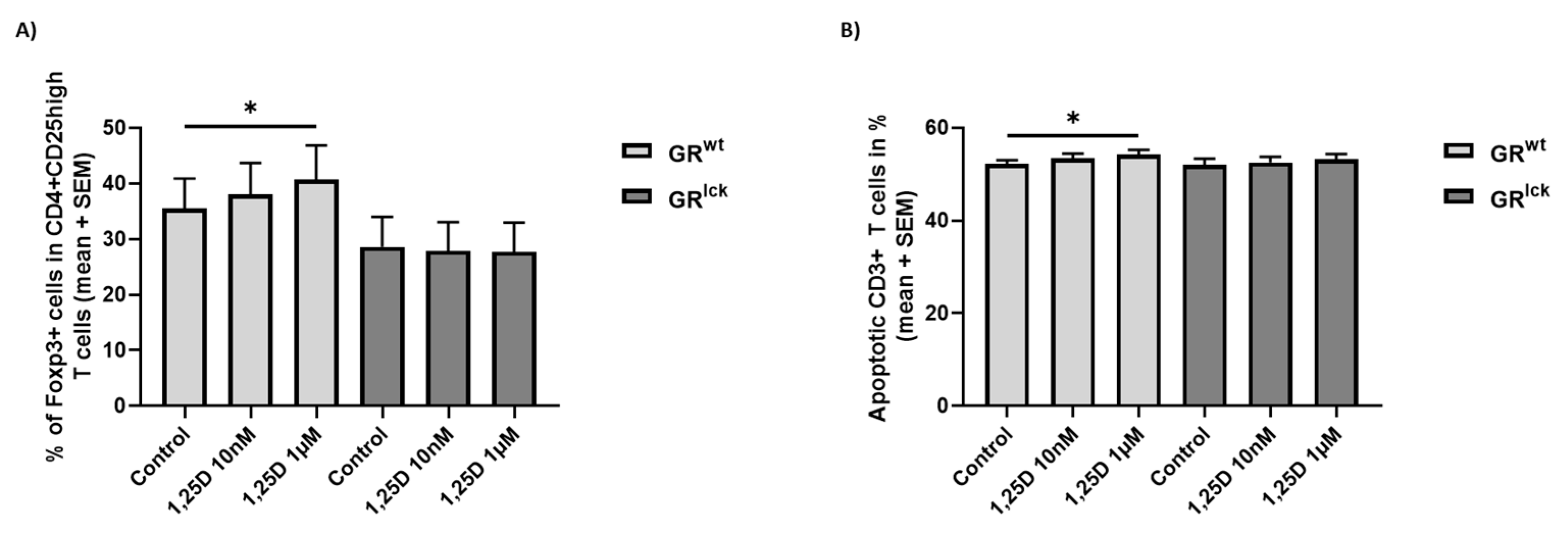
3.2. Calcitriol Induces Treg Differentiation and Increases the Apoptosis of CD3+ T Cells from WT but Not GRlck Mice In Vitro
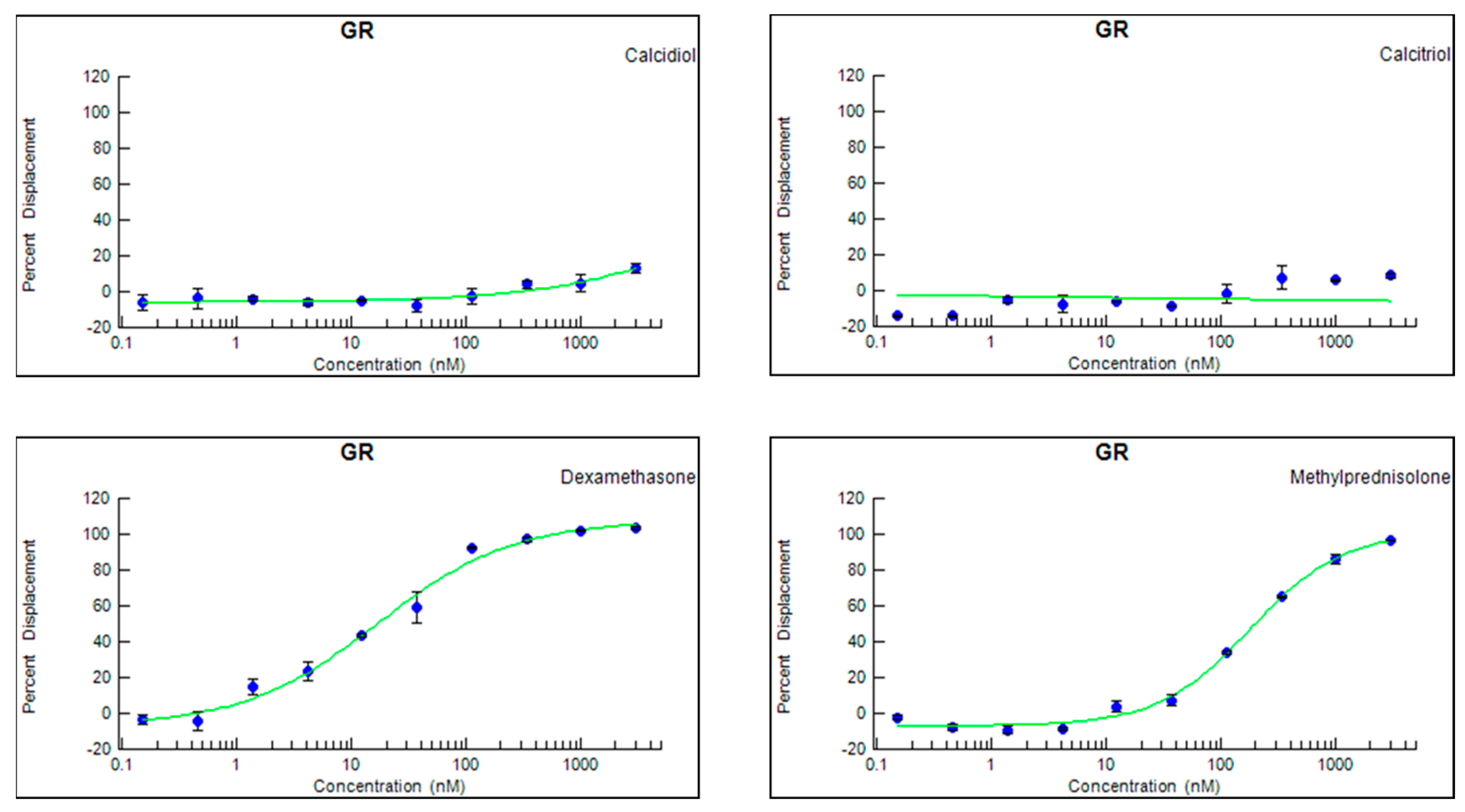
3.3. Neither Calcitriol nor Calcidiol Bind to a Human Recombinant GR
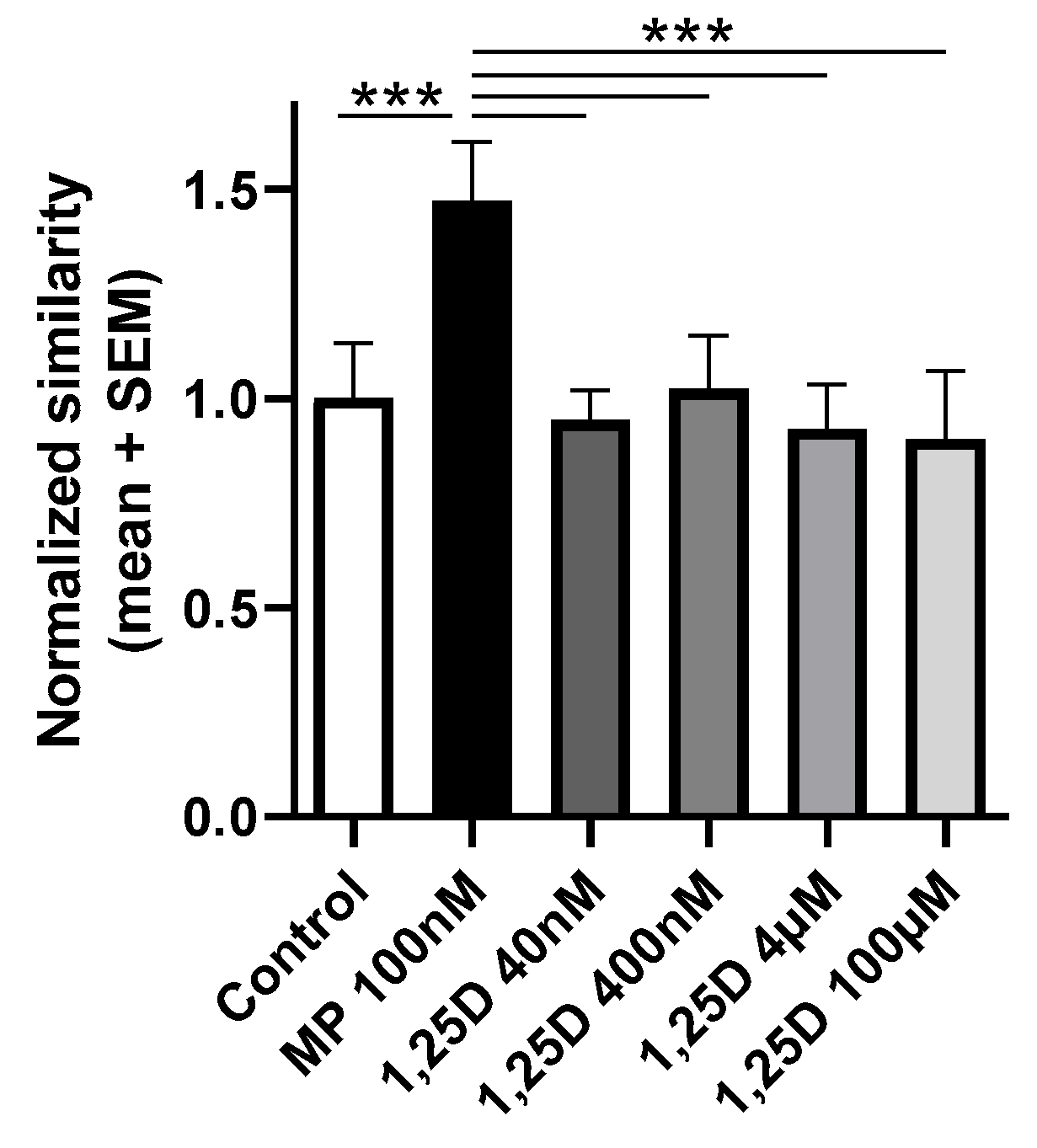
3.4. Calcitriol Does Not Induce the Nuclear Translocation of the GR In Vitro
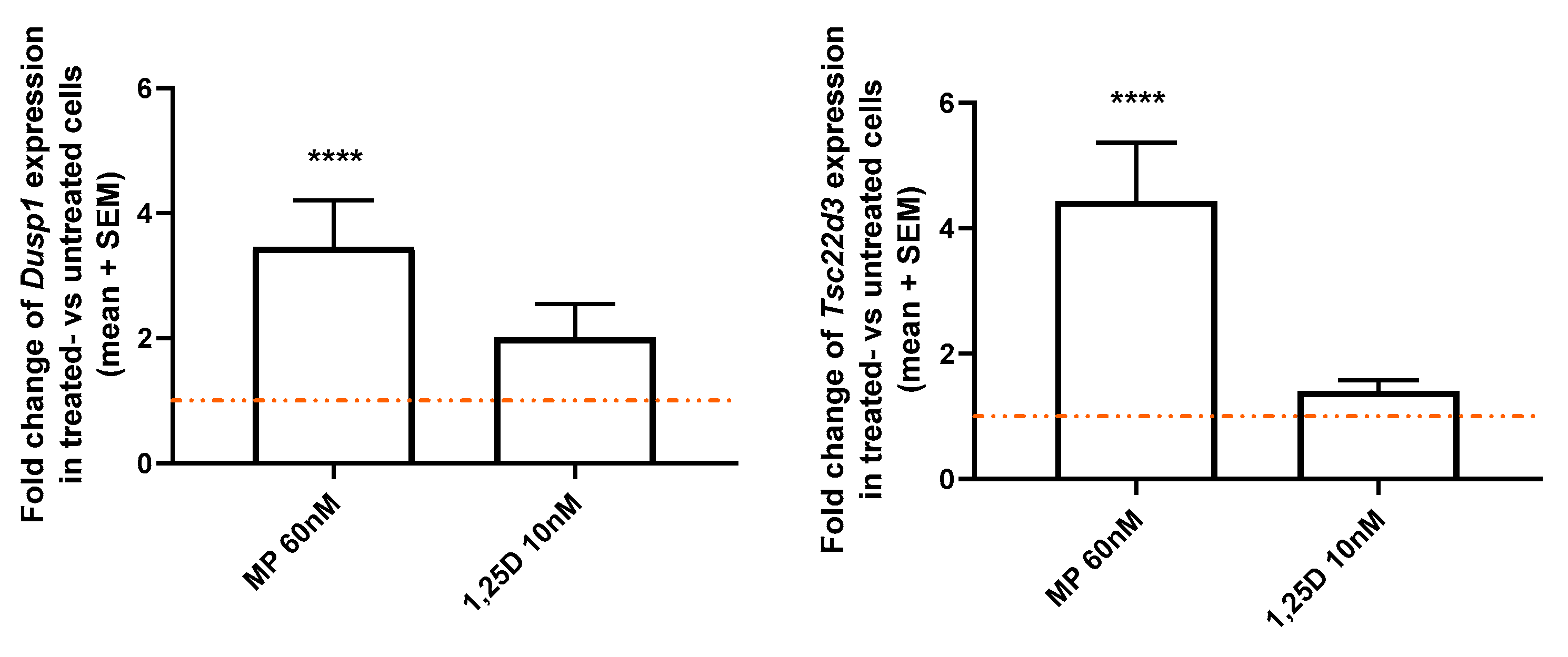
3.5. Calcitriol Does Not Modulate the Expression of Two GR-Induced Genes, Dusp1 and Tsc22d3, in CD3+ T Cells from WT Mice In Vitro
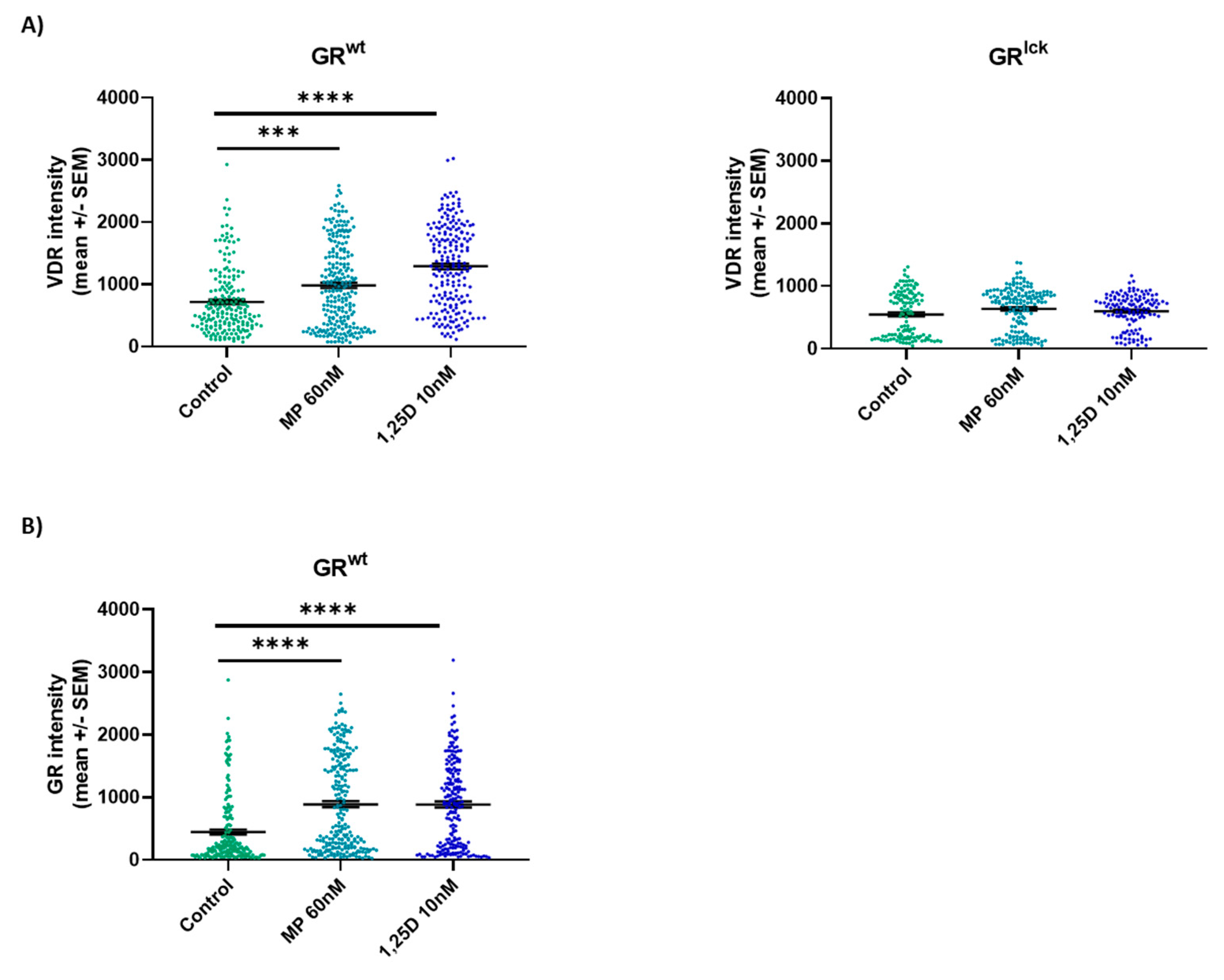
3.6. Calcitriol Enhances VDR Protein Expression in CD3+ T Cells from WT but Not GRlck Mice In Vitro
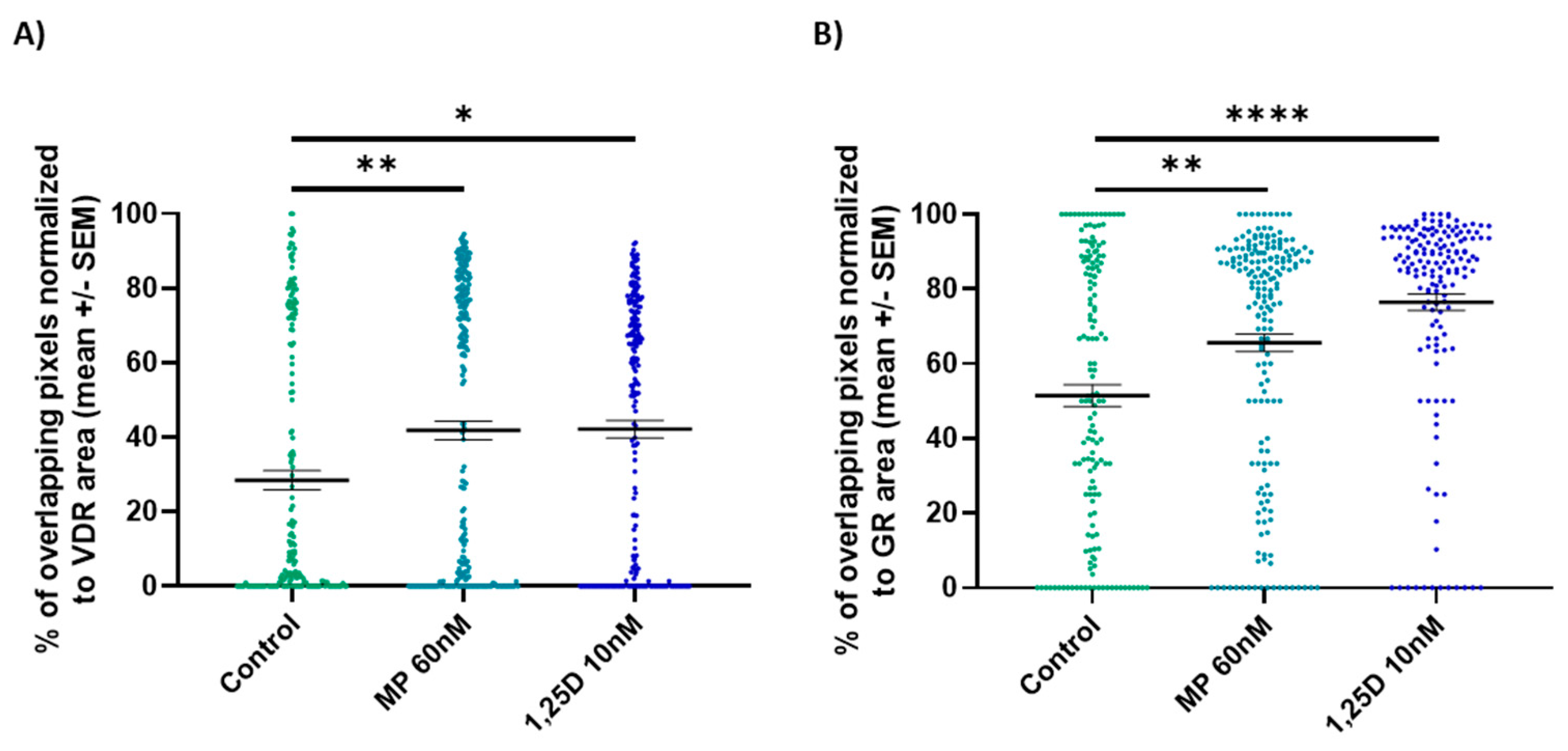
3.7. Calcitriol Induces the Nuclear Co-Localization of the GR and VDR in CD3+ T Cells from WT Mice In Vitro
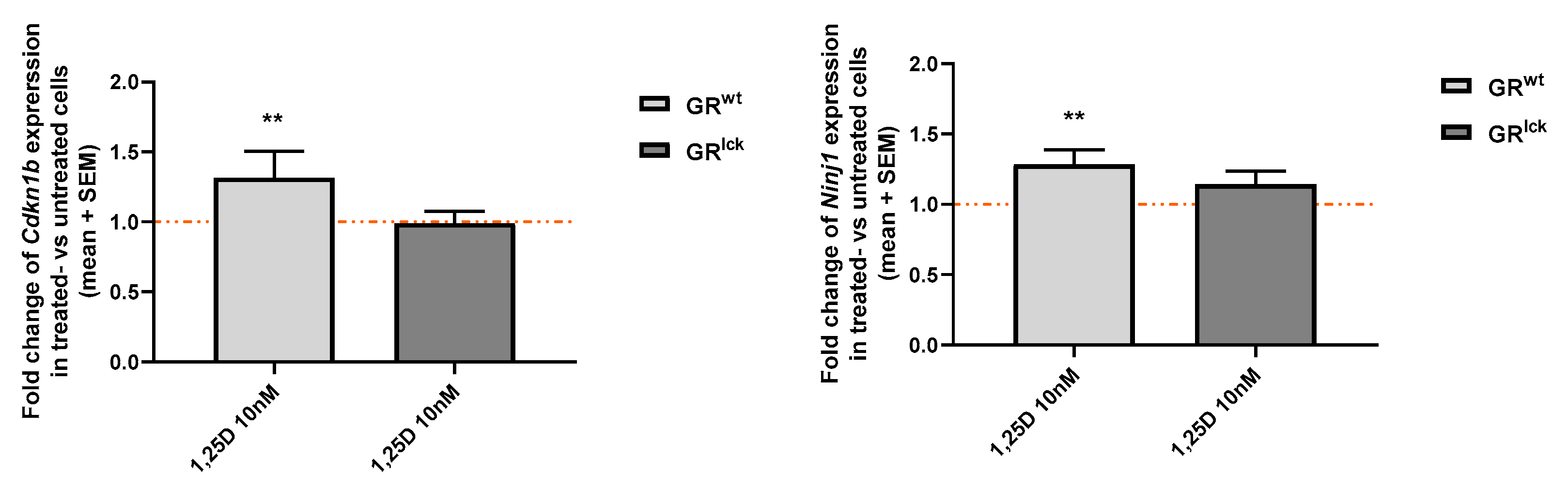
3.8. Calcitriol Enhances the Expression of Two VDR-Induced Genes, Cdkn1b and Ninj1, in CD3+ T Cells from WT but Not GRlck Mice In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Vandevyver, S.; Dejager, L.; Libert, C. On the trail of the glucocorticoid receptor: Into the nucleus and back. Traffic 2012, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Joanny, E.; Ding, Q.; Gong, L.; Kong, P.; Saklatvala, J.; Clark, A.R. Anti-inflammatory effects of selective glucocorticoid receptor modulators are partially dependent on up-regulation of dual specificity phosphatase 1. Br. J. Pharmacol. 2012, 165, 1124–1136. [Google Scholar] [CrossRef]
