Shared Molecular Pathways in Glaucoma and Other Neurodegenerative Diseases: Insights from RNA-Seq Analysis and miRNA Regulation for Promising Therapeutic Avenues
Abstract
:1. Introduction
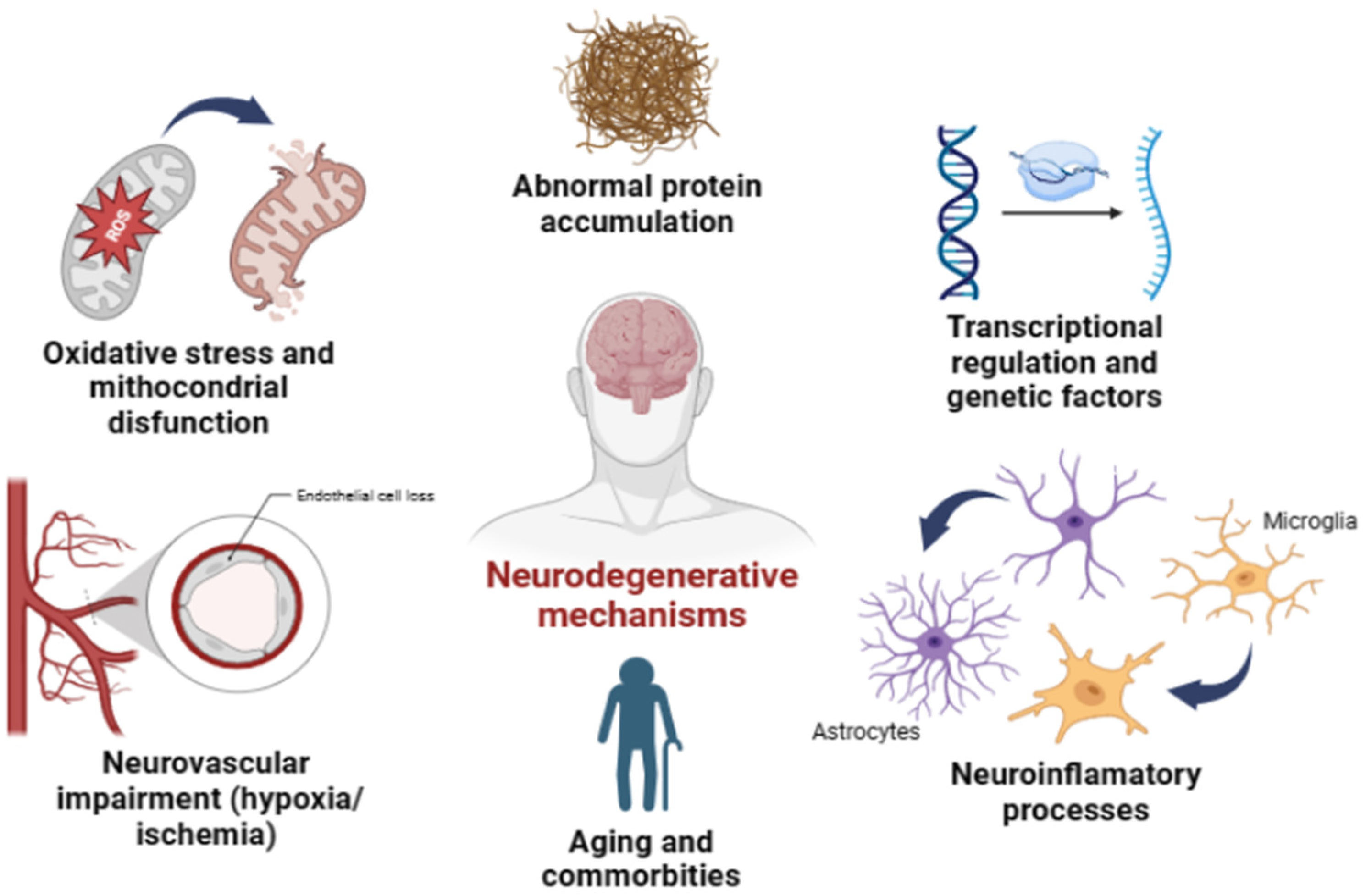
2. Glaucoma Similarity Hallmarks with Other Neurodegenerative Diseases
3. RNA-Seq Analysis as a Tool to Search for Similar Pathway Regulation Mechanisms in Neurodegenerative Diseases
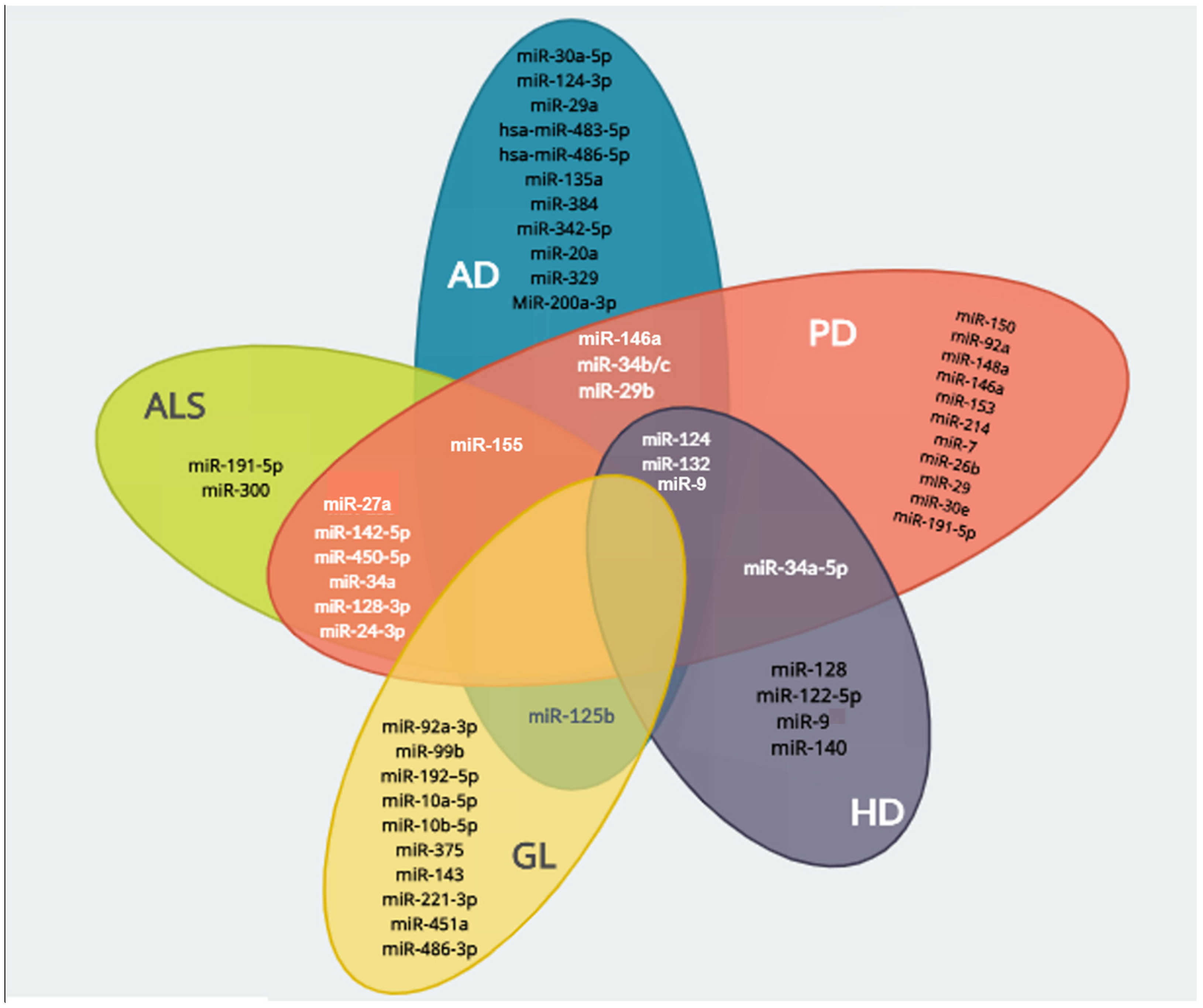
4. miRNAs as Potential Therapeutic Agents in Neurodegenerative Disorders
| Disease | miRNA | Targeted Genes | Physiological Process | Reference |
|---|---|---|---|---|
| Alzheimer’s disease | miR-124 | BACE1 | Reduction of Aβ oligomers production and neuroprotection | [81] |
| miR-34b | VAMP2, SYT1, BCL2 | Regulation of presynaptic activity and anti-apoptotic mechanisms | [111] | |
| miR-30a | BDNF | Regulation of synaptic plasticity and neuroprotective mechanisms | [112,113] | |
| miR-34c | P53, SIRT1 | Neuronal development | ||
| miR-125b | BACE1 | Attenuated Aβ toxicity in Aβ-treated N2a cells via targeting BACE1 | [114] | |
| miR-132 | No reports | Prevents the apoptosis of neurons and is involved in the regulation of synaptic plasticity, learning and memory, as well as in reducing tau hyperphosphorylation | [19,115,116] | |
| miR-124 | No reports | Regulates the hyperphosphorylation of the tau protein in cell cultures via the PI3K/AKT/GSK-3 pathway | [94] | |
| miR-29a miR-29b | BACE1 | Regulation of APP and beta-site APP-cleaving enzyme 1 (BACE1) | [117] | |
| hsa-miR-483 hsa-miR-486 | MAPK1/3 | Direct repression of Erk1/2 and reduction of tau phosphorylation | [118,119] | |
| miR-135a miR-384 | No reports | Repression of BACE-1 and APP-cleavage | [120] | |
| miR-342 | ANK3 | Regulation of neurogenesis and neuroprotection in an AD mouse model | [121] | |
| miR-20a | BCL2, MEF2D, MAP3K12 | Regulation of gene expression during brain development. | [122] | |
| miR-329 | No reports | Regulation of activity-dependent dendritic outgrowth of hippocampal neurons | ||
| MiR-200a | BACE1, PRKACB | Reduces Aβ accumulation and tau hyperphosphorylation, respectively | [123] | |
| Parkinson’s disease | miR-150 | AKT3 | Downregulation of the proinflammatory cytokines IL-1β, IL-6 and TNF-α | [114] |
| miR-124 | No reports | Attenuates microglia activation, improves survival of dopaminergic neurons and reduces α-synuclein aggregation | [98,99] | |
| miR-132 miR-92a miR-27a miR-148a | GLRX | Regulates the activation and loss of microglial cells | [111,124] | |
| miR-146a | No reports | Regulation of anti-inflammatory mechanisms | [104] | |
| miR-153 miR-214 miR-34b/c miR-7 | No reports | Downregulation of α-synuclein expression, preventing its neurotoxicity | [125,126,127,128] | |
| miR-26b | TAK1, TAB3 | Suppressing neuroinflammation by downregulating the activators of NF-Kβ | [129] | |
| miR-34a | D1, SIRT1, BCL-2 | Neuronal development, brain ageing metabolic regulation p53/miR-34a/SIRT1 pathway | [114] | |
| miR-29 | No reports | Regulates T helper 1 (Th1) differentiation and targets the transcription factors T-bet and Eomes, resulting in the repression of IFN-γ production | [130] | |
| mir-29b | BAD, BIM, BID, BIK/NBK, BNIP3, BLK, HRK, NOXA, and EGL-1 | Regulates neuronal survival by targeting genes in the pro-apoptotic BH3-only family to inhibit apoptosis | [131] | |
| miR-30e | NLRP3 | Regulates neuroinflammation by reducing the inflammatory cytokines TNF-α, COX-2 and iNOS | [132] | |
| Amyotrophic Lateral Sclerosis | miR-128 | THY1 | p53 Pro-apoptotic pathway regulation | [133,134] |
| miR-191 | BDNF | Neuronal and immune cell apoptosis regulation | [133] | |
| miR-24 | BIM, PUMA | Thy1/ Thy2 balance regulation | ||
| miR-27a miR-155 miR-142 | No reports | Regulation and control of oxidative stress | [135,136] | |
| miR-300 | VASH2 | Neuron differentiation | [137] | |
| miR-450b | SOX2, PTPRZ1 | Neuron differentiation and neurogenesis regulation | ||
| miR-34a | XIAP | Protective against oxidative stress-induced apoptosis through SIRT1 | [5,92] | |
| Huntington’s disease | miR-124a | No reports | Regulator of neuronal differentiation and morphology | [100,138] |
| miR-128 | HTT | Neuronal survival, metabolic pathways, particularly cholesterol (affected by mutant HTT) | [139] | |
| miR-122 | AACS, ADAM10, BCL2 | |||
| miR-132 | ITPKB | Neuronal development and survival | [111,140] | |
| miR-9 | HTT, CoREST | Increases the expression of RE1 in leukocytes | [89] | |
| miR-140 | ADAM10 | Synaptic function regulation | [90] | |
| miR-34a | D1, SIRT1, BCL-2 | Neuronal development and brain ageing metabolic regulation p53/miR-34a/SIRT1 pathway | [141] | |
| Glaucoma | miR-92a | KALRN | Axonal guidance signaling, Ephrin B signaling, synaptogenesis signaling pathway | [142] |
| miR-99b | No reports | Regulation of autophagy, senescence, neuroinflammation | [143] | |
| miR-125b | LIN28B | Adhesion, tight junctions and TGF-β signaling | [141] | |
| miR-192 miR-10a miR-10b | No reports | Neurogenesis, aging, apoptosis and autophagy | [144] | |
| miR-375 | BDNF | |||
| miR-143 | LMO4 | Regulation of autophagy, apoptosis, senescence, neuroinflammation | [145] | |
