Sperm Concentration Improvement May Be a Parameter Predicting Efficacy of FSH Therapy of Male Idiopathic Infertility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Andrological Diagnostic Work-Up
2.3. Data Collection
2.4. Ethical
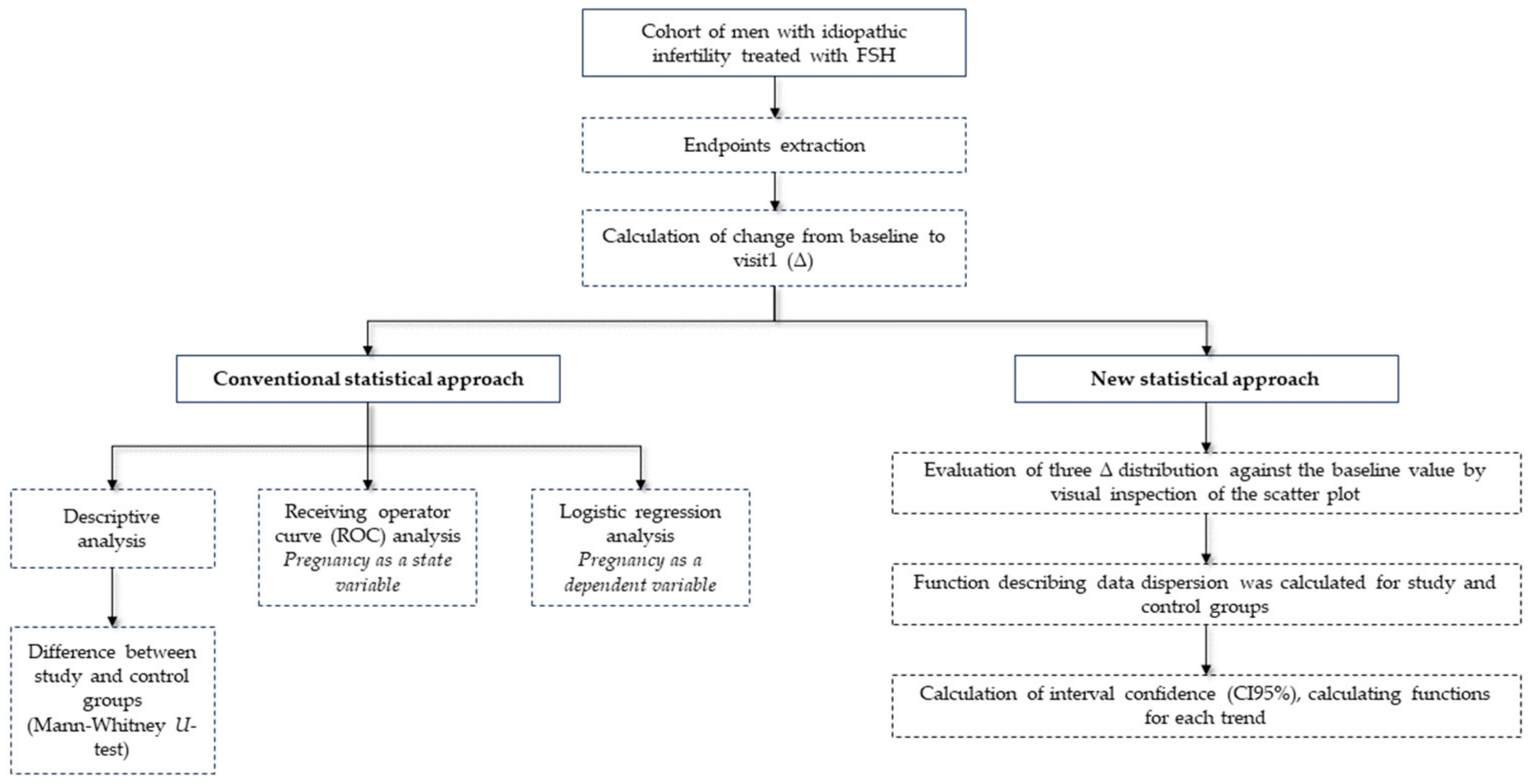
2.5. Statistical Analysis
3. Results
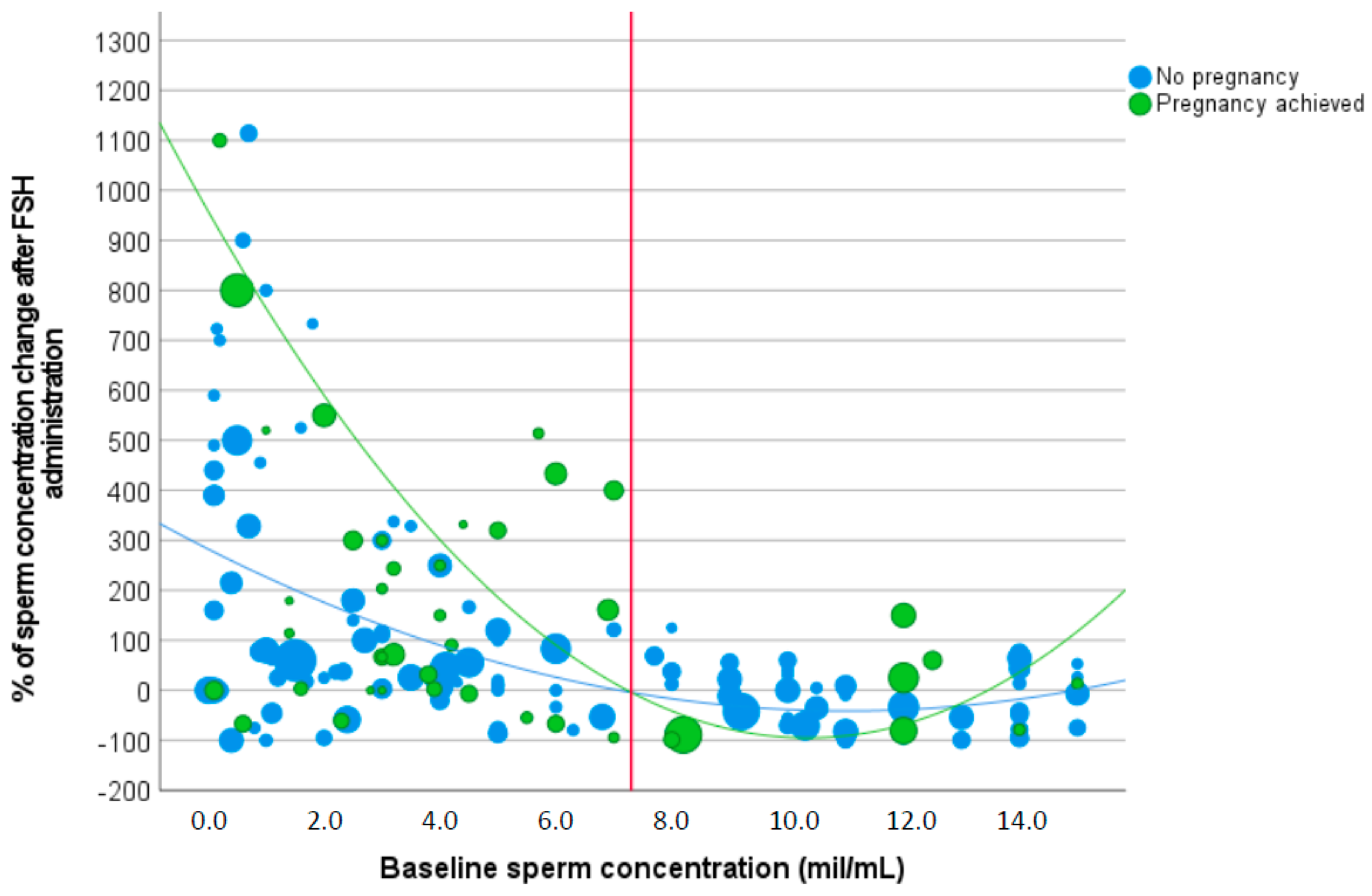
3.1. Sperm Concentration
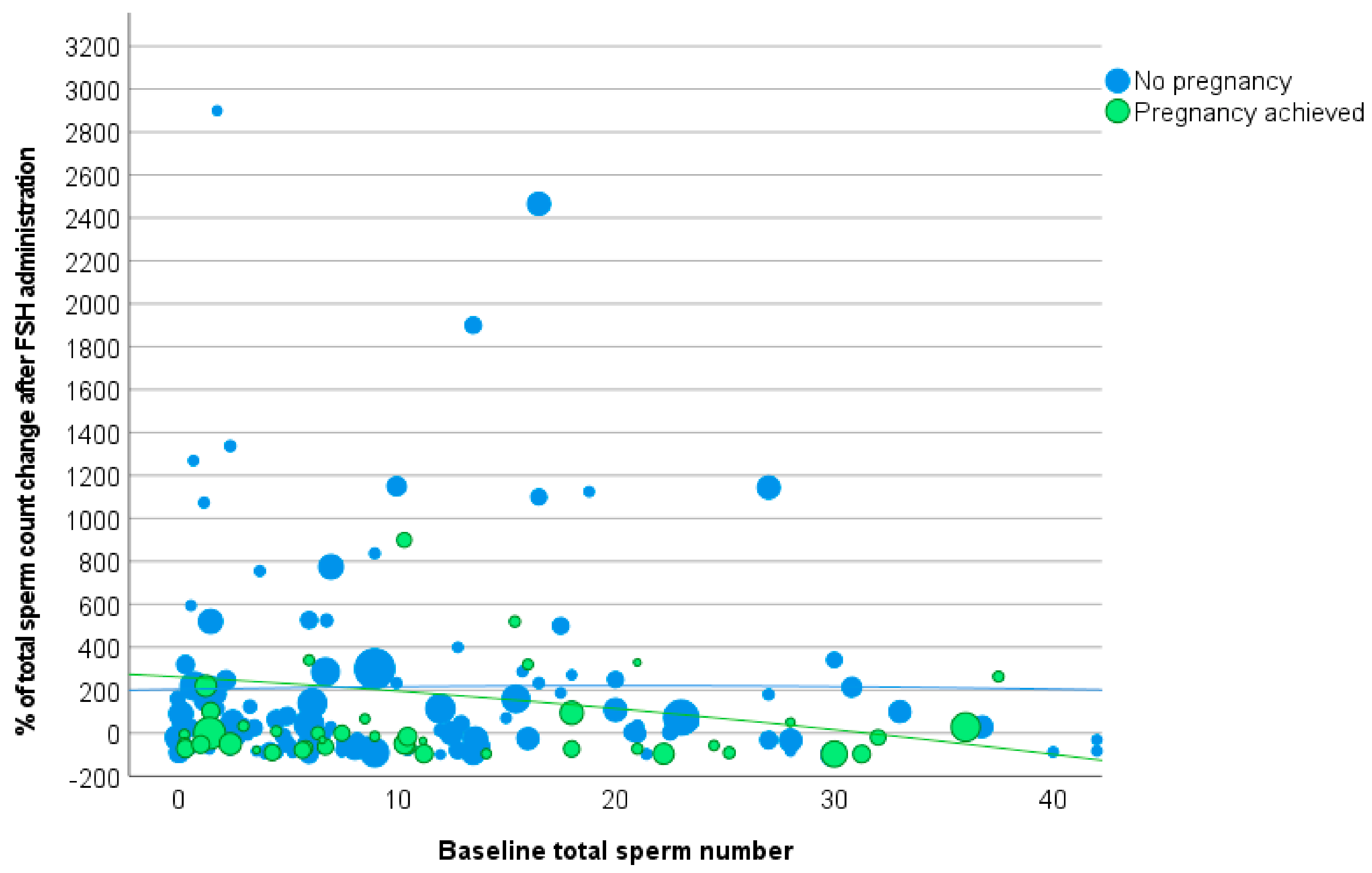
3.2. Total Sperm Count
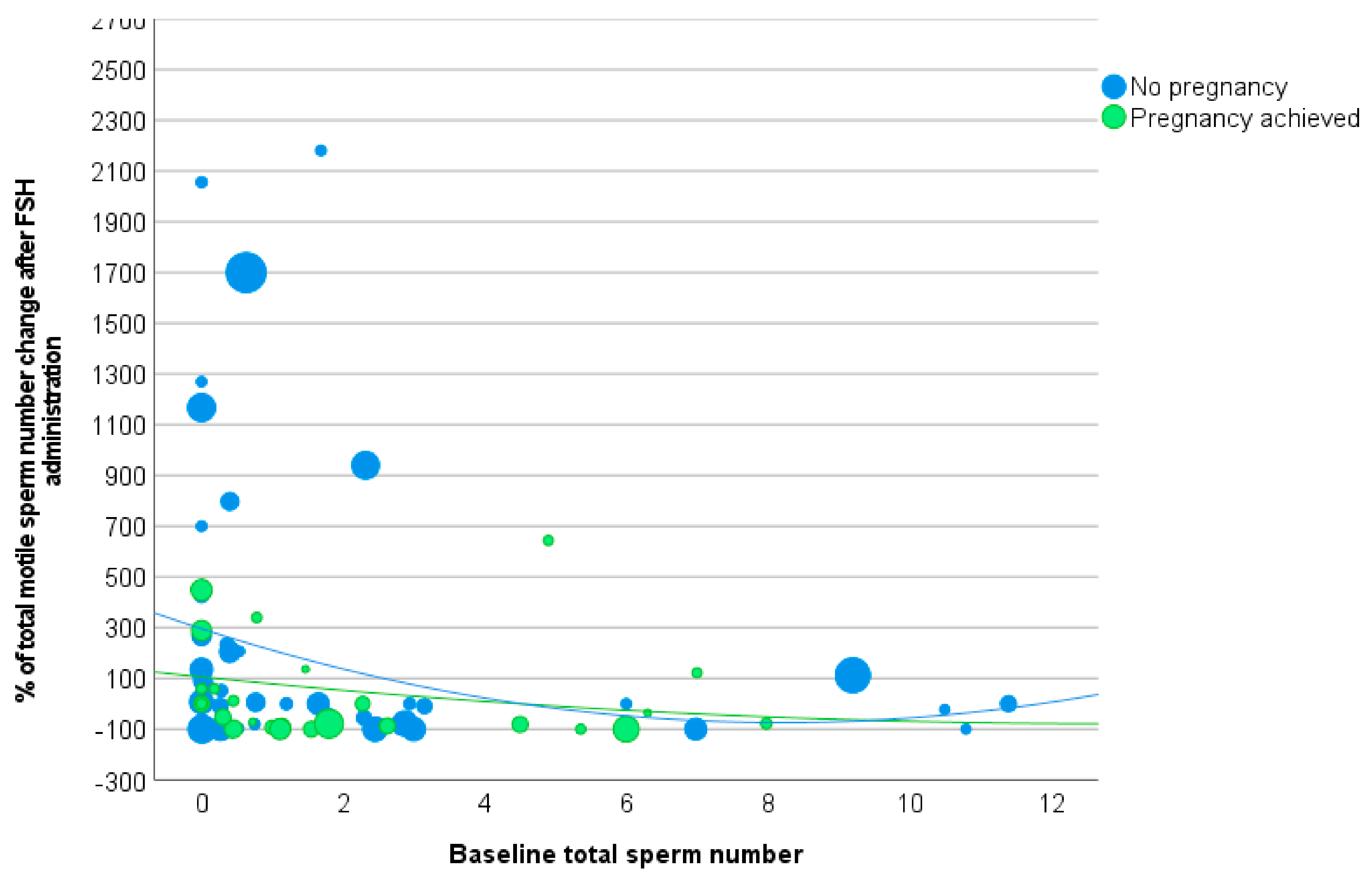
3.3. Total Motile Sperm Number
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jonge, C.; Barratt, C.L.R. The present crisis in male reproductive health: An urgent need for a political, social, and research roadmap. Andrology 2019, 7, 762–768. [Google Scholar] [CrossRef]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef]
- Rastrelli, G.; Corona, G.; Mannucci, E.; Maggi, M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: A meta-analytic study. Andrology 2014, 2, 794–808. [Google Scholar] [CrossRef]
- Santi, D.; Corona, G. Primary and secondary hypogonadism. In Endocrinology of the Testis and Male Reproduction; Simoni, M., Huhtaniemi, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, F.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41, 1107–1122. [Google Scholar] [CrossRef]
- Ashkenazi, J.; Bar-Hava, I.; Farhi, J.; Levy, T.; Feldberg, D.; Orvieto, R.; Ben-Rafael, Z. The role of purified follicle stimulating hormone therapy in the male partner before intracytoplasmic sperm injection. Fertil. Steril. 1999, 72, 670–673. [Google Scholar] [CrossRef]
- Baccetti, B.; Piomboni, P.; Bruni, E.; Capitani, S.; Gambera, L.; Moretti, E.; Sterzik, K.; Strehler, E. Effect of follicle-stimulating hormone on sperm quality and pregnancy rate. Asian J. Androl. 2004, 6, 133–137. [Google Scholar] [PubMed]
