The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids
Abstract
1. Introduction
2. Materials and Methods
2.1. Formation of COs from hPSCs
2.2. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.3. Immunofluorescence Staining
2.4. Western Blotting
2.5. Transmission Electron Microscopy (TEM)
2.6. Beating Analysis Using Captured Videos
2.7. Whole-Cell Patch Clamp Recordings
2.8. Ca2+ Transient Analysis
2.9. Image Rendering
2.10. RNA Sequencing (RNA-Seq) Analysis
2.11. Pharmacologic Reagents
2.12. Statistical Analysis
3. Results
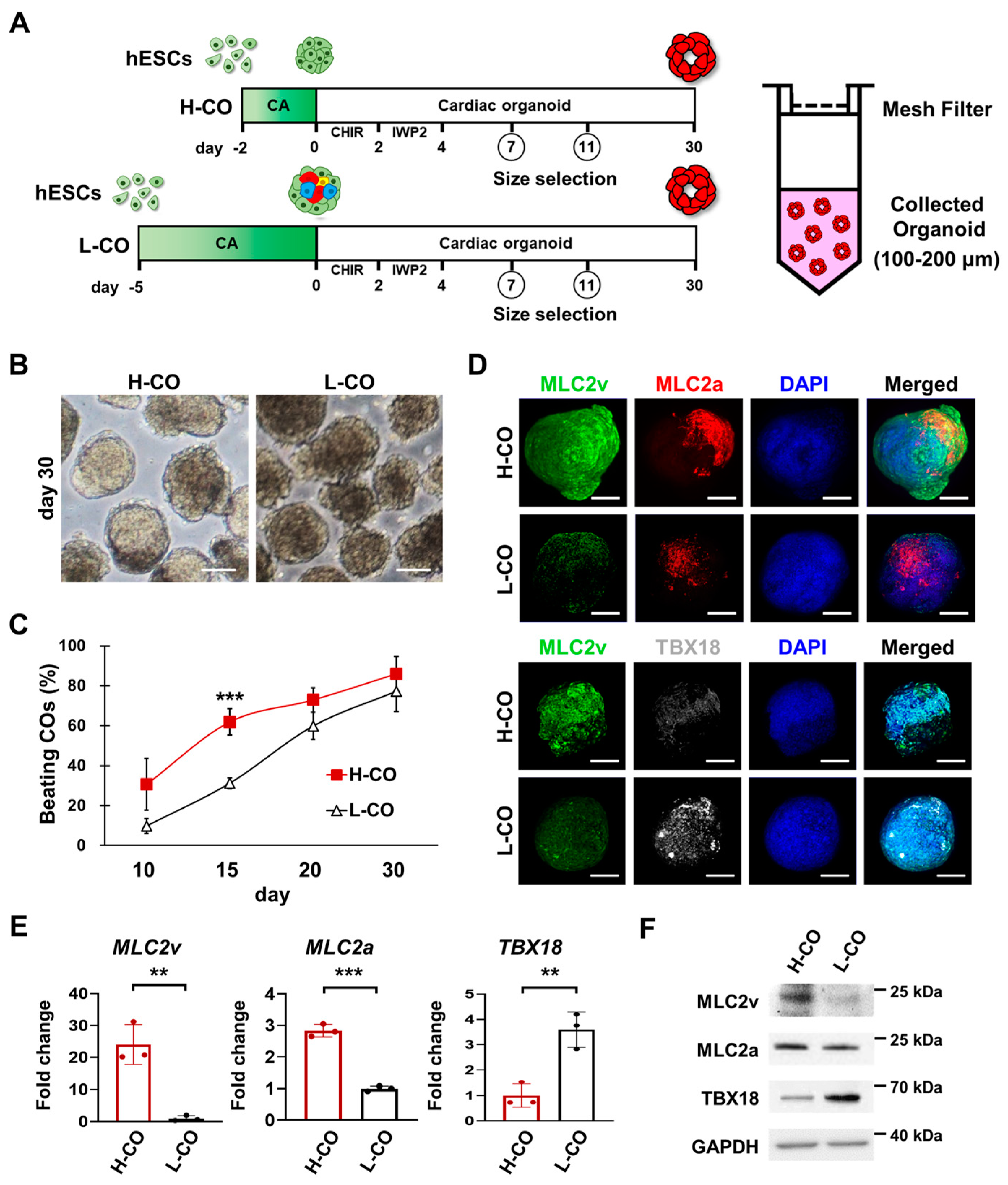
3.1. CAs Formed for 2 Days Generate More Ventricular-like and Atrial-like CMs Than Those Formed for 5 Days
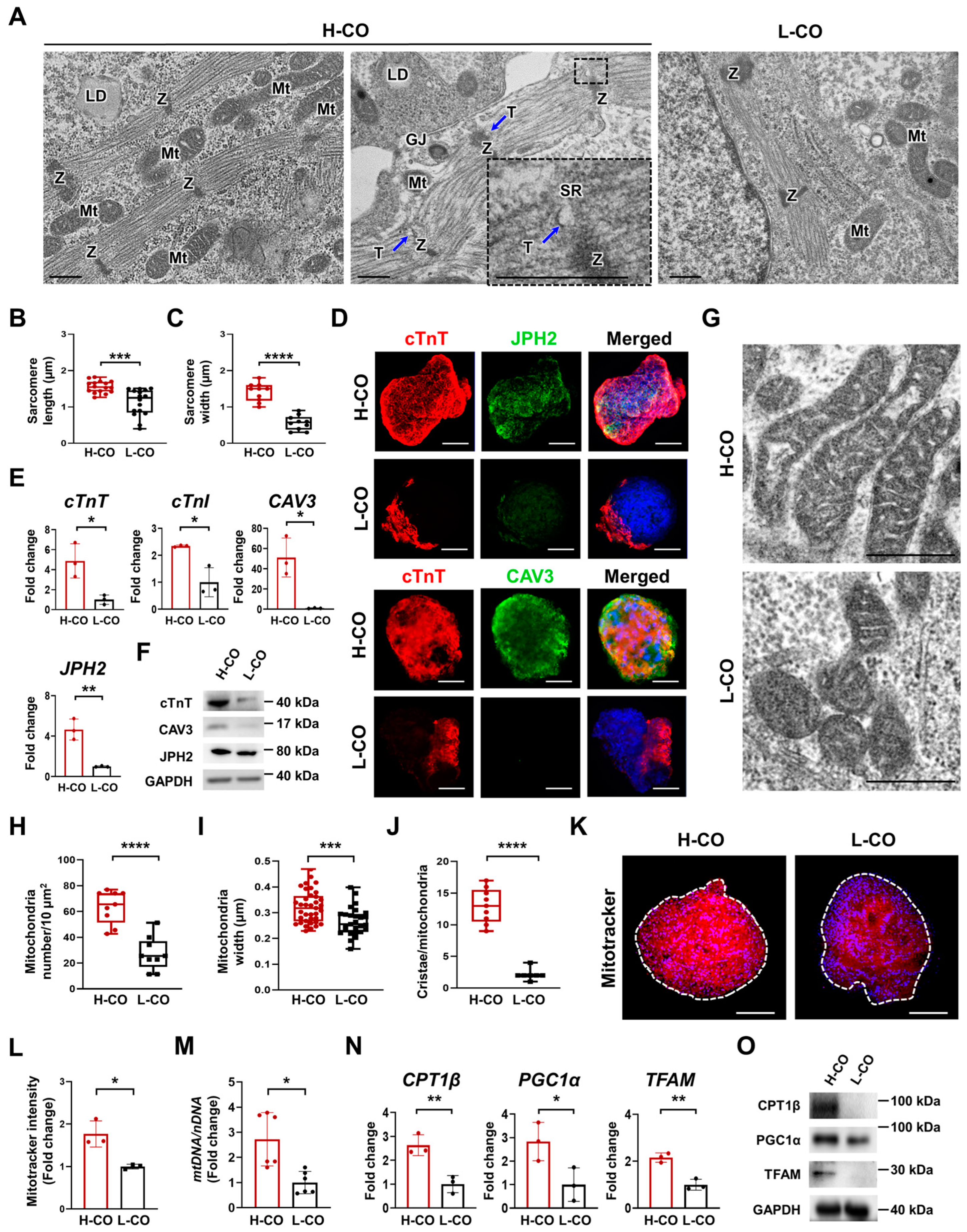
3.2. Structural and Metabolic Maturation Are Increased in H-COs Compared with L-COs
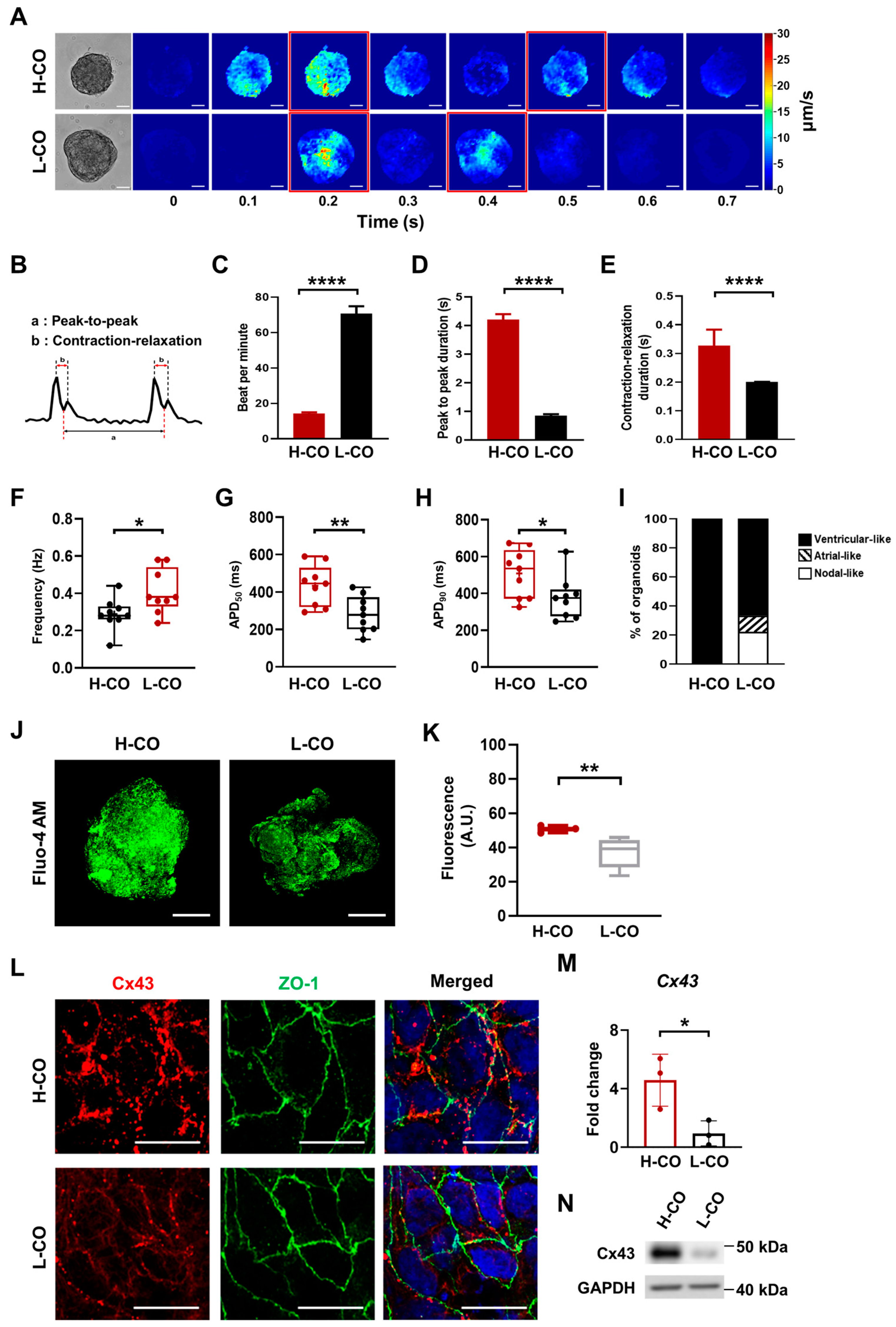
3.3. Beating and Electrophysiological Properties of H-COs Indicate Ventricular-like CMs
3.4. Junctional Structures between CMs Are Better Aligned in H-COs Than in L-COs
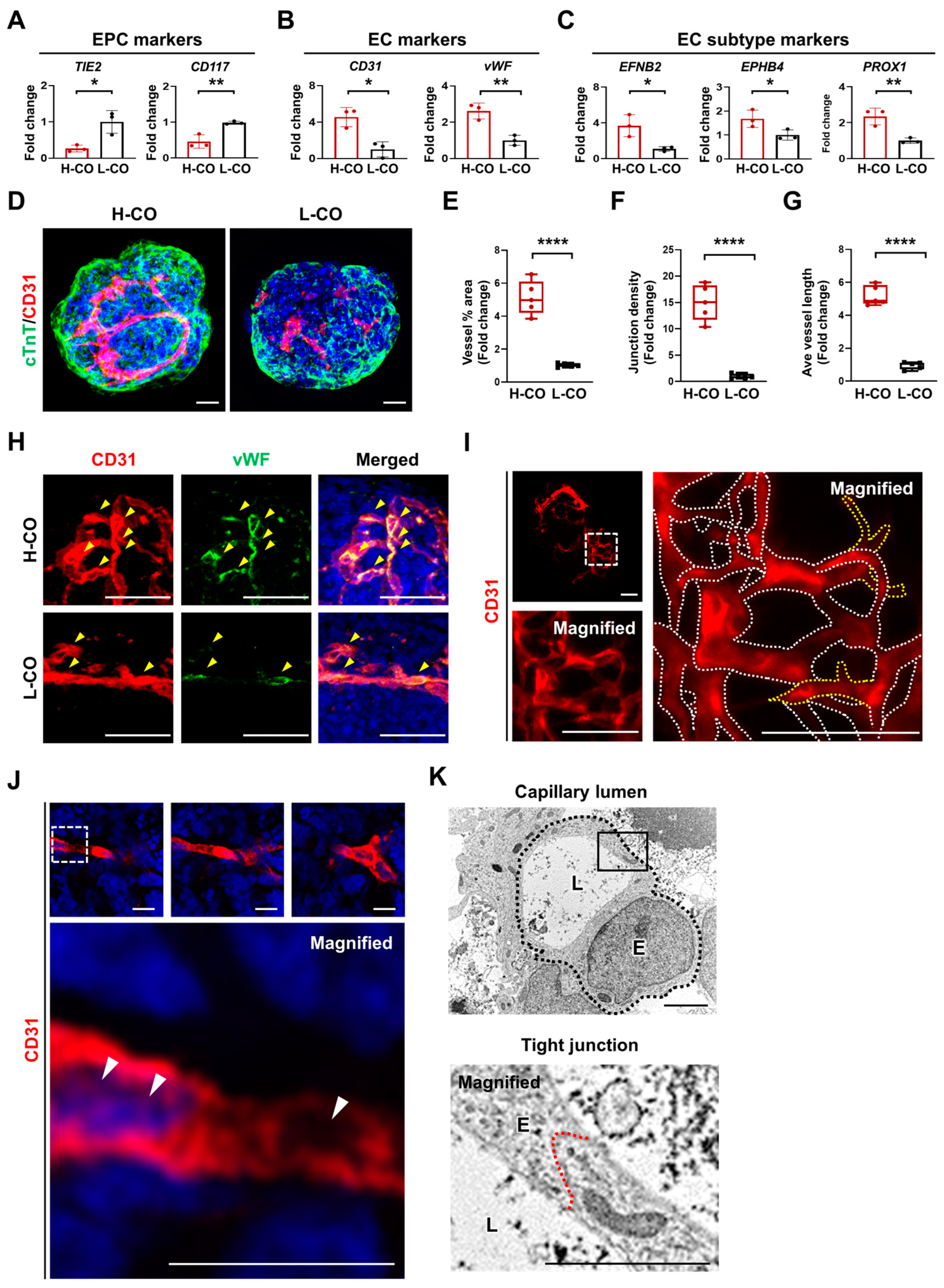
3.5. Formation of a Capillary Network with a Lumen Is Predominantly Found in H-COs
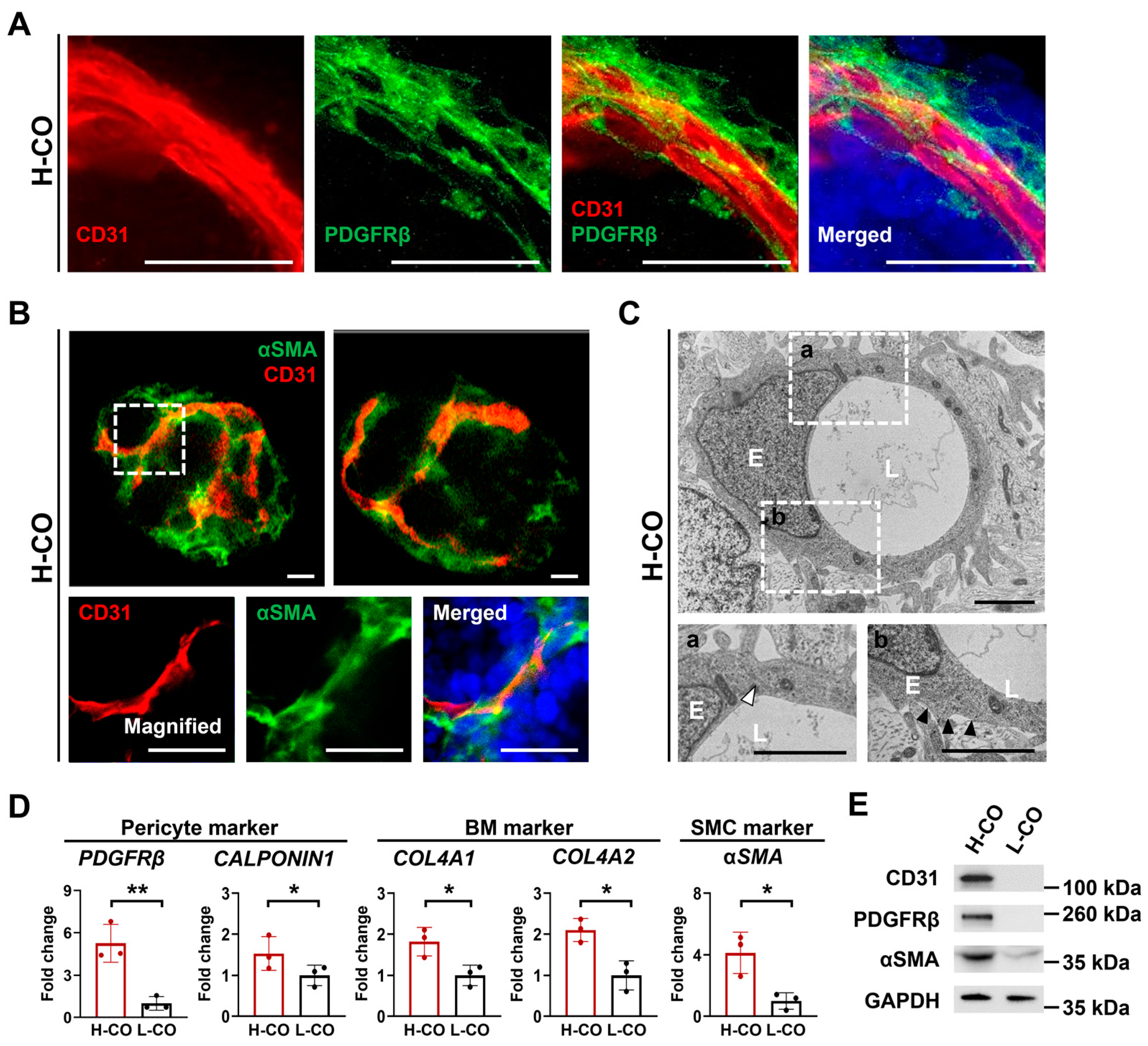
3.6. Mature Vessels Covered by Pericytes, SMCs, and a BM Are Formed in H-COs
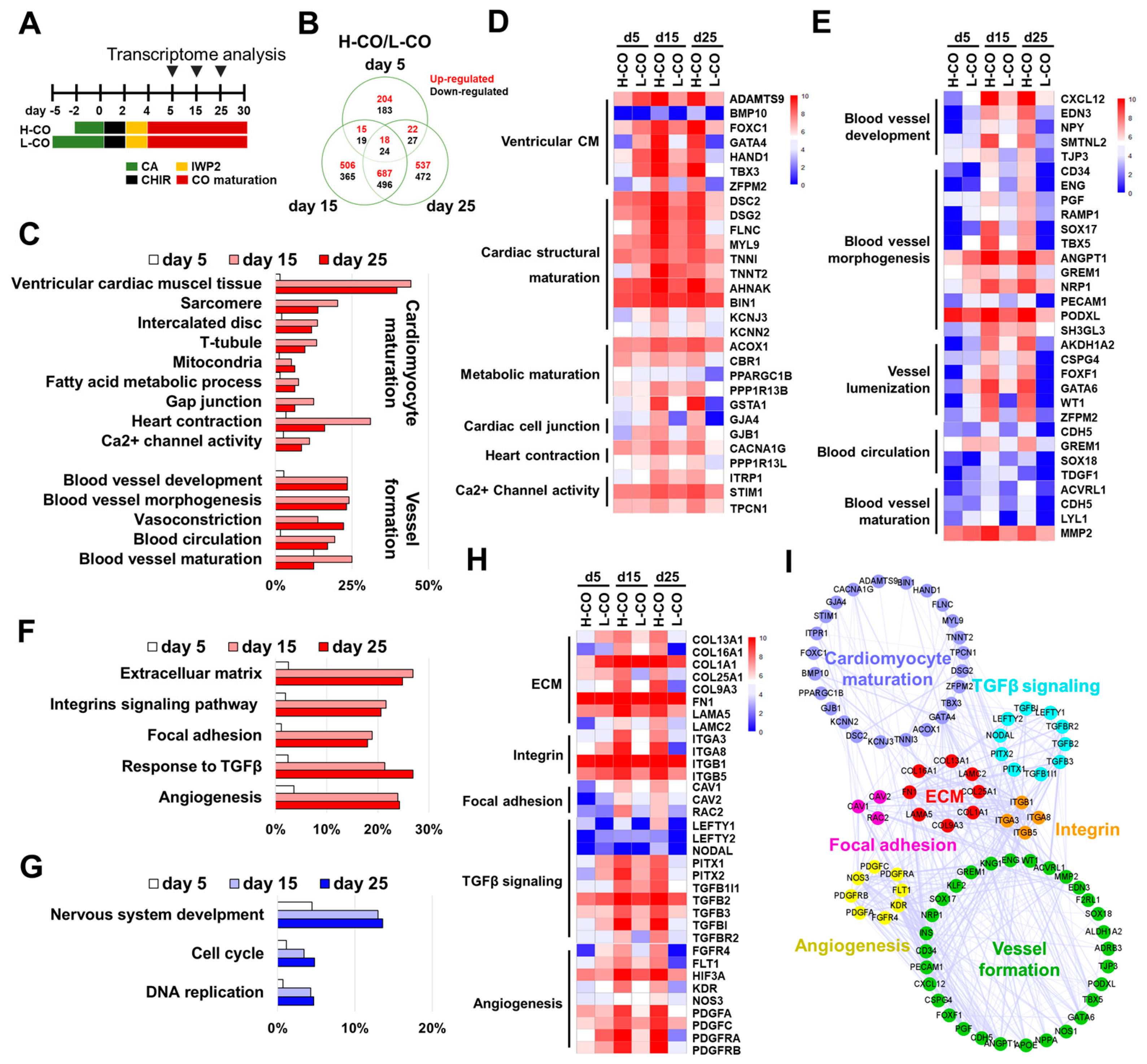
3.7. Transcriptional Profiling Reveals CM Maturation and Vessel Formation in H-COs at the Molecular Level
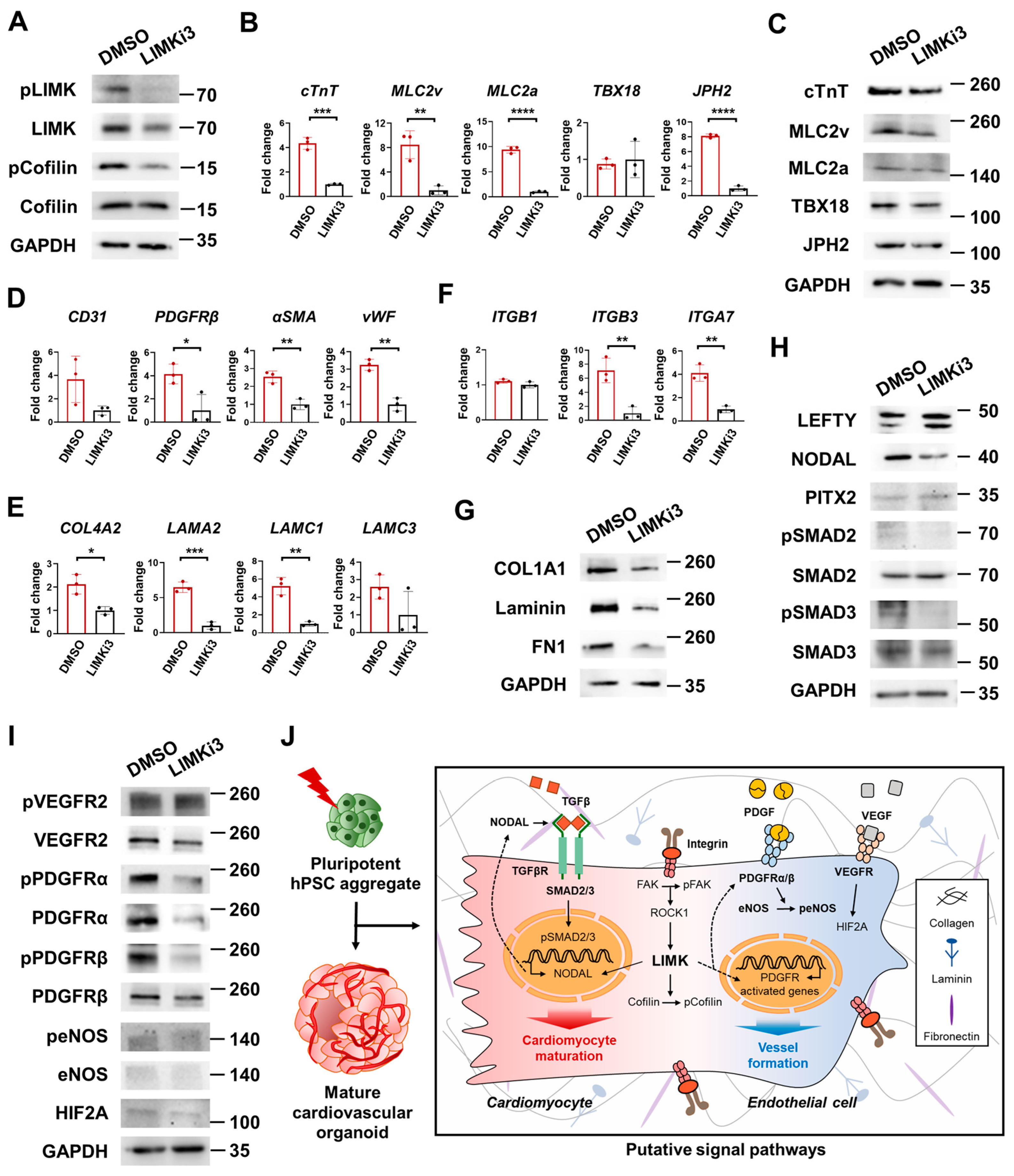
3.8. Activation of ROCK-LIMK-pCofilin, LEFTY-NODAL, pVEGFR, pPDGFR, and peNOS Pathways via ECM–Integrin Interactions Led to CM Maturation and Vessel Formation in COs
3.9. LIMK/Cofilin Signaling Pathways Play Critical Roles in CM Maturation and Vessel Formation in H-COs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, J.; Lee, H.; Rah, W.; Chang, H.J.; Yoon, Y.S. From engineered heart tissue to cardiac organoid. Theranostics 2022, 12, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Matthys, O.B.; Joy, D.A.; Kauss, M.A.; Natarajan, V.; Lai, M.H.; Turaga, D.; Blair, A.P.; Alexanian, M.; Bruneau, B.G.; et al. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 2021, 28, 2137–2152.e6. [Google Scholar] [CrossRef] [PubMed]
