Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients
Abstract
1. Introduction
2. Materials and Methods
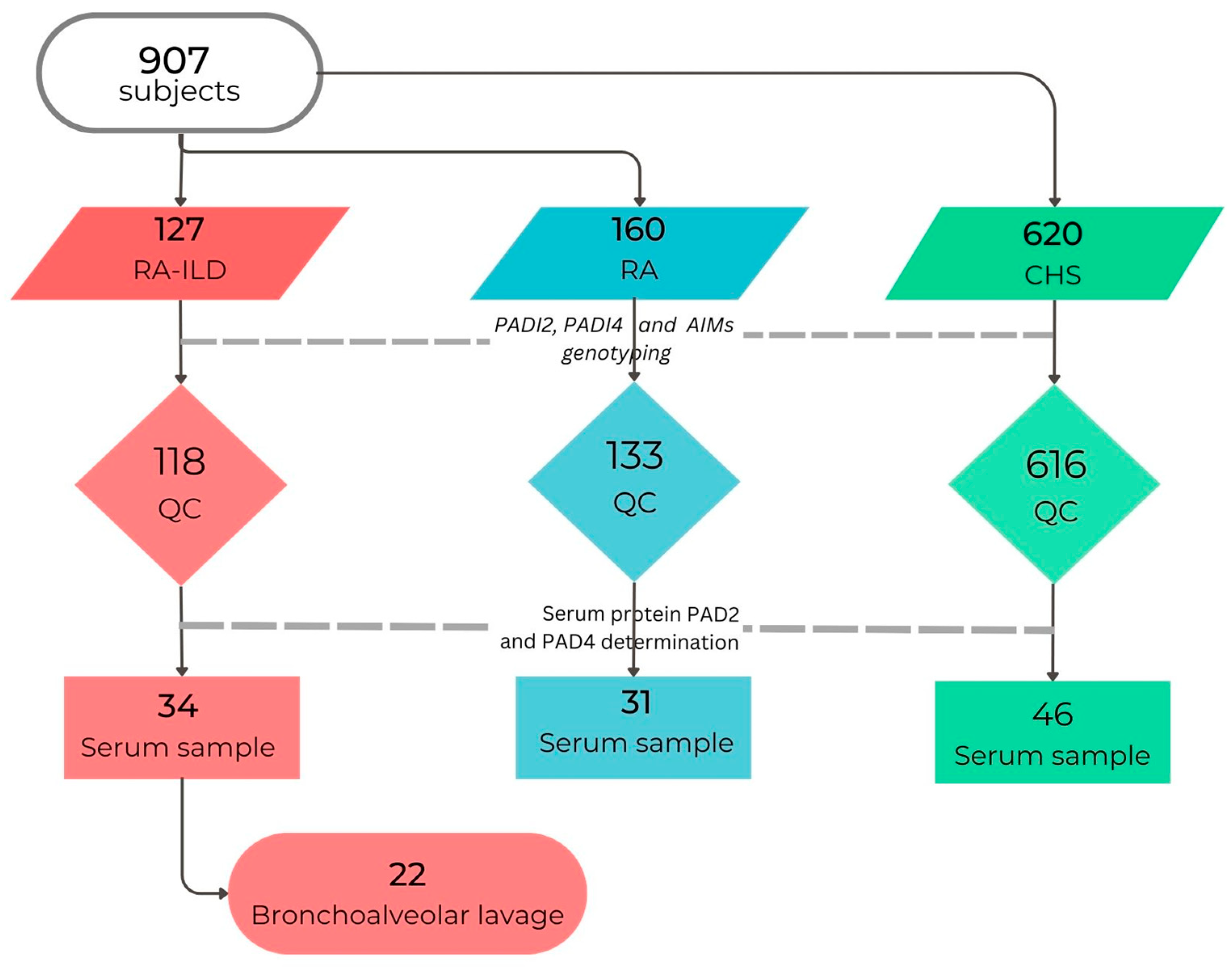
2.1. Subjects, Material, and Methods
2.2. Ethical Statement
2.3. Study Groups
2.4. HRCT Scan Pattern
2.5. Biological Samples Collection
2.6. Genotyping
2.7. Ancestry Informative Markers
2.8. Determination of PAD2 and PAD4 Proteins
2.9. Statistical Analysis
3. Results
3.1. Demographic and Clinical Data
3.2. Genetic Susceptibility Analysis
3.3. Comparison of Rheumatoid Arthritis and Clinically Healthy Subjects
3.4. Comparison of RA-ILD and RA Groups
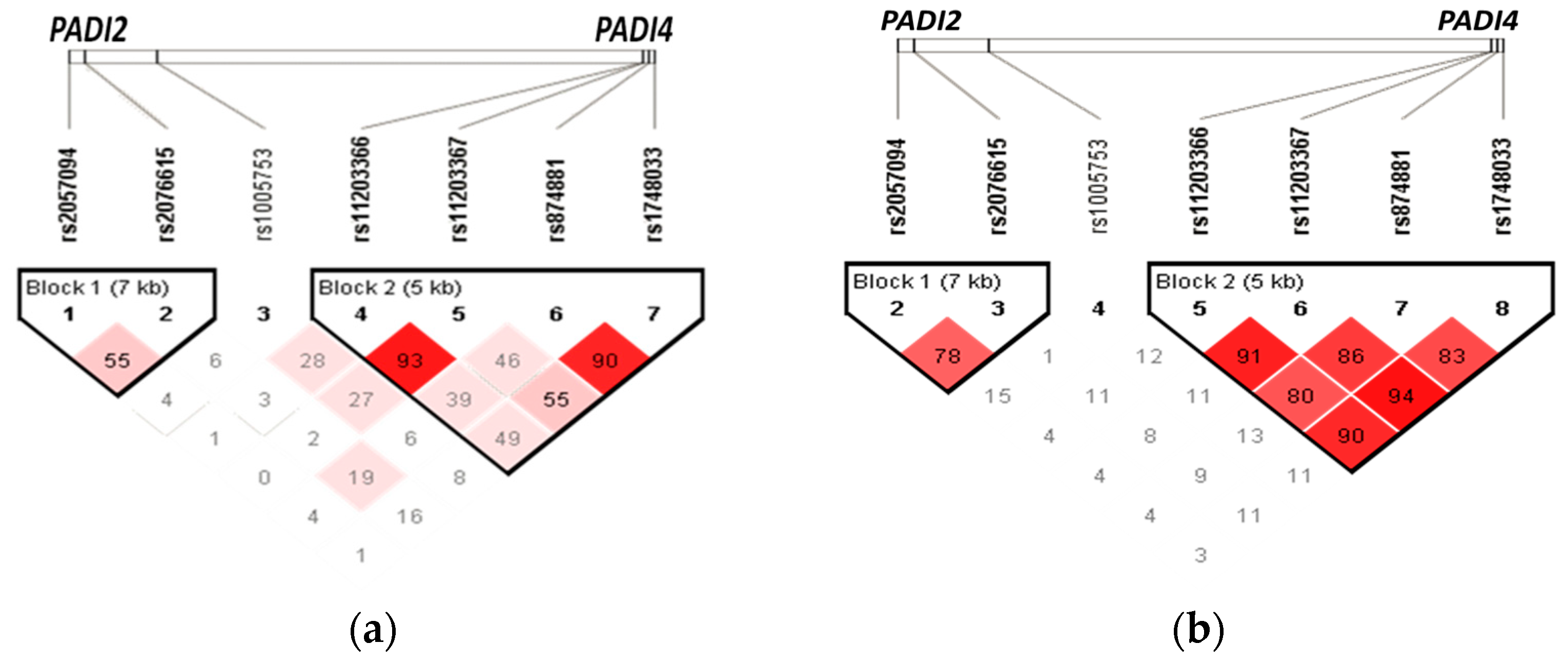
3.5. Haplotypes
3.6. PAD2 and PAD4 Protein Levels
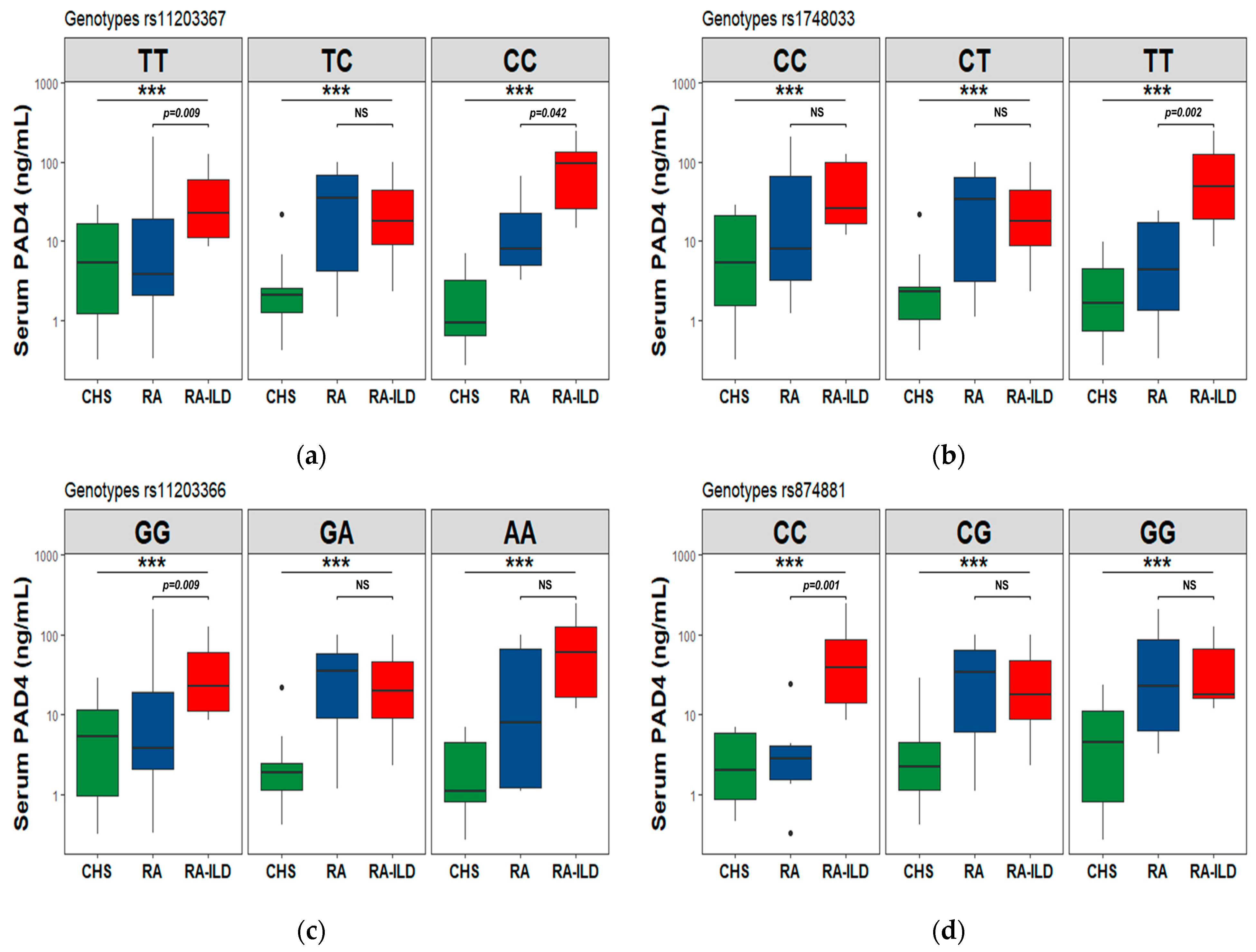
3.7. PAD2 and PAD4 Levels in the Codominant Genetic Model
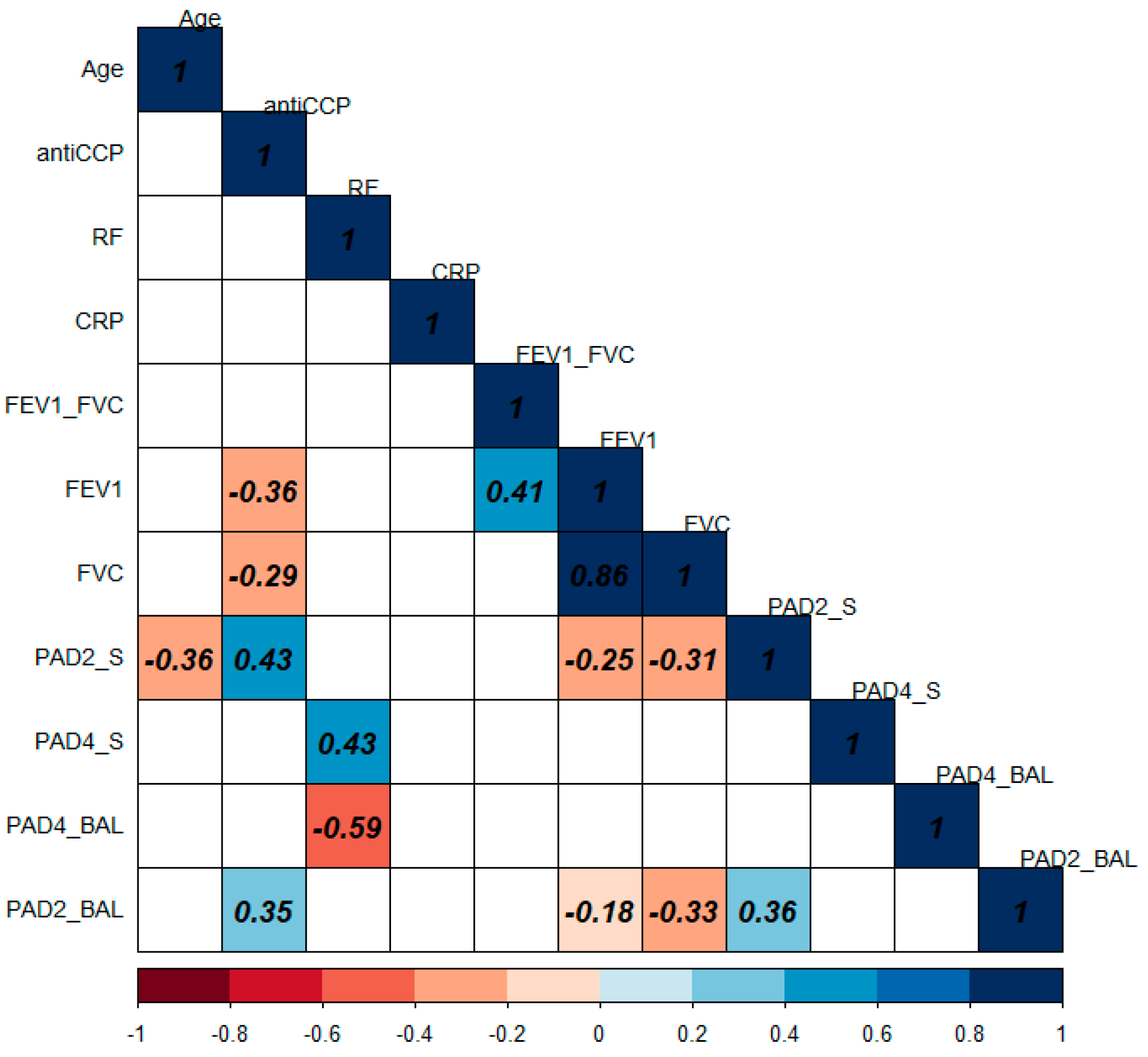
3.8. Correlation of PAD2 and PAD4 Levels with Clinical and Biochemical Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, T.J.; Dellaripa, P.F.; Batra, K.; Frits, M.L.; Iannaccone, C.K.; Hatabu, H.; Nishino, M.; Weinblatt, M.E.; Ascherman, D.P.; Washko, G.R.; et al. Functional Impact of a Spectrum of Interstitial Lung Abnormalities in Rheumatoid Arthritis. Chest 2014, 146, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Bilgici, A.; Ulusoy, H.; Kuru, O.; Celenk, C.; Ünsal, M.; Danacı, M. Pulmonary Involvement in Rheumatoid Arthritis. Rheumatol. Int. 2005, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Hensvold, A.H.; Magnusson, P.K.E.; Joshua, V.; Hansson, M.; Israelsson, L.; Ferreira, R.; Jakobsson, P.; Holmdahl, R.; Hammarström, L.; Malmström, V.; et al. Environmental and Genetic Factors in the Development of Anticitrullinated Protein Antibodies (ACPAs) and ACPA-Positive Rheumatoid Arthritis: An Epidemiological Investigation in Twins. Ann. Rheum. Dis. 2015, 74, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Lugli, E.B.; Correia, R.E.S.M.; Fischer, R.; Lundberg, K.; Bracke, K.R.; Montgomery, A.B.; Kessler, B.M.; Brusselle, G.G.; Venables, P.J. Expression of Citrulline and Homocitrulline Residues in the Lungs of Non-Smokers and Smokers: Implications for Autoimmunity in Rheumatoid Arthritis. Arthritis Res. Ther. 2015, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.W.; van Venrooij, W.J.; Pruijn, G.J.M. PAD, a Growing Family of Citrullinating Enzymes: Genes, Features and Involvement in Disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Solomon, J.J.; Matson, S.; Kelmenson, L.B.; Chung, J.H.; Hobbs, S.B.; Rosas, I.O.; Dellaripa, P.F.; Doyle, T.J.; Poli, S.; Esposito, A.J.; et al. IgA Antibodies Directed Against Citrullinated Protein Antigens Are Elevated in Patients with Idiopathic Pulmonary Fibrosis. Chest 2019, 157, 1513–1521. [Google Scholar] [CrossRef]
- Roos, K.; Martinsson, K.; Ziegelasch, M.; Sommarin, Y.; Svärd, A.; Skogh, T.; Kastbom, A. Circulating Secretory IgA Antibodies against Cyclic Citrullinated Peptides in Early Rheumatoid Arthritis Associate with Inflammatory Activity and Smoking. Arthritis Res. Ther. 2016, 18, 119. [Google Scholar] [CrossRef]