- Bartoov, B.; Eltes, F.; Lunenfeld, E.; Har-Even, D.; Lederman, H.; Lunenfeld, B. Sperm quality of subfertile males before and after treatment with human follicle-stimulating hormone. Fertil. Steril. 1994, 61, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Ben-Rafael, Z.; Farhi, J.; Feldberg, D.; Bartoov, B.; Kovo, M.; Eltes, F.; Ashkenazi, J. Follicle-stimulating hormone treatment for men with idiopathic oligoteratoasthenozoospermia before in vitro fertilization: The impact on sperm microstructure and fertilization potential. Fertil. Steril. 2000, 73, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Caroppo, E.; Niederberger, C.; Vizziello, G.M.; D’Amato, G. Recombinant human follicle-stimulating hormone as a pretreatment for idiopathic oligoasthenoteratozoospermic patients undergoing intracytoplasmic sperm injection. Fertil. Steril. 2003, 80, 1398–1403. [Google Scholar] [CrossRef]
- Colacurci, N.; Monti, M.G.; Fornaro, F.; Izzo, G.; Izzo, P.; Trotta, C.; Mele, D.; De Franciscis, P. Recombinant human FSH reduces sperm DNA fragmentation in men with idiopathic oligoasthenoteratozoospermia. J. Androl. 2012, 33, 588–593. [Google Scholar] [CrossRef]
- Ding, Y.M.; Zhang, X.J.; Li, J.P.; Chen, S.S.; Zhang, R.T.; Tan, W.L.; Shi, X.J. Treatment of idiopathic oligozoospermia with recombinant human follicle-stimulating hormone: A prospective, randomized, double-blind, placebo-controlled clinical study in Chinese population. Clin. Endocrinol. 2015, 83, 866–871. [Google Scholar] [CrossRef]
