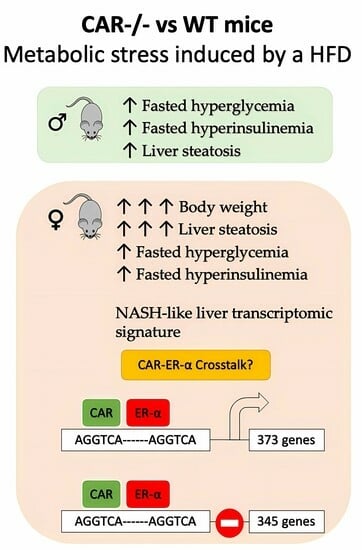
CAR Protects Females from Diet-Induced Steatosis and Associated Metabolic Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Oral Glucose Tolerance Tests
2.3. Liver Histological Sections and Scoring
2.4. Hepatic Neutral Lipids Analysis
2.5. Plasma Analysis
2.6. Microarray and qPCR Gene Expression Studies
2.7. Brain Immunohistochemistry
2.8. Data Representation and Statistical Analysis
3. Results
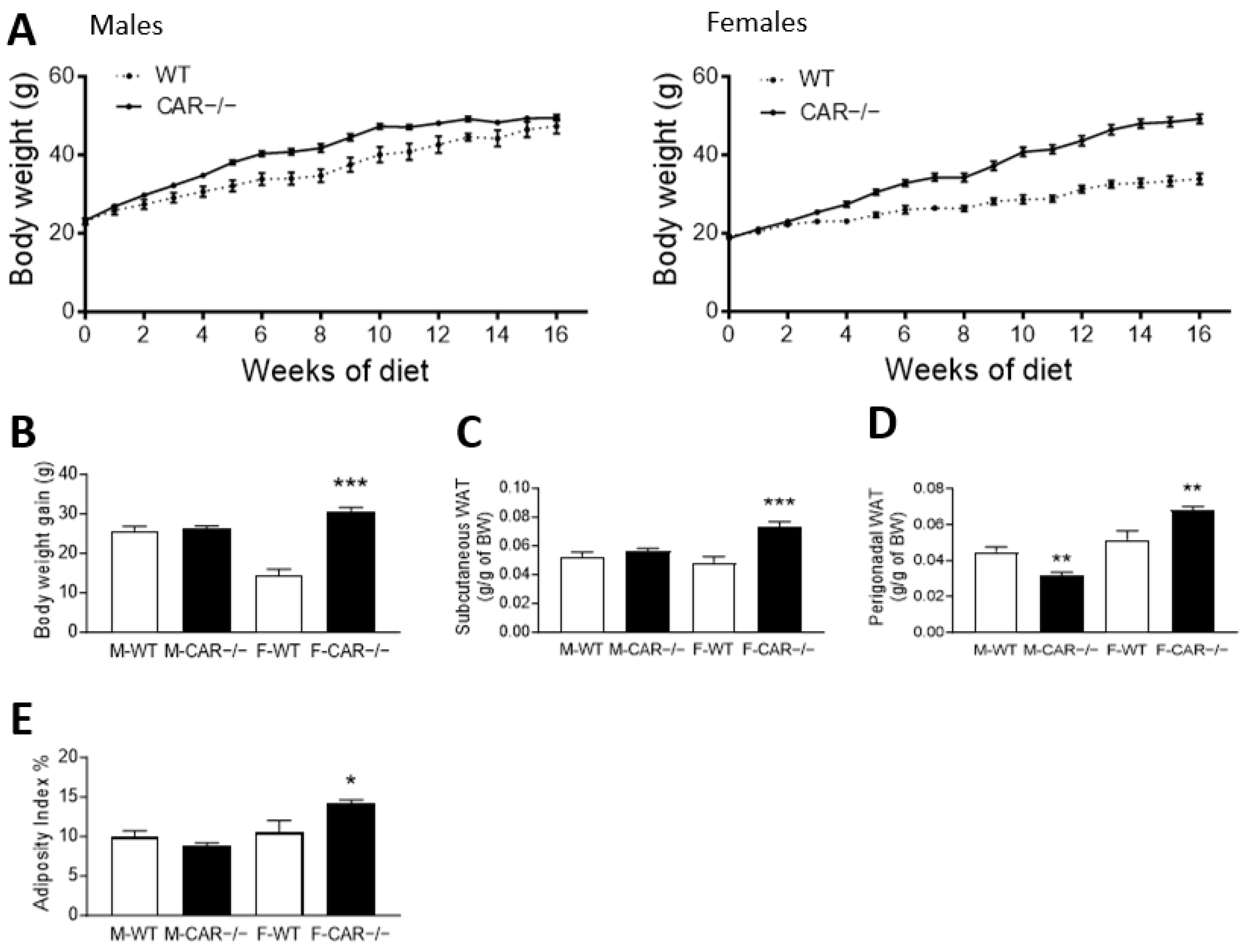
3.1. CAR Deletion Exacerbates HFD-Induced Body Weight Gain Only in Female Mice
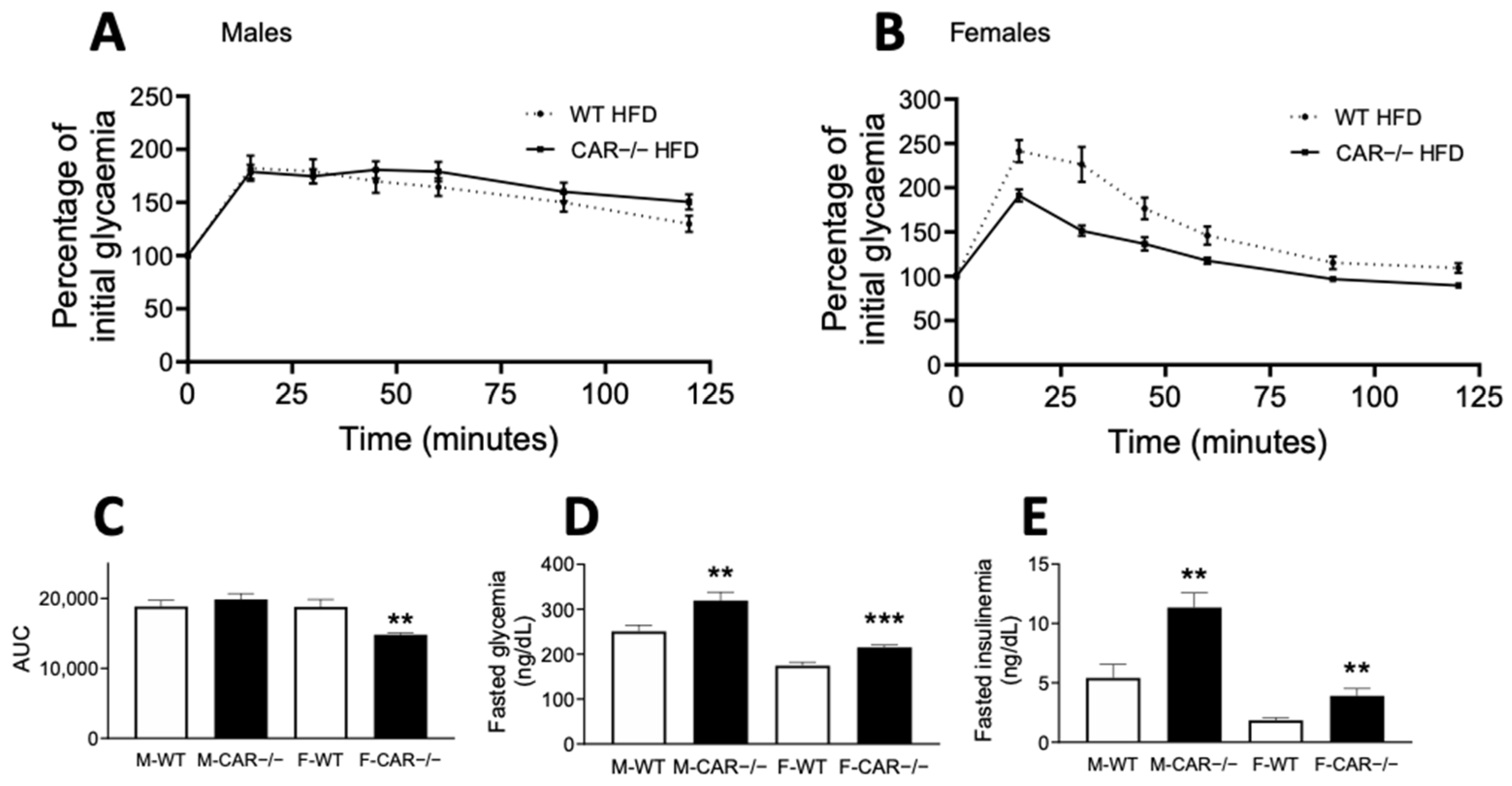
3.2. CAR Deletion Exacerbates HFD-Induced Hyperglycemia and Hyperinsulinemia
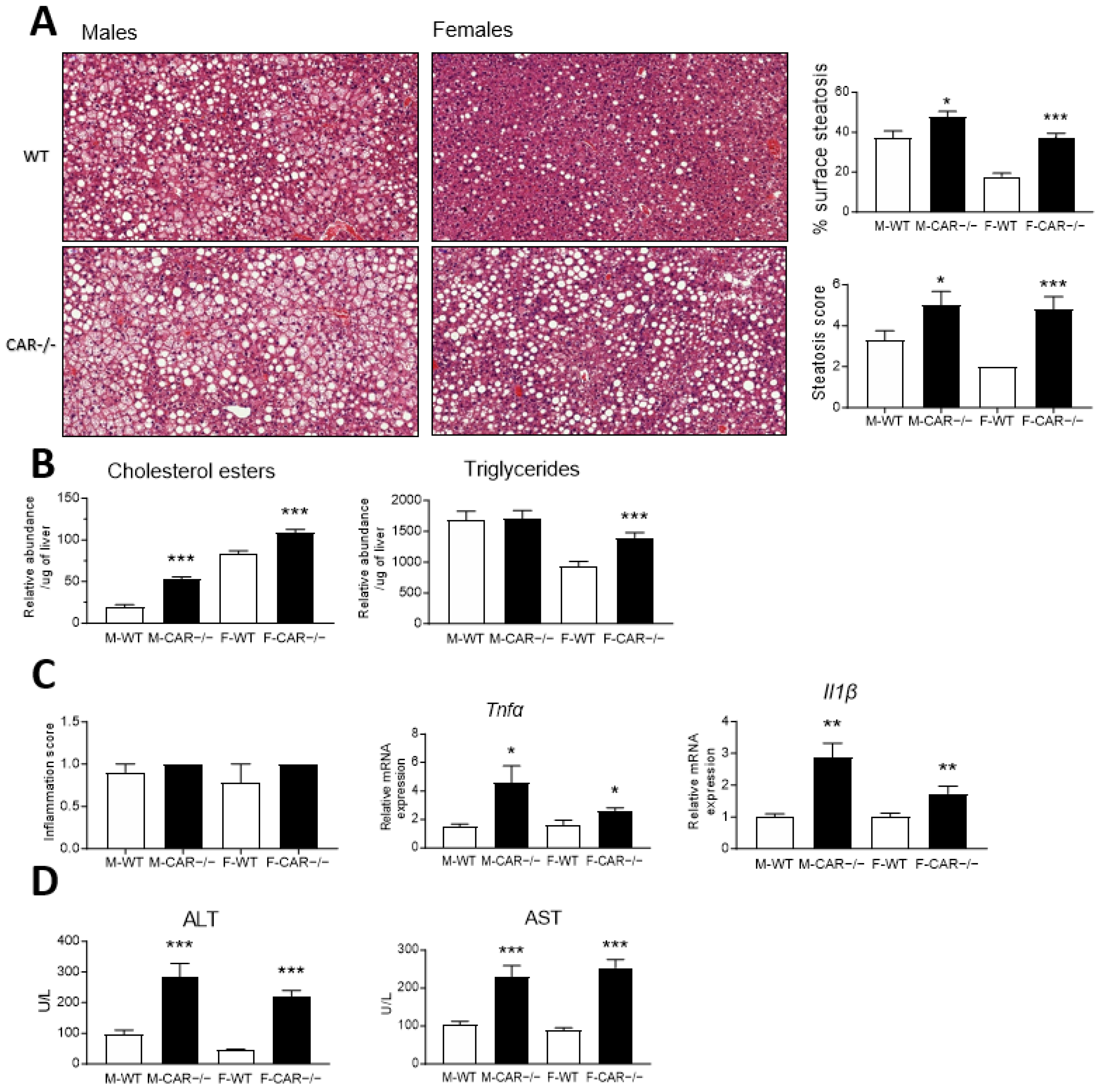
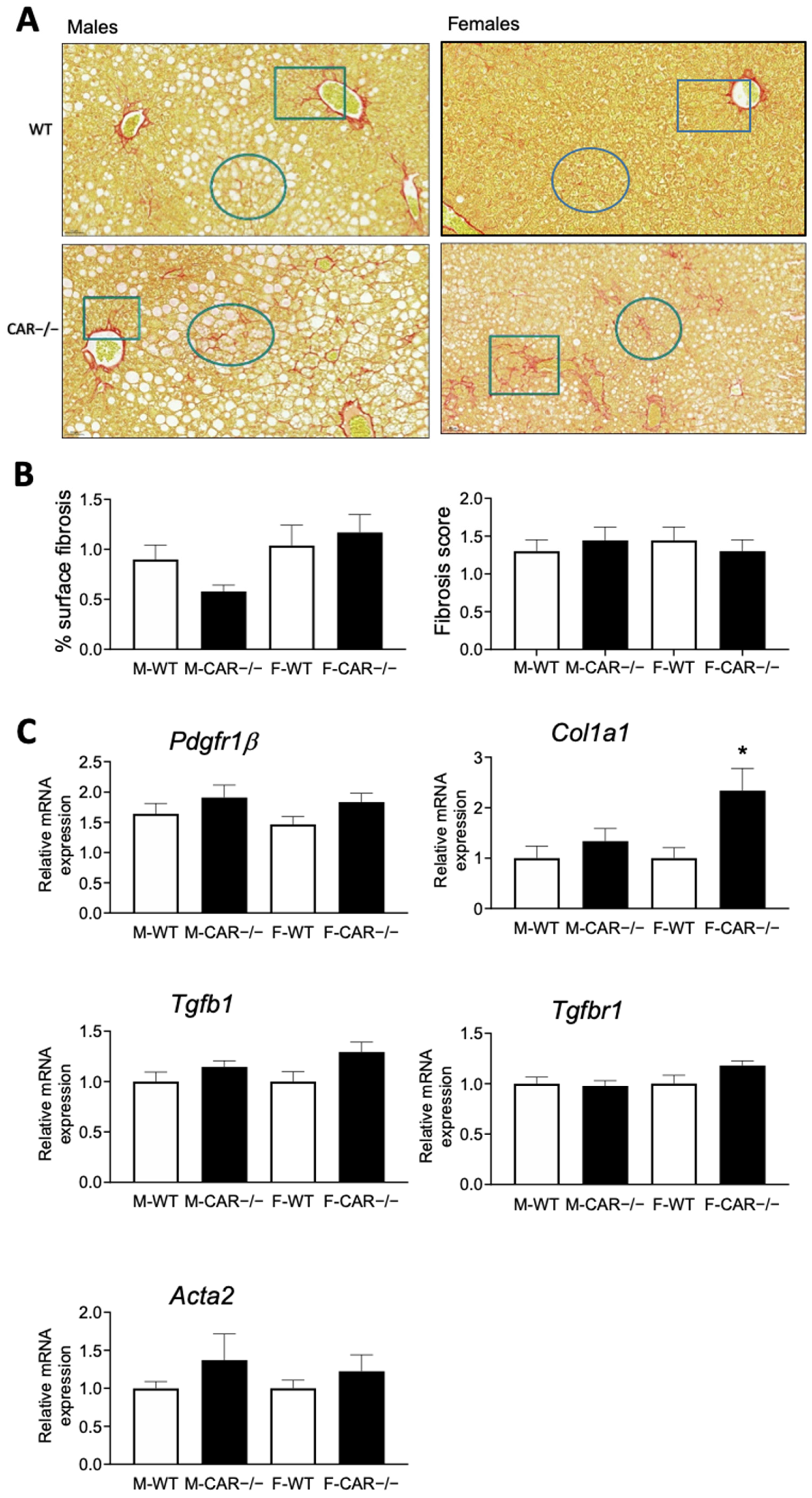
3.3. CAR Deletion Exacerbates HFD-Induced Hepatic Steatosis and Liver Injury
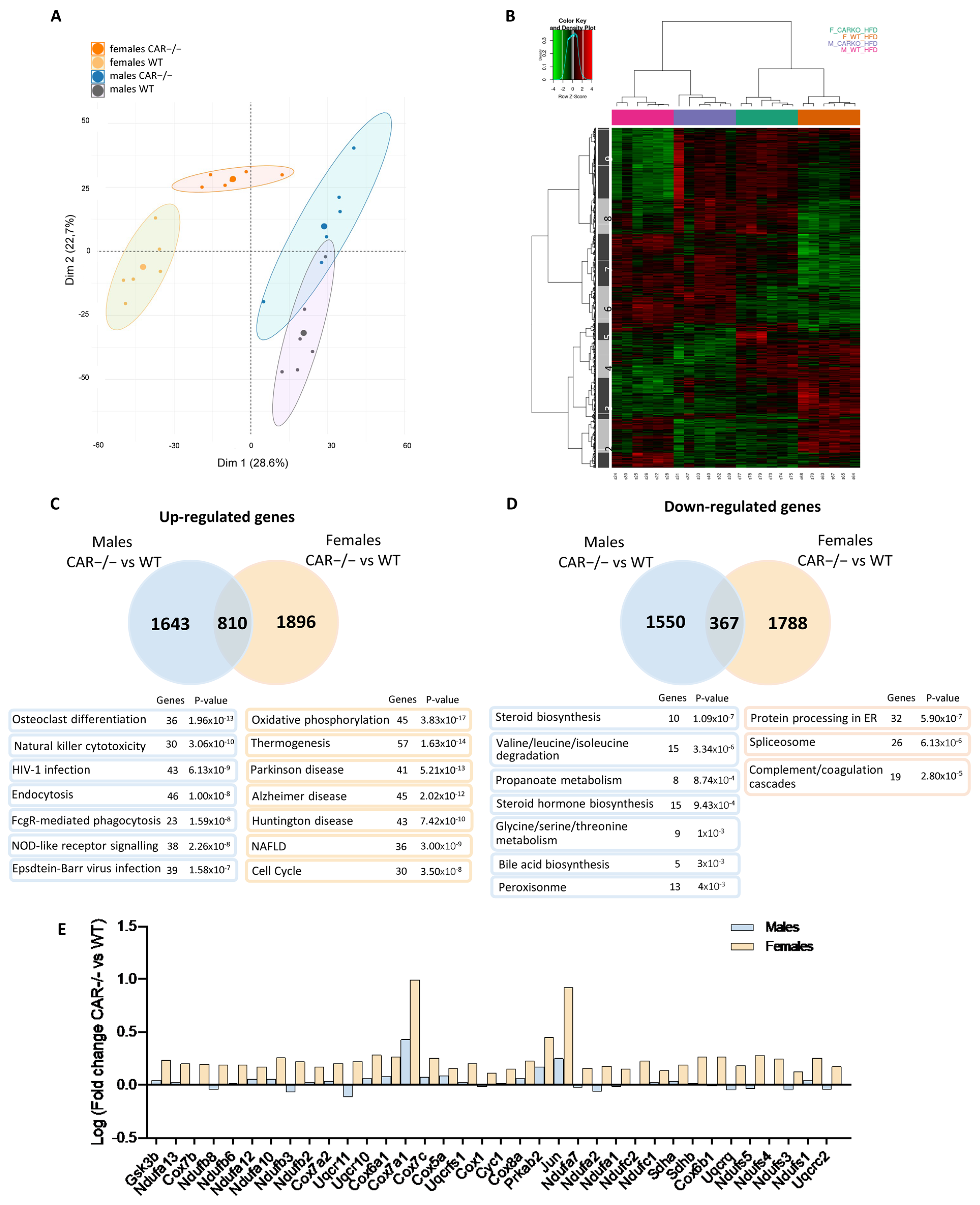
3.4. Dimorphic Impact of CAR Deletion on Hepatic Transcriptome
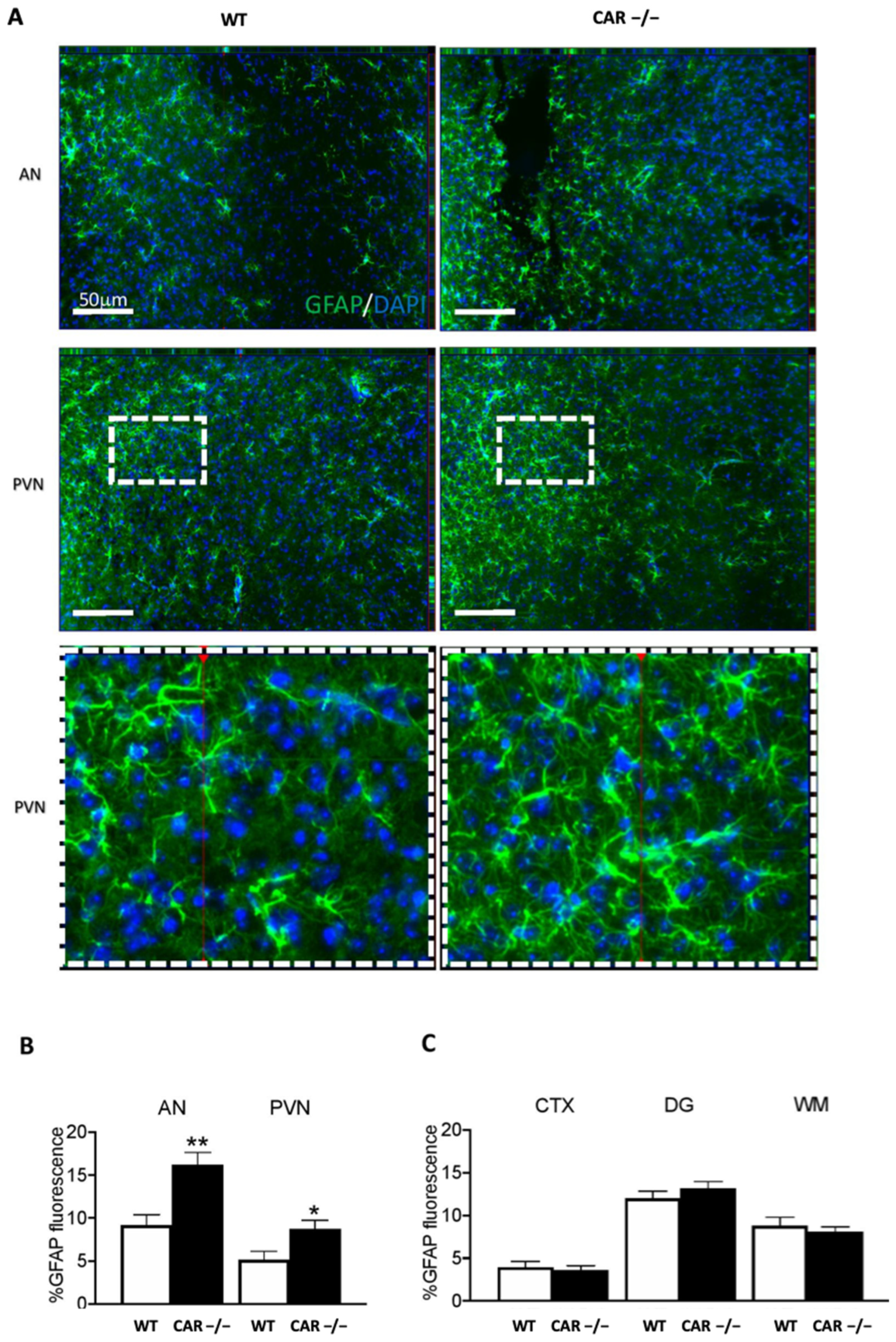
