Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage
Abstract
1. Introduction
2. Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
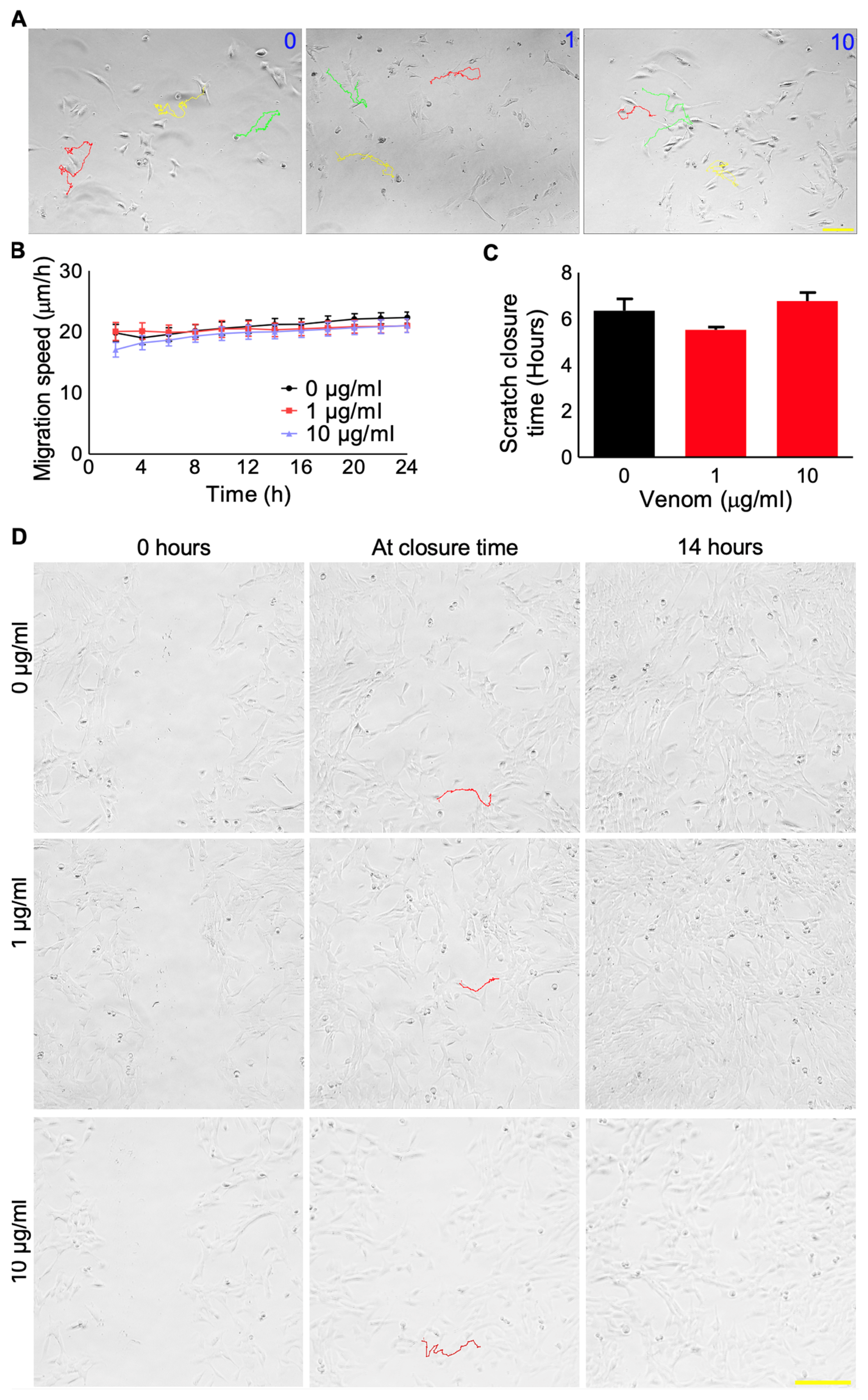
2.4. Cell Migration Assay
2.5. Scratch Assay
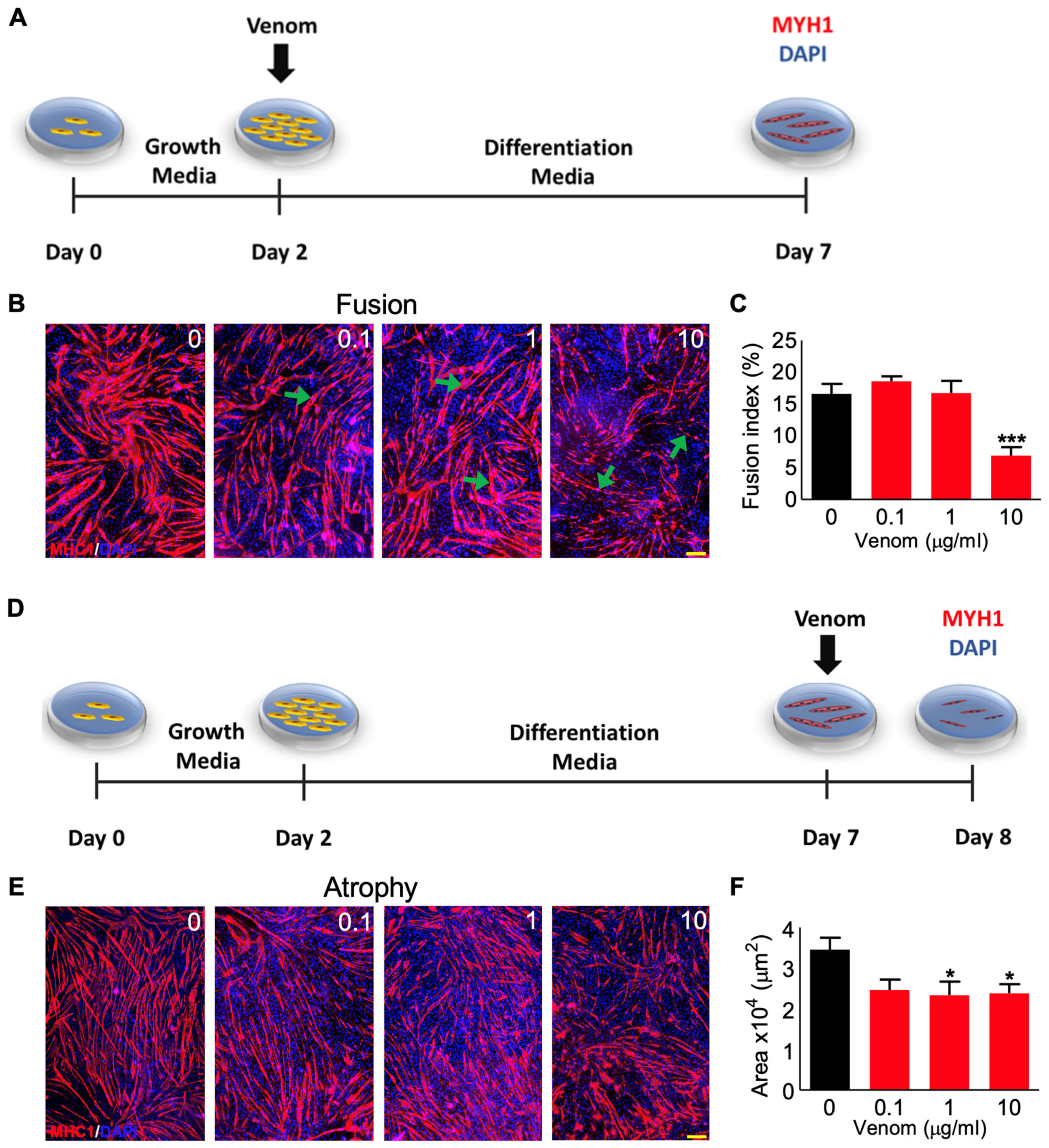
2.6. Fusion Assay
2.7. Atrophy Assay
2.8. Immunocytochemistry
2.9. Enzymatic Assays
2.10. SDS-PAGE and Silver Staining
2.11. Venom-Induced Muscle Damage in Mice
2.12. Histological Staining
2.13. Immunohistochemistry of TA Muscle
2.14. Statistical Analysis
3. Results
3.1. Biochemical Characterisation of P. regalis Venom
3.2. P. regalis Venom Does Not Affect the Survival and Migration of Myoblasts
3.3. P. regalis Venom Affects the Fusion of Myoblasts into Myotubes and Induces Atrophy
3.4. Administration of P. regalis Venom in TA Muscle Induces Muscle Loss in Mice
3.5. P. regalis Venom Induces Fibrosis and Infiltration of IgG in TA Muscle at Early Time Points
3.6. Muscle Regeneration following P. regalis Venom-Induced Damage
3.7. P. regalis Venom Affects Neuromuscular Junctions
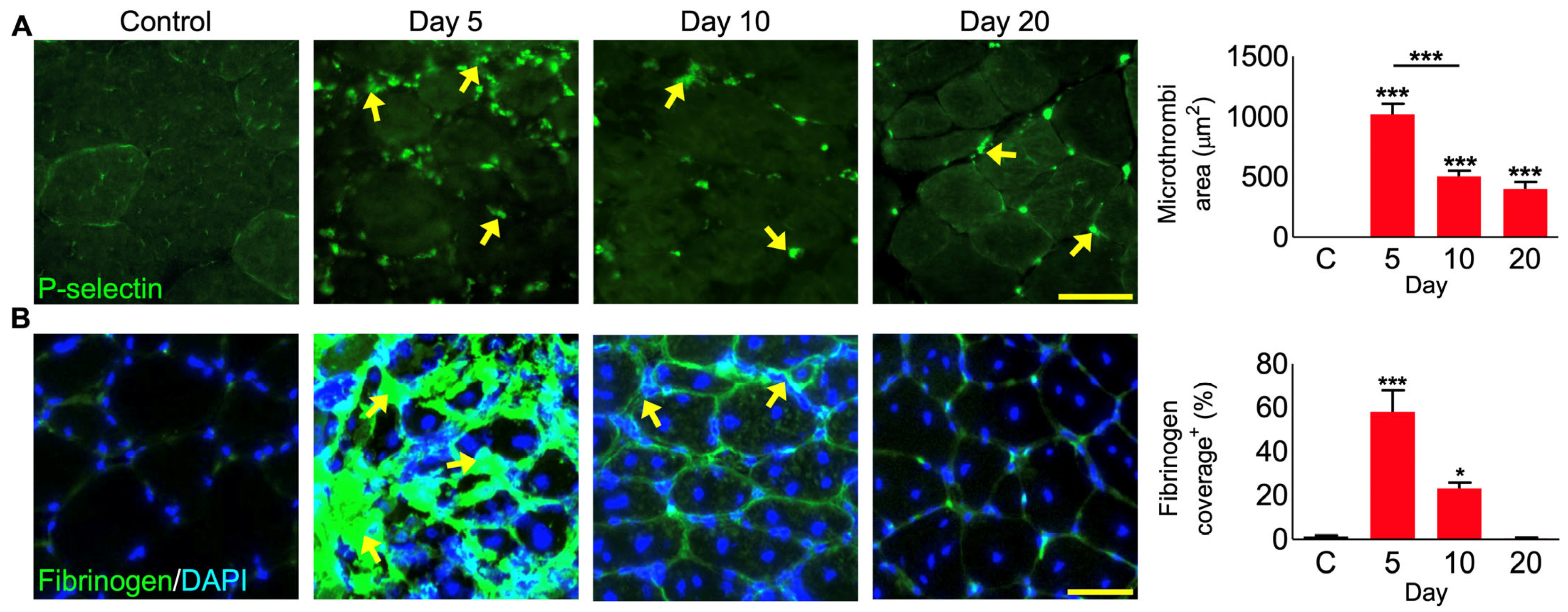
3.8. P. regalis Venom Induces Bleeding and Microthrombi Formation in Damaged Muscle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, J.; von Dechend, M.; Mordasini, R.; Ceschi, A.; Nentwig, W. A verified spider bite and a review of the literature confirm Indian ornamental tree spiders (Poecilotheria species) as underestimated theraphosids of medical importance. Toxicon 2014, 77, 73–77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jegaraj, K.; Saurabh, R.; Rakesh, P. Spider bite from South India. J. Postgrad. Med. 2014, 60, 216–217. [Google Scholar] [CrossRef]
- Thirunarayanan, T. Ethnobotanical Survey on Folk Medicine in the Management of Animal Bite Poisons in the Forest Tract of Salem Region of Tamil Nadu, India. Int. J. Pharmacol. Clin. Sci. 2013, 2, 41–46. [Google Scholar]
- de Haro, L.; Jouglard, J. The dangers of pet tarantulas: Experience of the Marseilles Poison Centre. J. Toxicol. Clin. Toxicol. 1998, 36, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Hauke, T.J.; Herzig, V. Love bites—Do venomous arachnids make safe pets? Toxicon 2021, 190, 65–72. [Google Scholar] [CrossRef]