- Drakhlis, L.; Biswanath, S.; Farr, C.M.; Lupanow, V.; Teske, J.; Ritzenhoff, K.; Franke, A.; Manstein, F.; Bolesani, E.; Kempf, H.; et al. Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol. 2021, 39, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, Y.J.; Son, M.Y.; Oh, M.S.; Kim, J.; Ryu, B.; Kang, K.R.; Baek, J.; Chung, G.; Woo, D.H.; et al. Generation of human iPSCs derived heart organoids structurally and functionally similar to heart. Biomaterials 2022, 290, 121860. [Google Scholar] [CrossRef]
- Song, M.H.; Choi, S.C.; Noh, J.M.; Joo, H.J.; Park, C.Y.; Cha, J.J.; Ahn, T.H.; Ko, T.H.; Choi, J.I.; Na, J.E.; et al. LEFTY-PITX2 signaling pathway is critical for generation of mature and ventricular cardiac organoids in human pluripotent stem cell-derived cardiac mesoderm cells. Biomaterials 2021, 278, 121133. [Google Scholar] [CrossRef]
- Lewis-Israeli, Y.R.; Wasserman, A.H.; Gabalski, M.A.; Volmert, B.D.; Ming, Y.; Ball, K.A.; Yang, W.; Zou, J.; Ni, G.; Pajares, N.; et al. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun. 2021, 12, 5142. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef]
- Flaherty, M.P.; Kamerzell, T.J.; Dawn, B. Wnt signaling and cardiac differentiation. Prog. Mol. Biol. Transl. Sci. 2012, 111, 153–174. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Chung, B.G.; Ortmann, D.; Hattori, N.; Moeller, H.C.; Khademhosseini, A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc. Natl. Acad. Sci. USA 2009, 106, 16978–16983. [Google Scholar] [CrossRef]
