Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder
Abstract
1. Introduction
2. Materials and Methods
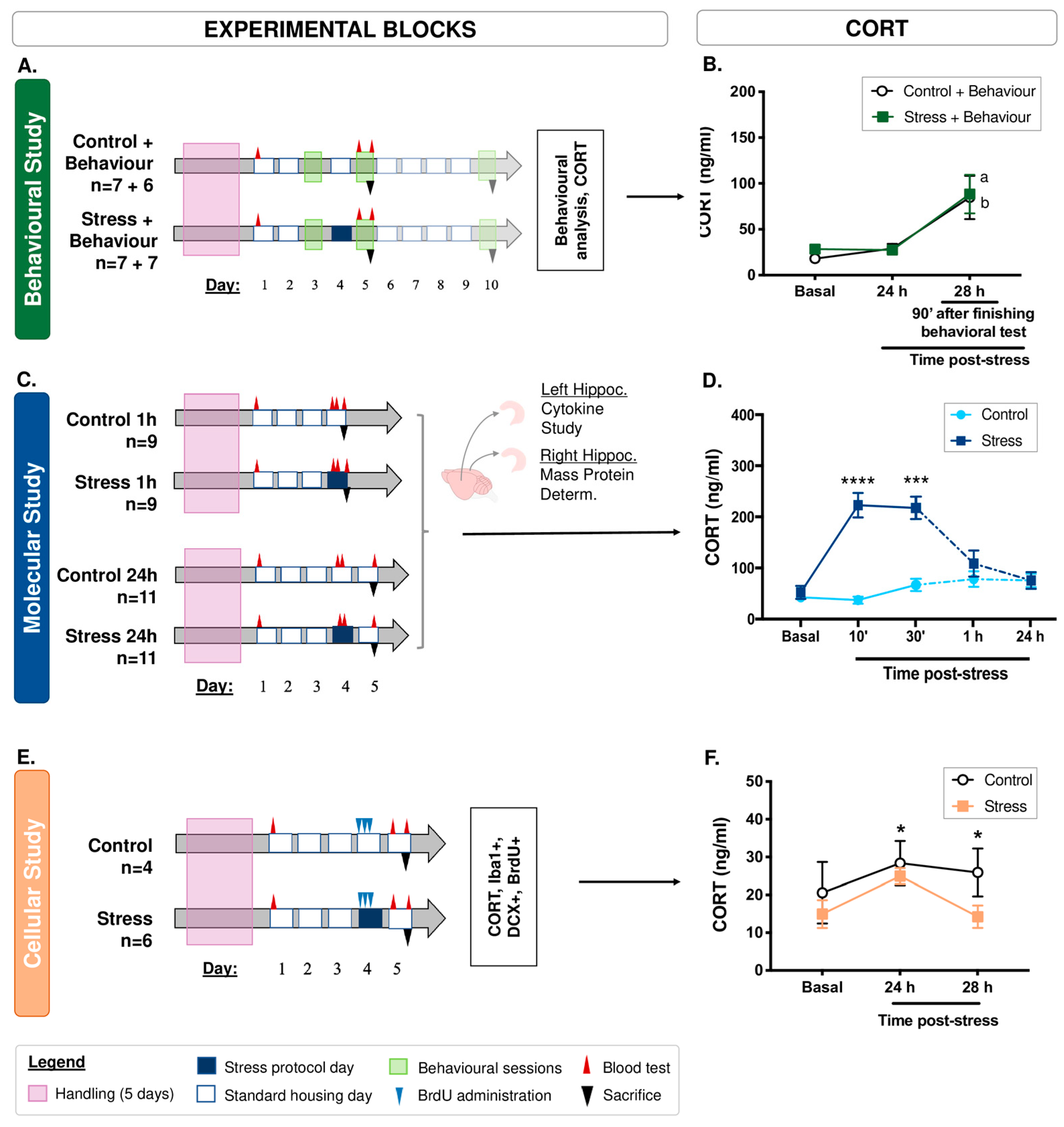
2.1. Animals and General Procedure
2.2. Stress Procedure
2.3. Corticosterone Measurement
2.4. Behavioural Testing
2.4.1. Open Field Test (OFT)
2.4.2. Saccharin Preference Test (SPT)
2.4.3. Elevated Plus Maze (EPM)
2.4.4. Tail Suspension Test (TST)
2.5. Hippocampal Molecular Analysis
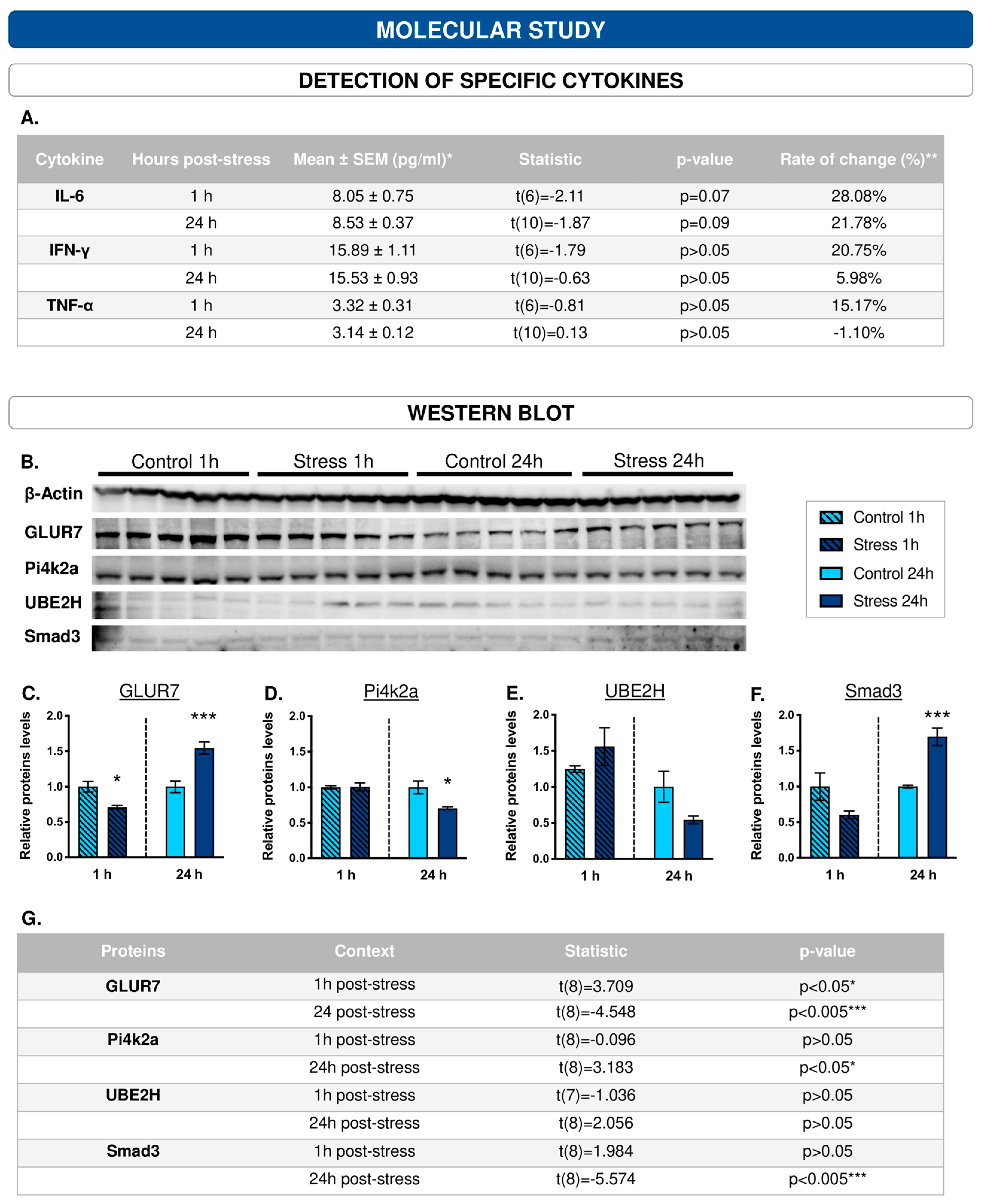
2.5.1. Cytokine Measurement
2.5.2. Mass Protein Determination: Hippocampal Protein Profile
2.5.3. Western Blotting
2.6. Histology
2.6.1. Immunolabelling
2.6.2. Morphological Analysis of Iba1+ and DCX+ Cells and Cell Count
2.7. Statistical Analysis
Principal Components Analysis (PCA)
3. Results
3.1. CORT Analyses
3.2. Basal Locomotor and Exploratory Activity Measured by OFT
3.3. WIRS Causes Anhedonic Behavior
3.4. WIRS Did Not Affect the Parameters Assessed by the EPM
3.5. Application of WIRS-Type Acute Stress Does Not Have Negative Consequences on Passive Stress-Coping Behaviour
3.6. WIRS Increased Anhedonic Behaviour and Some Parameters Related to Discomfort and Anxiety
3.7. Among the Cytokines Studied, IL-6 Appears to Be the Most Sensitive to the Effects of ‘Stress’
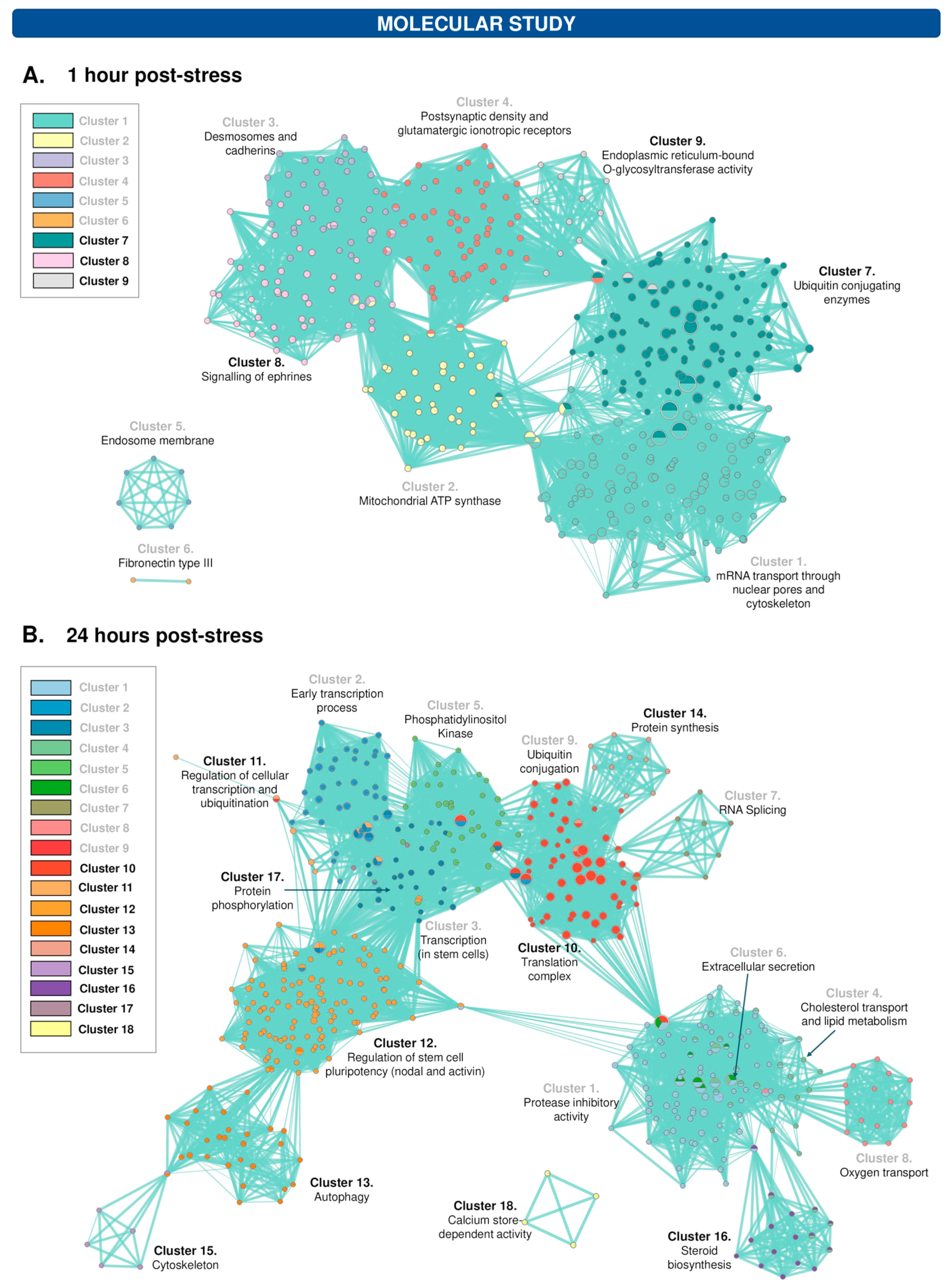
3.8. The Protein Profile of the Hippocampus Varies after 1 h and 24 h of WIRS Exposure
3.9. The Microglia of the Dentate Gyrus Responded with Morphological Changes after Exposure to WIRS
3.10. WIRS Decreased the Number of DCX+ Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Dunlop, B.W.; Kaye, J.L.; Youngner, C.; Rothbaum, B. Assessing Treatment-Resistant Posttraumatic Stress Disorder: The Emory Treatment Resistance Interview for PTSD (E-TRIP). Behav. Sci. 2014, 4, 511–527. [Google Scholar] [CrossRef]
- Rytwinski, N.K.; Scur, M.D.; Feeny, N.C.; Youngstrom, E.A. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: A meta-analysis. J. Trauma. Stress 2013, 26, 299–309. [Google Scholar] [CrossRef]
- Tipps, M.E.; Raybuck, J.D.; Lattal, K.M. Substance abuse, memory, and post-traumatic stress disorder. Neurobiol. Learn. Mem. 2014, 112, 87–100. [Google Scholar] [CrossRef]
- Staniaszek, K.; Cyniak-Cieciura, M.; Zawadzki, B. Posttraumatic stress disorder symptom profiles—The role of temperament, traumatization, and cognitive factors. Pers. Individ. Differ. 2022, 193, 111595. [Google Scholar] [CrossRef]
- Goldstein, R.B.; Smith, S.M.; Chou, S.P.; Saha, T.D.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Huang, B.; Grant, B.F. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 1137–1148. [Google Scholar] [CrossRef]
- Koenen, K.C.; Ratanatharathorn, A.; Ng, L.; McLaughlin, K.A.; Bromet, E.J.; Stein, D.J.; Karam, E.G.; Meron Ruscio, A.; Benjet, C.; Scott, K.; et al. Posttraumatic stress disorder in the World Mental Health Surveys. Psychol. Med. 2017, 47, 2260–2274. [Google Scholar] [CrossRef]
- Golitaleb, M.; Mazaheri, E.; Bonyadi, M.; Sahebi, A. Prevalence of Post-traumatic Stress Disorder After Flood: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 890671. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Stork, O.; Schmidt, M.V. Animal models of PTSD: A challenge to be met. Mol. Psychiatry 2019, 24, 1135–1156. [Google Scholar] [CrossRef]