- Van Delft, M.A.M.; Van Der Woude, D.; Toes, R.E.M.; Trouw, L.A. Secretory Form of Rheumatoid Arthritis-Associated Autoantibodies in Serum Are Mainly of the IgM Isotype, Suggesting a Continuous Reactivation of Autoantibody Responses at Mucosal Surfaces. Ann. Rheum. Dis. 2019, 78, 146–148. [Google Scholar] [CrossRef]
- Sharma, M.; Damgaard, D.; Senolt, L.; Svensson, B.; Bay-Jensen, A.C.; Nielsen, C.H.; Hägglund, P. Expanding the Citrullinome of Synovial Fibrinogen from Rheumatoid Arthritis Patients. J. Proteom. 2019, 208, 103484. [Google Scholar] [CrossRef]
- Samara, K.D.; Trachalaki, A.; Tsitoura, E.; Koutsopoulos, A.V.; Lagoudaki, E.D.; Lasithiotaki, I.; Margaritopoulos, G.; Pantelidis, P.; Bibaki, E.; Siafakas, N.M.; et al. Upregulation of Citrullination Pathway: From Autoimmune to Idiopathic Lung Fibrosis. Respir. Res. 2017, 18, 218. [Google Scholar] [CrossRef]
- Makrygiannakis, D.; Hermansson, M.; Ulfgren, A.-K.; Nicholas, A.P.; Zendman, A.J.W.; Eklund, A.; Grunewald, J.; Skold, C.M.; Klareskog, L.; Catrina, A.I. Smoking Increases Peptidylarginine Deiminase 2 Enzyme Expression in Human Lungs and Increases Citrullination in BAL Cells. Ann. Rheum. Dis. 2008, 67, 1488–1492. [Google Scholar] [CrossRef]
- van Gaalen, F.A.; Linn-Rasker, S.P.; van Venrooij, W.J.; de Jong, B.A.; Breedveld, F.C.; Verweij, C.L.; Toes, R.E.; Huizinga, T.W. Autoantibodies to Cyclic Citrullinated Peptides Predict Progression to Rheumatoid Arthritis in Patients with Undifferentiated Arthritis: A Prospective Cohort Study. Arthritis Rheum. 2004, 50, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kochi, Y.; Shoda, H.; Seri, Y.; Fujio, K.; Sawada, T.; Yamada, R.; Yamamoto, K. Decreased Severity of Experimental Autoimmune Arthritis in Peptidylarginine Deiminase Type 4 Knockout Mice. BMC Musculoskelet. Disord. 2016, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Wilches, L.V.; Guerrero, J. Timolol-Induced Interstitial Lung Disease. Respir. Med. Case Rep. 2015, 15, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Cano-Jiménez, E.; Molina-Molina, M.; Ramírez, J.; Aliaga, J.L.; Sánchez, M.; Xaubet, A. Diffuse Interstitial Lung Disease Related to Peribronchiolar Metaplasia. Arch. Bronconeumol. 2009, 45, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.P.; Dong, S.K.; Park, I.N.; Se, J.J.; Kitaichi, M.; Nicholson, A.G.; Colby, T.V. Prognosis of Fibrotic Interstitial Pneumonia: Idiopathic versus Collagen Vascular Disease-Related Subtypes. Am. J. Respir. Crit. Care Med. 2007, 175, 705–711. [Google Scholar] [CrossRef]
- Kim, E.J.; Elicker, B.M.; Maldonado, F.; Webb, W.R.; Ryu, J.H.; Van Uden, J.H.; Lee, J.S.; King, T.E.; Collard, H.R. Usual Interstitial Pneumonia in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Eur. Respir. J. 2010, 35, 1322–1328. [Google Scholar] [CrossRef]