- Mohr, J.C.; Zhang, J.; Azarin, S.M.; Soerens, A.G.; de Pablo, J.J.; Thomson, J.A.; Lyons, G.E.; Palecek, S.P.; Kamp, T.J. The microwell control of embryoid body size in order to regulate cardiac differentiation of human embryonic stem cells. Biomaterials 2010, 31, 1885–1893. [Google Scholar] [CrossRef]
- Jiang, B.; Xiang, Z.; Ai, Z.; Wang, H.; Li, Y.; Ji, W.; Li, T. Generation of cardiac spheres from primate pluripotent stem cells in a small molecule-based 3D system. Biomaterials 2015, 65, 103–114. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Loskill, P.; Huebsch, N.; Koo, S.; Svedlund, F.L.; Marks, N.C.; Hua, E.W.; Grigoropoulos, C.P.; Conklin, B.R.; et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 2015, 6, 7413. [Google Scholar] [CrossRef]
- Hofbauer, P.; Jahnel, S.M.; Papai, N.; Giesshammer, M.; Deyett, A.; Schmidt, C.; Penc, M.; Tavernini, K.; Grdseloff, N.; Meledeth, C.; et al. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021, 184, 3299–3317.e22. [Google Scholar] [CrossRef] [PubMed]
- Vermij, S.H.; Abriel, H.; van Veen, T.A. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc. Res. 2017, 113, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Israeli-Rosenberg, S.; Manso, A.M.; Okada, H.; Ross, R.S. Integrins and integrin-associated proteins in the cardiac myocyte. Circ. Res. 2014, 114, 572–586. [Google Scholar] [CrossRef]
- Pagliarosi, O.; Picchio, V.; Chimenti, I.; Messina, E.; Gaetani, R. Building an Artificial Cardiac Microenvironment: A Focus on the Extracellular Matrix. Front. Cell Dev. Biol. 2020, 8, 559032. [Google Scholar] [CrossRef]
- Meno, C.; Saijoh, Y.; Fujii, H.; Ikeda, M.; Yokoyama, T.; Yokoyama, M.; Toyoda, Y.; Hamada, H. Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature 1996, 381, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ivanovitch, K.; Soro-Barrio, P.; Chakravarty, P.; Jones, R.A.; Bell, D.M.; Mousavy Gharavy, S.N.; Stamataki, D.; Delile, J.; Smith, J.C.; Briscoe, J. Ventricular, atrial, and outflow tract heart progenitors arise from spatially and molecularly distinct regions of the primitive streak. PLoS Biol. 2021, 19, e3001200. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Tukur, H.A.; Aljumaah, R.S.; Sindi, R.A. Rocking the Boat: The Decisive Roles of Rho Kinases During Oocyte, Blastocyst, and Stem Cell Development. Front. Cell Dev. Biol. 2020, 8, 616762. [Google Scholar] [CrossRef]
- Kilian, L.S.; Voran, J.; Frank, D.; Rangrez, A.Y. RhoA: A dubious molecule in cardiac pathophysiology. J. Biomed. Sci. 2021, 28, 33. [Google Scholar] [CrossRef]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-Kinase in the Cardiovascular System. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Manetti, F. Recent advances in the rational design and development of LIM kinase inhibitors are not enough to enter clinical trials. Eur. J. Med. Chem. 2018, 155, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Chen, Y.C.; Kao, Y.H.; Wu, T.J.; Chen, S.A.; Chen, Y.J. Extracellular matrix of collagen modulates intracellular calcium handling and electrophysiological characteristics of HL-1 cardiomyocytes with activation of angiotensin II type 1 receptor. J. Card. Fail. 2011, 17, 82–90. [Google Scholar] [CrossRef]
- Edalat, S.G.; Jang, Y.; Kim, J.; Park, Y. Collagen Type I Containing Hybrid Hydrogel Enhances Cardiomyocyte Maturation in a 3D Cardiac Model. Polymers 2019, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Tefft, B.J.; Spoon, D.B.; Simari, R.D. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014, 10, 2877–2893. [Google Scholar] [CrossRef] [PubMed]
- Kuivaniemi, H.; Tromp, G. Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 2019, 707, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Kontaridis, M.I. Physiology of Cardiac Development: From Genetics to Signaling to Therapeutic Strategies. Curr. Opin. Physiol. 2018, 1, 123–139. [Google Scholar] [CrossRef]
