Opposing Roles of Blood-Borne Monocytes and Tissue-Resident Macrophages in Limbal Stem Cell Damage after Ocular Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Model of Corneal Alkali Injury
2.2. Rabbit Model of Chemical Injury
2.3. Anterior Chamber pH Elevation with Cannulation
2.4. Monocyte Fate Mapping Using a Chimera
2.5. Tissue-Resident Macrophage Depletion Using CSF1R Inhibitor
2.6. Ex Vivo Corneal Culture Experiments
2.7. Immunohistochemical Analysis
2.8. In Situ Hybridization
2.9. Microscopy
2.10. Flow Cytometry
2.11. TUNEL Labeling and Quantitation of DNA Fragmentation
2.12. Image Quantification
2.13. Statistics
3. Results
3.1. Limbal Stem Cell Damage Occurs Even in the Absence of Direct Injury by Caustic Agent
3.2. Anterior Chamber pH Elevation Is an Alternative Mediator of Limbal Stem Cell Damage
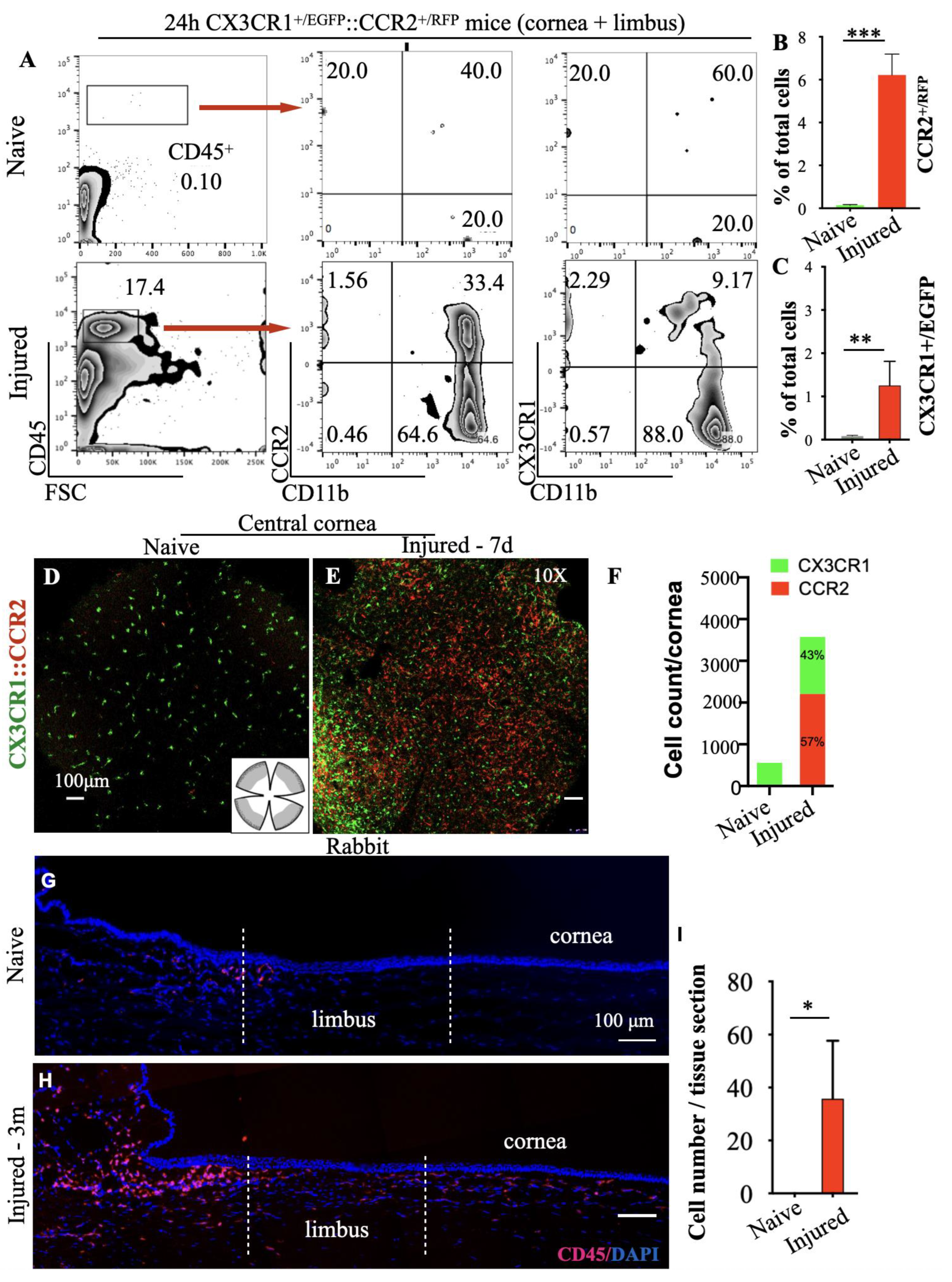
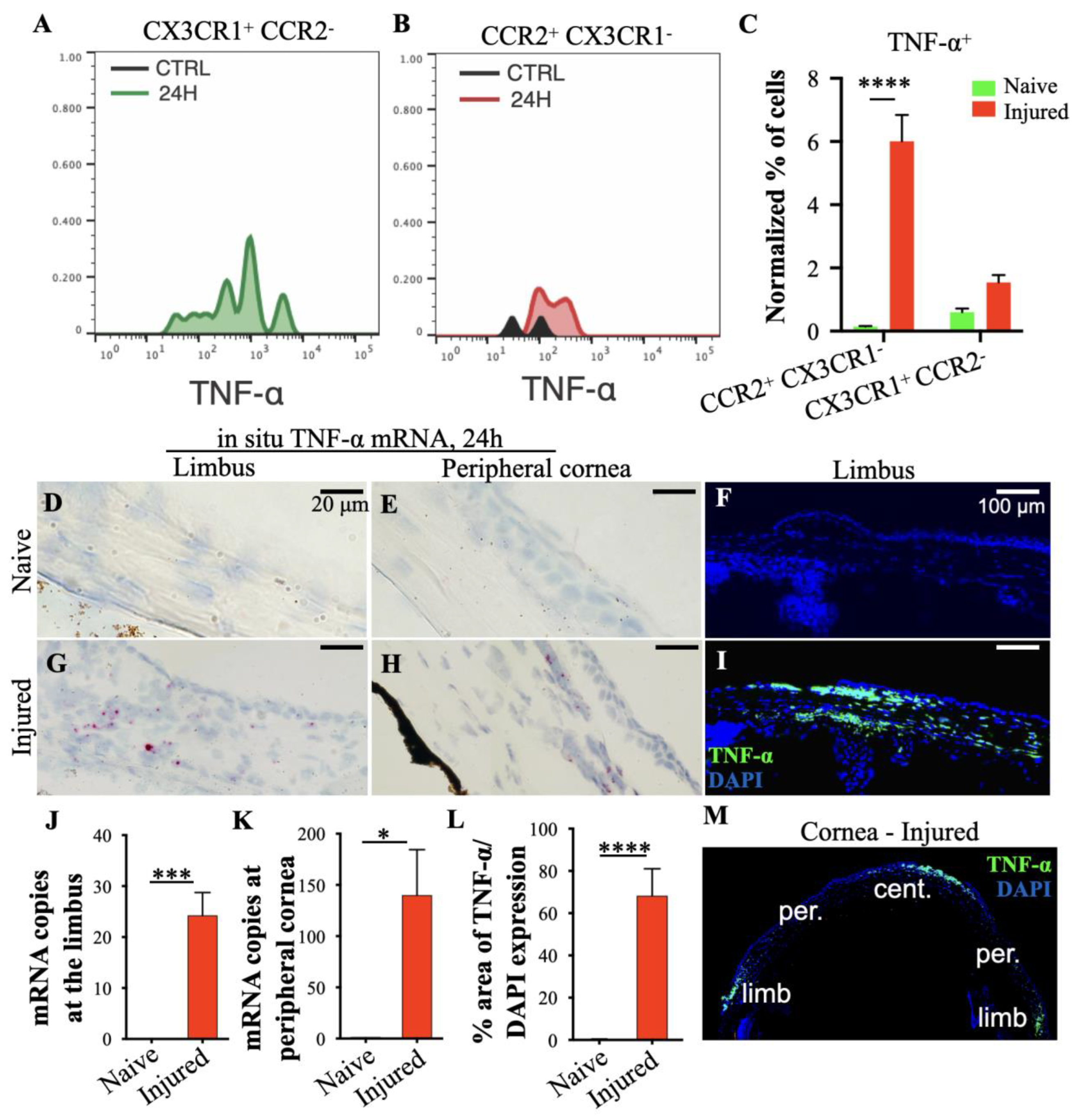
3.3. Peripheral Inflammatory Monocytes Are Key Mediators of Limbal Stem Cell Damage
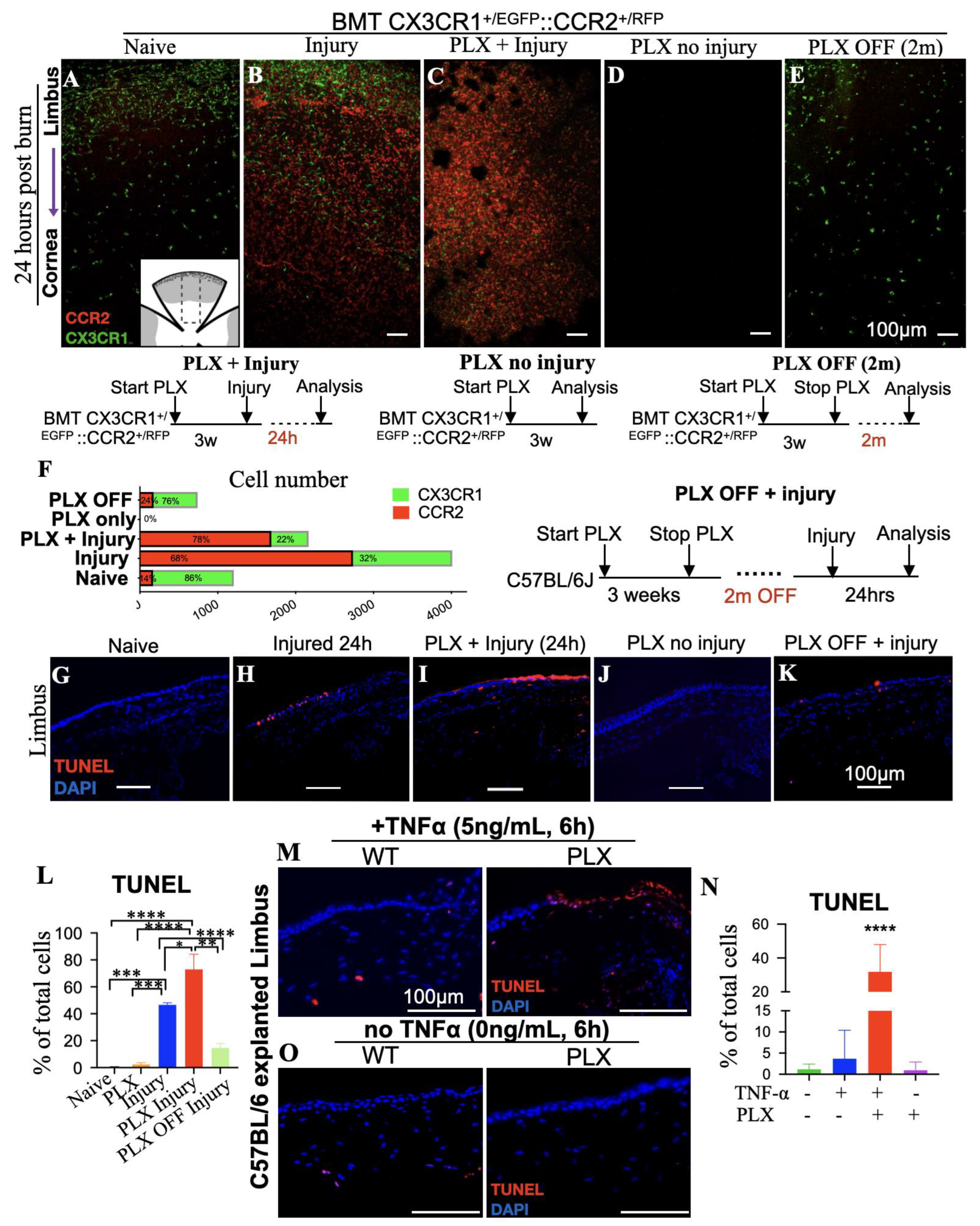
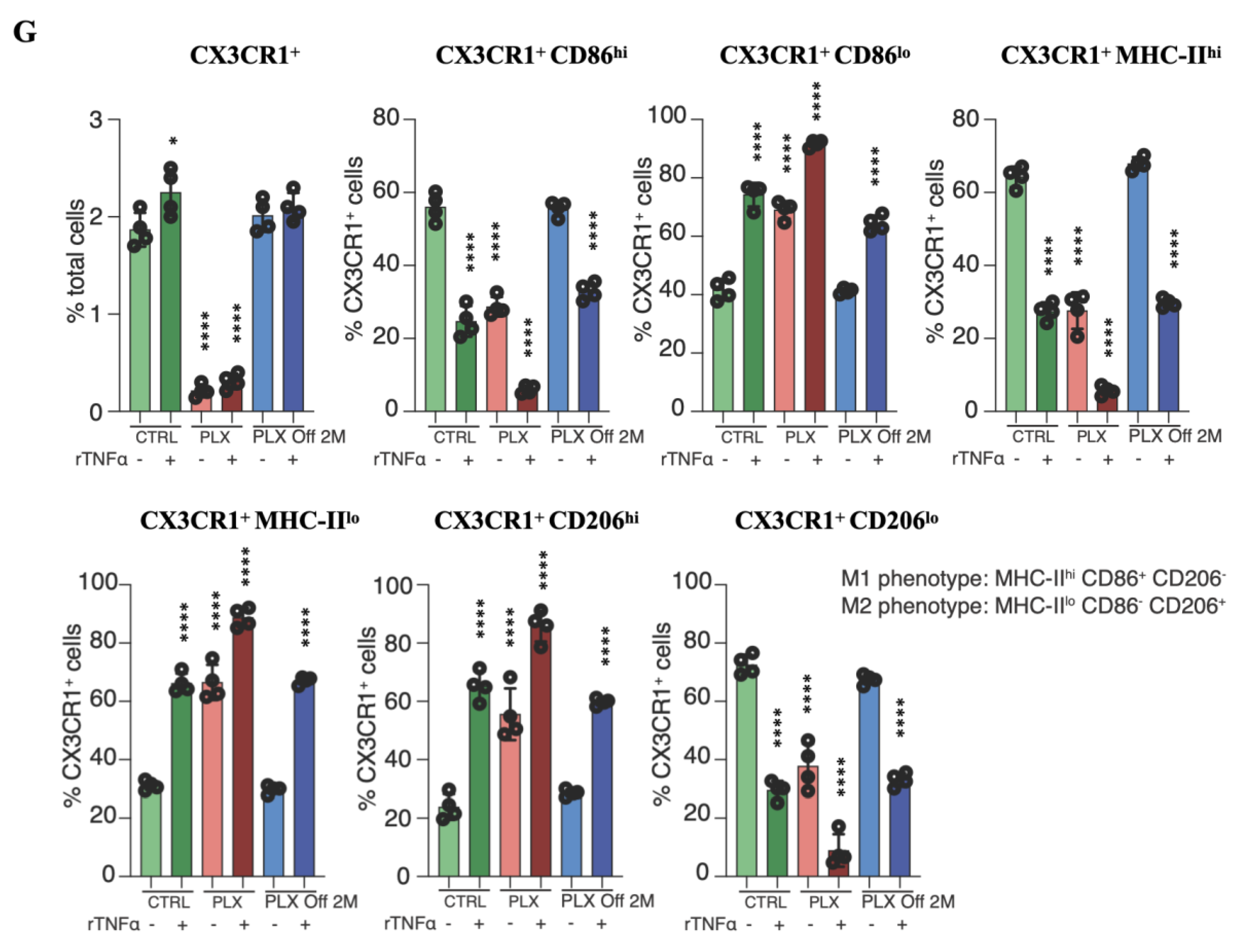
3.4. Tissue-Resident Macrophages Are Key Regulators of Limbal Stem Cell Injury
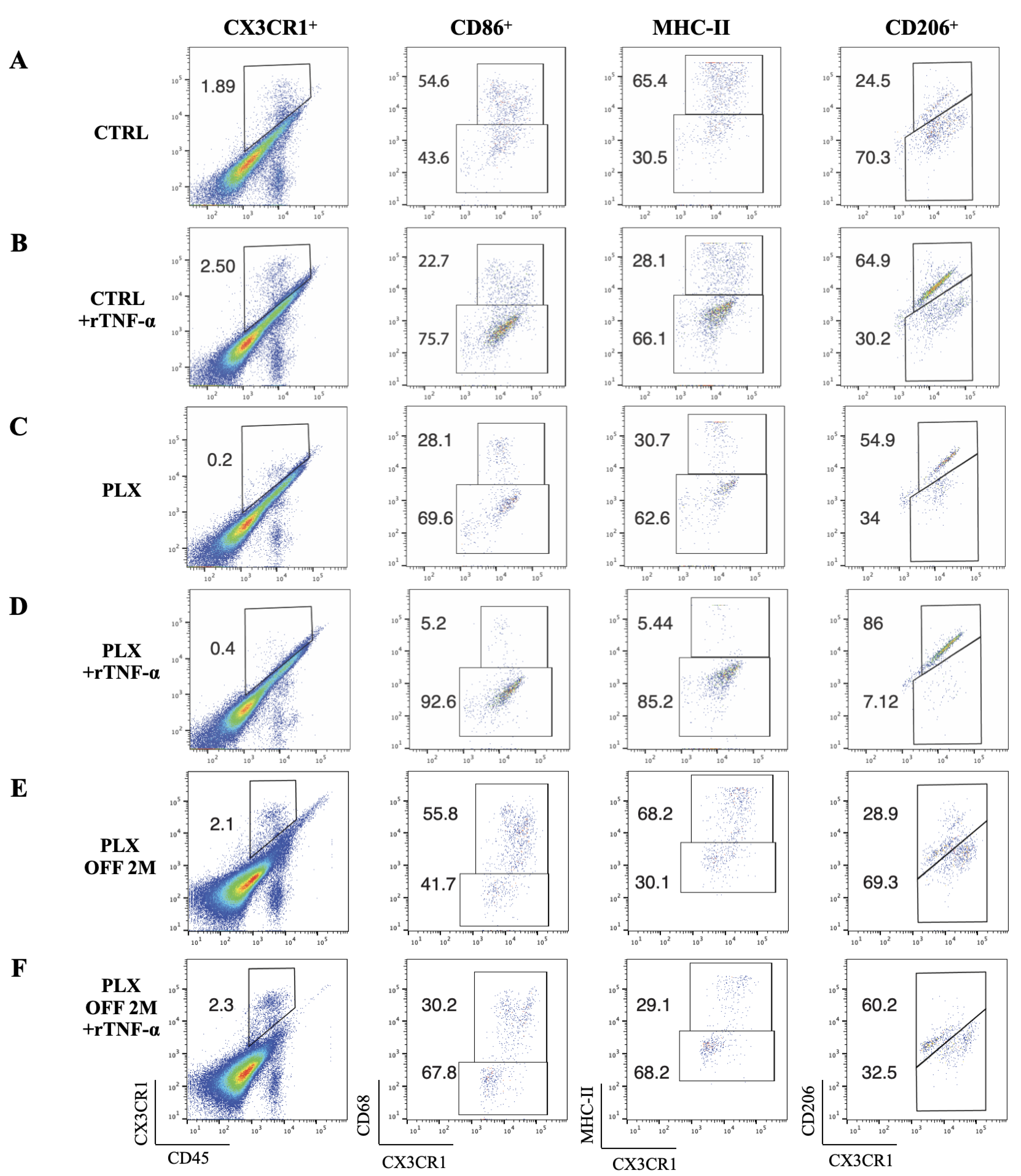
3.5. Depletion of Tissue-Resident Macrophages Sensitizes LSCs to TNF-α-Mediated Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dohlman, C.H.; Cade, F.; Pfister, R. Chemical burns to the eye: Paradigm shifts in treatment. Cornea 2011, 30, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.; Singh, A. Corneal epithelial wound healing. Br. J. Ophthalmol. 1994, 78, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1442–1443. [Google Scholar]
