Human and Pig Pluripotent Stem Cells: From Cellular Products to Organogenesis and Beyond
Abstract
:1. Introduction
2. Cellular Products
2.1. Advanced Stage Diabetes
2.2. Macular Degeneration
3. Human or Humanized Pig Organ Generation
3.1. Chimera Formation in Rodents PSC
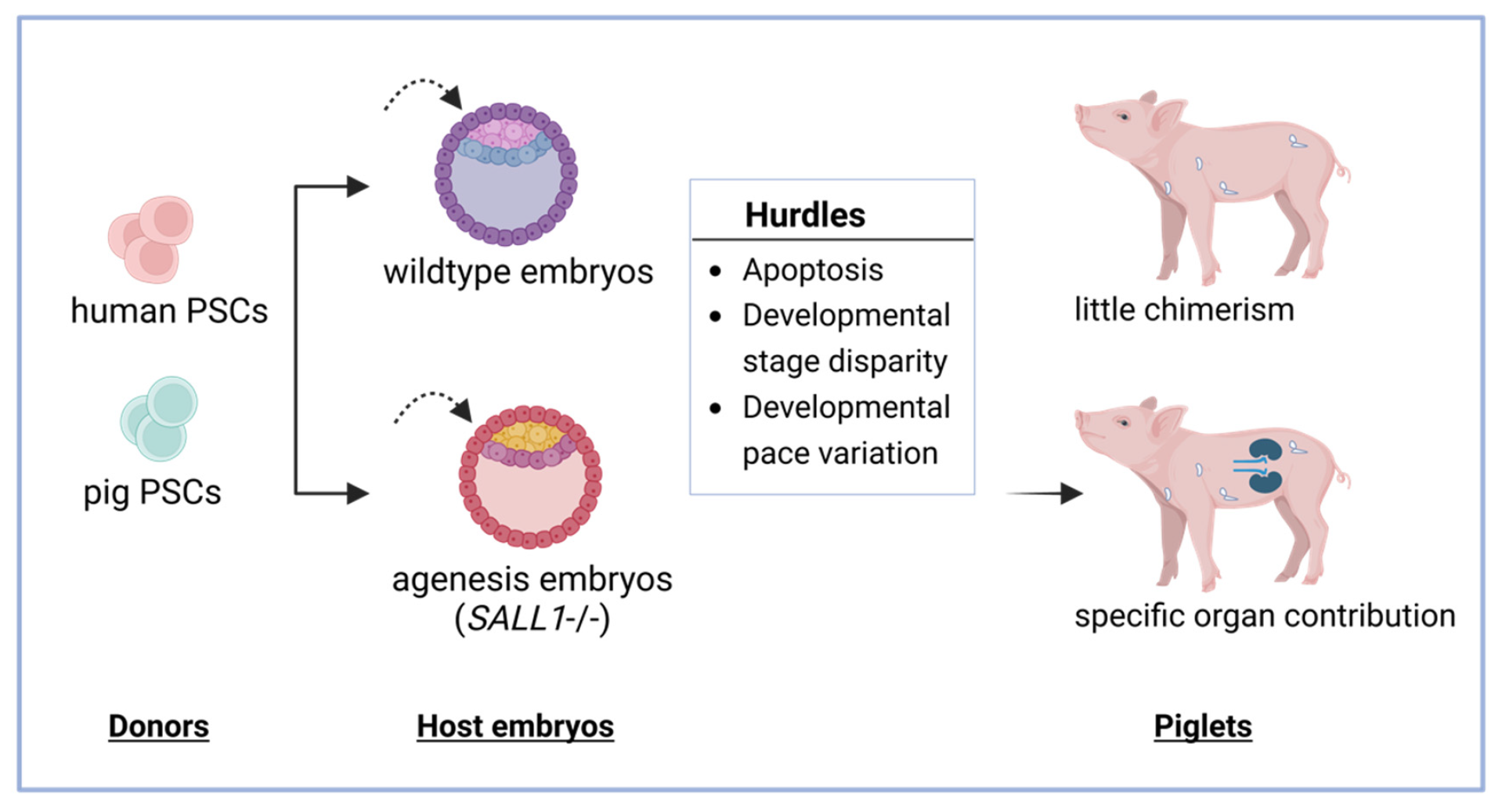
3.2. Chimera Formation in Animals Using Human and Pig PSC
3.2.1. Apoptosis
3.2.2. Developmental Disparity
3.2.3. Developmental Pace Variations
4. Humanized Pig PSCs for Xenotransplantation
4.1. Brief History of Xenotransplantation
4.2. Gene Modification Strategies
4.3. Cell Sources for Xenotransplantation
4.3.1. Pig Embryos
4.3.2. Pig Fibroblasts
4.3.3. Pig Pluripotent Stem Cells
4.4. Generation of Humanized Pigs through Cloning Techniques
4.5. Acquisition of Humanized Pig Organs through Chimera Formation
4.6. Production of Humanized Pigs via Blastocyst-like Structure Development
5. Ethics and Other Concerns
6. Conclusions
Funding
Conflicts of Interest
References
- Evans, M.; Kaufman, M. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.; Itskovitz-Eldor, J.; Shapiro, S.; Waknitz, M.; Swiergiel, J.; Marshall, V.; Jones, J. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Gao, X.; Nowak-Imialek, M.; Chen, X.; Chen, D.; Herrmann, D.; Ruan, D.; Chen, A.C.H.; Eckersley-Maslin, M.A.; Ahmad, S.; Lee, Y.L.; et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019, 21, 687–699. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, X.; Zheng, Y.; Wang, Z.; Zhao, G.; Ren, J.; Zhang, J.; Wu, J.; Wu, B.; Chen, Y.; et al. Establishment of bovine expanded potential stem cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2018505118. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.; Xu, J.; Wang, J.; Wu, J.; Shi, C.; Xu, Y.; Dong, J.; Wang, C.; Lai, W.; et al. Derivation of Pluripotent Stem Cells with In Vivo Embryonic and Extraembryonic Potency. Cell 2017, 169, 243–257.e25. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ryan, D.; Wang, W.; Tsang, J.; Lan, G.; Masaki, H.; Gao, X.; Antunes, L.; Yu, Y.; Zhu, Z.; et al. Establishment of mouse expanded potential stem cells. Nature 2017, 550, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, S.; Xu, Y.; Lyu, Y.; Wang, J.; Du, Y.; Sun, Y.; Liu, H.; Zhou, H.; Lai, W.; et al. Chemically defined and xeno-free culture condition for human extended pluripotent stem cells. Nat. Commun. 2021, 12, 3017. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, J.; Ayyash, M.; Shani, T.; Manor, Y.; Gafni, O.; Kalma, Y.; Aguilera-Castrejon, A.; Zerbib, M.; Amir, H.; Sheban, D. Others Tripartite inhibition of SRC-WNT-PKC signalling consolidates human naïve pluripotency. bioRxiv 2020. [Google Scholar] [CrossRef]
- Markmann, J.; Naji, A.; Rickels, M.; Alba, M.; Marigowda, G.; Ross, L.; Wang, C.; Pagliuca, F.; Sanna, B.; Kean, L.; et al. 259-OR: Stem cell–derived, fully differentiated islet cells for type 1 diabetes. Diabetes 2022, 71, 259-OR. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-grade stem cell–derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Kashani, A.; Lebkowski, J.; Hinton, D.; Zhu, D.; Faynus, M.; Chen, S.; Rahhal, F.; Avery, R.; Salehi-Had, H.; Chan, C.; et al. Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration. Stem Cell Rep. 2022, 17, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Maeng, G.; Das, S.; Greising, S.; Gong, W.; Singh, B.; Kren, S.; Mickelson, D.; Skie, E.; Gafni, O.; Sorensen, J.; et al. Humanized skeletal muscle in MYF5/MYOD/MYF6-null pig embryos. Nat. Biomed. Eng. 2021, 5, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Koyano-Nakagawa, N.; Gafni, O.; Maeng, G.; Singh, B.; Rasmussen, T.; Pan, X.; Choi, K.; Mickelson, D.; Gong, W.; et al. Generation of human endothelium in pig embryos deficient in ETV2. Nat. Biotechnol. 2020, 38, 297–302. [Google Scholar] [CrossRef]