- Baharvand, H.; Azarnia, M.; Parivar, K.; Ashtiani, S.K. The effect of extracellular matrix on embryonic stem cell-derived cardiomyocytes. J. Mol. Cell Cardiol. 2005, 38, 495–503. [Google Scholar] [CrossRef]
- Yousif, L.F.; Di Russo, J.; Sorokin, L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh Migr. 2013, 7, 101–110. [Google Scholar] [CrossRef]
- Hallmann, R.; Horn, N.; Selg, M.; Wendler, O.; Pausch, F.; Sorokin, L.M. Expression and function of laminins in the embryonic and mature vasculature. Physiol. Rev. 2005, 85, 979–1000. [Google Scholar] [CrossRef]
- Lee, J.; Sutani, A.; Kaneko, R.; Takeuchi, J.; Sasano, T.; Kohda, T.; Ihara, K.; Takahashi, K.; Yamazoe, M.; Morio, T.; et al. In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commun. 2020, 11, 4283. [Google Scholar] [CrossRef] [PubMed]
- Fässler, R.; Rohwedel, J.; Maltsev, V.; Bloch, W.; Lentini, S.; Guan, K.; Gullberg, D.; Hescheler, J.; Addicks, K.; Wobus, A.M. Differentiation and integrity of cardiac muscle cells are impaired in the absence of beta 1 integrin. J. Cell Sci. 1996, 109 Pt 13, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Herron, T.J.; Rocha, A.M.; Campbell, K.F.; Ponce-Balbuena, D.; Willis, B.C.; Guerrero-Serna, G.; Liu, Q.; Klos, M.; Musa, H.; Zarzoso, M.; et al. Extracellular Matrix-Mediated Maturation of Human Pluripotent Stem Cell-Derived Cardiac Monolayer Structure and Electrophysiological Function. Circ. Arrhythm. Electrophysiol. 2016, 9, e003638. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Ou, D.B.; Wei, T.; Ding, L.; Liu, X.T.; Hu, X.L.; Li, X.; Zheng, Q.S. Collagen/β(1) integrin interaction is required for embryoid body formation during cardiogenesis from murine induced pluripotent stem cells. BMC Cell Biol. 2013, 14, 5. [Google Scholar] [CrossRef]
- Wang, Y.G.; Samarel, A.M.; Lipsius, S.L. Laminin acts via beta 1 integrin signalling to alter cholinergic regulation of L-type Ca2+ current in cat atrial myocytes. J. Physiol. 2000, 526 Pt 1, 57–68. [Google Scholar] [CrossRef]