- Shanbhag, S.S.; Saeed, H.N.; Paschalis, E.I.; Chodosh, J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: A systematic review. Br. J. Ophthalmol. 2017, 102, 1114–1121. [Google Scholar] [CrossRef]
- Williams, K.A.; Brereton, H.M.; Aggarwal, R.; Sykes, P.J.; Turner, D.R.; Russ, G.R.; Coster, D.J. Use of DNA Polymorphisms and the Polymerase Chain Reaction to Examine the Survival of a Human Limbal Stem Cell Allograft. Am. J. Ophthalmol. 1995, 120, 342–350. [Google Scholar] [CrossRef]
- Santos, M.S.; Gomes, J.A.P.; Hofling-Lima, A.L.; Rizzo, L.V.; Romano, A.C.; Belfort, R. Survival Analysis of Conjunctival Limbal Grafts and Amniotic Membrane Transplantation in Eyes With Total Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2005, 140, 223.e221–223.e229. [Google Scholar] [CrossRef]
- Shimazaki, J.; Yang, H.-Y.; Tsubota, K. Amniotic Membrane Transplantation for Ocular Surface Reconstruction in Patients with Chemical and Thermal Burns. Ophthalmology 1997, 104, 2068–2076. [Google Scholar] [CrossRef]
- Cade, F.; Paschalis, E.I.; Regatieri, C.V.; Vavvas, D.G.; Dana, R.; Dohlman, C.H. Alkali burn to the eye: Protection using TNF-α inhibition. Cornea 2014, 33, 382–389. [Google Scholar] [CrossRef]
- Paschalis, E.I.; Zhou, C.; Lei, F.; Scott, N.; Kapoulea, V.; Robert, M.-C.; Vavvas, D.; Dana, R.; Chodosh, J.; Dohlman, C.H. Mechanisms of Retinal Damage after Ocular Alkali Burns. Am. J. Pathol. 2017, 187, 1327–1342. [Google Scholar] [CrossRef]