- Simoni, M.; Santi, D.; Negri, L.; Hoffmann, I.; Muratori, M.; Baldi, E.; Cambi, M.; Marcou, M.; Greither, T.; Baraldi, E.; et al. Treatment with human, recombinant FSH improves sperm DNA fragmentation in idiopathic infertile men depending on the FSH receptor polymorphism p.N680S: A pharmacogenetic study. Hum. Reprod. 2016, 31, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Kamischke, A.; Behre, H.M.; Bergmann, M.; Simoni, M.; Schafer, T.; Nieschlag, E. Recombinant human follicle stimulating hormone for treatment of male idiopathic infertility: A randomized, double-blind, placebo-controlled, clinical trial. Hum. Reprod. 1998, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Matorras, R.; Perez, C.; Corcostegui, B.; Pijoan, J.I.; Ramon, O.; Delgado, P.; Rodriguez-Escudero, F.J. Treatment of the male with follicle-stimulating hormone in intrauterine insemination with husband’s spermatozoa: A randomized study. Hum. Reprod. 1997, 12, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Paradisi, R.; Busacchi, P.; Seracchioli, R.; Porcu, E.; Venturoli, S. Effects of high doses of recombinant human follicle-stimulating hormone in the treatment of male factor infertility: Results of a pilot study. Fertil. Steril. 2006, 86, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Garolla, A.; Ambrosini, G.; Ferlin, A. Treatment of male idiopathic infertility with recombinant human follicle-stimulating hormone: A prospective, controlled, randomized clinical study. Fertil. Steril. 2005, 84, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Ferlin, A.; Rossato, M. Use of recombinant human follicle-stimulating hormone in the treatment of male factor infertility. Fertil. Steril. 2002, 77, 238–244. [Google Scholar] [CrossRef]
- Foresta, C.; Selice, R.; Moretti, A.; Pati, M.A.; Carraro, M.; Engl, B.; Garolla, A. Gonadotropin administration after gonadotropin-releasing-hormone agonist: A therapeutic option in severe testiculopathies. Fertil. Steril. 2009, 92, 1326–1332. [Google Scholar] [CrossRef]