- Zheng, Y.; Garrett, M.E.; Sun, D.; Clarke-Rubright, E.K.; Haswell, C.C.; Maihofer, A.X.; Elman, J.A.; Franz, C.E.; Lyons, M.J.; Kremen, W.S.; et al. Trauma and posttraumatic stress disorder modulate polygenic predictors of hippocampal and amygdala volume. Transl. Psychiatry 2021, 11, 637. [Google Scholar] [CrossRef]
- Conrad, C.D. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008, 19, 395–411. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar]
- Kuan, P.F.; Clouston, S.; Yang, X.; Kotov, R.; Bromet, E.; Luft, B.J. Molecular linkage between post-traumatic stress disorder and cognitive impairment: A targeted proteomics study of World Trade Center responders. Transl. Psychiatry 2020, 10, 269. [Google Scholar] [CrossRef] [PubMed]
- Catlow, B.J.; Song, S.; Paredes, D.A.; Kirstein, C.L.; Sanchez-Ramos, J. Effects of psilocybin on hippocampal neurogenesis and extinction of trace fear conditioning. Exp. Brain Res. 2013, 228, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Malberg, J.E. Implications of adult hippocampal neurogenesis in antidepressant action. J. Psychiatry Neurosci. 2004, 29, 196–205. [Google Scholar]
- Sherin, J.E.; Nemeroff, C.B. Post-traumatic stress disorder: The neurobiological impact of psychological trauma. Dialogues Clin. Neurosci. 2011, 13, 263–278. [Google Scholar] [CrossRef]
- Carmi, L.; Fostick, L.; Burshtein, S.; Cwikel-Hamzany, S.; Zohar, J. PTSD treatment in light of DSM-5 and the “golden hours” concept. CNS Spectr. 2016, 21, 279–282. [Google Scholar] [CrossRef]
- Adamec, R.; Burton, P.; Blundell, J.; Murphy, D.L.; Holmes, A. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav. Brain Res. 2006, 170, 126–140. [Google Scholar] [CrossRef]
- Miyata, S.; Koyama, Y.; Takemoto, K.; Yoshikawa, K.; Ishikawa, T.; Taniguchi, M.; Inoue, K.; Aoki, M.; Hori, O.; Katayama, T.; et al. Plasma corticosterone activates SGK1 and induces morphological changes in oligodendrocytes in corpus callosum. PLoS ONE 2011, 6, e19859. [Google Scholar] [CrossRef] [PubMed]
- Ohgidani, M.; Kato, T.A.; Sagata, N.; Hayakawa, K.; Shimokawa, N.; Sato-Kasai, M.; Kanba, S. TNF-α from hippocampal microglia induces working memory deficits by acute stress in mice. Brain Behav. Immun. 2016, 55, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernández, R.D.; Nieto-Quero, A.; Gómez-Salas, F.J.; Chun, J.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pérez-Martín, M.; Pedraza, C. Effects of genetic deletion versus pharmacological blockade of the LPA1 receptor on depression like behaviour and related brain functional activity. Dis. Models Mech. 2018, 11, dmm035519. [Google Scholar] [CrossRef]
- Moreno-Fernández, R.D.; Rosell-Valle, C.; Bacq, A.; Zanoletti, O.; Cifuentes, M.; Pérez-Martín, M.; Gavito, A.L.; García-Fernández, M.I.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; et al. LPA1 receptor and chronic stress: Effects on behaviour and the genes involved in the hippocampal excitatory/inhibitory balance. Neuropharmacology 2020, 164, 107896. [Google Scholar] [CrossRef]
- Tabbai, S.; Moreno-Fernández, R.D.; Zambrana-Infantes, E.; Nieto-Quero, A.; Chun, J.; García-Fernández, M.I.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Oliveira, T.G.; et al. Effects of the LPA1 Receptor Deficiency and Stress on the Hippocampal LPA Species in Mice. Front. Mol. Neurosci. 2019, 12, 146. [Google Scholar] [CrossRef]
- Moreno-Fernández, R.D.; Pérez-Martín, M.; Castilla-Ortega, E.; Rosell Del Valle, C.; García-Fernández, M.I.; Chun, J.; Estivill-Torrús, G.; Rodríguez de Fonseca, F.; Santín, L.J.; Pedraza, C. maLPA1-null mice as an endophenotype of anxious depression. Transl. Psychiatry 2017, 7, e1077. [Google Scholar] [CrossRef] [PubMed]