- Wu, J.; Platero-Luengo, A.; Sakurai, M.; Sugawara, A.; Gil, M.; Yamauchi, T.; Suzuki, K.; Bogliotti, Y.; Cuello, C.; Valencia, M.; et al. Interspecies Chimerism with Mammalian Pluripotent Stem Cells. Cell 2017, 168, 473–486.e15. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Zhang, J.; Tang, Q.; Yu, D.; Gao, S.; Gao, D.; Liu, P.; Guo, J.; Hai, T.; Gao, J.; et al. Generation and characterization of stable pig pregastrulation epiblast stem cell lines. Cell Res. 2021, 32, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhu, Y.; Ma, Y.; Zhao, B.; Fan, N.; Li, Y.; Song, H.; Chu, S.; Ouyang, Z.; Zhang, Q.; et al. BMI1 enables interspecies chimerism with human pluripotent stem cells. Nat. Commun. 2018, 9, 4649. [Google Scholar] [CrossRef]
- Zheng, C.; Hu, Y.; Sakurai, M.; Pinzon-Arteaga, C.; Li, J.; Wei, Y.; Okamura, D.; Ravaux, B.; Barlow, H.; Yu, L.; et al. Cell competition constitutes a barrier for interspecies chimerism. Nature 2021, 592, 272–276. [Google Scholar] [CrossRef]
- Huang, Y.; Osorno, R.; Tsakiridis, A.; Wilson, V. In Vivo Differentiation Potential of Epiblast Stem Cells Revealed by Chimeric Embryo Formation. Cell Rep. 2012, 2, 1571–1578. [Google Scholar] [CrossRef]
- Zhong, C.; Li, R.; Belmonte, J. Transcriptomic profiling fuels the derivation of stable pig epiblast stem cells. Cell Res. 2022, 32, 329–330. [Google Scholar] [CrossRef]
- Kinoshita, M.; Kobayashi, T.; Planells, B.; Klisch, D.; Spindlow, D.; Masaki, H.; Bornelöv, S.; Stirparo, G.; Matsunari, H.; Uchikura, A.; et al. Pluripotent stem cells related to embryonic disc exhibit common self-renewal requirements in diverse livestock species. Development 2021, 148, dev199901. [Google Scholar] [CrossRef]
- Veres, A.; Faust, A.L.; Bushnell, H.L.; Engquist, E.N.; Kenty, J.H.-R.; Harb, G.; Poh, Y.-C.; Sintov, E.; Gürtler, M.; Pagliuca, F.W.; et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 2019, 569, 368–373. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.; Xu, Y.; et al. An Effective Approach to Prevent Immune Rejection of Human ESC-Derived Allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Limnios, I.; Chau, Y.; Skabo, S.; Surrao, D.; O’Neill, H. Efficient differentiation of human embryonic stem cells to retinal pigment epithelium under defined conditions. Stem Cell Res. Ther. 2021, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Su, B.; Jiao, L.; Xu, Z.; Zhang, C.; Nie, J.; Gao, M.; Zhang, Y.; Jin, Z. Transplantation of GMP-grade human iPSC-derived retinal pigment epithelial cells in rodent model: The first pre-clinical study for safety and efficacy in China. Ann. Transl. Med. 2021, 9, 245. [Google Scholar] [CrossRef]
- Stanzel, B.; Liu, Z.; Somboonthanakij, S.; Wongsawad, W.; Brinken, R.; Eter, N.; Corneo, B.; Holz, F.; Temple, S.; Stern, J.; et al. Human RPE Stem Cells Grown into Polarized RPE Monolayers on a Polyester Matrix Are Maintained after Grafting into Rabbit Subretinal Space. Stem Cell Rep. 2014, 2, 64–77. [Google Scholar] [CrossRef]