- Selice, R.; Garolla, A.; Pengo, M.; Caretta, N.; Ferlin, A.; Foresta, C. The response to FSH treatment in oligozoospermic men depends on FSH receptor gene polymorphisms. Int. J. Androl. 2011, 34, 306–312. [Google Scholar] [CrossRef]
- Knuth, U.A.; Honigl, W.; Bals-Pratsch, M.; Schleicher, G.; Nieschlag, E. Treatment of severe oligospermia with human chorionic gonadotropin/human menopausal gonadotropin: A placebo-controlled, double blind trial. J. Clin. Endocrinol. Metab. 1987, 65, 1081–1087. [Google Scholar] [CrossRef]
- Verdi, A.; Nasr-Esfahani, M.H.; Forouzanfar, M.; Tavalaee, M. The Effect of Recombinant Human Follicle-Stimulating Hormone on Sperm Quality, Chromatin Status and Clinical Outcomes of Infertile Oligozoospermic Men Candidate for Intracytoplasmic Sperm Injection: A Randomized Clinical Trial. Int. J. Fertil. Steril. 2021, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aljuhayman, A.; Almardawi, A.; Fallatah, M.; Alhathal, N. Efficacy of follicle-stimulating hormone as a treatment of severe idiopathic oligospermia: A retrospective study. Urol. Ann. 2020, 12, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Vinanzi, C.; Selice, R.; Garolla, A.; Frigo, A.C.; Foresta, C. Toward a pharmacogenetic approach to male infertility: Polymorphism of follicle-stimulating hormone beta-subunit promoter. Fertil. Steril. 2011, 96, 1344–1349.e2. [Google Scholar] [CrossRef] [PubMed]
- Foresta, C.; Bettella, A.; Merico, M.; Garolla, A.; Plebani, M.; Ferlin, A.; Rossato, M. FSH in the treatment of oligozoospermia. Mol. Cell. Endocrinol. 2000, 161, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Granata, A.R.; Simoni, M. Follicle-stimulating hormone treatment of male idiopathic infertility improves pregnancy rate: A meta-analysis. Endocr. Connect. 2015, 4, R46–R58. [Google Scholar] [CrossRef] [PubMed]