- Harkin, A.; Houlihan, D.D.; Kelly, J.P. Reduction in preference for saccharin by repeated unpredictable stress in mice and its prevention by imipramine. J. Psychopharmacol. 2002, 16, 115–123. [Google Scholar] [CrossRef]
- Sclafani, A. Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: Experience attenuates strain differences. Physiol. Behav. 2007, 90, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J.; Matar, M.A.; Zeev, K.; Loewenthal, U.; Richter-Levin, G. Setting apart the affected: The use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacology 2004, 29, 1962–1970. [Google Scholar] [CrossRef]
- Moore, N.L.; Gauchan, S.; Genovese, R.F. Differential severity of anxiogenic effects resulting from a brief swim or underwater trauma in adolescent male rats. Pharmacol. Biochem. Behav. 2012, 102, 264–268. [Google Scholar] [CrossRef]
- Richter-Levin, G. Acute and long-term behavioral correlates of underwater trauma--potential relevance to stress and post-stress syndromes. Psychiatry Res. 1998, 79, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.B.; Jaffee, M.S.; Jorge, R.E. Posttraumatic Stress Disorder and Anxiety-Related Conditions. Behav. Neurol. Psychiatry 2021, 27, 1738–1763. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.Z.; Li, X.; Chen, Z.; Benedek, D.M.; Fullerton, C.S.; Wynn, G.; Biomarker Team; Ursano, R.J. Potential chemokine biomarkers associated with PTSD onset, risk and resilience as well as stress responses in US military service members. Transl. Psychiatry 2020, 10, 31. [Google Scholar] [CrossRef]
- Dozio, V.; Sanchez, J.C. Profiling the proteomic inflammatory state of human astrocytes using DIA mass spectrometry. J. Neuroinflamm. 2018, 15, 331. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; García-Diaz, B.; Llebrez-Zayas, P.; López-Barroso, D.; Santín, L.; Pedraza, C.; Smith-Fernández, A.; Fernández-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Arrabal, S.; Cifuentes, M.; Grondona, J.M.; Pérez-Martín, M.; Rubio, L.; Vargas, A.; Serrano, A.; Pavón, F.J.; Suárez, J.; et al. Localization of the cannabinoid CB1 receptor and the 2-AG synthesizing (DAGLα) and degrading (MAGL, FAAH) enzymes in cells expressing the Ca(2+)-binding proteins calbindin, calretinin, and parvalbumin in the adult rat hippocampus. Front. Neuroanat. 2014, 8, 56. [Google Scholar] [CrossRef]
- Davis, B.M.; Salinas-Navarro, M.; Cordeiro, M.F.; Moons, L.; De Groef, L. Characterizing microglia activation: A spatial statistics approach to maximize information extraction. Sci. Rep. 2017, 7, 1576. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Balaban, C.D.; Ogburn, S.W.; Warshafsky, S.G.; Ahmed, A.; Yates, B.J. Identification of neural networks that contribute to motion sickness through principal components analysis of fos labeling induced by galvanic vestibular stimulation. PLoS ONE 2014, 9, e86730. [Google Scholar] [CrossRef]
- Sanchez, K.; Darling, J.S.; Kakkar, R.; Wu, S.L.; Zentay, A.; Lowry, C.A.; Fonken, L.K. Mycobacterium vaccae immunization in rats ameliorates features of age-associated microglia activation in the amygdala and hippocampus. Sci. Rep. 2022, 12, 2165. [Google Scholar] [CrossRef]
