Sexual Dimorphism in the Mechanism of Pain Central Sensitization
Abstract
1. Introduction
2. Pain Central Sensitization
3. Sexual Dimorphism of Pain in Clinical Studies
4. Sexual Dimorphism of Pain in Pre-Clinical Studies
4.1. Sexual Dimorphic Pain Responses in Various Animal Models
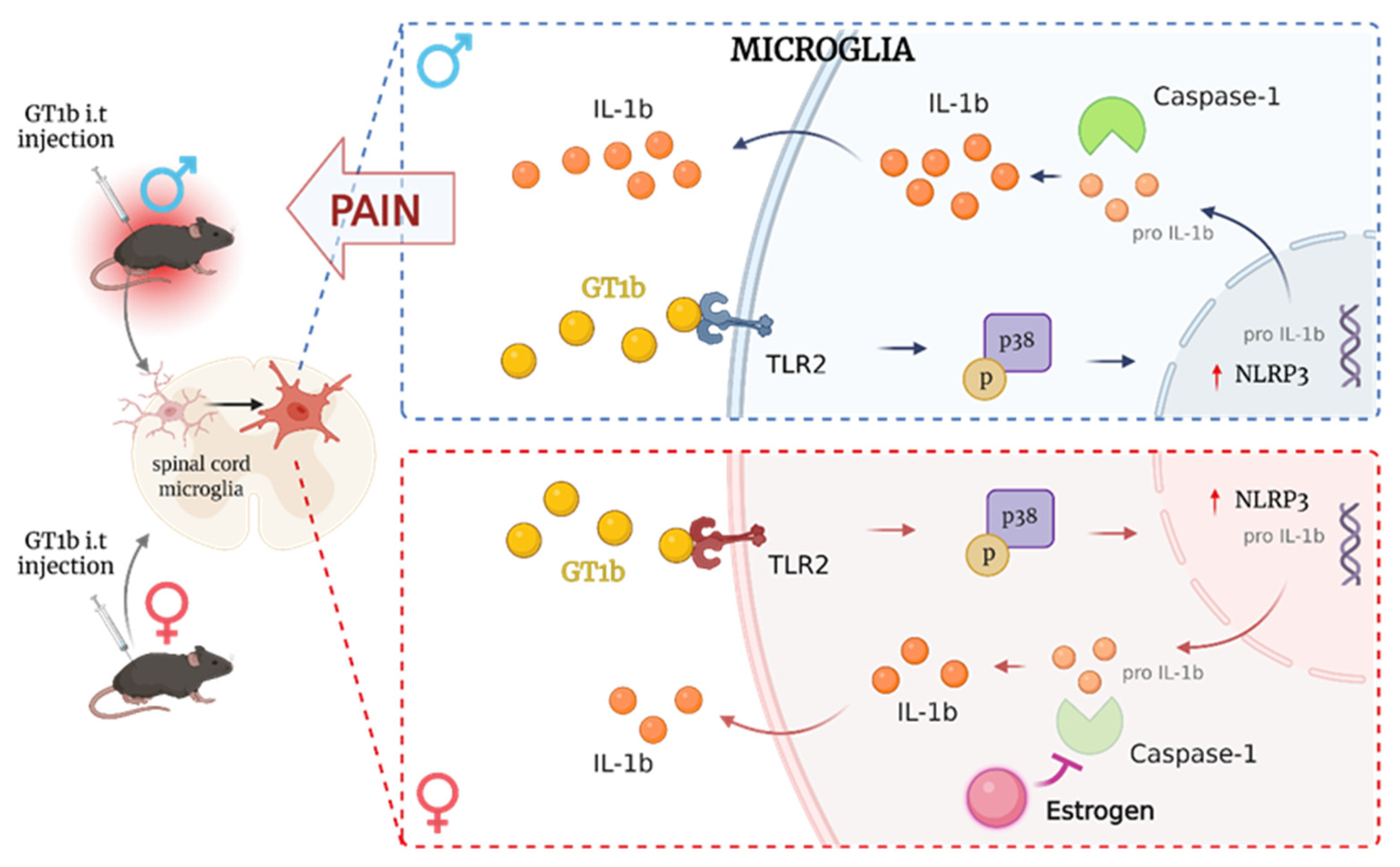
4.2. Factors of the Sexually Dimorphic Pain Mechanisms
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Melzack, R. From the gate to the neuromatrix. Pain 1999, 82, S121–S126. [Google Scholar] [CrossRef]
- Treede, R.-D.; Rief, W.; Barke, A.; Aziz, Q.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Evers, S.; Finnerup, N.B.; First, M.B.; et al. Chronic pain as a symptom or a disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. [Google Scholar] [CrossRef]
- Woolf, C.J. Capturing Novel Non-opioid Pain Targets. Biol. Psychiatry 2020, 87, 74–81. [Google Scholar] [CrossRef]
- Rusman, T.; van Vollenhoven, R.F.; van der Horst-Bruinsma, I.E. Gender Differences in Axial Spondyloarthritis: Women Are Not So Lucky. Curr. Rheumatol. Rep. 2018, 20, 35. [Google Scholar] [CrossRef]
- Butcher, B.; Carmody, J. Sex differences in analgesic response to ibuprofen are influenced by expectancy: A randomized, crossover, balanced placebo-designed study. Eur. J. Pain 2012, 16, 1005–1013. [Google Scholar] [CrossRef]
- Benyamin, R.; Trescot, A.M.; Datta, S.; Buenaventura, R.; Adlaka, R.; Sehgal, N.; Glaser, S.E.; Vallejo, R. Opioid complications and side effects. Pain Physician 2008, 11, S105–S120. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex differences in pain and pain inhibition: Multiple explanations of a controversial phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Berkley, K.J. Sex differences in pain. Behav. Brain Sci. 1997, 20, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Navratilova, E.; Fillingim, R.B.; Porreca, F. Sexual dimorphism in functional pain syndromes. Sci. Transl. Med. 2021, 13, eabj7180. [Google Scholar] [CrossRef]
- Boccella, S.; Guida, F.; De Logu, F.; De Gregorio, D.; Mazzitelli, M.; Belardo, C.; Iannotta, M.; Serra, N.; Nassini, R.; Novellis, V.; et al. Ketones and pain: Unexplored role of hydroxyl carboxylic acid receptor type 2 in the pathophysiology of neuropathic pain. FASEB J. 2019, 33, 1062–1073. [Google Scholar] [CrossRef]
- Averitt, D.L.; Eidson, L.N.; Doyle, H.H.; Murphy, A.Z. Neuronal and glial factors contributing to sex differences in opioid modulation of pain. Neuropsychopharmacology 2019, 44, 155–165. [Google Scholar] [CrossRef]