- Ahmed, N.; Pinkham, M.; Warrell, D.A. Symptom in search of a toxin: Muscle spasms following bites by Old World tarantula spiders (Lampropelma nigerrimum, Pterinochilus murinus, Poecilotheria regalis) with review. QJM 2009, 102, 851–857. [Google Scholar] [CrossRef]
- Andreev-Andrievskiy, A.; Popova, A.; Lagereva, E.; Osipov, D.; Berkut, A.; Grishin, E.; Vassilevski, A. Pharmacological analysis of Poecilotheria spider venoms in mice provides clues for human treatment. Toxicon 2017, 138, 59–67. [Google Scholar] [CrossRef]
- Williams, J.; Vaiyapuri, S. The value of venom. Biologist 2023, 70, 14–17. [Google Scholar]
- Dumont, N.A.; Bentzinger, C.F.; Sincennes, M.C.; Rudnicki, M.A. Satellite Cells and Skeletal Muscle Regeneration. Compr. Physiol. 2015, 5, 1027–1059. [Google Scholar] [CrossRef]
- Joanisse, S.; Nederveen, J.P.; Snijders, T.; McKay, B.R.; Parise, G. Skeletal Muscle Regeneration, Repair and Remodelling in Aging: The Importance of Muscle Stem Cells and Vascularization. Gerontology 2017, 63, 91–100. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Ferri, R.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem. 2004, 48, 223–233. [Google Scholar] [CrossRef]
- Bajaj, P.; Reddy, B.; Millet, L.; Wei, C.; Zorlutuna, P.; Bao, G.; Bashir, R. Patterning the differentiation of C2C12 skeletal myoblasts. Integr. Biol. 2011, 3, 897–909. [Google Scholar] [CrossRef]
- Schöneich, C.; Dremina, E.; Galeva, N.; Sharov, V. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis 2014, 19, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, M.; Yang, Z.; Nakagawa, K.; Maruyama, J.; Xu, X.; Sarkar, A.; Ichimura, A.; Nasu, Y.; Ozawa, T.; Iwasa, H.; et al. A new cell-based assay to evaluate myogenesis in mouse myoblast C2C12 cells. Exp. Cell Res. 2015, 336, 171–181. [Google Scholar] [CrossRef]
- Menezes, M.C.; Kitano, E.S.; Bauer, V.C.; Oliveira, A.K.; Cararo-Lopes, E.; Nishiyama, M.Y.; Zelanis, A.; Serrano, S.M.T. Early response of C2C12 myotubes to a sub-cytotoxic dose of hemorrhagic metalloproteinase HF3 from Bothrops jararaca venom. J. Proteom. 2019, 198, 163–176. [Google Scholar] [CrossRef]
- Jang, M.; Scheffold, J.; Rost, L.M.; Cheon, H.; Bruheim, P. Serum-free cultures of C2C12 cells show different muscle phenotypes which can be estimated by metabolic profiling. Sci. Rep. 2022, 12, 827. [Google Scholar] [CrossRef]