- Hodge, R.D.; Kowalczyk, T.D.; Wolf, S.A.; Encinas, J.M.; Rippey, C.; Enikolopov, G.; Kempermann, G.; Hevner, R.F. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 2008, 28, 3707–3717. [Google Scholar] [CrossRef]
- Mann, S.K.; Marwaha, R. Posttraumatic Stress Disorder; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Torrisi, S.A.; Leggio, G.M.; Drago, F.; Salomone, S. Therapeutic Challenges of Post-traumatic Stress Disorder: Focus on the Dopaminergic System. Front. Pharmacol. 2019, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Logue, M.W.; van Rooij, S.J.H.; Dennis, E.L.; Davis, S.L.; Hayes, J.P.; Stevens, J.S.; Densmore, M.; Haswell, C.C.; Ipser, J.; Koch, S.B.J.; et al. Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol. Psychiatry 2018, 83, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Adamec, R.; Head, D.; Blundell, J.; Burton, P.; Berton, O. Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol. Behav. 2006, 8, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Holly, E.N.; Miczek, K.A. Capturing Individual Differences: Challenges in Animal Models of Posttraumatic Stress Disorder and Drug Abuse. Biol. Psychiatry 2015, 78, 816–818. [Google Scholar] [CrossRef][Green Version]
- Vinograd, M.; Stout, D.M.; Risbrough, V.B. Anhedonia in Posttraumatic Stress Disorder: Prevalence, Phenotypes, and Neural Circuitry. Curr. Top. Behav. Neurosci. 2022, 58, 185–199. [Google Scholar] [CrossRef]
- Feeny, N.C.; Zoellner, L.A.; Fitzgibbons, L.A.; Foa, E.B. Exploring the roles of emotional numbing, depression, and dissociation in PTSD. J. Trauma. Stress 2000, 13, 489–498. [Google Scholar] [CrossRef]
- Boscarino, J.A. Posttraumatic stress disorder and physical illness: Results from clinical and epidemiologic studies. Ann. N. Y. Acad. Sci. 2004, 1032, 141–153. [Google Scholar] [CrossRef]
- Dai, W.; Kaminga, A.C.; Tan, H.; Wang, J.; Lai, Z.; Wu, X.; Xiong, Y.; Deng, J.; Liu, A. Comorbidity of post-traumatic stress disorder and anxiety in flood survivors: Prevalence and shared risk factors. Medicine 2017, 96, e7994. [Google Scholar] [CrossRef]
- Zatzick, D.F.; Jurkovich, G.J.; Gentilello, L.; Wisner, D.; Rivara, F.P. Posttraumatic stress, problem drinking, and functional outcomes after injury. Arch. Surg. 2002, 137, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Lee, H. Systemic Administration of Curcumin Affect Anxiety-Related Behaviors in a Rat Model of Posttraumatic Stress Disorder via Activation of Serotonergic Systems. Evid. Based Complement. Alternat. Med. 2018, 2018, 9041309. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Matar, M.A.; Vainer, E.; Zohar, J.; Kaplan, Z.; Cohen, H. Significance of the orexinergic system in modulating stress-related responses in an animal model of post-traumatic stress disorder. Transl. Psychiatry 2020, 10, 10. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wei, C.; Wang, H.; Sui, N.; Kirouac, G.J. Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol. Biochem. Behav. 2010, 95, 121–128. [Google Scholar] [CrossRef]
- Almeida, F.B.; Pinna, G.; Barros, H.M.T. The Role of HPA Axis and Allopregnanolone on the Neurobiology of Major Depressive Disorders and PTSD. Int. J. Mol. Sci. 2021, 22, 5495. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Moussa, F.; Moustafa, A.; Ayoub, D.R. Cortisol level in depressed patients and its relation with suicidal risk and anhedonia. Egypt. J. Neurol. Psychiatr. Neurosurg. 2016, 53, 193–199. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Wong, A. The hypothalamic-pituitary-adrenal axis in PTSD: Pathophysiology and treatment interventions. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 89, 361–379. [Google Scholar] [CrossRef]