- Paulin, F.; Doyle, T.J.; Fletcher, E.A.; Ascherman, D.P.; Rosas, I.O. Rheumatoid Arthritis-Associated Interstitial Lung Disease and Idiopathic Pulmonary Fibrosis: Shared Mechanistic and Phenotypic Traits Suggest Overlapping Disease Mechanisms. Rev. Investig. Clin. 2015, 67, 280–286. [Google Scholar]
- Cha, S.; Choi, C.-B.; Han, T.-U.; Kang, C.P.; Kang, C.; Bae, S.-C. Association of Anti–Cyclic Citrullinated Peptide Antibody Levels with PADI4 Haplotypes in Early Rheumatoid Arthritis and with Shared Epitope Alleles in Very Late Rheumatoid Arthritis. Arthritis Rheum. 2007, 56, 1454–1463. [Google Scholar] [CrossRef]
- György, B.; Tóth, E.; Tarcsa, E.; Falus, A.; Buzás, E.I. Citrullination: A Posttranslational Modification in Health and Disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef]
- Pérez-Padilla, R.; Valdivia, G.; Muiño, A.; López, M.V.; Márquez, M.N.; Montes de Oca, M.; Tálamo, C.; Lisboa, C.; Pertuzé, J.; Jardim, J.R.B.; et al. Spirometric Reference Values in 5 Large Latin American Cities for Subjects Aged 40 Years or Over. Arch. Bronconeumol. (Eng. Ed.) 2006, 42, 317–325. [Google Scholar] [CrossRef]
- Sood, A.; Dawson, B.K.; Henkle, J.Q.; Hopkins-Price, P.; Qualls, C. Effect of Change of Reference Standard to NHANES III on Interpretation of Spirometric “Abnormality”. Int. J. Chronic Obstr. Pulm. Dis. 2007, 2, 361–367. [Google Scholar]
- Demedts, M.; Costabel, U. ATS/ERS International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. Eur. Respir. J. 2002, 19, 794–796. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General Considerations for Lung Function Testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of Spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Silva-Zolezzi, I.; Hidalgo-Miranda, A.; Estrada-Gil, J.; Fernandez-Lopez, J.C.; Uribe-Figueroa, L.; Contreras, A.; Balam-Ortiz, E.; Bosque-Plata, L.; Velazquez-Fernandez, D.; Lara, C.; et al. Analysis of Genomic Diversity in Mexican Mestizo Populations to Develop Genomic Medicine in Mexico. Proc. Natl. Acad. Sci. USA 2009, 106, 8611–8616. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.; Daly, M.J.; et al. PLINK: A Toolset for Whole-Genome Association and Population-Based Linkage Analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and Visualization of LD and Haplotype Maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast Model-Based Estimation of Ancestry in Unrelated Individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal Components Analysis Corrects for Stratification in Genome-Wide Association Studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Levine, D.; Shen, J.; Gogarten, S.M.; Laurie, C.; Weir, B.S. A High-Performance Computing Toolset for Relatedness and Principal Component Analysis of SNP Data. Bioinformatics 2012, 28, 3326–3328. [Google Scholar] [CrossRef] [PubMed]