- Williams, H.F.; Mellows, B.A.; Mitchell, R.; Sfyri, P.; Layfield, H.J.; Salamah, M.; Vaiyapuri, R.; Collins-Hooper, H.; Bicknell, A.B.; Matsakas, A.; et al. Mechanisms underpinning the permanent muscle damage induced by snake venom metalloprotease. PLoS Negl. Trop. Dis. 2019, 13, e0007041. [Google Scholar] [CrossRef]
- Damico, D.C.; da Cruz Höfling, M.A.; Cintra, M.; Leonardo, M.B.; Calgarotto, A.K.; da Silva, S.L.; Marangoni, S. Pharmacological study of edema and myonecrosis in mice induced by venom of the bushmaster snake (Lachesis muta muta) and its basic Asp49 phospholipase A(2) (LmTX-I). Protein J. 2008, 27, 384–391. [Google Scholar] [CrossRef]
- Layfield, H.J.; Williams, H.F.; Ravishankar, D.; Mehmi, A.; Sonavane, M.; Salim, A.; Vaiyapuri, R.; Lakshminarayanan, K.; Vallance, T.M.; Bicknell, A.B.; et al. Repurposing Cancer Drugs Batimastat and Marimastat to Inhibit the Activity of a Group I Metalloprotease from the Venom of the Western Diamondback Rattlesnake, Crotalus atrox. Toxins 2020, 12, 309. [Google Scholar] [CrossRef] [PubMed]
- Vaiyapuri, S.; Hutchinson, E.G.; Ali, M.S.; Dannoura, A.; Stanley, R.G.; Harrison, R.A.; Bicknell, A.B.; Gibbins, J.M. Rhinocetin, a venom-derived integrin-specific antagonist inhibits collagen-induced platelet and endothelial cell functions. J. Biol. Chem. 2012, 287, 26235–26244. [Google Scholar] [CrossRef]
- Hatakeyama, D.M.; Tasima, L.J.; Bravo-Tobar, C.A.; Serino-Silva, C.; Tashima, A.K.; Rodrigues, C.F.B.; Aguiar, W.D.S.; Galizio, N.D.C.; de Lima, E.O.V.; Kavazoi, V.K.; et al. Venom complexity of Bothrops atrox (common lancehead) siblings. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20200018. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Fasoli, E.; Sanz, L.; Boschetti, E.; Righetti, P.G. Exploring the venom proteome of the western diamondback rattlesnake, Crotalus atrox, via snake venomics and combinatorial peptide ligand library approaches. J. Proteome Res. 2009, 8, 3055–3067. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.R.; Mendes, B.; Patino, R.S.P.; Pico, J.; Laines, J.; Teran, M.; Mogollon, N.G.S.; Zaruma-Torres, F.; Caldeira, C.; da Silva, S.L. Assessing the stability of historical and desiccated snake venoms from a medically important Ecuadorian collection. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 230, 108702. [Google Scholar] [CrossRef] [PubMed]