- Zhou, C.; Robert, M.-C.; Kapoulea, V.; Lei, F.; Stagner, A.M.; Jakobiec, F.A.; Dohlman, C.H.; Paschalis, E.I. Sustained Subconjunctival Delivery of Infliximab Protects the Cornea and Retina Following Alkali Burn to the Eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 96–105. [Google Scholar] [CrossRef]
- Zhou, C.; Lei, F.; Sharma, J.; Hui, P.-C.; Wolkow, N.; Dohlman, C.H.; Vavvas, D.G.; Chodosh, J.; Paschalis, E.I. Sustained Inhibition of VEGF and TNF-α Achieves Multi-Ocular Protection and Prevents Formation of Blood Vessels after Severe Ocular Trauma. Pharmaceutics 2023, 15, 2059. [Google Scholar] [CrossRef]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Kapoulea, V.; Thanos, A.; Dana, R.; Vavvas, D.G.; Chodosh, J.; Dohlman, C.H. The Role of Microglia and Peripheral Monocytes in Retinal Damage after Corneal Chemical Injury. Am. J. Pathol. 2018, 188, 1580–1596. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Kapoulea, V.; Dana, R.; Chodosh, J.; Vavvas, D.G.; Dohlman, C.H. Permanent neuroglial remodeling of the retina following infiltration of CSF1R inhibition-resistant peripheral monocytes. Proc. Natl. Acad. Sci. USA 2018, 115, E11359–E11368. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, E.I.; Lei, F.; Zhou, C.; Chen, X.N.; Kapoulea, V.; Hui, P.-C.; Dana, R.; Chodosh, J.; Vavvas, D.G.; Dohlman, C.H. Microglia Regulate Neuroglia Remodeling in Various Ocular and Retinal Injuries. J. Immunol. (Baltim. Md. 1950) 2019, 202, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lei, F.; Zhou, C.; Chodosh, J.; Wang, L.; Huang, Y.; Dohlman, C.H.; Paschalis, E.I. Glaucoma after ocular surgery or trauma: The role of infiltrating monocytes and their response to cytokine inhibitors. Am. J. Pathol. 