| miR-221 | No reports | TGF-β and neurotrophin signaling | [146] | |
| miR-451a | No reports | Adhesion, tight junctions and TGF-β signaling | [147] | |
| miR-486 | LMCM, LMX1B, PTPN1 | TGF-β signaling regulation | [146] | |
| TXNRD2 | Antioxidant action of vitamin C, mitochondrial dysfunction, thioredoxin pathway, vitamin C transport | |||
| miR-124 miR-204 | No reports | Development and maintenance of retinal cells in adult mice. | [95] | |
| miR-29 | p53 | TGF-β signaling regulation and antioxidant effects. | [97] |
5. Challenges and Potential Solutions for miRNA Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative Diseases and Oxidatives Stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Simonato, M.; Bennett, J.; Boulis, N.M.; Castro, M.G.; Fink, D.J.; Goins, W.F.; Gray, S.J.; Lowenstein, P.R.; Vandenberghe, L.H.; Wilson, T.J.; et al. Progress in Gene Therapy for Neurological Disorders. Nat. Rev. Neurol. 2013, 9, 277–291. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative Diseases Target Large-Scale Human Brain Networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef]
- Matheus, F.C.; Rial, D.; Real, J.I.; Lemos, C.; Ben, J.; Guaita, G.O.; Pita, I.R.; Sequeira, A.C.; Pereira, F.C.; Walz, R.; et al. Decreased Synaptic Plasticity in the Medial Prefrontal Cortex Underlies Short-Term Memory Deficits in 6-OHDA-Lesioned Rats. Behav. Brain Res. 2016, 301, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Hanson, P.S.; Morris, C.M. SIRT1 Ameliorates Oxidative Stress Induced Neural Cell Death and Is Down-Regulated in Parkinson’s Disease. BMC Neurosci. 2017, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pires, E.N.; Ferreira, S.T.; Linden, R. Roles of Glutamate Receptors in a Novel in Vitro Model of Early, Comorbid Cerebrovascular, and Alzheimer’s Diseases. J. Neurochem. 2021, 156, 539–552. [Google Scholar] [CrossRef]
- Gupta, N.; Fong, J.; Ang, L.C.; Yücel, Y.H. Retinal Tau Pathology in Human Glaucomas. Can. J. Ophthalmol. 2008, 43, 53–60. [Google Scholar] [CrossRef]
- Moon, J.Y.; Kim, H.J.; Park, Y.H.; Park, T.K.; Park, E.C.; Kim, C.Y.; Lee, S.H. Association between Open-Angle Glaucoma and the Risks of Alzheimer’s and Parkinson’s Diseases in South Korea: A 10-Year Nationwide Cohort Study. Sci. Rep. 2018, 24, 11161. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Dias, M.S.; Luo, X.; Ribas, V.T.; Petrs-Silva, H.; Koch, J.C. The Role of Axonal Transport in Glaucoma. Int. J. Mol. Sci. 2022, 23, 3935. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; Volume 214, ISBN 9789241516570. [Google Scholar]
- Swenor, B.K.; Ehrlich, J.R. Ageing and Vision Loss: Looking to the Future. Lancet Glob. Health 2021, 9, e385–e386. [Google Scholar] [CrossRef]
- Bayer, A.U.; Keller, O.N.; Ferrari, F.; Maag, K.P. Association of Glaucoma with Neurodegenerative Diseases with Apoptotic Cell Death: Alzheimer’s Disease and Parkinson’s Disease. Am. J. Ophthalmol. 2002, 133, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, V.; Rezania, F.; Trounce, I.A.; Crowston, J.G. Oxidative Stress and Mitochondrial Dysfunction in Glaucoma. Curr. Opin. Pharmacol. 2013, 13, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Llorens, F.; Thüne, K.; Martí, E.; Kanata, E.; Dafou, D.; Díaz-Lucena, D.; Vivancos, A.; Shomroni, O.; Zafar, S.; Schmitz, M.; et al. Regional and Subtype-Dependent MiRNA Signatures in Sporadic Creutzfeldt-Jakob Disease Are Accompanied by Alterations in MiRNA Silencing Machinery and Biogenesis. PLoS Pathog. 2018, 14, e1006802. [Google Scholar] [CrossRef]
- Kumar, A.; Kopra, J.; Varendi, K.; Porokuokka, L.L.; Panhelainen, A.; Kuure, S.; Marshall, P.; Karalija, N.; Härma, M.A.; Vilenius, C.; et al. GDNF Overexpression from the Native Locus Reveals Its Role in the Nigrostriatal Dopaminergic System Function. PLoS Genet. 2015, 11, e1005710. [Google Scholar] [CrossRef]
- Islam, M.R.; Kaurani, L.; Berulava, T.; Heilbronner, U.; Budde, M.; Centeno, T.P.; Elerdashvili, V.; Zafieriou, M.; Benito, E.; Sertel, S.; et al. A MicroRNA Signature That Correlates with Cognition and Is a Target against Cognitive Decline. EMBO Mol. Med. 2021, 13, e13659. [Google Scholar] [CrossRef] [PubMed]
- Cogswell, J.P.; Ward, J.; Taylor, I.A.; Waters, M.; Shi, Y.; Cannon, B.; Kelnar, K.; Kemppainen, J.; Brown, D.; Chen, C.; et al. Identification of MiRNA Changes in Alzheimer’s Disease Brain and CSF Yields Putative Biomarkers and Insights into Disease Pathways. J. Alzheimer’s Dis. 2008, 14, 27–41. [Google Scholar] [CrossRef]
- Lau, P.; Bossers, K.; Janky, R.; Salta, E.; Frigerio, C.S.; Barbash, S.; Rothman, R.; Sierksma, A.S.R.; Thathiah, A.; Greenberg, D.; et al. Alteration of the MicroRNA Network during the Progression of Alzheimer’s Disease. EMBO Mol. Med. 2013, 5, 1613–1634. [Google Scholar] [CrossRef]
- Kanach, C.; Blusztajn, J.K.; Fischer, A.; Delalle, I. MicroRNAs as Candidate Biomarkers for Alzheimer’s Disease. Non-Coding RNA 2021, 7, 8. [Google Scholar] [CrossRef]