- Liu, Z.; Parikh, B.; Tan, Q.; Wong, D.; Ong, K.; Yu, W.; Seah, I.; Holder, G.; Hunziker, W.; Tan, G.; et al. Surgical Transplantation of Human RPE Stem Cell-Derived RPE Monolayers into Non-Human Primates with Immunosuppression. Stem Cell Rep. 2021, 16, 237–251. [Google Scholar] [CrossRef]
- Schwartz, S.; Regillo, C.; Lam, B.; Eliott, D.; Rosenfeld, P.; Gregori, N.; Hubschman, J.; Davis, J.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef]
- Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.; Ahmado, A.; Vernon, A.; Daniels, J.; Nommiste, B.; Hasan, S.; et al. Phase 1 clinical study of an embryonic stem cell–derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H. Ocular barriers as a double-edged sword: Preventing and facilitating drug delivery to the retina. Drug Deliv. Transl. Res. 2022, 13, 547–567. [Google Scholar] [CrossRef] [PubMed]
- Petrus-Reurer, S.; Kumar, P.; Sánchez, S.; Aronsson, M.; André, H.; Bartuma, H.; Reyes, A.; Nandrot, E.; Kvanta, A.; Lanner, F. Preclinical safety studies of human embryonic stem cell-derived retinal pigment epithelial cells for the treatment of age-related macular degeneration. Stem Cells Transl. Med. 2020, 9, 936–953. [Google Scholar] [CrossRef] [PubMed]
- Petrus-Reurer, S.; Winblad, N.; Kumar, P.; Gorchs, L.; Chrobok, M.; Wagner, A.; Bartuma, H.; Lardner, E.; Aronsson, M.; Plaza Reyes, A.; et al. Generation of Retinal Pigment Epithelial Cells Derived from Human Embryonic Stem Cells Lacking Human Leukocyte Antigen Class I and II. Stem Cell Rep. 2020, 14, 648–662. [Google Scholar] [CrossRef]
- Kanemura, H.; Go, M.; Shikamura, M.; Nishishita, N.; Sakai, N.; Kamao, H.; Mandai, M.; Morinaga, C.; Takahashi, M.; Kawamata, S. Tumorigenicity Studies of Induced Pluripotent Stem Cell (iPSC)-Derived Retinal Pigment Epithelium (RPE) for the Treatment of Age-Related Macular Degeneration. PLoS ONE 2014, 9, e85336. [Google Scholar] [CrossRef] [PubMed]
- Kuijk, E.; Jager, M.; Roest, B.; Locati, M.; Hoeck, A.; Korzelius, J.; Janssen, R.; Besselink, N.; Boymans, S.; Boxtel, R.; et al. The mutational impact of culturing human pluripotent and adult stem cells. Nat. Commun. 2020, 11, 2493. [Google Scholar] [CrossRef] [PubMed]
- Hasaart, K.; Manders, F.; Ubels, J.; Verheul, M.; Roosmalen, M.; Groenen, N.; Oka, R.; Kuijk, E.; Sousa Lopes, S.; Boxtel, R. Human induced pluripotent stem cells display a similar mutation burden as embryonic pluripotent cells in vivo. iScience 2022, 25, 103736. [Google Scholar] [CrossRef] [PubMed]
- Avior, Y.; Lezmi, E.; Eggan, K.; Benvenisty, N. Cancer-Related Mutations Identified in Primed Human Pluripotent Stem Cells. Cell Stem Cell 2021, 28, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Head, S.; Çelik, M.; Kappetein, A. Mechanical versus bioprosthetic aortic valve replacement. Eur. Heart J. 2017, 38, 2183–2191. [Google Scholar] [CrossRef]
- Gardner, R. Mouse Chimaeras obtained by the Injection of Cells into the Blastocyst. Nature 1968, 220, 596–597. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sato, H.; Kato-Itoh, M.; Goto, T.; Hara, H.; Sanbo, M.; Mizuno, N.; Kobayashi, T.; Yanagida, A.; Umino, A.; et al. Interspecies organogenesis generates autologous functional islets. Nature 2017, 542, 191–196. [Google Scholar] [CrossRef]