- Bereshchenko, O.; Migliorati, G.; Bruscoli, S.; Riccardi, C. Glucocorticoid-Induced Leucine Zipper: A Novel Anti-inflammatory Molecule. Front. Pharmacol. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, H.M.; Gold, R.; Luhder, F. Glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Expert Rev. Neurother. 2006, 6, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Schweingruber, N.; Fischer, H.J.; Fischer, L.; van den Brandt, J.; Karabinskaya, A.; Labi, V.; Villunger, A.; Kretzschmar, B.; Huppke, P.; Simons, M.; et al. Chemokine-mediated redirection of T cells constitutes a critical mechanism of glucocorticoid therapy in autoimmune CNS responses. Acta Neuropathol. 2014, 127, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Gold, R.; Schönrock, L.; Zettl, U.K.; Hartung, H.-P.; Toyka, K.V. T-cell apoptosis in situ in experimental autoimmune encephalomyelitis following methylprednisolone pulse therapy. Brain 2000, 123, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Oppenheim, J.J.; Winkler-Pickett, R.T.; Ortaldo, J.R.; Howard, O.M. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3(+)CD4(+)CD25(+) T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006, 36, 2139–2149. [Google Scholar] [CrossRef]
- Wüst, S.; van den Brandt, J.; Tischner, D.; Kleiman, A.; Tuckermann, J.P.; Gold, R.; Lühder, F.; Reichardt, H.M. Peripheral T cells are the therapeutic targets of glucocorticoids in experimental autoimmune encephalomyelitis. J. Immunol. 2008, 180, 8434–8443. [Google Scholar] [CrossRef]
- He, C.S.; Aw Yong, X.H.; Walsh, N.P.; Gleeson, M. Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 2016, 22, 42–64. [Google Scholar]