- Lu, Z.; Mathew, S.; Chen, J.; Hadziselimovic, A.; Palamuttam, R.; Hudson, B.G.; Fässler, R.; Pozzi, A.; Sanders, C.R.; Zent, R. Implications of the differing roles of the β1 and β3 transmembrane and cytoplasmic domains for integrin function. Elife 2016, 5, e18633. [Google Scholar] [CrossRef] [PubMed]
- Rossier, O.; Octeau, V.; Sibarita, J.B.; Leduc, C.; Tessier, B.; Nair, D.; Gatterdam, V.; Destaing, O.; Albigès-Rizo, C.; Tampé, R.; et al. Integrins β1 and β3 exhibit distinct dynamic nanoscale organizations inside focal adhesions. Nat. Cell Biol. 2012, 14, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Béguin, E.P.; Janssen, E.F.J.; Hoogenboezem, M.; Meijer, A.B.; Hoogendijk, A.J.; van den Biggelaar, M. Flow-induced Reorganization of Laminin-integrin Networks Within the Endothelial Basement Membrane Uncovered by Proteomics. Mol. Cell Proteom. 2020, 19, 1179–1192. [Google Scholar] [CrossRef]
- Kumar, A.; Novoselov, V.; Celeste, A.J.; Wolfman, N.M.; ten Dijke, P.; Kuehn, M.R. Nodal signaling uses activin and transforming growth factor-beta receptor-regulated Smads. J. Biol. Chem. 2001, 276, 656–661. [Google Scholar] [CrossRef]
- Saha, S.; Ji, L.; de Pablo, J.J.; Palecek, S.P. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys. J. 2008, 94, 4123–4133. [Google Scholar] [CrossRef]
- Müller, P.; Rogers, K.W.; Jordan, B.M.; Lee, J.S.; Robson, D.; Ramanathan, S.; Schier, A.F. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science 2012, 336, 721–724. [Google Scholar] [CrossRef]
- Vargas-Valderrama, A.; Messina, A.; Mitjavila-Garcia, M.T.; Guenou, H. The endothelium, a key actor in organ development and hPSC-derived organoid vascularization. J. Biomed. Sci. 2020, 27, 67. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 2003, 9, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gürke, L.; Briquez, P.S.; Larsson, H.M.; Gianni-Barrera, R.; et al. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front. Bioeng. Biotechnol. 