2020, 190, 2056–2066. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.B.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2015, 511, 353–357. [Google Scholar] [CrossRef]
- Ferrari, G.; Bignami, F.; Giacomini, C.; Franchini, S.; Rama, P. Safety and Efficacy of Topical Infliximab in a Mouse Model of Ocular Surface ScarringSafety and Efficacy of Topical Infliximab. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1680–1688. [Google Scholar] [CrossRef]
- Lei, F.; Cui, N.; Zhou, C.; Chodosh, J.; Vavvas, D.G.; Paschalis, E.I. CSF1R inhibition by a small-molecule inhibitor is not microglia specific; affecting hematopoiesis and the function of macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 23336–23338. [Google Scholar] [CrossRef]
- Lei, F.; Cui, N.; Zhou, C.; Chodosh, J.; Vavvas, D.G.; Paschalis, E.I. Reply to Green and Hume: Nonmicroglia peripheral immune effects of short-term CSF1R inhibition with PLX5622. Proc. Natl. Acad. Sci. USA 2021, 118, e2020660118. [Google Scholar] [CrossRef]
- Dagher, N.N.; Najafi, A.R.; Kayala, K.M.N.; Elmore, M.R.P.; White, T.E.; Medeiros, R.; West, B.L.; Green, K.N. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflamm. 2015, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Hatch, K.M.; Dana, R. The structure and function of the limbal stem cell and the disease states associated with limbal stem cell deficiency. Int. Ophthalmol. Clin. 2009, 49, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Hughes, W.F., Jr. Alkali burns of the eye; review of the literature and summary of present knowledge. Arch. Ophthalmol. 1946, 35, 423–449. [Google Scholar] [CrossRef]