- García-Arredondo, A.; Rodríguez-Rios, L.; Díaz-Peña, L.F.; Vega-Ángeles, R. Pharmacological characterization of venoms from three theraphosid spiders: Poecilotheria regalis, Ceratogyrus darlingi and Brachypelma epicureanum. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sartore, S.; Gorza, L.; Schiaffino, S. Fetal myosin heavy chains in regenerating muscle. Nature 1982, 298, 294–296. [Google Scholar] [CrossRef]
- Guiraud, S.; Edwards, B.; Squire, S.E.; Moir, L.; Berg, A.; Babbs, A.; Ramadan, N.; Wood, M.J.; Davies, K.E. Embryonic myosin is a regeneration marker to monitor utrophin-based therapies for DMD. Hum. Mol. Genet. 2019, 28, 307–319. [Google Scholar] [CrossRef]
- Gao, Q.Q.; McNally, E.M. The Dystrophin Complex: Structure, Function, and Implications for Therapy. Compr. Physiol. 2015, 5, 1223–1239. [Google Scholar] [CrossRef]
- Gullberg, D.; Tiger, C.F.; Velling, T. Laminins during muscle development and in muscular dystrophies. Cell Mol. Life Sci. 1999, 56, 442–460. [Google Scholar] [CrossRef] [PubMed]
- Shigemoto, K.; Kubo, S.; Mori, S.; Yamada, S.; Akiyoshi, T.; Miyazaki, T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr. Gerontol. Int. 2010, 10 (Suppl. 1), S137–S147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Cohen, M.W. Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle. J. Physiol. 1974, 237, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Kasher, R.; Nicolas, A.; Guss, J.M.; Balass, M.; Fridkin, M.; Smit, A.B.; Brejc, K.; Sixma, T.K.; Katchalski-Katzir, E.; et al. The binding site of acetylcholine receptor as visualized in the X-ray structure of a complex between alpha-bungarotoxin and a mimotope peptide. Neuron 2001, 32, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Latroche, C.; Gitiaux, C.; Chrétien, F.; Desguerre, I.; Mounier, R.; Chazaud, B. Skeletal Muscle Microvasculature: A Highly Dynamic Lifeline. Physiology 2015, 30, 417–427. [Google Scholar] [CrossRef]