- Vaz Fragoso, C.A. Epidemiology of Lung Disease in Older Persons. Clin. Geriatr. Med. 2017, 33, 491–501. [Google Scholar] [CrossRef]
- Peláez-Ballestas, I.; Sanin, L.H.; Moreno-Montoya, J.; Alvarez-Nemegyei, J.; Burgos-Vargas, R.; Garza-Elizondo, M.; Rodríguez-Amado, J.; Goycochea-Robles, M.V.; Madariaga, M.; Zamudio, J.; et al. Epidemiology of the Rheumatic Diseases in Mexico. A Study of 5 Regions Based on the COPCORD Methodology. J. Rheumatol. 2011, 38, 3–6. [Google Scholar] [CrossRef]
- Patterson, K.C.; Shah, R.J.; Porteous, M.K.; Christie, J.D.; D’Errico, C.A.; Chadwick, M.; Triano, M.J.; Deshpande, C.; Rossman, M.D.; Litzky, L.A.; et al. Interstitial Lung Disease in the Elderly. Chest 2017, 151, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, D.; Senolt, L.; Nielsen, C.H. Increased Levels of Peptidylarginine Deiminase 2 in Synovial Fluid from Anti-CCP-Positive Rheumatoid Arthritis Patients: Association with Disease Activity and Inflammatory Markers. Rheumatology 2016, 55, 918–927. [Google Scholar] [CrossRef]
- Arnoux, F.; Mariot, C.; Peen, E.; Lambert, N.C.; Balandraud, N.; Roudier, J.; Isabelle, A. Peptidyl Arginine Deiminase Immunization Induces Anticitrullinated Protein Antibodies in Mice with Particular MHC Types. Proc. Natl. Acad. Sci. USA 2017, 114, E10169–E10177. [Google Scholar] [CrossRef]
- Giles, J.T.; Darrah, E.; Danoff, S.; Johnson, C.; Andrade, F.; Rosen, A.; Bathon, J.M. Association of Cross-Reactive Antibodies Targeting Peptidyl-Arginine Deiminase 3 and 4 with Rheumatoid Arthritis-Associated Interstitial Lung Disease. PLoS ONE 2014, 9, e98794. [Google Scholar] [CrossRef]
- Shamah-Levy, T.; Vielma-Orozco, E.; Heredia-Hernández, O.; Romero-Martínez, M.; Mojica-Cuevas, J.; Cuevas-Nasu, L.; Santaella-Castell, J.; Rivera-Dommarco, J. Encuesta Nacional de Salud y Nutrición 2018–2019; Resultados Nacionales; Instituto Nacional de Salud Pública: Cuernavaca, México, 2020; ISBN 978-607-511-205-3. [Google Scholar]
- Regalado, J.; Pérez-Padilla, R.; Sansores, R.; Páramo-Ramirez, J.I.; Brauer, M.; Paré, P.; Vedal, S. The Effect of Biomass Burning on Respiratory Symptoms and Lung Function in Rural Mexican Women. Am. J. Respir. Crit. Care Med. 2006, 174, 901–905. [Google Scholar] [CrossRef]
- Bruce, N.; Perez-padilla, R.; Albalak, R. Indoor Air Pollution in Developing Countries: A Major Environmental and Public Health Challenge. Spec. Theme-Environ. Health 2000, 78, 1078–1092. [Google Scholar]
- Ramirez-Venegas, A.; Sansores, R.H.; Quintana-Carrillo, R.; Velázquez-Uncal, M.; Hernandez-Zenteno, R.J.; Sánchez-Romero, C.; Velazquez-Montero, A.; Flores-Trujillo, F. Forced Expiratory Volume in One Second Decline in Patients with Chronic Obstructive Pulmonary Disease Associated with Biomass Exposure. Am. J. Respir. Crit. Care Med. 2014, 190, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Soto, M.A.; Rodríguez-Rodríguez, M.; Pérez-Pérez, M.E.; Daza-Benitez, L.; Bollain-y-Goytia, J.J.; Carrillo-Jiménez, M.A.; Avalos-Díaz, E.; Herrera-Esparza, R. Potential Protein Targets of the Peptidylarginine Deiminase 2 and Peptidylarginine Deiminase 4 Enzymes in Rheumatoid Synovial Tissue and Its Possible Meaning. Eur. J. Rheumatol. 