- Hamanaka, S.; Umino, A.; Sato, H.; Hayama, T.; Yanagida, A.; Mizuno, N.; Kobayashi, T.; Kasai, M.; Suchy, F.; Yamazaki, S.; et al. Generation of Vascular Endothelial Cells and Hematopoietic Cells by Blastocyst Complementation. Stem Cell Rep. 2018, 11, 988–997. [Google Scholar] [CrossRef]
- Goto, T.; Hara, H.; Sanbo, M.; Masaki, H.; Sato, H.; Yamaguchi, T.; Hochi, S.; Kobayashi, T.; Nakauchi, H.; Hirabayashi, M. Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat. Commun. 2019, 10, 451. [Google Scholar] [CrossRef]
- Coppiello, G.; Barlabé, P.; Moya-Jódar, M.; Abizanda, G.; Barreda, C.; Iglesias, E.; Linares, J.; Arellano-Viera, E.; Ruiz-Villalba, A.; Larequi, E.; et al. In vivo generation of heart and vascular system by blastocyst complementation. bioRxiv 2022. [Google Scholar] [CrossRef]
- Kitahara, A.; Ran, Q.; Oda, K.; Yasue, A.; Abe, M.; Ye, X.; Sasaoka, T.; Tsuchida, M.; Sakimura, K.; Ajioka, Y.; et al. Generation of Lungs by Blastocyst Complementation in Apneumic Fgf10-Deficient Mice. Cell Rep. 2020, 31, 107626. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kubara, K.; Ishii, S.; Li, P.; Dairiki, R.; Hihara, T.; Ishizuka, Y.; Izumi, Y.; Kumai, M.; Kamisako, T.; et al. In vitro and in vivo functions of T cells produced in complemented thymi of chimeric mice generated by blastocyst complementation. Sci. Rep. 2022, 12, 3242. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Yamaguchi, T.; Hamanaka, S.; Kato-Itoh, M.; Yamazaki, Y.; Ibata, M.; Sato, H.; Lee, Y.-S.; Usui, J.-I.; Knisely, A.S.; et al. Generation of Rat Pancreas in Mouse by Interspecific Blastocyst Injection of Pluripotent Stem Cells. Cell 2010, 142, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Estevez, M.; Crane, A.; Rodriguez-Villamil, P.; Ongaratto, F.; You, Y.; Steevens, A.; Hill, C.; Goldsmith, T.; Webster, D.; Sherry, L.; et al. Liver development is restored by blastocyst complementation of HHEX knockout in mice and pigs. Stem Cell Res. Ther. 2021, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, J.; Li, Z.; Qin, G.; Zhang, N.; Hai, T.; Hong, Q.; Zheng, Q.; Zhang, Y.; Song, R.; et al. Rescuing ocular development in an anophthalmic pig by blastocyst complementation. EMBO Mol. Med. 2018, 10, e8861. [Google Scholar] [CrossRef] [PubMed]
- Steevens, A.; Griesbach, M.; You, Y.; Dutton, J.; Low, W.; Santi, P. Generation of inner ear sensory neurons using blastocyst complementation in a Neurog1+/−-deficient mouse. Stem Cell Res. Ther. 2021, 12, 122. [Google Scholar] [CrossRef]
- Kobayashi, T.; Goto, T.; Oikawa, M.; Sanbo, M.; Yoshida, F.; Terada, R.; Niizeki, N.; Kajitani, N.; Kazuki, K.; Kazuki, Y.; et al. Blastocyst complementation using Prdm14-deficient rats enables efficient germline transmission and generation of functional mouse spermatids in rats. Nat. Commun. 2021, 12, 1328. [Google Scholar] [CrossRef]
- Tan, T.; Wu, J.; Si, C.; Dai, S.; Zhang, Y.; Sun, N.; Zhang, E.; Shao, H.; Si, W.; Yang, P.; et al. Chimeric contribution of human extended pluripotent stem cells to monkey embryos ex vivo. Cell 2021, 184, 2020–2032.e14. [Google Scholar] [CrossRef]