- Norman, A.W. The history of the discovery of vitamin D and its daughter steroid hormone. Ann. Nutr. Metab. 2012, 61, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D: A millenium perspective. J. Cell. Biochem. 2003, 88, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Miclea, A.; Bagnoud, M.; Chan, A.; Hoepner, R. A Brief Review of the Effects of Vitamin D on Multiple Sclerosis. Front. Immunol. 2020, 11, 781. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S. Chapter 13—Vitamin D: Biology, Actions, and Clinical Implications. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 283–328. [Google Scholar] [CrossRef]
- Galoppin, M.; Kari, S.; Soldati, S.; Pal, A.; Rival, M.; Engelhardt, B.; Astier, A.; Thouvenot, E. Full spectrum of vitamin D immunomodulation in multiple sclerosis: Mechanisms and therapeutic implications. Brain Commun. 2022, 4, fcac171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shih, D.Q.; Zhang, X. Mechanisms underlying effects of 1,25-Dihydroxyvitamin D3 on the Th17 cells. Eur. J. Microbiol. Immunol. 2013, 3, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kim, S.H.; Lee, N.; Lee, W.W.; Hwang, K.A.; Shin, M.S.; Lee, S.H.; Kim, W.U.; Kang, I. 1,25-Dihyroxyvitamin D3 promotes FOXP3 expression via binding to vitamin D response elements in its conserved noncoding sequence region. J. Immunol. 2012, 188, 5276–5282. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2016, 7, 697. [Google Scholar] [CrossRef]
- Spach, K.M.; Nashold, F.E.; Dittel, B.N.; Hayes, C.E. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 6030–6037. [Google Scholar] [CrossRef]
- Nashold, F.E.; Miller, D.J.; Hayes, C.E. 1,25-dihydroxyvitamin D3 treatment decreases macrophage accumulation in the CNS of mice with experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000, 103, 171–179. [Google Scholar] [CrossRef]