- Miñones-Moyano, E.; Porta, S.; Escaramís, G.; Rabionet, R.; Iraola, S.; Kagerbauer, B.; Espinosa-Parrilla, Y.; Ferrer, I.; Estivill, X.; Martí, E. MicroRNA Profiling of Parkinson’s Disease Brains Identifies Early Downregulation of MiR-34b/c Which Modulate Mitochondrial Function. Hum. Mol. Genet. 2011, 20, 3067–3078. [Google Scholar] [CrossRef]
- Martí, E.; Pantano, L.; Bañez-Coronel, M.; Llorens, F.; Miñones-Moyano, E.; Porta, S.; Sumoy, L.; Ferrer, I.; Estivill, X. A Myriad of MiRNA Variants in Control and Huntington’s Disease Brain Regions Detected by Massively Parallel Sequencing. Nucleic Acids Res. 2010, 38, 7219–7235. [Google Scholar] [CrossRef] [PubMed]
- Jindal, V. Glaucoma: An Extension of Various Chronic Neurodegenerative Disorders. Mol. Neurobiol. 2013, 48, 186–189. [Google Scholar] [CrossRef]
- Marchesi, N.; Fahmideh, F.; Boschi, F.; Pascale, A.; Barbieri, A. Ocular Neurodegenerative Diseases: Interconnection between Retina and Cortical Areas. Cells 2021, 10, 2394. [Google Scholar] [CrossRef]
- Snyder, P.J.; Alber, J.; Alt, C.; Bain, L.J.; Bouma, B.E.; Bouwman, F.H.; DeBuc, D.C.; Campbell, M.C.W.; Carrillo, M.C.; Chew, E.Y.; et al. Retinal Imaging in Alzheimer’s and Neurodegenerative Diseases. Alzheimer’s Dement. 2021, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Calkins, D.J. The Neurovascular Unit in Glaucomatous Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 452. [Google Scholar] [CrossRef] [PubMed]
- Calkins, D.J. Adaptive Responses to Neurodegenerative Stress in Glaucoma. Prog. Retin. Eye Res. 2021, 84, 100953. [Google Scholar] [CrossRef]
- Weinreb, R.N. Ocular Hypertension: Defining Risks and Clinical Options. Am. J. Ophthalmol. 2004, 138, S1–S2. [Google Scholar] [CrossRef]
- Tamm, E.R.; Ethier, C.R.; Dowling, J.E.; Downs, C.; Ellisman, M.H.; Fisher, S.; Fortune, B.; Fruttiger, M.; Jakobs, T.; Lewis, G.; et al. Biological Aspects of Axonal Damage in Glaucoma: A Brief Review. Exp. Eye Res. 2017, 157, 5–12. [Google Scholar] [CrossRef]
- Lawlor, M.; Danesh-Meyer, H.; Levin, L.A.; Davagnanam, I.; De Vita, E.; Plant, G.T. Glaucoma and the Brain: Trans-Synaptic Degeneration, Structural Change, and Implications for Neuroprotection. Surv. Ophthalmol. 2018, 63, 296–306. [Google Scholar] [CrossRef]
- Rojas, P.; Ramírez, A.I.; Fernández-Albarral, J.A.; López-Cuenca, I.; Salobrar-García, E.; Cadena, M.; Elvira-Hurtado, L.; Salazar, J.J.; de Hoz, R.; Ramírez, J.M. Amyotrophic Lateral Sclerosis: A Neurodegenerative Motor Neuron Disease With Ocular Involvement. Front. Neurosci. 2020, 14, 566858. [Google Scholar] [CrossRef]
- Soldatov, V.O.; Kukharsky, M.S.; Belykh, A.E.; Sobolev, A.M.; Deykin, A.V. Retinal Damage in Amyotrophic Lateral Sclerosis: Underlying Mechanisms. Eye Brain 2021, 3, 131–146. [Google Scholar] [CrossRef] [PubMed]
- O’Bryhim, B.E.; Apte, R.S.; Kung, N.; Coble, D.; Van Stavern, G.P. Association of Preclinical Alzheimer Disease with Optical Coherence Tomographic Angiography Findings. JAMA Ophthalmol. 2018, 136, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, D.; Ji, J.; Wang, Y.; Zhang, R. Central Retina Changes in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2021, 268, 4646–4654. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, S.J.; Lehman, D.M.; Kerrigan-Baumrind, L.A.; Merges, C.A.; Pease, M.E.; Kerrigan, D.F.; Ransom, N.L.; Tahzib, N.G.; Reitsamer, H.A.; Levkovitch-Verbin, H.; et al. Caspase Activation and Amyloid Precursor Protein Cleavage in Rat Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1077–1087. [Google Scholar]
- Indrieri, A.; Pizzarelli, R.; Franco, B.; De Leonibus, E. Dopamine, Alpha-Synuclein, and Mitochondrial Dysfunctions in Parkinsonian Eyes. Front. Neurosci. 2020, 14, 567129. [Google Scholar] [CrossRef]
- Sugiyama, K.; Tomita, G.; Kitazawa, Y.; Onda, E.; Shinohara, H. Ki Ho Park The Associations of Optic Disc Hemorrhage with Retinal Nerve Fiber Layer Defect and Peripapillary Atrophy in Normal-Tension Glaucoma. Ophthalmology 1997, 104, 1926–1933. [Google Scholar] [CrossRef]
- Schmidl, D.; Resch, H.; Rensch, F.; Hommer, A.; Vass, C.; Luksch, A.; Garhofer, G.; Jonas, J.; Schmetterer, L. Correlation of Optic Disc Morphology and Ocular Perfusion Parameters in Patients with Primary Open Angle Glaucoma. Acta Ophthalmol. 2008, 86, e544–e549. [Google Scholar] [CrossRef]
- Nitta, K.; Sugiyama, K.; Wajima, R.; Tachibana, G.; Yamada, Y. Associations between Changes in Radial Peripapillary Capillaries and Occurrence of Disc Hemorrhage in Normal-Tension Glaucoma. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1963–1970. [Google Scholar] [CrossRef]