2015, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Bai, L.; Zhou, J.; Chen, H.; Zhang, L. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system enhance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018, 323, 19–32. [Google Scholar] [CrossRef]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef]
- Stratman, A.N.; Schwindt, A.E.; Malotte, K.M.; Davis, G.E. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood 2010, 116, 4720–4730. [Google Scholar] [CrossRef]
- Wimmer, R.A.; Leopoldi, A.; Aichinger, M.; Wick, N.; Hantusch, B.; Novatchkova, M.; Taubenschmid, J.; Hämmerle, M.; Esk, C.; Bagley, J.A.; et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019, 565, 505–510. [Google Scholar] [CrossRef]
- Downes, N.L.; Laham-Karam, N.; Kaikkonen, M.U.; Ylä-Herttuala, S. Differential but Complementary HIF1α and HIF2α Transcriptional Regulation. Mol. Ther. 2018, 26, 1735–1745. [Google Scholar] [CrossRef]
- Skuli, N.; Majmundar, A.J.; Krock, B.L.; Mesquita, R.C.; Mathew, L.K.; Quinn, Z.L.; Runge, A.; Liu, L.; Kim, M.N.; Liang, J.; et al. Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J. Clin. Investig. 2012, 122, 1427–1443. [Google Scholar] [CrossRef]
- Durán, W.N.; Breslin, J.W.; Sánchez, F.A. The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 2010, 87, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Stinnett, A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arter. Thromb. Vasc. Biol. 2008, 28, 1606–1613. [Google Scholar] [CrossRef]
- Varzideh, F.; Pahlavan, S.; Ansari, H.; Halvaei, M.; Kostin, S.; Feiz, M.S.; Latifi, H.; Aghdami, N.; Braun, T.; Baharvand, H. Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 2019, 192, 537–550. [Google Scholar] [CrossRef]