- Wang, F.; Yin, Y.; Ye, X.; Liu, K.; Zhu, H.; Wang, L.; Chiourea, M.; Okuka, M.; Ji, G.; Dan, J.; et al. Molecular insights into the heterogeneity of telomere reprogramming in induced pluripotent stem cells. Cell Res. 2011, 22, 757–768. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.; Liu, Y.; Fu, Y.; Gao, S.; Gong, P.; Wang, H.; Zhou, Z.; Zeng, M.; Wu, Z.; et al. Telomere heterogeneity linked to metabolism and pluripotency state revealed by simultaneous analysis of telomere length and RNA-seq in the same human embryonic stem cell. BMC Biol. 2017, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H.; Kato-Itoh, M.; Takahashi, Y.; Umino, A.; Sato, H.; Ito, K.; Yanagida, A.; Nishimura, T.; Yamaguchi, T.; Hirabayashi, M.; et al. Inhibition of Apoptosis Overcomes Stage-Related Compatibility Barriers to Chimera Formation in Mouse Embryos. Cell Stem Cell 2016, 19, 587–592. [Google Scholar] [CrossRef]
- Zalzman, M.; Falco, G.; Sharova, L.; Nishiyama, A.; Thomas, M.; Lee, S.; Stagg, C.; Hoang, H.; Yang, H.; Indig, F.; et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010, 464, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, H.; Jiang, H.; Ren, Y.; Yu, X.; Qiu, J.; Stablewski, A.; Zhang, B.; Buck, M.; Feng, J. Transient inhibition of mTOR in human pluripotent stem cells enables robust formation of mouse-human chimeric embryos. Sci. Adv. 2020, 6, eaaz0298. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef]
- Li, J.; Zhu, Q.; Cao, J.; Liu, Y.; Lu, Y.; Sun, Y.; Li, Q.; Huang, Y.; Shang, S.; Bian, X.; et al. Cynomolgus monkey embryo model captures gastrulation and early pregnancy. Cell Stem Cell 2023, 30, 362–377.e7. [Google Scholar] [CrossRef]
- Keshet, G.; Benvenisty, N. Large-scale analysis of imprinting in naive human pluripotent stem cells reveals recurrent aberrations and a potential link to FGF signaling. Stem Cell Rep. 2021, 16, 2520–2533. [Google Scholar] [CrossRef]
- Yang, L.; Church, G.; Zhao, H.; Huang, L.; Gao, Y.; Wei, H.; Yang, G. Porcine germline genome engineering. Proc. Natl. Acad. Sci. USA 2021, 118, e2004836117. [Google Scholar] [CrossRef]
- Yang, Y.; Sykes, M. Xenotransplantation: Current status and a perspective on the future. Nat. Rev. Immunol. 2007, 7, 519–531. [Google Scholar] [CrossRef]
- Varki, A. Multiple changes in sialic acid biology during human evolution. Glycoconj. J. 2008, 26, 231–245. [Google Scholar] [CrossRef]
- Mercer, W.; Shields, M.; Amin, M.; Sauve, G.; Appella, E.; Romano, J.; Ullrich, S. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc. Natl. Acad. Sci. USA 1990, 87, 6166–6170. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Koren, E.; Oriol, R. Oligosaccharides and Discordant Xenotransplantation. Immunol. Rev. 1994, 141, 31–58. [Google Scholar] [CrossRef] [PubMed]
- Petkov, S.; Glage, S.; Nowak-Imialek, M.; Niemann, H. Long-Term Culture of Porcine Induced Pluripotent Stem-Like Cells Under Feeder-Free Conditions in the Presence of Histone Deacetylase Inhibitors. Stem Cells Dev. 2016, 25, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Tian, F.; Ma, X.; Zhang, Y.; Zhang, C.; Zeng, W.; Zhang, Y.; Lin, Y. PDIA3 regulates trophoblast apoptosis and proliferation in preeclampsia via the MDM2/p53 pathway. Reproduction 2020, 160, 293–305. [Google Scholar] [CrossRef]
- Phelps, C.; Koike, C.; Vaught, T.; Boone, J.; Wells, K.; Chen, S.; Ball, S.; Specht, S.; Polejaeva, I.; Monahan, J.; et al. Production of α1, 3-Galactosyltransferase-Deficient Pigs. Science 2003, 299, 411–414. [Google Scholar] [CrossRef]
- Griesemer, A.; Hirakata, A.; Shimizu, A.; Moran, S.; Tena, A.; Iwaki, H.; Ishikawa, Y.; Schule, P.; Arn, J.; Robson, S.; et al. Results of Gal-Knockout Porcine Thymokidney Xenografts. Am. J. Transplant. 2009, 9, 2669–2678. [Google Scholar] [CrossRef]