3.5. CAR Deletion Associates with Hypothalamic Astrogliosis in HFD-Fed Females: Initial Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.; Kim, W.R.; Kim, H.J.; Therneau, T.M. Association Between Noninvasive Fibrosis Markers and Mortality Among Adults With Nonalcoholic Fatty Liver Disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef] [PubMed]
- Qatanani, M.; Zhang, J.; Moore, D.D. Role of the Constitutive Androstane Receptor in Xenobiotic-Induced Thyroid Hormone Metabolism. Endocrinology 2005, 146, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yeuh, M.-F.; Radominska-Pandya, A.; Saini, S.P.S.; Negishi, Y.; Bottroff, B.S.; Cabrera, G.Y.; Tukey, R.H.; Evans, R.M. Control of Steroid, Heme, and Carcinogen Metabolism by Nuclear Pregnane X Receptor and Constitutive Androstane Receptor. Proc. Natl. Acad. Sci. USA 2003, 100, 4150–4155. [Google Scholar] [CrossRef]
- Lynch, C.; Pan, Y.; Li, L.; Heyward, S.; Moeller, T.; Swaan, P.W.; Wang, H. Activation of the Constitutive Androstane Receptor Inhibits Gluconeogenesis without Affecting Lipogenesis or Fatty Acid Synthesis in Human Hepatocytes. Toxicol. Appl. Pharmacol. 2014, 279, 33–42. [Google Scholar] [CrossRef]