- Wax, M.B. The Case for Autoimmunity in Glaucoma. Exp. Eye Res. 2011, 93, 187–190. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Gilbert, H.Y.; Yang, Y.; Yang, Z.; Berlinicke, C.; Li, Z.; Zaverucha-do-Valle, C.; He, H.; Petkovag, V.; et al. Oncomodulin Links Inflammation to Optic Nerve Regeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 19587–19592. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Li, Y.; Irwin, N.; Fischer, D.; Harvey, A.R.; Benowitz, L.I. Macrophage-Derived Factors Stimulate Optic Nerve Regeneration. J. Neurosci. 2003, 23, 2284–2293. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin Is a Macrophage-Derived Signal for Axon Regeneration in Retinal Ganglion Cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Yang, F.; Morihara, T.; Teter, B.; Ubeda, O.; Rostaing, P.; Triller, A.; Salem, N.; Ashe, K.H.; et al. Docosahexaenoic Acid Protects from Dendritic Pathology in an Alzheimer’s Disease Mouse Model. Neuron 2004, 43, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Calon, F.; Lim, G.P.; Morihara, T.; Yang, F.; Ubeda, O.; Salem, N.; Frautschy, S.A.; Cole, G.M. Dietary N-3 Polyunsaturated Fatty Acid Depletion Activates Caspases and Decreases NMDA Receptors in the Brain of a Transgenic Mouse Model of Alzheimer’s Disease. Eur. J. Neurosci. 2005, 22, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J.M. Effects of Nutrients (in Food) on the Structure and Function of the Nervous System: Update on Dietary Requirements for Brain. Part 2: Macronutrients. J. Nutr. Health Aging 2006, 10, 386–399. [Google Scholar]
- Bousquet, M.; Saint-Pierre, M.; Julien, C.; Salem, N.; Cicchetti, F.; Calon, F. Beneficial Effects of Dietary Omega-3 Polyunsaturated Fatty Acid on Toxin-induced Neuronal Degeneration in an Animal Model of Parkinson’s Disease. FASEB J. 2008, 22, 1213–1225. [Google Scholar] [CrossRef]
- Spain, R.I.; Liu, L.; Zhang, X.; Jia, Y.; Tan, O.; Bourdette, D.; Huang, D. Optical Coherence Tomography Angiography Enhances the Detection of Optic Nerve Damage in Multiple Sclerosis. Br. J. Ophthalmol. 2018, 102, 520–524. [Google Scholar] [CrossRef]
- Faissner, S.; Gold, R. Progressive Multiple Sclerosis: Latest Therapeutic Developments and Future Directions. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419878323. [Google Scholar] [CrossRef]
- Vitek, M.P.; Brown, C.M.; Colton, C.A. APOE Genotype-Specific Differences in the Innate Immune Response. Neurobiol. Aging 2009, 30, 1350–1360. [Google Scholar] [CrossRef]
- Tulloch, J.; Leong, L.; Thomson, Z.; Chen, S.; Lee, E.G.; Keene, C.D.; Millard, S.P.; Yu, C.E. Glia-Specific APOE Epigenetic Changes in the Alzheimer’s Disease Brain. Brain Res. 2018, 1698, 179–186. [Google Scholar] [CrossRef]
- Chai, A.B.; Lam, H.H.J.; Kockx, M.; Gelissen, I.C. Apolipoprotein E Isoform-Dependent Effects on the Processing of Alzheimer’s Amyloid-β. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 158980. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Letcher, S.M.; Igo, R.P.; Cooke Bailey, J.N.; Pasquale, L.R.; Haines, J.L.; Butovsky, O.; Wiggs, J.L. Association of APOE with Primary Open-Angle Glaucoma Suggests a Protective Effect for APOE Ε4. Investig. Ophthalmol. Vis. Sci. 2020, 61, 3. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.A.; Inman, C.E.; Wargel, Z.M.; Dube, U.; Freeberg, B.M.; Galluppi, A.; Haines, J.N.; Dhavale, D.D.; Miller, R.; Choudhury, F.A.; et al. APOE Genotype Regulates Pathology and Disease Progression in Synucleinopathy. Sci. Transl. Med. 2020, 12, eaay3069. [Google Scholar] [CrossRef] [PubMed]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the Parkin Gene Cause Autosomal Recessive Juvenile Parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef]
- Valente, E.M.; Bentivoglio, A.R.; Dixon, P.H.; Ferraris, A.; Lalongo, T.; Frontali, M.; Albanese, A.; Wood, N.W. Localization of a Novel Locus for Autosomal Recessive Early-Onset Parkinsonism, PARK6, on Human Chromosome 1p35–P36. Am. J. Hum. Genet. 2001, 68, 895–900. [Google Scholar] [CrossRef]
- Malpartida, A.B.; Williamson, M.; Narendra, D.P.; Wade-Martins, R.; Ryan, B.J. Mitochondrial Dysfunction and Mitophagy in Parkinson’s Disease: From Mechanism to Therapy. Trends Biochem. Sci. 2021, 46, 329–343. [Google Scholar] [CrossRef]
- Tribble, J.R.; Vasalauskaite, A.; Redmond, T.; Young, R.D.; Hassan, S.; Fautsch, M.P.; Sengpiel, F.; Williams, P.A.; Morgan, J.E. Midget Retinal Ganglion Cell Dendritic and Mitochondrial Degeneration Is an Early Feature of Human Glaucoma. Brain Commun. 2019, 1, fcz035. [Google Scholar] [CrossRef]
- Takihara, Y.; Inatani, M.; Eto, K.; Inoue, T.; Kreymerman, A.; Miyake, S.; Ueno, S.; Nagaya, M.; Nakanishi, A.; Iwao, K.; et al. In Vivo Imaging of Axonal Transport of Mitochondria in the Diseased and Aged Mammalian CNS. Proc. Natl. Acad. Sci. USA 2015, 112, 10515–10520. [Google Scholar] [CrossRef]
- Hvozda Arana, A.G.; Lasagni Vitar, R.M.; Reides, C.G.; Calabró, V.; Marchini, T.; Lerner, S.F.; Evelson, P.A.; Ferreira, S.M. Mitochondrial Function Is Impaired in the Primary Visual Cortex in an Experimental Glaucoma Model. Arch. Biochem. Biophys. 2021, 701, 108815. [Google Scholar] [CrossRef]
- Redina, O.E.; Babenko, V.N. Advances of Brain Transcriptomics. Genes 2022, 13, 1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A Revolutionary Tool for Transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.; Lee, S.J.V. Advances in Transcriptome Analysis of Human Brain Aging. Exp. Mol. Med. 2020, 52, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics Technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef] [PubMed]
- Arneson, D.; Zhang, Y.; Yang, X.; Narayanan, M. Shared Mechanisms among Neurodegenerative Diseases: From Genetic Factors to Gene Networks. J. Genet. 2018, 97, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Burns, T.C.; Morgan, A.A.; Khatri, P. Integrated Multi-Cohort Transcriptional Meta-Analysis of Neurodegenerative Diseases. Acta Neuropathol. Commun. 2014, 2, 93. [Google Scholar] [CrossRef]