- Shim, J.; Ko, N.; Kim, H.; Lee, Y.; Lee, J.; Jin, D.; Kim, H.; Choi, K. Human immune reactivity of GGTA1/CMAH/A3GALT2 triple knockout Yucatan miniature pigs. Transgenic Res. 2021, 30, 619–634. [Google Scholar] [CrossRef]
- Niu, D.; Wei, H.; Lin, L.; George, H.; Wang, T.; Lee, I.; Zhao, H.; Wang, Y.; Kan, Y.; Shrock, E.; et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 2017, 357, 1303–1307. [Google Scholar] [CrossRef]
- Ma, D.; Hirose, T.; Lassiter, G.; Sasaki, H.; Rosales, I.; Coe, T.; Rickert, C.; Matheson, R.; Colvin, R.; Qin, W.; et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am. J. Transplant. 2022, 22, 46–57. [Google Scholar] [CrossRef]
- Yue, Y.; Xu, W.; Kan, Y.; Zhao, H.; Zhou, Y.; Song, X.; Wu, J.; Xiong, J.; Goswami, D.; Yang, M.; et al. Extensive germline genome engineering in pigs. Nat. Biomed. Eng. 2020, 5, 134–143. [Google Scholar] [CrossRef]
- Le, Q.; Wittayarat, M.; Namula, Z.; Lin, Q.; Takebayashi, K.; Hirata, M.; Tanihara, F.; Do, L.; Otoi, T. Multiple gene editing in porcine embryos using a combination of microinjection, electroporation, and transfection methods. Vet. World 2022, 15, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Mianné, J.; Codner, G.; Caulder, A.; Fell, R.; Hutchison, M.; King, R.; Stewart, M.; Wells, S.; Teboul, L. Analysing the outcome of CRISPR-aided genome editing in embryos: Screening, genotyping and quality control. Methods 2017, 121–122, 68–76. [Google Scholar] [CrossRef]
- Mackenzie, M.; Fower, A.; Allan, A.; Codner, G.; Bunton-Stasyshyn, R.; Teboul, L. Genotyping Genome-Edited Founders and Subsequent Generation. In Methods In Molecular Biology; Springer: Berlin/Heidelberg, Germany, 2023; pp. 103–134. [Google Scholar] [CrossRef]
- Yen, S.; Zhang, M.; Deng, J.; Usman, S.; Smith, C.; Parker-Thornburg, J.; Swinton, P.; Martin, J.; Behringer, R. Somatic mosaicism and allele complexity induced by CRISPR/Cas9 RNA injections in mouse zygotes. Dev. Biol. 2014, 393, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Yoon, S.; Koh, Y.; Seo, Y.; Kim, K. Maintenance of genome integrity and active homologous recombination in embryonic stem cells. Exp. Mol. Med. 2020, 52, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.; Chen, J.; Shang, Z.; Dou, H.; Ji, G.; Zou, Q.; Wu, L.; He, L.; Wang, F.; Liu, K.; et al. Piglets cloned from induced pluripotent stem cells. Cell Res. 2012, 23, 162–166. [Google Scholar] [CrossRef]
- Zhao, J.; Ross, J.; Hao, Y.; Spate, L.; Walters, E.; Samuel, M.; Rieke, A.; Murphy, C.; Prather, R. Significant Improvement in Cloning Efficiency of an Inbred Miniature Pig by Histone Deacetylase Inhibitor Treatment after Somatic Cell Nuclear Transfer1. Biol. Reprod. 2009, 81, 525–530. [Google Scholar] [CrossRef]
- Jin, L.; Zhu, H.; Guo, Q.; Li, X.; Zhang, Y.; Zhang, G.; Xing, X.; Xuan, M.; Luo, Q.; Yin, X.; et al. PCI-24781 can improve in vitro and in vivo developmental capacity of pig somatic cell nuclear transfer embryos. Biotechnol. Lett. 2016, 38, 1433–1441. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, X.; Xie, W.; Zhou, Y.; Li, D.; Zhou, Y.; Zhu, J.; Yuan, T.; Lai, L.; Pang, D.; et al. Vitamin C enhances in vitro and in vivo development of porcine somatic cell nuclear transfer embryos. Biochem. Biophys. Res. Commun. 2011, 411, 397–401. [Google Scholar] [CrossRef]