2016, 3, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Bromberg, R.; Deane, K.D.; Lahey, L.J.; Derber, L.A.; Chandra, P.E.; Edison, J.D.; Gilliland, W.R.; Tibshirani, R.J.; Norris, J.M.; et al. Autoantibody Epitope Spreading in the Pre-Clinical Phase Predicts Progression to Rheumatoid Arthritis. PLoS ONE 2012, 7, e35296. [Google Scholar] [CrossRef]
- Karlson, E.W.; Chang, S.-C.; Cui, J.; Chibnik, L.B.; Fraser, P.A.; De Vivo, I.; Costenbader, K.H. Gene-Environment Interaction between HLA-DRB1 Shared Epitope and Heavy Cigarette Smoking in Predicting Incident Rheumatoid Arthritis. Ann. Rheum. Dis. 2010, 69, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kolfenbach, J.R.; Deane, K.D.; Derber, L.A.; Colin, I.; Donnell, O.; Gilliland, W.R.; Edison, J.D.; Rosen, A.; Darrah, E.; Norris, J.M.; et al. Autoimmunity to Peptidyl Arginine Deiminase Type 4 Precedes Clinical Onset of Rheumatoid Arthritis. Arthritis Rheum 2010, 62, 2633–2639. [Google Scholar] [CrossRef]
- Too, C.L.; Murad, S.; Dhaliwal, J.S.; Larsson, P.T.; Jiang, X.; Ding, B.; Alfredsson, L.; Klareskog, L.; Padyukov, L. Polymorphisms in Peptidylarginine Deiminase (PADI) Associate with Rheumatoid Arthritis in Diverse Asian Populations: Evidence from MyEIRA Study and Meta-Analysis. Arthritis Res. Ther. 2012, 14, R250. [Google Scholar] [CrossRef]
- Chang, X.; Xia, Y.; Pan, J.; Meng, Q.; Zhao, Y.; Yan, X. PADI2 Is Significantly Associated with Rheumatoid Arthritis. PLoS ONE 2013, 8, e81259. [Google Scholar] [CrossRef]
- Bang, S.-Y.; Han, T.-U.; Choi, C.-B.; Sung, Y.-K.; Bae, S.-C.; Kang, C. Peptidyl Arginine Deiminase Type IV (PADI4) Haplotypes Interact with Shared Epitope Regardless of Anti-Cyclic Citrullinated Peptide Antibody or Erosive Joint Status in Rheumatoid Arthritis: A Case Control Study. Arthritis Res. Ther. 2010, 12, R115. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamada, R.; Chang, X.; Tokuhiro, S.; Sawada, T.; Suzuki, M.; Nagasaki, M.; Nakayama-Hamada, M.; Kawaida, R.; Ono, M.; et al. Functional Haplotypes of PADI4, Encoding Citrullinating Enzyme Peptidylarginine Deiminase 4, Are Associated with Rheumatoid Arthritis. Nat. Genet. 2003, 34, 395–402. [Google Scholar] [CrossRef]
- Panati, K.; Pal, S.; Rao, K.V.; Reddy, V.D. Association of Single Nucleotide Polymorphisms (SNPs) of PADI4 Gene with Rheumatoid Arthritis (RA) in Indian Population. Genes Genet. Syst. 2012, 87, 191–196. [Google Scholar] [CrossRef][Green Version]
- Guzmán-Guzmán, I.P.; Reyes-Castillo, Z.; Muñoz-Barrios, S.; Ruiz-Noa, Y.; Martínez-Bonilla, G.E.; Parra-Rojas, I.; Palafox-Sánchez, C.A.; Muñoz-Valle, J.F. Polymorphisms and Functional Haplotype in PADI4: Further Evidence for Contribution on Rheumatoid Arthritis Susceptibility and Anti-Cyclic Citrullinated Peptide Antibodies in a Western Mexican Population. Immunol. Lett. 2015, 163, 214–220. [Google Scholar] [CrossRef]