- Goldberg, M.F.; Bron, A. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc. 1982, 80, 155. [Google Scholar] [PubMed]
- Schofield, R. The stem cell system. Biomed. Pharmacother. Biomed. Pharmacother. 1983, 37, 375–380. [Google Scholar] [PubMed]
- Tseng, S.C. Regulation and clinical implications of corneal epithelial stem cells. Mol. Biol. Rep. 1996, 23, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Wolosin, J.M.; Xiong, X.; Schutte, M.; Stegman, Z.; Tieng, A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog. Retin. Eye Res. 2000, 19, 223–255. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Burgess, M.; Wicks, K.; Gardasevic, M.; Mace, K.A. Cx3CR1 Expression Identifies Distinct Macrophage Populations That Contribute Differentially to Inflammation and Repair. ImmunoHorizons 2019, 3, 262–273. [Google Scholar] [CrossRef]
- Vestergaard, C.; Just, H.; Baumgartner Nielsen, J.; Thestrup-Pedersen, K.; Deleuran, M. Expression of CCR2 on Monocytes and Macrophages in Chronically Inflamed Skin in Atopic Dermatitis and Psoriasis. Acta Derm.-Venereol. 2004, 84, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chauhan, S.K.; Zhang, Q.; Dana, R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch. Ophthalmol. 2009, 127, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhao, X.; Wang, Y.; Zhang, X.; Chen, X.; Xu, C.; Yuan, Z.