- Gao, J.; He, J.; Zhai, Y.; Wada, T.; Xie, W. The Constitutive Androstane Receptor Is an Anti-Obesity Nuclear Receptor That Improves Insulin Sensitivity. J. Biol. Chem. 2009, 284, 25984–25992. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and Validation of a Histological Scoring System for Nonalcoholic Fatty Liver Disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Régnier, M.; Polizzi, A.; Lippi, Y.; Fouché, E.; Michel, G.; Lukowicz, C.; Smati, S.; Marrot, A.; Lasserre, F.; Naylies, C.; et al. Insights into the Role of Hepatocyte PPARα Activity in Response to Fasting. Mol. Cell. Endocrinol. 2017, 471, 75–88. [Google Scholar] [CrossRef]
- Lukowicz, C.; Ellero-Simatos, S.; Régnier, M.; Oliviero, F.; Lasserre, F.; Polizzi, A.; Montagner, A.; Smati, S.; Boudou, F.; Lenfant, F.; et al. Dimorphic Metabolic and Endocrine Disorders in Mice Lacking the Constitutive Androstane Receptor. Sci. Rep. 2019, 9, 20169. [Google Scholar] [CrossRef]
- Huber, W.; Carey, V.J.; Gentleman, R.; Anders, S.; Carlson, M.; Carvalho, B.S.; Bravo, H.C.; Davis, S.; Gatto, L.; Girke, T.; et al. Orchestrating High-Throughput Genomic Analysis with Bioconductor. Nat. Methods 2015, 12, 115–121. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Åstrand, M.; Speed, T.P. A Comparison of Normalization Methods for High Density Oligonucleotide Array Data Based on Variance and Bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Benjamini, Y. Discovering the False Discovery Rate. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2010, 72, 405–416. [Google Scholar] [CrossRef]
- Boussadia, B.; Gangarossa, G.; Mselli-Lakhal, L.; Rousset, M.-C.; de Bock, F.; Lassere, F.; Ghosh, C.; Pascussi, J.-M.; Janigro, D.; Marchi, N. Lack of CAR Impacts Neuronal Function and Cerebrovascular Integrity in Vivo. Exp. Neurol. 2016, 283, 39–48. [Google Scholar] [CrossRef]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex Differences in Metabolic Regulation and Diabetes Susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Saha, P.K.; Huang, W.; Chen, W.; Abu-Elheiga, L.A.; Wakil, S.J.; Stevens, R.D.; Ilkayeva, O.; Newgard, C.B.; Chan, L.; et al. Activation of Nuclear Receptor CAR Ameliorates Diabetes and Fatty Liver Disease. Proc. Natl. Acad. Sci. USA 2009, 106, 18831–18836. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.I.; Kim, K.M.; Choi, I.; Pratley, R.E.; Lee, Y.-H. Improved glucose tolerance with restored expression of glucose transporter 4 in C57BL/6 mice after a long period of high-fat diet feeding. Anim. Cells Syst. 2014, 18, 197–203. [Google Scholar] [CrossRef]
- Takizawa, D.; Kakizaki, S.; Horiguchi, N.; Yamazaki, Y.; Tojima, H.; Mori, M. Constitutive Active/Androstane Receptor Promotes Hepatocarcinogenesis in a Mouse Model of Non-Alcoholic Steatohepatitis. Carcinogenesis 2011, 32, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Kettner, N.M.; Voicu, H.; Finegold, M.J.; Coarfa, C.; Sreekumar, A.; Putluri, N.; Katchy, C.A.; Lee, C.; Moore, D.D.; Fu, L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell 2016, 30, 909–924. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef]
- Pancani, T.; Anderson, K.L.; Brewer, L.D.; Kadish, I.; DeMoll, C.; Landfield, P.W.; Blalock, E.M.; Porter, N.M.; Thibault, O. Effect of High-Fat Diet on Metabolic Indices, Cognition, and Neuronal Physiology in Aging F344 Rats. Neurobiol. Aging 2013, 34, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.; Allaman, I.; Magistretti, P.J. Brain Energy Metabolism: Focus on Astrocyte-Neuron Metabolic Cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Thompson, M.M.; Lippert, R.N.; Blackwell, T.S.; Yull, F.E.; Ellacott, K.L.J. Evidence for a Novel Functional Role of Astrocytes in the Acute Homeostatic Response to High-Fat Diet Intake in Mice. Mol. Metab. 2014, 4, 58–63. [Google Scholar] [CrossRef]
- Kumar, R.; Mota, L.C.; Litoff, E.J.; Rooney, J.P.; Tyler Boswell, W.; Courter, E.; Henderson, C.M.; Hernandez, J.P.; Christopher Corton, J.; Moore, D.D.; et al. Compensatory Changes in CYP Expression in Three Different Toxicology Mouse Models: CAR-Null, Cyp3a-Null, and Cyp2b9/10/13-Null Mice. PLoS ONE 2017, 12, e0174355. [Google Scholar] [CrossRef] [PubMed]