- Simoni, M.; Santi, D. FSH Treatment of male idiopathic infertility: Time for a paradigm change. Andrology 2019, 8, 535–544. [Google Scholar] [CrossRef]
- Cannarella, R.; La Vignera, S.; Condorelli, R.A.; Mongioi, L.M.; Calogero, A.E. FSH dosage effect on conventional sperm parameters: A meta-analysis of randomized controlled studies. Asian J. Androl. 2019, 22, 309–316. [Google Scholar] [CrossRef]
- Fatemi, H.; Bilger, W.; Denis, D.; Griesinger, G.; La Marca, A.; Longobardi, S.; Mahony, M.; Yin, X.; D’Hooghe, T. Dose adjustment of follicle-stimulating hormone (FSH) during ovarian stimulation as part of medically-assisted reproduction in clinical studies: A systematic review covering 10 years (2007–2017). Reprod. Biol. Endocrinol. RB&E 2021, 19, 68. [Google Scholar] [CrossRef]
- Prodromidou, A.; Anagnostou, E.; Mavrogianni, D.; Liokari, E.; Dimitroulia, E.; Drakakis, P.; Loutradis, D. Past, Present, and Future of Gonadotropin Use in Controlled Ovarian Stimulation During Assisted Reproductive Techniques. Cureus 2021, 13, e15663. [Google Scholar] [CrossRef]
- Racca, A.; Drakopoulos, P.; Neves, A.R.; Polyzos, N.P. Current Therapeutic Options for Controlled Ovarian Stimulation in Assisted Reproductive Technology. Drugs 2020, 80, 973–994. [Google Scholar] [CrossRef]
- Romeo, M.; Spaggiari, G.; Nuzzo, F.; Granata, A.R.M.; Simoni, M.; Santi, D. Follicle-stimulating hormone effectiveness in male idiopathic infertility: What happens in daily practice? Andrology 2022, 11, 478–488. [Google Scholar] [CrossRef] [PubMed]
- AIFA: Note 74. 2010. Available online: http://www.agenziafarmaco.gov.it/it/content/nota-74 (accessed on 31 March 2023).