- Chassagnon, I.R.; McCarthy, C.A.; Chin, Y.K.-Y.; Pineda, S.S.; Keramidas, A.; Mobli, M.; Pham, V.; De Silva, T.M.; Lynch, J.W.; Widdop, R.E.; et al. Potent neuroprotection after stroke afforded by a double-knot spider-venom peptide that inhibits acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA 2017, 114, 3750–3755. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.J.; Liu, Y.Y.; Li, H.; Guo, L.X.; Liu, Z.H.; Shi, X.L.; Hu, M. In vitro potential of Lycosin-I as an alternative antimicrobial drug for treatment of multidrug-resistant Acinetobacter baumannii infections. Antimicrob. Agents Chemother. 2014, 58, 6999–7002. [Google Scholar] [CrossRef]
- Reis, P.V.M.; Boff, D.; Verly, R.M.; Melo-Braga, M.N.; Cortés, M.E.; Santos, D.M.; Pimenta, A.M.C.; Amaral, F.A.; Resende, J.M.; de Lima, M.E. LyeTxI-b, a Synthetic Peptide Derived From Lycosa erythrognatha Spider Venom, Shows Potent Antibiotic Activity in Vitro and in Vivo. Front. Microbiol. 2018, 9, 667. [Google Scholar] [CrossRef]
- Garcia, F.; Villegas, E.; Espino-Solis, G.P.; Rodriguez, A.; Paniagua-Solis, J.F.; Sandoval-Lopez, G.; Possani, L.D.; Corzo, G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. 2013, 66, 3–10. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601). Expert. Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A. Snake venom metalloproteinases: Their role in the pathogenesis of local tissue damage. Biochimie 2000, 82, 841–850. [Google Scholar] [CrossRef]
- Trevisan-Silva, D.; Gremski, L.H.; Chaim, O.M.; da Silveira, R.B.; Meissner, G.O.; Mangili, O.C.; Barbaro, K.C.; Gremski, W.; Veiga, S.S.; Senff-Ribeiro, A. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles). Biochimie 2010, 92, 21–32. [Google Scholar] [CrossRef]
- Nagaraju, S.; Girish, K.S.; Fox, J.W.; Kemparaju, K. ‘Partitagin’ a hemorrhagic metalloprotease from Hippasa partita spider venom: Role in tissue necrosis. Biochimie 2007, 89, 1322–1331. [Google Scholar] [CrossRef]
- Sannaningaiah, D.; Subbaiah, G.K.; Kempaiah, K. Pharmacology of spider venom toxins. Toxin Rev. 2014, 33, 206–220. [Google Scholar] [CrossRef]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Lopes, P.H.; Berg, C.W.v.d.; Tambourgi, D.V. Sphingomyelinases D From Loxosceles Spider Venoms and Cell Membranes: Action on Lipid Rafts and Activation of Endogenous Metalloproteinases. Front. Pharmacol. 2020, 11, 36. [Google Scholar] [CrossRef]