- Gandjbakhch, F.; Fajardy, I.; Ferré, B.; Dubucquoi, S.; Flipo, R.-M.; Roger, N.; Solau-Gervais, E. A Functional Haplotype of PADI4 Gene in Rheumatoid Arthritis: Positive Correlation in a French Population. J. Rheumatol. 2009, 36, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M.; Padyukov, L.; Remmers, E.F.; Purcell, S.; Lee, A.T.; Karlson, E.W.; Wolfe, F.; Kastner, D.L.; Alfredsson, L.; Altshuler, D.; et al. Replication of Putative Candidate-Gene Associations with Rheumatoid Arthritis in >4000 Samples from North America and Sweden: Association of Susceptibility with PTPN22, CTLA4, and PADI4. Am. J. Hum. Genet. 2005, 77, 1044–1060. [Google Scholar] [CrossRef]
- Chen, R.; Wei, Y.; Cai, Q.; Duan, S.; Ren, D.; Shen, J.; He, D.; Fang, M.; Lv, K.; Cheng, N.; et al. The PADI4 Gene Does Not Contribute to Genetic Susceptibility to Rheumatoid Arthritis in Chinese Han Population. Rheumatol. Int. 2011, 31, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Han, J. Expression of Peptidylarginine Deiminase Type 4 (PAD4) in Various Tumors. Mol. Carcinog. 2006, 45, 183–196. [Google Scholar] [CrossRef]
- Hancock, D.B.; Artigas, M.S.; Gharib, S.A.; Henry, A.; Manichaikul, A.; Ramasamy, A.; Loth, D.W.; Imboden, M.; Koch, B.; McArdle, W.L.; et al. Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function. PLoS Genet. 2012, 8, e1003098. [Google Scholar] [CrossRef] [PubMed]
- Kochi, Y.; Thabet, M.M.; Suzuki, A.; Okada, Y.; Daha, N.A.; Toes, R.E.M.; Huizinga, T.W.J.; Myouzen, K.; Kubo, M.; Yamada, R.; et al. PADI4 Polymorphism Predisposes Male Smokers to Rheumatoid Arthritis. Ann. Rheum. Dis. 2011, 70, 512–515. [Google Scholar] [CrossRef]
- Andrade, F.; Darrah, E.; Gucek, M.; Cole, R.N.; Rosen, A.; Zhu, X. Autocitrullination of Human Peptidyl Arginine Deiminase Type 4 Regulates Protein Citrullination during Cell Activation. Arthritis Rheum 2010, 62, 1630–1640. [Google Scholar] [CrossRef]
- Arita, K.; Shimizu, T.; Hashimoto, H.; Hidaka, Y.; Yamada, M.; Sato, M. Structural Basis for Histone N-Terminal Recognition by Human Peptidylarginine Deiminase 4. Proc. Natl. Acad. Sci. USA 2006, 103, 5291–5296. [Google Scholar] [CrossRef]
| RA-ILD (n = 118) | RA (n = 133) | CHS (n = 616) | p-Value * | |
|---|---|---|---|---|
| Male, n (%) | 38 (32.20) | 10 (7.52) | 358 (58%) | <0.01 |
| Age (years) | 61.50 (53–67) | 54 (44–62) | 49.24 (42–56) | <0.01 |
| RA onset (years) | 52 (42–59) | 48 (38–59) | NA | <0.01 |
| Biochemical data | ||||
| anti-CCP (IU/mL) | 150.49 (70.93–201) | 261 (130–345.6) | NA | <0.01 |
| Rheumatoid Factor (IU/mL) | 322 (64.17–761) | 182 (55.15–656) | NA | 0.153 |
| C-Reactive protein (mg/dL) | 1.65 (0.52–3.61) | 1.39 (0.40–3.30) | NA | 0.625 |