- Rastrelli, G.; Cipriani, S.; Craparo, A.; De Vincentis, S.; Granata, A.R.M.; Spaggiari, G.; Simoni, M.; Maggi, M.; Santi, D. The physician’s gender influences the results of the diagnostic workup for erectile dysfunction. Andrology 2020, 8, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Spaggiari, G.; Romeo, M.; Ebert, R.; Corradini, F.; Baraldi, C.; Granata, A.R.M.; Rochira, V.; Simoni, M.; Gavioli, L.; et al. Qualitative and quantitative analysis of doctor-patient interactions during andrological consultations. Andrology 2022, 10, 1240–1249. [Google Scholar] [CrossRef]
- Bonde, J.P.; Ernst, E.; Jensen, T.K.; Hjollund, N.H.; Kolstad, H.; Henriksen, T.B.; Scheike, T.; Giwercman, A.; Olsen, J.; Skakkebaek, N.E. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.G.; Ziebe, S.; Jørgensen, N.; Petersen, J.H.; Skakkebaek, N.E.; Andersen, A.N. Time to pregnancy in relation to semen quality assessed by CASA before and after sperm separation. Hum. Reprod. 2002, 17, 173–177. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.; Lynch, C.D.; Kim, S.; Maisog, J.M.; Gore-Langton, R.; Eisenberg, M.L.; Chen, Z. Semen quality and time to pregnancy: The Longitudinal Investigation of Fertility and the Environment Study. Fertil. Steril. 2014, 101, 453–462. [Google Scholar] [CrossRef]
- Lamb, D.J.; Marinaro, J.A. Semen parameter prediction of pregnancy. Fertil. Steril. 2023, S0015-0282. [Google Scholar] [CrossRef]
- Zinaman, M.J.; Brown, C.C.; Selevan, S.G.; Clegg, E.D. Semen quality and human fertility: A prospective study with healthy couples. J. Androl. 2000, 21, 145–153. [Google Scholar]
- Keihani, S.; Verrilli, L.E.; Zhang, C.; Presson, A.P.; Hanson, H.A.; Pastuszak, A.W.; Johnstone, E.B.; Hotaling, J.M. Semen parameter thresholds and time-to-conception in subfertile couples: How high is high enough? Hum. Reprod. 2021, 36, 2121–2133. [Google Scholar] [CrossRef]
- Muthigi, A.; Jahandideh, S.; Bishop, L.A.; Naeemi, F.K.; Shipley, S.K.; O’Brien, J.E.; Shin, P.R.; Devine, K.; Tanrikut, C. Clarifying the relationship between total motile sperm counts and intrauterine insemination pregnancy rates. Fertil. Steril. 2021, 115, 1454–1460. [Google Scholar] [CrossRef]
- Villani, M.T.; Morini, D.; Spaggiari, G.; Falbo, A.I.; Melli, B.; La Sala, G.B.; Romeo, M.; Simoni, M.; Aguzzoli, L.; Santi, D. Are sperm parameters able to predict the success of assisted reproductive technologies? A retrospective analysis of over 22000 ART cycles. Andrology 2021, 10, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Belladelli, F.; Chen, T.; Glover, F.; Mulloy, E.A.; Kasman, A.M.; Sciarra, A.; Salciccia, S.; Canale, V.; Maggi, M.; et al. The association of impaired semen quality and pregnancy rates in assisted reproduction technology cycles: Systematic review and meta-analysis. Andrologia 2022, 54, e14409. [Google Scholar] [CrossRef]
- Casarini, L.; Crépieux, P.; Reiter, E.; Lazzaretti, C.; Paradiso, E.; Rochira, V.; Brigante, G.; Santi, D.; Simoni, M. FSH for the Treatment of Male Infertility. Int. J. Mol. Sci. 2020, 21, 2270. [Google Scholar] [CrossRef] [PubMed]
- Lispi, M.; Drakopoulos, P.; Spaggiari, G.; Caprio, F.; Colacurci, N.; Simoni, M.; Santi, D. Testosterone Serum Levels Are Related to Sperm DNA Fragmentation Index Reduction after FSH Administration in Males with Idiopathic Infertility. Biomedicines 2022, 10, 2599. [Google Scholar] [CrossRef]
- Björndahl, L.; Barratt, C.L.R.; Mortimer, D.; Agarwal, A.; Aitken, R.J.; Alvarez, J.G.; Aneck-Hahn, N.; Arver, S.; Baldi, E.; Bassas, L.; et al. Standards in semen examination: Publishing reproducible and reliable data based on high-quality methodology. Hum. Reprod. 2022, 37, 2497–2502. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Duca, Y.; Mongioi, L.M.; Cannarella, R.; Giacone, F.; Calogero, A.E. FSH therapy for idiopathic male infertility: Four schemes are better than one. Aging Male Off. J. Int. Soc. Study Aging Male 2020, 23, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; La Vignera, S.; Condorelli, R.A.; Rago, R.; Barone, N.; Vicari, E.; Calogero, A.E. Follicle-stimulating hormone treatment in normogonadotropic infertile men. Nat. Rev. Urol. 2013, 10, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Selice, R.; Engl, B.; Bertoldo, A.; Menegazzo, M.; Finos, L.; Lenzi, A.; Foresta, C. Spermatid count as a predictor of response to FSH therapy. Reprod. Biomed. Online 2014, 29, 102–112. [Google Scholar] [CrossRef]