- Guo, Z.; Lv, L.; Liu, D.; Fu, B. Effects of trichostatin A on pig SCNT blastocyst formation rate and cell number: A meta-analysis. Res. Vet. Sci. 2018, 117, 161–166. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; Wang, Y.; Nie, Y.; Zhang, C.; Xu, Y.; Zhang, X.; Lu, Y.; Wang, Z.; Poo, M.; et al. Cloning of Macaque Monkeys by Somatic Cell Nuclear Transfer. Cell 2018, 172, 881–887.e7. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Wang, C.; Gao, Y.; Gao, R.; Kou, X.; Zhao, Y.; Li, J.; Wu, Y.; Xiu, W.; et al. Identification of key factors conquering developmental arrest of somatic cell cloned embryos by combining embryo biopsy and single-cell sequencing. Cell Discov. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakano, K.; Uchikura, A.; Matsunari, H.; Yashima, S.; Umeyama, K.; Takayanagi, S.; Sakuma, T.; Yamamoto, T.; Morita, S.; et al. Anephrogenic phenotype induced by SALL1 gene knockout in pigs. Sci. Rep. 2019, 9, 8016. [Google Scholar] [CrossRef] [PubMed]
- Rivron, N.; Frias-Aldeguer, J.; Vrij, E.; Boisset, J.; Korving, J.; Vivié, J.; Truckenmüller, R.; Oudenaarden, A.; Blitterswijk, C.; Geijsen, N. Blastocyst-like structures generated solely from stem cells. Nature 2018, 557, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, Y.; Duan, J.; Schmitz, D.; Sakurai, M.; Wang, L.; Wang, K.; Zhao, S.; Hon, G.; Wu, J. Blastocyst-like structures generated from human pluripotent stem cells. Nature 2021, 591, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, J.; Schröder, J.; Aberkane, A.; Ouyang, J.; Mohenska, M.; Lim, S.; Sun, Y.; Chen, J.; Sun, G.; et al. Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 2021, 591, 627–632. [Google Scholar] [CrossRef]
- Kagawa, H.; Javali, A.; Khoei, H.; Sommer, T.; Sestini, G.; Novatchkova, M.; Reimer, Y.; Castel, G.; Bruneau, A.; Maenhoudt, N.; et al. Human blastoids model blastocyst development and implantation. Nature 2021, 601, 600–605. [Google Scholar] [CrossRef]
- Pinzón-Arteaga, C.; Wang, Y.; Wei, Y.; Li, L.; Orsi, A.; Scatolin, G.; Liu, L.; Sakurai, M.; Ye, J.; Yu, L.; et al. Bovine blastocyst like structures derived from stem cell cultures. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Kögel, J.; Marckmann, G. “Xenotransplantation challenges us as a society”. EMBO Rep. 2020, 21, e50274. [Google Scholar] [CrossRef]
- Fraser, D.; Weary, D.; Pajor, E.; Milligan, B. A Scientific Conception of Animal Welfare that Reflects Ethical Concerns. Anim. Welf. 1997, 6, 187–205. [Google Scholar] [CrossRef]
- Rashid, T.; Kobayashi, T.; Nakauchi, H. Revisiting the Flight of Icarus: Making Human Organs from PSCs with Large Animal Chimeras. Cell Stem Cell 2014, 15, 406–409. [Google Scholar] [CrossRef]

| Author | Donor–Host Relationship | Donor PSC Type | Donor Cell Culture Condition | Host Embryo Type | Lineage Contribution | PSC Contribution Percentage | Germline Transmission |
|---|---|---|---|---|---|---|---|
| Yang, Y. et al. (2017) [5] | Mouse–mouse | Mouse EPSCs | LCDM medium | Mouse 8C embryo, WT | At mouse E17.5, to both embryonic and extra-embryonic lineages | 1% to 19% | Yes |
| Human–mouse | Human EPSCs | LCDM medium | Mouse 8C embryo, WT | In vitro cultured mouse blastocyst, to both ICM or TE part | 14.70% | No | |
| Wu, J. et al. (2017) [14] | Rat–mouse | Rat naïve ESC | rat ESC medium | Mouse blastocysts | 3-week-old rat-mouse chimera, to brain, heart, intestine, kidney, lung, pancreas, stomach, liver etc, no germline reported | From 0.1% to 10% across different tissues | Not reported |