- Aerni, A.; Traber, R.; Hock, C.; Roozendaal, B.; Schelling, G.; Papassotiropoulos, A.; Nitsch, R.M.; Schnyder, U.; de Quervain, D.J. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am. J. Psychiatry 2004, 161, 1488–1490. [Google Scholar] [CrossRef]
- Zohar, J.; Juven-Wetzler, A.; Sonnino, R.; Cwikel-Hamzany, S.; Balaban, E.; Cohen, H. New insights into secondary prevention in post-traumatic stress disorder. Dialogues Clin. Neurosci. 2011, 13, 301–309. [Google Scholar] [CrossRef]
- Li, Y.P.; Mikrani, R.; Hu, Y.F.; Faran Ashraf Baig, M.M.; Abbas, M.; Akhtar, F.; Xu, M. Research progress of phosphatidylinositol 4-kinase and its inhibitors in inflammatory diseases. Eur. J. Pharmacol. 2021, 907, 174300. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.E.; Girgenti, M.J.; Davis, M.T.; Pietrzak, R.H.; DellaGioia, N.; Nabulsi, N.; Matuskey, D.; Southwick, S.; Duman, R.S.; Carson, R.E.; et al. Traumatic Stress Brain Study Group. Altered metabotropic glutamate receptor 5 markers in PTSD: In vivo and postmortem evidence. Proc. Natl. Acad. Sci. USA 2017, 114, 8390–8395. [Google Scholar] [CrossRef]
- Schiffer, H.H.; Heinemann, S.F. Association of the human kainate receptor GluR7 gene (GRIK3) with recurrent major depressive disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007, 144B, 20–26. [Google Scholar] [CrossRef]
- Xu, W.; Yao, X.; Zhao, F.; Zhao, H.; Cheng, Z.; Yang, W.; Cui, R.; Xu, S.; Li, B. Changes in Hippocampal Plasticity in Depression and Therapeutic Approaches Influencing These Changes. Neural Plast. 2020, 2020, 8861903. [Google Scholar] [CrossRef] [PubMed]
- Höflich, A.; Michenthaler, P.; Kasper, S.; Lanzenberger, R. Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 2019, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, L.P.; Qin, X.H.; Li, S.J.; Zhang, M.; Wang, Q.; Hu, H.H.; Fang, Y.Y.; Gao, Y.B.; Li, X.W.; et al. Astrocytic adenosine 5’-triphosphate release regulates the proliferation of neural stem cells in the adult hippocampus. Stem Cells 2013, 31, 1633–1643. [Google Scholar] [CrossRef]
- Compagnucci, C.; Piemonte, F.; Sferra, A.; Piermarini, E.; Bertini, E. The cytoskeletal arrangements necessary to neurogenesis. Oncotarget 2016, 7, 19414–19429. [Google Scholar] [CrossRef]
- Espósito, M.S.; Piatt, V.C.; Laplagne, D.A.; Morgenstern, N.A.; Ferrari, C.C.; Pitossi, F.J.; Schinder, A.F. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J. Neurosci. 2005, 25, 10074–10086. [Google Scholar] [CrossRef]
- Fournier, N.M.; Lee, B.; Banasr, M.; Elsayed, M.; Duman, R.S. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology 2012, 63, 642–652. [Google Scholar] [CrossRef]
- Leeson, H.C.; Kasherman, M.A.; Chan-Ling, T.; Lovelace, M.D.; Brownlie, J.C.; Toppinen, K.M.; Gu, B.J.; Weible, M.W. P2X7 Receptors Regulate Phagocytosis and Proliferation in Adult Hippocampal and SVZ Neural Progenitor Cells: Implications for Inflammation in Neurogenesis. Stem Cells 2018, 36, 1764–1777. [Google Scholar] [CrossRef] [PubMed]