- Lake, J.; Storm, C.S.; Makarious, M.B.; Bandres-Ciga, S. Genetic and Transcriptomic Biomarkers in Neurodegenerative Diseases: Current Situation and the Road Ahead. Cells 2021, 10, 1030. [Google Scholar] [CrossRef]
- Gatz, M.; Reynolds, C.A.; Fratiglioni, L.; Johansson, B.; Mortimer, J.A.; Berg, S.; Fiske, A.; Pedersen, N.L. Role of Genes and Environments for Explaining Alzheimer Disease. Arch. Gen. Psychiatry 2006, 63, 168–174. [Google Scholar] [CrossRef]
- Van Cauwenberghe, C.; Van Broeckhoven, C.; Sleegers, K. The Genetic Landscape of Alzheimer Disease: Clinical Implications and Perspectives. Genet. Med. 2016, 18, 421–430. [Google Scholar] [CrossRef]
- Hamza, T.H.; Payami, H. The Heritability of Risk and Age at Onset of Parkinson’s Disease after Accounting for Known Genetic Risk Factors. J. Hum. Genet. 2010, 55, 241–243. [Google Scholar] [CrossRef]
- Bertram, L.; Tanzi, R.E. The Genetic Epidemiology of Neurodegenerative Disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Pasinelli, P.; Brown, R.H. Molecular Biology of Amyotrophic Lateral Sclerosis: Insights from Genetics. Nat. Rev. Neurosci. 2006, 7, 710–723. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Viswanathan, A.C. Are we ready for genetic testing for primary open-angle glaucoma? Eye 2018, 32, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Struebing, F.L.; Geisert, E.E. Commonalities of Optic Nerve Injury and Glaucoma-Induced Neurodegeneration: Insights from Transcriptome-Wide Studies. Exp. Eye Res. 2021, 207, 108571. [Google Scholar] [CrossRef]
- Li, P.; Nie, Y.; Yu, J. An Effective Method to Identify Shared Pathways and Common Factors among Neurodegenerative Diseases. PLoS ONE 2015, 10, e0143045. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Nhung Nguyen, T.P.; Kumar, M.; Fedele, E.; Bonanno, G.; Bonifacino, T. MicroRNA Alteration, Application as Biomarkers, and Therapeutic Approaches in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 4718. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The MiR-124 Regulates the Expression of BACE1/β-Secretase Correlated with Cell Death in Alzheimer’s Disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef]
- Kou, X.; Chen, D.; Chen, N. The Regulation of MicroRNAs in Alzheimer’s Disease. Front. Neurol. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Cosín-Tomás, M.; Antonell, A.; Lladó, A.; Alcolea, D.; Fortea, J.; Ezquerra, M.; Lleó, A.; Martí, M.J.; Pallàs, M.; Sanchez-Valle, R.; et al. Plasma MiR-34a-5p and MiR-545-3p as Early Biomarkers of Alzheimer’s Disease: Potential and Limitations. Mol. Neurobiol. 2017, 54, 5550–5562. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, P.; Wang, X.; Yao, J.; Zhuang, S. MiR-34a Deficiency in APP/PS1 Mice Promotes Cognitive Function by Increasing Synaptic Plasticity via AMPA and NMDA Receptors. Neurosci. Lett. 2018, 670, 94–104. [Google Scholar] [CrossRef]
- Li, H.; Yu, L.; Li, M.; Chen, X.; Tian, Q.; Jiang, Y.; Li, N. MicroRNA-150 Serves as a Diagnostic Biomarker and Is Involved in the Inflammatory Pathogenesis of Parkinson’s Disease. Mol. Genet. Genom. Med. 2020, 8, e1189. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lei, Z.; Sun, T. The Role of MicroRNAs in Neurodegenerative Diseases: A Review. Cell Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Higgs, P.G.; Lehman, N. The RNA World: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2015, 16, 7–17. [Google Scholar] [CrossRef]
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Gasser, T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607. [Google Scholar] [CrossRef]
- Chang, K.H.; Wu, Y.R.; Chen, C.M. Down-Regulation of MiR-9∗ in the Peripheral Leukocytes of Huntington’s Disease Patients. Orphanet J. Rare Dis. 2017, 12, 185. [Google Scholar] [CrossRef]
- Reed, E.R.; Latourelle, J.C.; Bockholt, J.H.; Bregu, J.; Smock, J.; Paulsen, J.S.; Myers, R.H. MicroRNAs in CSF as Prodromal Biomarkers for Huntington Disease in the PREDICT-HD Study. Neurology 2018, 90, e264–e272. [Google Scholar] [CrossRef]
- Liu, T.; Im, W.; Mook-Jung, I.; Kim, M. MicroRNA-124 Slows down the Progression of Huntington’s Disease by Promoting Neurogenesis in the Striatum. Neural Regen. Res. 2015, 10, 786–791. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. MiR-34a Repression of SIRT1 Regulates Apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- De Luna, N.; Turon-Sans, J.; Cortes-Vicente, E.; Carrasco-Rozas, A.; Illán-Gala, I.; Dols-Icardo, O.; Clarimón, J.; Lleó, A.; Gallardo, E.; Illa, I.; et al. Downregulation of MiR-335-5P in Amyotrophic Lateral Sclerosis Can Contribute to Neuronal Mitochondrial Dysfunction and Apoptosis. Sci. Rep. 2020, 10, 4308. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Xiang, Y.; Li, D.; Liang, J.; Zhang, X.; Zhou, F.; Qiao, M.; Nie, Y.; He, Y.; Cheng, J.; et al. MiR-124-3p Attenuates Hyperphosphorylation of Tau Proteininduced Apoptosis via Caveolin-1-PI3K/Akt/GSK3β Pathway in N2a/APP695swe Cells. Oncotarget 2017, 8, 24314–24326. [Google Scholar] [CrossRef]