- do Nascimento, S.M.; de Oliveira, U.C.; Nishiyama-Jr, M.Y.; Tashima, A.K.; Silva Junior, P.I.d. Presence of a neprilysin on Avicularia juruensis (Mygalomorphae: Theraphosidae) venom. Toxin Rev. 2021, 41, 370–379. [Google Scholar] [CrossRef]
- Balaji, R.A.; Sasaki, T.; Gopalakrishnakone, P.; Sato, K.; Kini, R.M.; Bay, B.H. Purification, structure determination and synthesis of covalitoxin-II, a short insect-specific neurotoxic peptide from the venom of the Coremiocnemis validus (Singapore tarantula). FEBS Lett. 2000, 474, 208–212. [Google Scholar] [CrossRef]
- Lee, C.K.; Chan, T.K.; Ward, B.C.; Howell, D.E.; Odell, G.V. The purification and characterization of a necrotoxin from tarantula, Dugesiella hentzi (Girard), venom. Arch. Biochem. Biophys. 1974, 164, 341–350. [Google Scholar] [CrossRef]
- Silva, L.M.; Silva, C.A.; Silva, A.; Vieira, R.P.; Mesquita-Ferrari, R.A.; Cogo, J.C.; Zamuner, S.R. Photobiomodulation Protects and Promotes Differentiation of C2C12 Myoblast Cells Exposed to Snake Venom. PLoS ONE 2016, 11, e0152890. [Google Scholar] [CrossRef]
- Bustillo, S.; Van de Velde, A.C.; Matzner Perfumo, V.; Gay, C.C.; Leiva, L.C. Apoptosis induced by a snake venom metalloproteinase from Bothrops alternatus venom in C2C12 muscle cells. Apoptosis 2017, 22, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Angulo, Y.; Rufini, S.; Cho, W.; Giglio, J.R.; Ohno, M.; Daniele, J.J.; Geoghegan, P.; Gutiérrez, J.M. Comparative study of the cytolytic activity of myotoxic phospholipases A2 on mouse endothelial (tEnd) and skeletal muscle (C2C12) cells in vitro. Toxicon 1999, 37, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Omran, M.A. Cytotoxic and apoptotic effects of scorpion Leiurus quinquestriatus venom on 293T and C2C12 eukaryotic cell line. J. Venom. Anim. Toxins Incl. Trop. Dis. 2003, 9, 2. [Google Scholar] [CrossRef]
- Inceoglu, B.; Lango, J.; Jing, J.; Chen, L.; Doymaz, F.; Pessah, I.N.; Hammock, B.D. One scorpion, two venoms: Prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc. Natl. Acad. Sci. USA 2003, 100, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Angulo, Y.; Lomonte, B. Differential susceptibility of C2C12 myoblasts and myotubes to group II phospholipase A2 myotoxins from crotalid snake venoms. Cell Biochem. Funct. 2005, 23, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Bolanos, P.; Calderon, J.C. Excitation-contraction coupling in mammalian skeletal muscle: Blending old and last-decade research. Front. Physiol. 2022, 13, 989796. [Google Scholar] [CrossRef]