- Oshida, K.; Vasani, N.; Waxman, D.J.; Corton, J.C. Disruption of STAT5b-Regulated Sexual Dimorphism of the Liver Transcriptome by Diverse Factors Is a Common Event. PLoS ONE 2016, 11, e0148308. [Google Scholar] [CrossRef]
- Abumrad, N.A. The Liver as a Hub in Thermogenesis. Cell Metab. 2017, 26, 454–455. [Google Scholar] [CrossRef]
- Guillaume, M.; Riant, E.; Fabre, A.; Raymond-Letron, I.; Buscato, M.; Davezac, M.; Tramunt, B.; Montagner, A.; Smati, S.; Zahreddine, R.; et al. Selective Liver Estrogen Receptor α Modulation Prevents Steatosis, Diabetes, and Obesity Through the Anorectic Growth Differentiation Factor 15 Hepatokine in Mice. Hepatol. Commun. 2019, 3, 908–924. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Jung, Y. Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Cells 2019, 8, 1259. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Y.; Wang, E.; Zhang, Z.; Xiong, X.; Zhang, H.; Lu, J.; Zheng, S.; Yang, J.; Xia, X.; et al. Hepatic Estrogen Receptor α Improves Hepatosteatosis through Upregulation of Small Heterodimer Partner. J. Hepatol. 2015, 63, 183–190. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliviero, F.; Klement, W.; Mary, L.; Dauwe, Y.; Lippi, Y.; Naylies, C.; Gayrard, V.; Marchi, N.; Mselli-Lakhal, L. CAR Protects Females from Diet-Induced Steatosis and Associated Metabolic Disorders. Cells 2023, 12, 2218. https://doi.org/10.3390/cells12182218
Oliviero F, Klement W, Mary L, Dauwe Y, Lippi Y, Naylies C, Gayrard V, Marchi N, Mselli-Lakhal L. CAR Protects Females from Diet-Induced Steatosis and Associated Metabolic Disorders. Cells. 2023; 12(18):2218. https://doi.org/10.3390/cells12182218
Chicago/Turabian StyleOliviero, Fabiana, Wendy Klement, Lucile Mary, Yannick Dauwe, Yannick Lippi, Claire Naylies, Véronique Gayrard, Nicola Marchi, and Laila Mselli-Lakhal. 2023. "CAR Protects Females from Diet-Induced Steatosis and Associated Metabolic Disorders" Cells 12, no. 18: 2218. https://doi.org/10.3390/cells12182218
APA StyleOliviero, F., Klement, W., Mary, L., Dauwe, Y., Lippi, Y., Naylies, C., Gayrard, V., Marchi, N., & Mselli-Lakhal, L. (2023). CAR Protects Females from Diet-Induced Steatosis and Associated Metabolic Disorders. Cells, 12(18), 2218. https://doi.org/10.3390/cells12182218