- Mayne, C.G.; Spanier, J.A.; Relland, L.M.; Williams, C.B.; Hayes, C.E. 1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2011, 41, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, R.; Bagnoud, M.; Pistor, M.; Salmen, A.; Briner, M.; Synn, H.; Schrewe, L.; Guse, K.; Ahmadi, F.; Demir, S.; et al. Vitamin D increases glucocorticoid efficacy via inhibition of mTORC1 in experimental models of multiple sclerosis. Acta Neuropathol. 2019, 138, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Marshall, T.G. Are statins analogues of vitamin D? Lancet 2006, 368, 1234. [Google Scholar] [CrossRef] [PubMed]
- Wüst, S.; Tischner, D.; John, M.; Tuckermann, J.P.; Menzfeld, C.; Hanisch, U.K.; van den Brandt, J.; Lühder, F.; Reichardt, H.M. Therapeutic and adverse effects of a non-steroidal glucocorticoid receptor ligand in a mouse model of multiple sclerosis. PLoS ONE 2009, 4, e8202. [Google Scholar] [CrossRef] [PubMed]
- Bagnoud, M.; Briner, M.; Remlinger, J.; Meli, I.; Schuetz, S.; Pistor, M.; Salmen, A.; Chan, A.; Hoepner, R. c-Jun N-Terminal Kinase as a Therapeutic Target in Experimental Autoimmune Encephalomyelitis. Cells 2020, 9, 2154. [Google Scholar] [CrossRef] [PubMed]
- Lodygin, D.; Odoardi, F.; Schläger, C.; Körner, H.; Kitz, A.; Nosov, M.; van den Brandt, J.; Reichardt, H.M.; Haberl, M.; Flügel, A. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat. Med. 2013, 19, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, S.D.; Lühder, F.; Wiegers, G.J.; Reichardt, H.M. A flow cytometric approach to study glucocorticoid receptor expression in immune cell subpopulations of genetically engineered mice. Immunol. Lett. 2021, 233, 68–79. [Google Scholar] [CrossRef]