| Variables | FSH-Treated Patients (n = 173) |
|---|---|
| Age (years) | 37.9 ± 6.2 (23.3–51.4) |
| Infertility duration (years) | 2.4 ± 3.1 |
| Primary infertility n (%) | 156 (90.2) |
| Total testosterone (ng/mL) | 5.5 ± 2.7 (2.1–11.8) |
| Luteinising hormone (LH) (IU/L) | 3.5 ± 1.4 (1.7–9.5) |
| Follicle-stimulating hormone (FSH) (IU/L) | 4.4 ± 2.0 (1.0–7.9) |
| Prolactin (ng/mL) | 12.9 ± 6.1 (1.5–30.9) |
| Semen volume (mL) | 2.5 ± 1.6 (0.3–11.5) |
| Sperm concentration (million/mL) | 5.5 ± 4.7 (0.1–14.9) |
| Progressive sperm motility (%) | 18.6 ± 16.3 (0.0–71.0) |
| Sperm with normal morphology (%) | 2.7 ± 5.6 (0.0–17.0) |
| Duration of FSH administration (months) | 8.0 ± 4.5 (2.0–12.0) |
| Pregnancies obtained n (%) | 48 (27.7) |
| Variables | Study Group (n = 48) | Control Group (n = 125) | p-Value |
|---|---|---|---|
| Age (years) | |||
| Increased sperm concentration | 35.7 ± 4.9 | 39.0 ± 6.9 | 0.064 |
| Not increased sperm concentration | 37.4 ± 4.7 | 37.9 ± 6.0 | 0.774 |
| p-value | 0.305 | 0.343 | |
| Total testosterone (ng/mL) | |||
| Increased sperm concentration | 5.7 ± 3.0 | 5.5 ± 2.4 | 0.750 |
| Not increased sperm concentration | 5.0 ± 1.6 | 5.5 ± 3.2 | 0.600 |
| p-value | 0.509 | 0.882 | |
| Luteinising hormone (LH) (IU/L) | |||
| Increased sperm concentration | 3.8 ± 1.0 | 3.4 ± 1.3 | 0.292 |
| Not increased sperm concentration | 4.3 ± 2.2 | 3.3 ± 1.3 | 0.091 |
| p-value | 0.374 | 0.701 | |
| Follicle-stimulating hormone (FSH) (IU/L) | |||
| Increased sperm concentration | 4.4 ± 1.4 | 4.3 ± 2.1 | 0.848 |
| Not increased sperm concentration | 5.0 ± 2.1 | 4.5 ± 2.0 | 0.491 |
| p-value | 0.332 | 0.589 | |
| Prolactin (ng/mL) | |||
| Increased sperm concentration | 13.1 ± 4.1 | 12.6 ± 6.1 | 0.824 |
| Not increased sperm concentration | 9.3 ± 4.7 | 13.8 ± 7.0 | 0.181 |
| p-value | 0.120 | 0.538 | |
| Baseline semen volume (mL) | |||
| Increased sperm concentration | 2.3 ± 1.3 | 2.5 ± 1.7 | 0.647 |
| Not increased sperm concentration | 3.5 ± 1.7 | 2.3 ± 1.8 | 0.065 |
| p-value | 0.018 | 0.704 | |
| Δ Total sperm count | |||
| Increased sperm concentration | 204.0 ± 939.5 | 237.2 ± 507.1 | 0.809 |
| Not increased sperm concentration | 57.5 ± 300.9 | 169.4 ± 441.5 | 0.391 |
| p-value | 0.568 | 0.443 | |
| Baseline progressive sperm motility (%) | |||
| Increased sperm concentration | 21.1 ± 17.8 | 17.4 ± 17.0 | 0.391 |
| Not increased sperm concentration | 21.6 ± 10.6 | 17.6 ± 16.0 | 0.429 |
| p-value | 0.933 | 0.957 | |
| Δ total motile sperm number (%) | |||
| Increased sperm concentration | 236.0 ± 939.5 | 237.2 ± 594.9 | 0.992 |
| Not increased sperm concentration | 13.8 ± 238.5 | 26.3 ± 222.9 | 0.882 |
| p-value | 0.490 | 0.086 | |
| Baseline sperm with normal morphology (%) | |||
| Increased sperm concentration | 4.9 ± 11.8 | 2.0 ± 2.7 | 0.237 |
| Not increased sperm concentration | 2.6 ± 4.0 | 2.3 ± 2.7 | 0.761 |
| p-value | 0.610 | 0.749 | |
| Duration of FSH administration (months) | |||
| Increased sperm concentration | 7.4 ± 4.2 | 8.1 ± 4.6 | 0.487 |
| Not increased sperm concentration | 7.9 ± 5.4 | 8.2 ± 4.3 | 0.814 |
| p-value | 0.751 | 0.854 | |
| Patients’ distribution n (%) | |||
| Increased sperm concentration | 35 (20.2) | 71 (41.0) | - |
| Not increased sperm concentration | 13 (7.5) | 54 (31.2) | - |
| p-value | - | - | 0.037 |
| Variables | Study Group (n = 48) | Control Group (n = 125) | p-Value |
|---|---|---|---|
| Age (years) | |||
| Baseline sperm concentration < 7.3 million/mL | 36.5 ± 5.0 | 37.6 ± 6.2 | 0.347 |
| Baseline sperm concentration > 7.3 million/mL | 35.0 ± 4.2 | 40.2 ± 6.9 | 0.088 |
| p-value | 0.409 | 0.082 | |
| Total testosterone (ng/mL) | |||
| Baseline sperm concentration < 7.3 million/mL | 5.4 ± 2.6 | 5.3 ± 2.8 | 0.832 |
| Baseline sperm concentration > 7.3 million/mL | 5.5 ± 2.7 | 5.9 ± 2.8 | 0.728 |