| Rodent–pig | Mouse/Rat naïve ESC | Mouse/rat ESC medium | Pig parthenogenesis blastocyst | At pig E21-28, no contribution | 0% | No | |
| Human–pig | Naïve or intermediate human iPSC | Naïve culture: 2iLD-hiPSCs, NHSM-hiPSCs, 4i-hiPSCs, intermediate: FAC medium | Pig parthenogenesis blastocyst | At pig E21-28, all three germ layers. 25–45% embryos showed chimerism, among chimera embryos, 75% showed growth retardation. | Not reported | No | |
| Human–cattle | Cattle IVF blastocyst | In vitro cultured cattle embryo at E9, 60–80% embryos with chimerism to ICM, not to TE. | Not reported | No | |||
| Huang, K. et al (2018) [16] | Human–mouse | Human primed PSC, BMI1-overexpression | mTeSR1 medium | Mouse morula or blastocysts | At mouse E10.5, all three germ layers | 7.50% | No |
| Zhi, M. et al. (2020) [15] | Human–mouse | Human naive PSC | mTOR inhibition pretreated; in 2iL plus insulin medium | Mouse blastocysts | At mouse E17.5, all three germ layers, red blood cells, liver, retina, not to germine | 0.1% to 4% | No |
| Maeng, G. et al. (2021) [12] | Human–pig | Human-primed WT and TP53-/- iPSC | mTeSR1 or TeSR-E8 medium | Porcine MYF5-/-MYOD-/- MYF6-/- cloned morula | At pig E27, preferred contribution to the myogenic lineages (muscle), not to neural and germline | 1:1000 to 1:100,000 | No |
| Das, S. et al. (2021) [13] | Human–pig | Human WT or BCL2 overexpression iPSC | mTeSR1 | Pig ETV2-null SCNT embryo | At pig E17-18, 81% embryos showed chimerism with BCL2-OE human cells; 52% with normal human cells. all endothelial cells with human origin | 1:2000 in BCL2-OE hiPSC, Less than 1:10,000 in WT hiPSC | No |
| Tan, T. et al. (2021) [49] | Human–monkey | Human EPSCs | LCDM medium | Monkey in vitro cultured embryos | In vitro culture until D19, to epiblast and hypoblast, undetected to trophoblast lineage (highest at D9 with 2%). | D19, 6% epiblast; 4% to hypoblast, 5% to trophoblast. | No |
| Zhi, M. et al. (2022) [15] | Pig–pig | Pig EpiSC | 3i/LAF medium | Porcine SCNT blastocysts at E5 | At pig E10, no contribution | 0% | No |
| Embryos | Fibroblasts | Embryonic Stem Cells | |
|---|---|---|---|
| Gene editing | One time editing | Simple editing | Complex editing |
| Genotyping | Mosaicism in nature, unclear results | Require serial dilution, clear results | Single colony genotyping, clear results |
| Passage number | No passage number | Limited, less than 20 passages | Unlimited passage number |
| Pig generation | In vivo production | SCNT | SCNT/ Blastoid, remain further validation |
| Off-spring healthy status | Healthy | Reprogramming issues | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Y.; Petersen, B.; Liu, P. Human and Pig Pluripotent Stem Cells: From Cellular Products to Organogenesis and Beyond. Cells 2023, 12, 2075. https://doi.org/10.3390/cells12162075
Xuan Y, Petersen B, Liu P. Human and Pig Pluripotent Stem Cells: From Cellular Products to Organogenesis and Beyond. Cells. 2023; 12(16):2075. https://doi.org/10.3390/cells12162075
Chicago/Turabian StyleXuan, Yiyi, Björn Petersen, and Pentao Liu. 2023. "Human and Pig Pluripotent Stem Cells: From Cellular Products to Organogenesis and Beyond" Cells 12, no. 16: 2075. https://doi.org/10.3390/cells12162075
APA StyleXuan, Y., Petersen, B., & Liu, P. (2023). Human and Pig Pluripotent Stem Cells: From Cellular Products to Organogenesis and Beyond. Cells, 12(16), 2075. https://doi.org/10.3390/cells12162075