| Erythrocyte sedimentation rate (mm/h) | 33 (24–36) | 29 (19.50–43) | NA | 0.891 |
| Lung Function | ||||
| FEV1 (%) | 55.50 (26–89) | 75.50 (64–99) | 99 (88–108) | <0.01 |
| FVC (%) | 66 (27–98) | 94.50 (75.5–113) | 97 (88–10.50) | <0.01 |
| FEV1/FVC (%) | 81.50 (30–103) | 83 (58–110) | 81 (77–85.60) | 0.04 |
| Exposure factors, n (%) | ||||
| Tobacco smoking (%) | 30 (25.42) | 20 (15.04) | 260 (42.19) | 0.04 |
| Tobacco index | 7.50 (2.40–20) | 2 (1.43–5.38) | 6 (3.20–9.0) | 0.02 |
| Biomass-burning smoke | 31 (26.27) | 20 (15.04) | 65 (10.55) | 0.01 |
| Birds | 21 (17.79) | 7 (5.26) | 37 (6.01) | <0.01 |
| Others | 16 (13.56) | 14 (10.53) | 68 (11.04) | 0.33 |
| No exposure | 29 (24.57) | 99 (74.44) | 319 (51.79) | <0.01 |
| PADI2 SNV | RA-ILD (n = 118) | RA (n = 133) | CHS (n = 616) | Arthritis++ vs. CHS | RA-ILD vs. RA | ||||
|---|---|---|---|---|---|---|---|---|---|
| F% | F% | F% | p-Value | OR | 95% CI | p-Value * | OR | 95% CI | |
| rs2057094 | |||||||||
| GG | 35.59 | 40.54 | 43.67 | 0.015 | 1 | 0.612 | |||
| GA | 23.73 | 22.52 | 30.19 | 0.88 | 0.60–1.30 | ||||
| AA | 40.68 | 36.94 | 26.14 | 1.71 | 1.19–2.44 | ||||
| G | 47.46 | 51.8 | 58.77 | 0.003 | 0.69 | 0.56–0.86 | 0.400 | ||
| A | 52.54 | 48.2 | 41.23 | 1.45 | 1.17–1.80 | ||||
| rs2076615 | |||||||||
| AA | 46.09 | 42.73 | 30.86 | 0.001 | 1 | 0.920 | |||
| AC | 50.43 | 53.64 | 63.86 | 0.57 | 0.41–0.78 | ||||
| CC | 03.48 | 03.64 | 05.28 | 0.47 | 0.21–1.05 | ||||
| A | 71.30 | 69.55 | 62.79 | 0.012 | 1.41 | 1.11–1.78 | 0.757 | ||
| C | 28.70 | 30.45 | 37.21 | 0.71 | 0.56–0.89 | ||||
| rs1005753 | |||||||||
| TT | 48.72 | 63.06 | 46.72 | 0.015 | 1 | 0.041 | 1 | ||
| TG | 44.44 | 35.14 | 44.10 | 0.76 | 0.55–1.04 | 1.63 | 0.95–2.82 | ||
| GG | 06.84 | 01.80 | 09.18 | 0.40 | 0.19–0.81 | 4.91 | 1.00–24.05 | ||
| T | 70.94 | 80.63 | 68.77 | 0.030 | 1.41 | 1.10–1.80 | 0.017 | 0.59 | 0.38–0.90 |
| G | 29.06 | 19.37 | 31.23 | 0.71 | 0.55–0.91 | 1.71 | 1.10–2.64 | ||
| PADI4 SNV | RA-ILD (n = 118) | RA (n = 133) | CHS (n = 616) | Arthritis++ vs. CHS | RA-ILD vs. RA | ||||
|---|---|---|---|---|---|---|---|---|---|
| F% | F% | F% | p-Value | OR | 95% CI | p-Value * | OR | 95% CI | |
| rs11203366 | |||||||||
| GG | 21.55 | 35.96 | 30.50 | 0.852 | 0.004 | 1 | |||
| GA | 52.59 | 50.00 | 47.00 | 1.76 | 0.95–3.25 | ||||
| AA | 25.86 | 14.04 | 20.90 | 3.08 | 1.40–6.74 | ||||
| G | 47.84 | 60.96 | 54.85 | 0.869 | 0.005 | 0.59 | 0.41–0.85 | ||
| A | 52.16 | 39.04 | 45.14 | 1.70 | 1.18–2.47 | ||||
| rs11203367 | |||||||||
| TT | 22.52 | 36.52 | 29.70 | 0.319 | 0.038 | 1 | |||
| TC | 59.46 | 51.30 | 48.30 | 1.88 | 1.02–3.45 | ||||
| CC | 18.02 | 12.17 | 20.60 | 2.40 | 1.03–5.58 | ||||
| T | 52.25 | 62.17 | 54.60 | 0.346 | 0.037 | 0.67 | 0.46–0.97 | ||
| C | 47.75 | 37.83 | 45.40 | 1.50 | 1.03–2.18 | ||||
| rs1748033 | |||||||||
| CC | 16.38 | 14.91 | 25.10 | 0.002 | 1 | 0.998 | |||
| CT | 50.00 | 51.57 | 49.80 | 1.64 | 1.08–2.50 | ||||