- Bereimipour, A.; Najafi, H.; Mirsane, E.S.; Moradi, S.; Satarian, L. Roles of miR-204 in retinal development and maintenance. Exp. Cell Res. 2021, 406, 112737. [Google Scholar] [CrossRef]
- Shahriari, F.; Satarian, L.; Moradi, S.; Zarchi, A.S.; Günther, S.; Kamal, A.; Totonchi, M.; Mowla, S.-J.; Braun, T.; Baharvand, H. MicroRNA profiling reveals important functions of miR-125b and let-7a during human retinal pigment epithelial cell differentiation. Exp. Eye Res. 2020, 190, 107883. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009, 15, 2488–2497. [Google Scholar] [PubMed]
- Yao, L.; Ye, Y.; Mao, H.; Lu, F.; He, X.; Lu, G.; Zhang, S. MicroRNA-124 Regulates the Expression of MEKK3 in the Inflammatory Pathogenesis of Parkinson’s Disease. J. Neuroinflamm. 2018, 15, 13. [Google Scholar] [CrossRef]
- Slota, J.A.; Booth, S.A. MicroRNAs in Neuroinflammation: Implications in Disease Pathogenesis, Biomarker Discovery and Therapeutic Applications. Non-Coding RNA 2019, 5, 35. [Google Scholar] [CrossRef]
- Das, E.; Jana, N.R.; Bhattacharyya, N.P. MicroRNA-124 Targets CCNA2 and Regulates Cell Cycle in STHdhQ111/HdhQ111 Cells. Biochem. Biophys. Res. Commun. 2013, 437, 217–224. [Google Scholar] [CrossRef]
- Saraiva, C.; Esteves, M.; Bernardino, L. MicroRNA: Basic Concepts and Implications for Regeneration and Repair of Neurodegenerative Diseases. Biochem. Pharmacol. 2017, 141, 118–131. [Google Scholar] [CrossRef]
- Sierksma, A.; Lu, A.; Salta, E.; Vanden Eynden, E.; Callaerts-Vegh, Z.; D’Hooge, R.; Blum, D.; Buée, L.; Fiers, M.; De Strooper, B. Deregulation of Neuronal MiRNAs Induced by Amyloid-β or TAU Pathology 11 Medical and Health Sciences 1109 Neurosciences. Mol. Neurodegener. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Caggiu, E.; Paulus, K.; Mameli, G.; Arru, G.; Sechi, G.P.; Sechi, L.A. Differential Expression of MiRNA 155 and MiRNA 146a in Parkinson’s Disease Patients. eNeurologicalSci 2018, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.A.; Hyrlov, K.H.; Elkjaer, M.L.; Thygesen, E.K.; Wlodarczyk, A.; Elbaek, K.J.; Aboo, C.; Okarmus, J.; Benedikz, E.; Reynolds, R.; et al. Absence of MiRNA-146a Differentially Alters Microglia Function and Proteome. Front. Immunol. 2020, 11, 1110. [Google Scholar] [CrossRef]
- Aloi, M.S.; Prater, K.E.; Sánchez, R.E.A.; Beck, A.; Pathan, J.L.; Davidson, S.; Wilson, A.; Keene, C.D.; de la Iglesia, H.; Jayadev, S.; et al. Microglia Specific Deletion of MiR-155 in Alzheimer’s Disease Mouse Models Reduces Amyloid-β Pathology but Causes Hyperexcitability and Seizures. J. Neuroinflamm. 2023, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. MicroRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J. Neurosci. 2016, 6, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Du, L.; Zhou, F.; Yuan, X.; Liu, X.; Zhang, L. Rosmarinic Acid Alleviates Inflammation, Apoptosis, and Oxidative Stress through Regulating MiR-155-5p in a Mice Model of Parkinson’s Disease. ACS Chem. Neurosci. 2020, 11, 3259–3266. [Google Scholar] [CrossRef]
- Briggs, C.E.; Wang, Y.; Kong, B.; Woo, T.U.W.; Iyer, L.K.; Sonntag, K.C. Midbrain Dopamine Neurons in Parkinson’s Disease Exhibit a Dysregulated MiRNA and Target-Gene Network. Brain Res. 2015, 1618, 111–121. [Google Scholar] [CrossRef]
- Essandoh, K.; Li, Y.; Huo, J.; Fan, G.C. MiRNA-Mediated Macrophage Polarization and Its Potential Role in the Regulation of Inflammatory Response. Shock 2016, 46, 122–131. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. MiR-212 and MiR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef]
- Jain, G.; Stuendl, A.; Rao, P.; Berulava, T.; Pena Centeno, T.; Kaurani, L.; Burkhardt, S.; Delalle, I.; Kornhuber, J.; Hüll, M.; et al. A Combined MiRNA–PiRNA Signature to Detect Alzheimer’s Disease. Transl. Psychiatry 2019, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Li, H.; Jiang, L.; Hermeking, H. The P53/MiR-34 Axis in Development and Disease. J. Mol. Cell Biol. 2014, 6, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, Y.; Wang, B.; Huang, J.; Li, Q. MiR-34a-5p and MiR-125b-5p Attenuate Aβ-Induced Neurotoxicity through Targeting BACE1. J. Neurol. Sci. 2020, 413, 116793. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rapp, J.; Rainone, S.; Hébert, S.S. MicroRNAs Underlying Memory Deficits in Neurodegenerative Disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 73, 79–86. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, Y.; Xie, W.; Liu, X.; Zhu, Y.; Zhu, Y. Nimodipine Attenuates Tau Phosphorylation at Ser396 via MiR-132/GSK-3β Pathway in Chronic Cerebral Hypoperfusion Rats. Eur. J. Pharmacol. 2018, 819, 1–8. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in Plasma and Cerebrospinal Fluid as Potential Markers for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef]
- Nagaraj, S.; Laskowska-Kaszub, K.; Debski, K.J.; Wojsiat, J.; Dabrowski, M.; Gabryelewicz, T.; Kuznicki, J.; Wojda, U. Profile of 6 MicroRNA in Blood Plasma Distinguish Early Stage Alzheimer’s Disease Patients from Non-Demented Subjects. Oncotarget 2017, 8, 16122–16143. [Google Scholar] [CrossRef]