-r.; Roberts, A.I.; Zhang, L.; Zheng, B.; et al. CCR2-Dependent Recruitment of Macrophages by Tumor-Educated Mesenchymal Stromal Cells Promotes Tumor Development and Is Mimicked by TNFα. Cell Stem Cell 2012, 11, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Lucas, T.; van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef]
- Sennlaub, F.; Auvynet, C.; Calippe, B.; Lavalette, S.; Poupel, L.; Hu, S.J.; Dominguez, E.; Camelo, S.; Levy, O.; Guyon, E.; et al. CCR2(+) monocytes infiltrate atrophic lesions in age-related macular disease and mediate photoreceptor degeneration in experimental subretinal inflammation in Cx3cr1 deficient mice. EMBO Mol. Med. 2013, 5, 1775–1793. [Google Scholar] [CrossRef]
- Evans, T.A.; Barkauskas, D.S.; Myers, J.T.; Hare, E.G.; You, J.Q.; Ransohoff, R.M.; Huang, A.Y.; Silver, J. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 2014, 254, 109–120. [Google Scholar] [CrossRef]
- Han, J.; Fan, Y.; Zhou, K.; Zhu, K.; Blomgren, K.; Lund, H.; Zhang, X.M.; Harris, R.A. Underestimated Peripheral Effects Following Pharmacological and Conditional Genetic Microglial Depletion. Int. J. Mol. Sci. 2020, 21, 8603. [Google Scholar] [CrossRef]
- Dohlman, C.H.; Zhou, C.; Lei, F.; Cade, F.; Regatieri, C.V.; Crnej, A.; Dohlman, J.G.; Shen, L.Q.; Paschalis, E.I. Glaucoma After Corneal Trauma or Surgery—A Rapid, Inflammatory, IOP-Independent Pathway. Cornea 2019, 38, 1589. [Google Scholar] [CrossRef]