- Rosas-Arellano, A.; Villalobos-González, J.B.; Palma-Tirado, L.; Beltrán, F.A.; Cárabez-Trejo, A.; Missirlis, F.; Castro, M.A. A simple solution for antibody signal enhancement in immunofluorescence and triple immunogold assays. Histochem. Cell Biol. 2016, 146, 421–430. [Google Scholar] [CrossRef]
- Mazaira, G.I.; Zgajnar, N.R.; Lotufo, C.M.; Daneri-Becerra, C.; Sivils, J.C.; Soto, O.B.; Cox, M.B.; Galigniana, M.D. The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl. Recept. Res. 2018, 5, 101320. [Google Scholar] [CrossRef]
- Carlberg, C.; Campbell, M.J. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids 2013, 78, 127–136. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure:function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef]
- Lemire, J.M.; Archer, D.C. 1,25-dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J. Clin. Investig. 1991, 87, 1103–1107. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Hayes, C.E.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc. Natl. Acad. Sci. USA 1996, 93, 7861–7864. [Google Scholar] [CrossRef] [PubMed]
- Hausler, D.; Torke, S.; Peelen, E.; Bertsch, T.; Djukic, M.; Nau, R.; Larochelle, C.; Zamvil, S.S.; Bruck, W.; Weber, M.S. High dose vitamin D exacerbates central nervous system autoimmunity by raising T-cell excitatory calcium. Brain 2019, 142, 2737–2755. [Google Scholar] [CrossRef] [PubMed]