- Nagaraj, S.; Want, A.; Laskowska-Kaszub, K.; Fesiuk, A.; Vaz, S.; Logarinho, E.; Wojda, U. Candidate Alzheimer’s Disease Biomarker Mir-483-5p Lowers Tau Phosphorylation by Direct Erk1/2 Repression. Int. J. Mol. Sci. 2021, 22, 3653. [Google Scholar] [CrossRef]
- Yang, T.T.; Liu, C.G.; Gao, S.C.; Zhang, Y.; Wang, P.C. The Serum Exosome Derived MicroRNA−135a, −193b, and −384 Were Potential Alzheimer’s Disease Biomarkers. Biomed. Environ. Sci. 2018, 31, 87–96. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Gu, M.; Zhang, Y. MiR-342-5p Decreases Ankyrin G Levels in Alzheimer’s Disease Transgenic Mouse Models. Cell Rep. 2014, 6, 264–270. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Niu, J.; Lu, K.; Xie, B.; Cui, D.; Xu, S. Screening of MicroRNAs Associated with Alzheimer’s Disease Using Oxidative Stress Cell Model and Different Strains of Senescence Accelerated Mice. J. Neurol. Sci. 2014, 338, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, J.; Wang, Q.; Jiang, H.; Zeng, L.; Li, Z.; Liu, R. MicroRNA-200a-3p Mediates Neuroprotection in Alzheimer-Related Deficits and Attenuates Amyloid-Beta Overproduction and Tau Hyperphosphorylation via Coregulating BACE1 and PRKACB. Front. Pharmacol. 2019, 10, 806. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Huang, M.; Chen, L. Mechanism of MiR-132-3p Promoting Neuroinflammation and Dopaminergic Neurodegeneration in Parkinson’s Disease. eNeuro 2022, 9, ENEURO.0393-21.2021. [Google Scholar] [CrossRef]
- Junn, E.; Lee, K.W.; Jeong, B.S.; Chan, T.W.; Im, J.Y.; Mouradian, M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA 2009, 106, 13052–13057. [Google Scholar] [CrossRef]
- Kabaria, S.; Choi, D.C.; Chaudhuri, A.D.; Mouradian, M.M.; Junn, E. Inhibition of miR-34b and miR-34c enhances alpha-synuclein expression in Parkinson’s disease. FEBS Lett. 2015, 589, 319–325. [Google Scholar] [CrossRef]
- O’Hara, D.M.; Pawar, G.; Kalia, S.K.; Kalia, L.V. LRRK2 and alpha-Synuclein: Distinct or Synergistic Players in Parkinson’s Disease? Front Neurosci. 2020, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Recasens, A.; Perier, C.; Sue, C.M. Role of MicroRNAs in the Regulation of α-Synuclein Expression: A Systematic Review. Front. Mol. Neurosci. 2016, 9, 128. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, R.; Zhou, L.; Zhu, Y.; Gong, J.; Zhuang, S.M. MicroRNA-26b Suppresses the NF-ΚB Signaling and Enhances the Chemosensitivity of Hepatocellular Carcinoma Cells by Targeting TAK1 and TAB3. Mol. Cancer 2014, 13, 35. [Google Scholar] [CrossRef]
- Steiner, D.F.; Thomas, M.F.; Hu, J.K.; Yang, Z.; Babiarz, J.E.; Allen, C.D.C.; Matloubian, M.; Blelloch, R.; Ansel, K.M. MicroRNA-29 Regulates T-Box Transcription Factors and Interferon-γ Production in Helper T Cells. Immunity 2011, 35, 169–181. [Google Scholar] [CrossRef]
- Kole, A.J.; Swahari, V.; Hammond, S.M.; Deshmukh, M. MiR-29b Is Activated during Neuronal Maturation and Targets BH3-Only Genes to Restrict Apoptosis. Genes Dev. 2011, 25, 125–130. [Google Scholar] [CrossRef]
- Li, D.; Yang, H.; Ma, J.; Luo, S.; Chen, S.; Gu, Q. MicroRNA-30e Regulates Neuroinflammation in MPTP Model of Parkinson’s Disease by Targeting Nlrp3. Hum. Cell 2018, 31, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Vistbakka, J.; Sumelahti, M.L.; Lehtimäki, T.; Elovaara, I.; Hagman, S. Evaluation of Serum MiR-191-5p, MiR-24-3p, MiR-128-3p, and MiR-376c-3 in Multiple Sclerosis Patients. Acta Neurol. Scand. 2018, 138, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Orlandi, E.; Rossetti, G.; Turatti, M.; Calabrese, M.; Gomez Lira, M.; Gajofatto, A. Upregulated Serum MiR-128-3p in Progressive and Relapse-Free Multiple Sclerosis Patients. Acta Neurol. Scand. 2020, 142, 511–516. [Google Scholar] [CrossRef]
- Li, C.; Wei, Q.; Gu, X.; Chen, Y.; Chen, X.; Cao, B.; Ou, R.; Shang, H. Decreased Glycogenolysis by MiR-338-3p Promotes Regional Glycogen Accumulation within the Spinal Cord of Amyotrophic Lateral Sclerosis Mice. Front. Mol. Neurosci. 2019, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Marzocchi, C.; Battistini, S. MicroRNAs as Biomarkers in Amyotrophic Lateral Sclerosis. Cells 2018, 7, 219. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.H.; El-Mehdawy, K.M.; Seleem, M.; El-Sawalhi, M.M.; Shaheen, A.A. Serum ROCK2, MiR-300 and MiR-450b-5p Levels in Two Different Clinical Phenotypes of Multiple Sclerosis: Relation to Patient Disability and Disease Progression. J. Neuroimmunol. 2020, 347, 577356. [Google Scholar] [CrossRef] [PubMed]
- Je, G.; Kim, Y.S. Mitochondrial ROS-Mediated Post-Transcriptional Regulation of α-Synuclein through MiR-7 and MiR-153. Neurosci. Lett. 2017, 661, 132–136. [Google Scholar] [CrossRef]
- Díez-Planelles, C.; Sánchez-Lozano, P.; Crespo, M.C.; Gil-Zamorano, J.; Ribacoba, R.; González, N.; Suárez, E.; Martínez-Descals, A.; Martínez-Camblor, P.; Álvarez, V.; et al. Circulating MicroRNAs in Huntington’s Disease: Emerging Mediators in Metabolic Impairment. Pharmacol. Res. 2016, 108, 102–110. [Google Scholar] [CrossRef]