| TT | 33.62 | 33.33 | 23.80 | 2.26 | 1.43–3.56 | ||||
| C | 41.38 | 40.79 | 50.66 | 0.002 | 0.68 | 0.55–0.84 | 0.925 | ||
| T | 58.62 | 59.21 | 49.34 | 1.47 | 1.18–1.83 | ||||
| rs874881 | |||||||||
| CC | 23.28 | 44.62 | 24.70 | 0.001 | 1 | 0.002 | 1 | ||
| CG | 59.48 | 42.31 | 51.50 | 0.64 | 0.46–0.90 | 2.69 | 1.51–4.80 | ||
| GG | 17.24 | 13.08 | 22.90 | 0.43 | 0.27–0.68 | 2.53 | 1.15–5.58 | ||
| C | 53.03 | 65.77 | 50.90 | 0.004 | 1.43 | 1.16–1.77 | 0.003 | 0.59 | 0.41–0.84 |
| G | 46.95 | 34.23 | 49.10 | 0.69 | 0.56–0.86 | 1.70 | 1.18–2.45 | ||
| PADI4 Haplotype | Arthritis++ (n = 251, HF%) | CHS (n = 616, HF%) | p | OR (95% CI) |
|---|---|---|---|---|
| GTTC | 41.60 | 35.10 | 0.018 | 1.40 (1.12–1.74) |
| ACCG | 33.30 | 30.80 | 0.501 | |
| GTCG | 9.00 | 13.10 | 0.021 | 0.65 (0.44–0.94) |
| ACTC | 7.00 | 10.60 | 0.029 | 0.64 (0.42–0.94) |
| GTCC | 2.40 | 4.00 | 0.318 | |
| ATTC | 2.30 | 1.10 | 0.215 | |
| GCCG | 2.20 | 1.20 | 0.285 | |
| ACTG | 0.40 | 1.80 | 0.009 | 0.12 (0.02–0.87) |
| PADI4 Haplotype | RA-ILD (n = 118, HF%) | RA (n = 133, HF%) | p | OR (95% CI) |
|---|---|---|---|---|
| GTTC | 39.80 | 47.40 | 0.107 | |
| ACCG | 37.70 | 32.20 | 0.222 | |
| GTCG | 5.00 | 9.90 | 0.051 | |
| ACTC | 7.20 | 2.90 | 0.038 | 2.64 (1.01–6.88) |
| ATTC | 2.30 | 2.50 | 0.889 | |
| GCCG | 2.00 | 2.40 | 0.795 | |
| GTCC | 2.40 | 1.40 | 0.445 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nava-Quiroz, K.J.; Rojas-Serrano, J.; Pérez-Rubio, G.; Buendia-Roldan, I.; Mejía, M.; Fernández-López, J.C.; Rodríguez-Henríquez, P.; Ayala-Alcantar, N.; Ramos-Martínez, E.; López-Flores, L.A.; et al. Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients. Cells 2023, 12, 2235. https://doi.org/10.3390/cells12182235
Nava-Quiroz KJ, Rojas-Serrano J, Pérez-Rubio G, Buendia-Roldan I, Mejía M, Fernández-López JC, Rodríguez-Henríquez P, Ayala-Alcantar N, Ramos-Martínez E, López-Flores LA, et al. Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients. Cells. 2023; 12(18):2235. https://doi.org/10.3390/cells12182235
Chicago/Turabian StyleNava-Quiroz, Karol J., Jorge Rojas-Serrano, Gloria Pérez-Rubio, Ivette Buendia-Roldan, Mayra Mejía, Juan Carlos Fernández-López, Pedro Rodríguez-Henríquez, Noé Ayala-Alcantar, Espiridión Ramos-Martínez, Luis Alberto López-Flores, and et al. 2023. "Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients" Cells 12, no. 18: 2235. https://doi.org/10.3390/cells12182235
APA StyleNava-Quiroz, K. J., Rojas-Serrano, J., Pérez-Rubio, G., Buendia-Roldan, I., Mejía, M., Fernández-López, J. C., Rodríguez-Henríquez, P., Ayala-Alcantar, N., Ramos-Martínez, E., López-Flores, L. A., Del Ángel-Pablo, A. D., & Falfán-Valencia, R. (2023). Molecular Factors in PAD2 (PADI2) and PAD4 (PADI4) Are Associated with Interstitial Lung Disease Susceptibility in Rheumatoid Arthritis Patients. Cells, 12(18), 2235. https://doi.org/10.3390/cells12182235