- Schütt, F.; Aretz, S.; Auffarth, G.U.; Kopitz, J. Moderately reduced ATP levels promote oxidative stress and debilitate autophagic and phagocytic capacities in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5354–5361. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Zabala, A.; Sierra-Torre, V.; Sierra, A. Assessing Autophagy in Microglia: A Two-Step Model to Determine Autophagosome Formation, Degradation, and Net Turnover. Front. Immunol. 2021, 11, 620602. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Van Eldik, L.J.; Schmitt, F.A.; Neltner, J.H.; Ighodaro, E.T.; Webster, S.J.; Patel, E.; Abner, E.L.; Kryscio, R.J.; Nelson, P.T. Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 2015, 3, 32. [Google Scholar] [CrossRef]
- Fernández-Arjona, M.D.M.; Grondona, J.M.; Granados-Durán, P.; Fernández-Llebrez, P.; López-Ávalos, M.D. Microglia Morphological Categorization in a Rat Model of Neuroinflammation by Hierarchical Cluster and Principal Components Analysis. Front. Cell. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef]
- Liu, L.; Kearns, K.N.; Eli, I.; Sharifi, K.A.; Soldozy, S.; Carlson, E.W.; Scott, K.W.; Sluzewski, M.F.; Acton, S.T.; Stauderman, K.A.; et al. Microglial Calcium Waves During the Hyperacute Phase of Ischemic Stroke. Stroke 2021, 52, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Naseer, S.; Abelleira-Hervas, L.; Savani, D.; de Burgh, R.; Aleksynas, R.; Donat, C.K.; Syed, N.; Sastre, M. Traumatic Brain Injury Leads to Alterations in Contusional Cortical miRNAs Involved in Dementia. Biomolecules 2022, 12, 1457. [Google Scholar] [CrossRef]
- Walker, F.R.; Nilsson, M.; Jones, K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets 2013, 14, 1262–1276. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Qiao, Y.; Lo, P.C.; Song, L.; Yang, Y.; Duan, L.; Wei, S.; Li, M.; Huang, S.; Zhang, B.; et al. Anti-depressive effects of Jiao-Tai-Wan on CORT-induced depression in mice by inhibiting inflammation and microglia activation. J. Ethnopharmacol. 2022, 283, 114717. [Google Scholar] [CrossRef]
- Picard, K.; St-Pierre, M.K.; Vecchiarelli, H.A.; Bordeleau, M.; Tremblay, M.È. Neuroendocrine, neuroinflammatory and pathological outcomes of chronic stress: A story of microglial remodeling. Neurochem. Int. 2021, 145, 104987. [Google Scholar] [CrossRef]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Surget, A.; Belzung, C. Adult hippocampal neurogenesis shapes adaptation and improves stress response: A mechanistic and integrative perspective. Mol. Psychiatry 2022, 27, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Conrad, C.D.; Wright, R.L.; McLaughlin, K.J. Stress and vulnerability to brain Damage. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier Ltd.: London, UK, 2009; pp. 481–488. [Google Scholar] [CrossRef]
- Kanatsou, S.; Fearey, B.C.; Kuil, L.E.; Lucassen, P.J.; Harris, A.P.; Seckl, J.R.; Krugers, H.; Joels, M. Overexpression of Mineralocorticoid Receptors Partially Prevents Chronic Stress-Induced Reductions in Hippocampal Memory and Structural Plasticity. PLoS ONE 2015, 10, e0142012. [Google Scholar] [CrossRef]
- Levone, B.R.; Cryan, J.F.; O’Leary, O.F. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol. Stress 2014, 1, 147–155. [Google Scholar] [CrossRef]
- Ishikawa, R.; Uchida, C.; Kitaoka, S.; Furuyashiki, T.; Kida, S. Improvement of PTSD-like behavior by the forgetting effect of hippocampal neurogenesis enhancer memantine in a social defeat stress paradigm. Mol. Brain 2019, 12, 68. [Google Scholar] [CrossRef] [PubMed]