- Langfelder, P.; Gao, F.; Wang, N.; Howland, D.; Kwak, S.; Vogt, T.F.; Aaronson, J.S.; Rosinski, J.; Coppola, G.; Horvath, S.; et al. MicroRNA Signatures of Endogenous Huntingtin CAG Repeat Expansion in Mice. PLoS ONE 2018, 13, e0190550. [Google Scholar] [CrossRef]
- Grossi, I.; Radeghieri, A.; Paolini, L.; Porrini, V.; Pilotto, A.; Padovani, A.; Marengoni, A.; Barbon, A.; Bellucci, A.; Pizzi, M.; et al. MicroRNA-34a-5p Expression in the Plasma and in Its Extracellular Vesicle Fractions in Subjects with Parkinson’s Disease: An Exploratory Study. Int. J. Mol. Med. 2021, 47, 533–546. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of Extracellular MiRNAs in the Aqueous Humor of Glaucoma Patients Assessed with a Microarray System. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.G.; Thoonen, R.; Jasien, J.V.; Grange, R.M.H.; Amin, K.; Wise, J.; Ozaki, M.; Ritch, R.; Malhotra, R.; Buys, E.S. Identification of Candidate MiRNA Biomarkers for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Seong, H.; Cho, H.-K.; Kee, C.; Song, D.H.; Cho, M.-C.; Kang, S.S. Profiles of MicroRNA in Aqueous Humor of Normal Tension Glaucoma Patients Using RNA Sequencing. Sci. Rep. 2021, 11, 19024. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Phillips, J.I.; Lozano, D.C.; Choe, T.E.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C.; Gattey, D.M.; Saugstad, J.A.; Keller, K.E. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2884–2890. [Google Scholar] [CrossRef]
- Hubens, W.H.G.; Krauskopf, J.; Beckers, H.J.M.; Kleinjans, J.C.S.; Webers, C.A.B.; Gorgels, T.G.M.F. Small RNA Sequencing of Aqueous Humor and Plasma in Patients with Primary Open-Angle Glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 24. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially Expressed MicroRNAs in the Aqueous Humor of Patients with Exfoliation Glaucoma or Primary Open-Angle Glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef]
- Sun, P.; Liu, D.Z.; Jickling, G.C.; Sharp, F.R.; Yin, K.J. MicroRNA-Based Therapeutics in Central Nervous System Injuries. J. Cereb. Blood Flow Metab. 2018, 38, 1125–1148. [Google Scholar] [CrossRef]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Hamzei Taj, S.; Kho, W.; Riou, A.; Wiedermann, D.; Hoehn, M. MiRNA-124 Induces Neuroprotection and Functional Improvement after Focal Cerebral Ischemia. Biomaterials 2016, 91, 151–165. [Google Scholar] [CrossRef]
- Calin, G.A.; Cimmino, A.; Fabbri, M.; Ferracin, M.; Wojcik, S.E.; Shimizu, M.; Taccioli, C.; Zanesi, N.; Garzon, R.; Aqeilan, R.I.; et al. MiR-15a and MiR-16-1 Cluster Functions in Human Leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 5166–5171. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 Induce Apoptosis by Targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef]
- Van Rooij, E.; Purcell, A.L.; Levin, A.A. Developing MicroRNA Therapeutics. Circ. Res. 2012, 110, 496–507. [Google Scholar] [CrossRef]
- Obad, S.; Dos Santos, C.O.; Petri, A.; Heidenblad, M.; Broom, O.; Ruse, C.; Fu, C.; Lindow, M.; Stenvang, J.; Straarup, E.M.; et al. Silencing of MicroRNA Families by Seed-Targeting Tiny LNAs. Nat. Genet. 2011, 43, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Van Roosbroeck, K.; Fanini, F.; Setoyama, T.; Ivan, C.; Rodriguez-Aguayo, C.; Fuentes-Mattei, E.; Xiao, L.; Vannini, I.; Redis, R.S.; D’Abundo, L.; et al. Combining Anti-MiR-155 with Chemotherapy for the Treatment of Lung Cancers. Clin. Cancer Res. 2017, 23, 2891–2904. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Valdecanas, D.; Niknam, S.; Peltier, H.J.; Diao, L.; Giri, U.; Komaki, R.; Calin, G.A.; Gomez, D.R.; Chang, J.Y.; et al. In Vivo Delivery of MiR-34a Sensitizes Lung Tumors to Radiation through RAD51 Regulation. Mol. Ther. Nucleic Acids 2015, 4, e270. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.D.; Staedel, C.; Zehnacker, L.; Benhida, R.; Darfeuille, F.; Duca, M. Targeting the Production of Oncogenic MicroRNAs with Multimodal Synthetic Small Molecules. ACS Chem. Biol. 2014, 9, 711–721. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasconcelos, C.F.M.; Ribas, V.T.; Petrs-Silva, H. Shared Molecular Pathways in Glaucoma and Other Neurodegenerative Diseases: Insights from RNA-Seq Analysis and miRNA Regulation for Promising Therapeutic Avenues. Cells 2023, 12, 2155. https://doi.org/10.3390/cells12172155
Vasconcelos CFM, Ribas VT, Petrs-Silva H. Shared Molecular Pathways in Glaucoma and Other Neurodegenerative Diseases: Insights from RNA-Seq Analysis and miRNA Regulation for Promising Therapeutic Avenues. Cells. 2023; 12(17):2155. https://doi.org/10.3390/cells12172155
Chicago/Turabian StyleVasconcelos, Carlos Franciney Moreira, Vinicius Toledo Ribas, and Hilda Petrs-Silva. 2023. "Shared Molecular Pathways in Glaucoma and Other Neurodegenerative Diseases: Insights from RNA-Seq Analysis and miRNA Regulation for Promising Therapeutic Avenues" Cells 12, no. 17: 2155. https://doi.org/10.3390/cells12172155
APA StyleVasconcelos, C. F. M., Ribas, V. T., & Petrs-Silva, H. (2023). Shared Molecular Pathways in Glaucoma and Other Neurodegenerative Diseases: Insights from RNA-Seq Analysis and miRNA Regulation for Promising Therapeutic Avenues. Cells, 12(17), 2155. https://doi.org/10.3390/cells12172155