- Hitsuda, A.; Dan, R.; Urakawa, A.; Hiraoka, Y.; Murakami, C.; Yamamoto, H.; Tanaka, A.R. 25-hydroxycholesterol-induced cell death via activation of ROCK/LIMK/cofilin axis in colorectal cancer cell spheroids. J. Steroid Biochem. Mol. Biol. 2022, 216, 106037. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, B.; Yang, L.; Zhao, Q.; Zhang, M.; Liu, X.; Zhou, C.; Wang, R.; Chen, H.; Wang, J.; et al. FMNL2 suppresses cell migration and invasion of breast cancer: A reduction of cytoplasmic p27 via RhoA/LIMK/Cofilin pathway. Cell Death Discov. 2022, 8, 155. [Google Scholar] [CrossRef]
- Wang, X.; Zou, S.; Ren, T.; Zhao, L.J.; Yu, L.F.; Li, X.Y.; Yan, X.; Zhang, L.J. Alantolactone suppresses the metastatic phenotype and induces the apoptosis of glioblastoma cells by targeting LIMK kinase activity and activating the cofilin/G-actin signaling cascade. Int. J. Mol. Med. 2021, 47, 68. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, J.-M.; Choi, S.-C.; Song, M.-H.; Kim, K.S.; Jun, S.; Park, J.H.; Kim, J.H.; Kim, K.; Ko, T.H.; Choi, J.-I.; et al. The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids. Cells 2023, 12, 2029. https://doi.org/10.3390/cells12162029
Noh J-M, Choi S-C, Song M-H, Kim KS, Jun S, Park JH, Kim JH, Kim K, Ko TH, Choi J-I, et al. The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids. Cells. 2023; 12(16):2029. https://doi.org/10.3390/cells12162029
Chicago/Turabian StyleNoh, Ji-Min, Seung-Cheol Choi, Myeong-Hwa Song, Kyung Seob Kim, Seongmin Jun, Jae Hyoung Park, Ju Hyeon Kim, Kyoungmi Kim, Tae Hee Ko, Jong-Il Choi, and et al. 2023. "The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids" Cells 12, no. 16: 2029. https://doi.org/10.3390/cells12162029
APA StyleNoh, J.-M., Choi, S.-C., Song, M.-H., Kim, K. S., Jun, S., Park, J. H., Kim, J. H., Kim, K., Ko, T. H., Choi, J.-I., Gim, J.-A., Kim, J.-H., Jang, Y., Park, Y., Na, J. E., Rhyu, I. J., & Lim, D.-S. (2023). The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix–Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids. Cells, 12(16), 2029. https://doi.org/10.3390/cells12162029