| Block | Stress (WIRS) | CORT (ELISA) | Behavioural Test (OFT, SPT, EPM, TST) | Molecular (Cytokines and Proteomic Profiles) | Cellular (Iba1+, BrdU+, DCX+) |
|---|---|---|---|---|---|
| I | + | + | + | − | − |
| II | + | + | − | + | - |
| III | + | + | − | − | + |
| Study | Context | Statistic | p-Value |
|---|---|---|---|
| Behavioural | 90 min after finishing behavioural procedures in both groups | Repeated-measures ANOVA: F(2, 26) = 14.30 | p < 0.0005 LSD: p < 0.005 |
| Molecular | 10 min post-stress 30 min post-stress 1 h post-stress 24 h post-stress | t(33) = −6.51 t(32) = −3.87 t(13) = −1.05 t(19) = −0.003 | p < 0.0005 p < 0.005 p > 0.05 p > 0.05 |
| Histological | 24 and 28 h post-stress | Repeated-measures ANOVA (for the environmental treatment factor): F(1, 4) = 11.83 | p < 0.05 LSD: p < 0.05 |
| Behavioural Test | Variable | Statistic | p-Value |
|---|---|---|---|
| OFT | Distance: periphery centre total Time: periphery centre Velocity | t(26) = −0.27 t(26) = −0.09 t(26) = −0.27 t(26) = −0.56 t(26) = −0.40 t(26) = −0.15 | p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 |
| SPT | 24 h post-stress | Repeated-measures ANOVA: F(1, 26) = 14.54 | p < 0.005 LSD: p < 0.0005 |
| 6 days after the end of the stressor | Repeated-measures ANOVA performed on the additional F(2, 22) = 5.94 | p < 0.01 LSD: p < 0.005 | |
| EPM | Anxiety index Time: centre open arms closed arms Frequency entry: centre open arms closed arms Time/frequency (open arms) | t(26) = 0.88 t(26) = 0.91 t(26) = −0.42 t(26) = 0.91 t(26) = −0.52 t(26) = −0.96 t(26) = −0.18 t(26) = 0.06 | p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p > 0.05 |
| TST | Immobility Energy PM | t(26) = 1.58 t(26) = −0.28 t(26) = −0.17 | p > 0.05 p > 0.05 p > 0.05 |
| Type of Expression | Cluster | Information * |
|---|---|---|
| Underexpression | Cluster 1 | Transport of messenger RNA (mRNA) through nuclear pores (40.28) Kinetochore, chromosome segregation and centromere (33.29) Nuclear membrane (9.23) |
| Cluster 2 | Mitochondrial ATP synthase complex (22.53) Ion transport, Huntington’s disease, Alzheimer’s and Parkinson’s (19.03) ATP synthesis (14.29) Myelin sheath (2.64) | |
| Cluster 3 | Desmosomes (10.7 y 6.13) Cadherins and calcium binding (7.71) Regulation of cell adhesion involved in cardiac muscle contraction (6.38) | |
| Cluster 4 | Glutamatergic ionotropic receptor and postsynaptic density (7.52) Glutamatergic synapses (5.98) Membrane-associated guanylate kinase (4.01) | |
| Cluster 5 | Endosome membrane (2.09) | |
| Cluster 6 | Fibronectin type III (1.86) | |
| Overexpression | Cluster 7 | Ubiquitin-conjugating enzymes (30.4) HECT domain, ubiquitin-binding enzyme-related domain (9.79) |
| Cluster 8 | Ephrines signalling (12.08) Ras and PI3K/Akt signalling (6.15) Cell differentiation and neurogenesis (3.27) Cell migration and angiogenesis (3.13) Development of dendritic spines, VEGFR and semaphorins (2.53) | |
| Cluster 9 | Endoplasmic reticulum-bound O-glycosyltransferase activity (3.38) |
| Type of Expression | Cluster | Information * |
|---|---|---|
| Underexpression | Cluster 1 | Protease inhibitor (7.73) Blood coagulation and haemostasis (6.23) Golgi Complex and ER (4.87) Cholesterol metabolism (4.64) |
| Cluster 2 | Early transcription process (35.19 and 10.6) | |
| Cluster 3 | Gene transcription (13.4) Gene transcription related to stem cell population maintenance (4.2) Exonuclease activity (3.97) Alternative splicing (3.87) | |