| p-value | 0.948 | 0.324 | |
| Luteinising hormone (LH) (IU/L) | |||
| Baseline sperm concentration < 7.3 million/mL | 3.9 ± 1.6 | 3.5 ± 1.4 | 0.283 |
| Baseline sperm concentration > 7.3 million/mL | 4.1 ± 1.0 | 3.3 ± 1.2 | 0.065 |
| p-value | 0.648 | 0.524 | |
| Follicle-stimulating hormone (FSH) (IU/L) | |||
| Baseline sperm concentration < 7.3 million/mL | 4.4 ± 1.6 | 4.3 ± 2.0 | 0.828 |
| Baseline sperm concentration > 7.3 million/mL | 5.0 ± 1.7 | 4.5 ± 2.1 | 0.506 |
| p-value | 0.377 | 0.699 | |
| Prolactin (ng/mL) | |||
| Baseline sperm concentration < 7.3 million/mL | 12.5 ± 3.4 | 12.8 ± 6.6 | 0.868 |
| Baseline sperm concentration > 7.3 million/mL | 11.2 ± 5.9 | 13.7 ± 6.5 | 0.362 |
| p-value | 0.584 | 0.639 | |
| Baseline semen volume (mL) | |||
| Baseline sperm concentration < 7.3 million/mL | 2.6 ± 1.5 | 2.6 ± 1.8 | 0.983 |
| Baseline sperm concentration > 7.3 million/mL | 2.9 ± 1.3 | 2.1 ± 1.5 | 0.185 |
| p-value | 0.619 | 0.137 | |
| Δ sperm concentration (%) | |||
| Baseline sperm concentration < 7.3 million/mL | 445.0 ± 781.8 | 164.5 ± 315.1 | 0.007 |
| Baseline sperm concentration > 7.3 million/mL | −9.8 ± 79.1 | −16.8 ± 59.3 | 0.752 |
| p-value | 0.021 | <0.001 | |
| Δ Total sperm count | |||
| Baseline sperm concentration < 7.3 million/mL | 174.8 ± 853.2 | 203.2 ± 465.8 | 0.818 |
| Baseline sperm concentration > 7.3 million/mL | 124.3 ± 411.1 | 217.0 ± 507.2 | 0.592 |
| p-value | 0.857 | 0.879 | |
| Baseline progressive sperm motility (%) | |||
| Baseline sperm concentration < 7.3 million/mL | 17.6 ± 14.7 | 15.0 ± 15.0 | 0.465 |
| Baseline sperm concentration > 7.3 million/mL | 32.3 ± 13.5 | 21.0 ± 17.9 | 0.083 |
| p-value | 0.012 | 0.087 | |
| Δ total motile sperm number (%) | |||
| Baseline sperm concentration < 7.3 million/mL | 198.5 ± 921.7 | 200.5 ± 578.9 | 0.990 |
| Baseline sperm concentration > 7.3 million/mL | 136.1 ± 362.0 | 60.8 ± 247.6 | 0.479 |
| p-value | 0.853 | 0.175 | |
| Baseline sperm with normal morphology (%) | |||
| Baseline sperm concentration < 7.3 million/mL | 1.6 ± 1.9 | 1.8 ± 2.2 | 0.832 |
| Baseline sperm concentration > 7.3 million/mL | 4.7 ± 5.9 | 2.5 ± 3.0 | 0.098 |
| p-value | 0.065 | 0.333 | |
| Duration of FSH administration (months) | |||
| Baseline sperm concentration < 7.3 million/mL | 6.7 ± 3.7 | 8.3 ± 4.5 | 0.065 |
| Baseline sperm concentration > 7.3 million/mL | 11.7 ± 6.2 | 8.0 ± 4.4 | 0.111 |
| p-value | 0.004 | 0.720 | |
| Patients’ distribution n (%) | |||
| Baseline sperm concentration < 7.3 million/mL | 38 (22.0) | 79 (45.7) | - |
| Baseline sperm concentration > 7.3 million/mL | 10 (5.8) | 46 (26.6) | - |
| p-value | - | - | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santi, D.; Spaggiari, G.; Dalla Valentina, L.; Romeo, M.; Nuzzo, F.; Serlenga, L.; Roli, L.; De Santis, M.C.; Trenti, T.; Granata, A.R.M.; et al. Sperm Concentration Improvement May Be a Parameter Predicting Efficacy of FSH Therapy of Male Idiopathic Infertility. Cells 2023, 12, 2236. https://doi.org/10.3390/cells12182236
Santi D, Spaggiari G, Dalla Valentina L, Romeo M, Nuzzo F, Serlenga L, Roli L, De Santis MC, Trenti T, Granata ARM, et al. Sperm Concentration Improvement May Be a Parameter Predicting Efficacy of FSH Therapy of Male Idiopathic Infertility. Cells. 2023; 12(18):2236. https://doi.org/10.3390/cells12182236
Chicago/Turabian StyleSanti, Daniele, Giorgia Spaggiari, Leonardo Dalla Valentina, Marilina Romeo, Federico Nuzzo, Lorenzo Serlenga, Laura Roli, Maria Cristina De Santis, Tommaso Trenti, Antonio R. M. Granata, and et al. 2023. "Sperm Concentration Improvement May Be a Parameter Predicting Efficacy of FSH Therapy of Male Idiopathic Infertility" Cells 12, no. 18: 2236. https://doi.org/10.3390/cells12182236
APA StyleSanti, D., Spaggiari, G., Dalla Valentina, L., Romeo, M., Nuzzo, F., Serlenga, L., Roli, L., De Santis, M. C., Trenti, T., Granata, A. R. M., & Simoni, M. (2023). Sperm Concentration Improvement May Be a Parameter Predicting Efficacy of FSH Therapy of Male Idiopathic Infertility. Cells, 12(18), 2236. https://doi.org/10.3390/cells12182236