- Matos, C.; Renner, K.; Peuker, A.; Schoenhammer, G.; Schreiber, L.; Bruss, C.; Eder, R.; Bruns, H.; Flamann, C.; Hoffmann, P.; et al. Physiological levels of 25-hydroxyvitamin D(3) induce a suppressive CD4(+) T cell phenotype not reflected in the epigenetic landscape. Scand. J. Immunol. 2022, 95, e13146. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Parastouei, K.; Mirshafiey, A.; Eshraghian, M.R.; Shiri-Shahsavar, M.R.; Solaymani-Mohammadi, F.; Chahardoli, R.; Alvandi, E.; Saboor-Yaraghi, A.A. The effect of 1, 25(OH)2 D3 (calcitriol) alone and in combination with all-trans retinoic acid on ROR-γt, IL-17, TGF-β, and FOXP3 gene expression in experimental autoimmune encephalomyelitis. Nutr. Neurosci. 2018, 21, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Fife, R.S.; Sledge, G.W., Jr.; Proctor, C. Effects of vitamin D3 on proliferation of cancer cells in vitro. Cancer Lett. 1997, 120, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, F.; Qu, H.; Wang, H.; Xiao, X.; Deng, H. 1, 25(OH)2D3 protects beta cell against high glucose-induced apoptosis through mTOR suppressing. Mol. Cell. Endocrinol. 2015, 414, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sutedja, E.K.; Amarassaphira, D.; Goenawan, H.; Susanti Pratiwi, Y.; Sylviana, N.; Setiabudiawan, B.; Suwarsa, O.; Tina Dewi Judistiani, R.; Supratman, U.; Lesmana, R. Calcitriol Inhibits Proliferation and Potentially Induces Apoptosis in B16-F10 Cells. Med. Sci. Monit. Basic Res. 2022, 28, e935139. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Nashold, F.E.; Spach, K.M.; Hayes, C.E. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J. Neurosci. Res. 2007, 85, 2480–2490. [Google Scholar] [CrossRef] [PubMed]