| Cluster 4 | Cholesterol transport (6.83) Lipidic metabolism (4.22) Lipoprotein metabolism (3.9) Lipid transport (3.81) Disulfide bonding and glycosylation (3.54) Extracellular región (3.42) Lipid homeostasis (2.58) | |
| Cluster 5 | Phosphorylation (7.56) Phosphatidylinositol Kinase (PIPK) (5.47) PI3K activity (4.76) Synthesis of phosphatidylinositols in the Golgi membrane (4.08) | |
| Cluster 6 | Extracellular secretion (2.91) | |
| Cluster 7 | RNA splicing (2.52) | |
| Cluster 8 | Oxygen transport (4.98) | |
| Cluster 9 | Ubiquitin conjugation (1.8) | |
| Overexpression | Cluster 10 | Translation initiation complex (70.1) Initiation of protein synthesis (27.85) Ribosomal proteins (27.51) Proteasome initiation complex (4.77) RNA binding to proteins (0.99) |
| Cluster 11 | Regulation of cellular transcription and ubiquitination (8.99) Endosomal transport (2.12) Cytoplasmic vesicle (1.91) | |
| Cluster 12 | Signalling pathway regulating stem cell pluripotency (nodal and activin) (13.92) TGF-β signalling (9.61 and 4.53) | |
| Cluster 13 | Autophagy (5.13) Autophagy regulation (4.81) | |
| Cluster 14 | Protein synthesis (2.97) | |
| Cluster 15 | Protein binding and cytoskeleton, in general (3.27) | |
| Cluster 16 | Steroid biosynthesis (3.91) | |
| Cluster 17 | Protein phosphorylation (1.62) | |
| Cluster 18 | Calcium store-dependent activity (2.47) |
| Study | Variable | Statistic | p-Value |
|---|---|---|---|
| Iba1+ Cells | Area | t(8) = 1.19 | p < 0.005 |
| Perimeter | t(8) = 1.16 | p < 0.005 | |
| Circularity | t(8) = −0.89 | p < 0.0005 | |
| Roundness | t(8) = −0.52 | p < 0.05 | |
| N° Iba1+/um2 | t(8) = 0.18 | p < 0.0005 | |
| Distance | t(8) = −0.84 | p < 0.05 | |
| RI | t(8) = 0.64 | p > 0.05 | |
| DCX+ Cells | Total DG | t(7) = 2.75 | p < 0.05 |
| Type A | t(7) = 3.06 | p < 0.05 | |
| Type B | t(7) = 0.27 | p > 0.05 | |
| Type C | t(7) = 1.05 | p > 0.05 | |
| BrdU/DCX+ Cells | t(4) = 3.59 | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieto-Quero, A.; Infantes-López, M.I.; Zambrana-Infantes, E.; Chaves-Peña, P.; Gavito, A.L.; Munoz-Martin, J.; Tabbai, S.; Márquez, J.; Rodríguez de Fonseca, F.; García-Fernández, M.I.; et al. Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder. Cells 2023, 12, 2290. https://doi.org/10.3390/cells12182290
Nieto-Quero A, Infantes-López MI, Zambrana-Infantes E, Chaves-Peña P, Gavito AL, Munoz-Martin J, Tabbai S, Márquez J, Rodríguez de Fonseca F, García-Fernández MI, et al. Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder. Cells. 2023; 12(18):2290. https://doi.org/10.3390/cells12182290
Chicago/Turabian StyleNieto-Quero, Andrea, María Inmaculada Infantes-López, Emma Zambrana-Infantes, Patricia Chaves-Peña, Ana L. Gavito, Jose Munoz-Martin, Sara Tabbai, Javier Márquez, Fernando Rodríguez de Fonseca, María Inmaculada García-Fernández, and et al. 2023. "Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder" Cells 12, no. 18: 2290. https://doi.org/10.3390/cells12182290
APA StyleNieto-Quero, A., Infantes-López, M. I., Zambrana-Infantes, E., Chaves-Peña, P., Gavito, A. L., Munoz-Martin, J., Tabbai, S., Márquez, J., Rodríguez de Fonseca, F., García-Fernández, M. I., Santín, L. J., Pedraza, C., & Pérez-Martín, M. (2023). Unveiling the Secrets of the Stressed Hippocampus: Exploring Proteomic Changes and Neurobiology of Posttraumatic Stress Disorder. Cells, 12(18), 2290. https://doi.org/10.3390/cells12182290