- Tsai, R.J.-F.; Tseng, S.C. Human allograft limbal transplantation for corneal surface reconstruction. Cornea 1994, 13, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.C.; Prabhasawat, P.; Barton, K.; Gray, T.; Meller, D. Amniotic membrane transplantation with or without limbal allografts for corneal surface reconstruction in patients with limbal stem cell deficiency. Arch. Ophthalmol. 1998, 116, 431–441. [Google Scholar] [CrossRef]
- Tsubota, K.; Satake, Y.; Kaido, M.; Shinozaki, N.; Shimmura, S.; Bissen-Miyajima, H.; Shimazaki, J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N. Engl. J. Med. 1999, 340, 1697–1703. [Google Scholar] [CrossRef]
- Rama, P.; Bonini, S.; Lambiase, A.; Golisano, O.; Paterna, P.; De Luca, M.; Pellegrini, G. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency1. Transplantation 2001, 72, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.A.P.; dos Santos, M.S.; Cunha, M.C.; de Nadai Barros, J.; de Sousa, L.B. Amniotic membrane transplantation for partial and total limbal stem cell deficiency secondary to chemical burn. Ophthalmology 2003, 110, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Secker, G.A.; Rajan, M.S.; Meligonis, G.; Dart, J.K.; Tuft, S.J.; Daniels, J.T. Ex Vivo Expansion and Transplantation of Limbal Epithelial Stem Cells. Ophthalmology 2008, 115, 1989–1997. [Google Scholar] [CrossRef]
- Tilkin, C.; Duchesne, B.; Camby, S. Holoclar®, an autologous stem cells graft for sight recovery after ocular burns. Rev. Med. Liege 2021, 76, 776–782. [Google Scholar]
- Oie, Y.; Hayashi, R.; Takagi, R.; Yamato, M.; Takayanagi, H.; Tano, Y.; Nishida, K. A novel method of culturing human oral mucosal epithelial cell sheet using post-mitotic human dermal fibroblast feeder cells and modified keratinocyte culture medium for ocular surface reconstruction. Br. J. Ophthalmol. 2010, 94, 1244–1250. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef]