- Esteve, E.; Mabrouk, K.; Dupuis, A.; Smida-Rezgui, S.; Altafaj, X.; Grunwald, D.; Platel, J.C.; Andreotti, N.; Marty, I.; Sabatier, J.M.; et al. Transduction of the scorpion toxin maurocalcine into cells. Evidence that the toxin crosses the plasma membrane. J. Biol. Chem. 2005, 280, 12833–12839. [Google Scholar] [CrossRef]
- Lukacs, B.; Sztretye, M.; Almassy, J.; Sarkozi, S.; Dienes, B.; Mabrouk, K.; Simut, C.; Szabo, L.; Szentesi, P.; De Waard, M.; et al. Charged surface area of maurocalcine determines its interaction with the skeletal ryanodine receptor. Biophys. J. 2008, 95, 3497–3509. [Google Scholar] [CrossRef]
- Spencer, M.J.; Croall, D.E.; Tidball, J.G. Calpains are activated in necrotic fibers from mdx dystrophic mice. J. Biol. Chem. 1995, 270, 10909–10914. [Google Scholar] [CrossRef]
- Sweeney, H.L.; Hammers, D.W. Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2018, 10, a023200. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The Calpain System. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Mattiello-Sverzuta, A.C.; da Cruz-Höfling, M.A. Toxin 2 (PhTx2), a neurotoxic fraction from Phoneutria nigriventer spider venom, causes acute morphological changes in mouse skeletal muscle. Toxicon 2000, 38, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef]
- Salamone, I.M.; Quattrocelli, M.; Barefield, D.Y.; Page, P.G.; Tahtah, I.; Hadhazy, M.; Tomar, G.; McNally, E.M. Intermittent glucocorticoid treatment enhances skeletal muscle performance through sexually dimorphic mechanisms. J. Clin. Investig. 2022, 132, e149828. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, J.; Liu, Y. Skeletal Muscle Regeneration in Cardiotoxin-Induced Muscle Injury Models. Int. J. Mol. Sci. 2022, 23, 13380. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.L.; Chiu, C.R.; Huang, W.N.; Wu, W.G. The role of sulfatide lipid domains in the membrane pore-forming activity of cobra cardiotoxin. Biochim. Biophys. Acta 2012, 1818, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Villegas, E.; Corzo, G. Pore-Forming Peptides from Spiders. Toxin Rev. 2005, 24, 345–357. [Google Scholar] [CrossRef]