- Spach, K.M.; Pedersen, L.B.; Nashold, F.E.; Kayo, T.; Yandell, B.S.; Prolla, T.A.; Hayes, C.E. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol. Genom. 2004, 18, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Phuc Le, P.; Friedman, J.R.; Schug, J.; Brestelli, J.E.; Parker, J.B.; Bochkis, I.M.; Kaestner, K.H. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet. 2005, 1, e16. [Google Scholar] [CrossRef] [PubMed]
- Helmuth, J.A.; Paul, G.; Sbalzarini, I.F. Beyond co-localization: Inferring spatial interactions between sub-cellular structures from microscopy images. BMC Bioinform. 2010, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Leung, D.Y.; Goleva, E. Vitamin D enhances glucocorticoid action in human monocytes: Involvement of granulocyte-macrophage colony-stimulating factor and mediator complex subunit 14. J. Biol. Chem. 2013, 288, 14544–14553. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zemel, M.B. 1Alpha, 25-dihydroxyvitamin D and corticosteroid regulate adipocyte nuclear vitamin D receptor. Int. J. Obes. 2008, 32, 1305–1311. [Google Scholar] [CrossRef][Green Version]
- Chen, T.L.; Hauschka, P.V.; Feldman, D. Dexamethasone increases 1,25-dihydroxyvitamin D3 receptor levels and augments bioresponses in rat osteoblast-like cells. Endocrinology 1986, 118, 1119–1126. [Google Scholar] [CrossRef]
- Shymanskyi, I.; Lisakovska, O.; Mazanova, A.; Labudzynskyi, D.; Veliky, M. Vitamin D3 Modulates Impaired Crosstalk Between RANK and Glucocorticoid Receptor Signaling in Bone Marrow Cells after Chronic Prednisolone Administration. Front. Endocrinol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Koetzier, S.C.; van Langelaar, J.; Wierenga-Wolf, A.F.; Melief, M.J.; Pol, K.; Musters, S.; Lubberts, E.; Dik, W.A.; Smolders, J.; van Luijn, M.M. Improving Glucocorticoid Sensitivity of Brain-Homing CD4(+) T Helper Cells by Steroid Hormone Crosstalk. Front. Immunol. 2022, 13, 893702. [Google Scholar] [CrossRef]