- Schwartz, G.S.; Holland, E.J. Iatrogenic Limbal Stem Cell Deficiency. Cornea 1998, 17, 31. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Lei, F.; Mittermaier, M.; Ksander, B.; Dana, R.; Dohlman, C.H.; Vavvas, D.G.; Chodosh, J.; Paschalis, E.I. Opposing Roles of Blood-Borne Monocytes and Tissue-Resident Macrophages in Limbal Stem Cell Damage after Ocular Injury. Cells 2023, 12, 2089. https://doi.org/10.3390/cells12162089
Zhou C, Lei F, Mittermaier M, Ksander B, Dana R, Dohlman CH, Vavvas DG, Chodosh J, Paschalis EI. Opposing Roles of Blood-Borne Monocytes and Tissue-Resident Macrophages in Limbal Stem Cell Damage after Ocular Injury. Cells. 2023; 12(16):2089. https://doi.org/10.3390/cells12162089
Chicago/Turabian StyleZhou, Chengxin, Fengyang Lei, Mirja Mittermaier, Bruce Ksander, Reza Dana, Claes H. Dohlman, Demetrios G. Vavvas, James Chodosh, and Eleftherios I. Paschalis. 2023. "Opposing Roles of Blood-Borne Monocytes and Tissue-Resident Macrophages in Limbal Stem Cell Damage after Ocular Injury" Cells 12, no. 16: 2089. https://doi.org/10.3390/cells12162089
APA StyleZhou, C., Lei, F., Mittermaier, M., Ksander, B., Dana, R., Dohlman, C. H., Vavvas, D. G., Chodosh, J., & Paschalis, E. I. (2023). Opposing Roles of Blood-Borne Monocytes and Tissue-Resident Macrophages in Limbal Stem Cell Damage after Ocular Injury. Cells, 12(16), 2089. https://doi.org/10.3390/cells12162089