- Okada, M.; Ortiz, E.; Corzo, G.; Possani, L.D. Pore-forming spider venom peptides show cytotoxicity to hyperpolarized cancer cells expressing K+ channels: A lentiviral vector approach. PLoS ONE 2019, 14, e0215391. [Google Scholar] [CrossRef]
- Rivera-de-Torre, E.; Palacios-Ortega, J.; Gavilanes, J.G.; Martínez-Del-Pozo, Á.; García-Linares, S. Pore-Forming Proteins from Cnidarians and Arachnids as Potential Biotechnological Tools. Toxins 2019, 11, 370. [Google Scholar] [CrossRef]
- Maden, M.; Brant, J.O.; Rubiano, A.; Sandoval, A.G.W.; Simmons, C.; Mitchell, R.; Collin-Hooper, H.; Jacobson, J.; Omairi, S.; Patel, K. Perfect chronic skeletal muscle regeneration in adult spiny mice, Acomys cahirinus. Sci. Rep. 2018, 8, 8920. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Liao, Q.; Meng, E.; Mwangi, J.; Lai, R.; Rong, M. LCTX-F2, a Novel Potentiator of Coagulation Factors from the Spider Venom of Lycosa singoriensis. Front. Pharmacol. 2020, 11, 896. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, N.J.; Alqallaf, A.; Mitchell, R.D.; Parnell, A.; Haidar, H.B.; Almeida, J.R.; Williams, J.; Vijayakumar, P.; Balogun, A.; Matsakas, A.; et al. Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage. Cells 2023, 12, 2074. https://doi.org/10.3390/cells12162074
Richards NJ, Alqallaf A, Mitchell RD, Parnell A, Haidar HB, Almeida JR, Williams J, Vijayakumar P, Balogun A, Matsakas A, et al. Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage. Cells. 2023; 12(16):2074. https://doi.org/10.3390/cells12162074
Chicago/Turabian StyleRichards, Nicholas J., Ali Alqallaf, Robert D. Mitchell, Andrew Parnell, Husain Bin Haidar, José R. Almeida, Jarred Williams, Pradeep Vijayakumar, Adedoyin Balogun, Antonios Matsakas, and et al. 2023. "Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage" Cells 12, no. 16: 2074. https://doi.org/10.3390/cells12162074
APA StyleRichards, N. J., Alqallaf, A., Mitchell, R. D., Parnell, A., Haidar, H. B., Almeida, J. R., Williams, J., Vijayakumar, P., Balogun, A., Matsakas, A., Trim, S. A., Patel, K., & Vaiyapuri, S. (2023). Indian Ornamental Tarantula (Poecilotheria regalis) Venom Affects Myoblast Function and Causes Skeletal Muscle Damage. Cells, 12(16), 2074. https://doi.org/10.3390/cells12162074