- Daniel, C.; Sartory, N.A.; Zahn, N.; Radeke, H.H.; Stein, J.M. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008, 324, 23–33. [Google Scholar] [CrossRef]
- Hidalgo, A.A.; Deeb, K.K.; Pike, J.W.; Johnson, C.S.; Trump, D.L. Dexamethasone enhances 1alpha,25-dihydroxyvitamin D3 effects by increasing vitamin D receptor transcription. J. Biol. Chem. 2011, 286, 36228–36237. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnoud, M.; Remlinger, J.; Massy, M.; Lodygin, D.; Salmen, A.; Chan, A.; Lühder, F.; Hoepner, R. In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling. Cells 2023, 12, 2291. https://doi.org/10.3390/cells12182291
Bagnoud M, Remlinger J, Massy M, Lodygin D, Salmen A, Chan A, Lühder F, Hoepner R. In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling. Cells. 2023; 12(18):2291. https://doi.org/10.3390/cells12182291
Chicago/Turabian StyleBagnoud, Maud, Jana Remlinger, Marine Massy, Dmitri Lodygin, Anke Salmen, Andrew Chan, Fred Lühder, and Robert Hoepner. 2023. "In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling" Cells 12, no. 18: 2291. https://doi.org/10.3390/cells12182291
APA StyleBagnoud, M., Remlinger, J., Massy, M., Lodygin, D., Salmen, A., Chan, A., Lühder, F., & Hoepner, R. (2023). In Vivo and In Vitro Evidence for an Interplay between the Glucocorticoid Receptor and the Vitamin D Receptor Signaling. Cells, 12(18), 2291. https://doi.org/10.3390/cells12182291