- Stephens, K.E.; Zhou, W.; Ji, Z.; Chen, Z.; He, S.; Ji, H.; Guan, Y.; Taverna, S.D. Sex differences in gene regulation in the dorsal root ganglion after nerve injury. BMC Genom. 2019, 20, 147. [Google Scholar] [CrossRef]
- Louw, A.; Nijs, J.; Puentedura, E.J. A clinical perspective on a pain neuroscience education approach to manual therapy. J. Man. Manip. Ther. 2017, 25, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.A.; Ma, Q.; De Koninck, Y. Normal and abnormal coding of somatosensory stimuli causing pain. Nat. Neurosci. 2014, 17, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Klasser, G.D.; Gremillion, H.A. Neuropathic orofacial pain patients in need of dental care. J. Can. Dent. Assoc. 2012, 78, c83. [Google Scholar]
- Ikeda, H.; Kiritoshi, T.; Murase, K. Contribution of Microglia and Astrocytes to the Central Sensitization, Inflammatory and Neuropathic Pain in the Juvenile Rat. Mol. Pain 2012, 8, 43. [Google Scholar] [CrossRef]
- Xu, Q.; Yaksh, T.L. A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr. Opin. Anaesthesiol. 2011, 24, 400–407. [Google Scholar] [CrossRef]
- Khammissa, R.A.G.; Ballyram, R.; Fourie, J.; Bouckaert, M.; Lemmer, J.; Feller, L. Selected pathobiological features and principles of pharmacological pain management. J. Int. Med. Res. 2020, 48, 0300060520903653. [Google Scholar] [CrossRef] [PubMed]
- Pickering, G.; Capriz-Ribière, F. Neuropathic pain in the elderly. Psychol. Neuro Psychiatr. Vieil. 2008, 6, 107–114. [Google Scholar]
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B. Neuropathic pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.E.; Ichesco, E.; Schrepf, A.; Halvorson, M.; Puiu, T.; Clauw, D.J.; Harris, R.E.; Harte, S.E. Relationships between brain metabolite levels, functional connectivity, and negative mood in urologic chronic pelvic pain syndrome patients compared to controls: A MAPP research network study. NeuroImage Clin. 2018, 17, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Barcelon, E.E.; Cho, W.-H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Zghoul, N.; Ross, E.L.; Edwards, R.R.; Ahmed, A.; Jamison, R.N. Prevalence of chronic pain with neuropathic characteristics: A randomized telephone survey among medical center patients in Kuwait. J. Pain Res. 2017, 10, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M.; Mogil, J.S.; Aloisi, A.M. Sex differences in pain and analgesia: The role of gonadal hormones. Eur. J. Pain 2004, 8, 397–411. [Google Scholar] [CrossRef]
- Craft, R.M. Modulation of pain by estrogens. Pain 2007, 132, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.R.; Stohler, C.S.; Nichols, T.E.; Bueller, J.A.; Koeppe, R.A.; Zubieta, J.-K. Pronociceptive and Antinociceptive Effects of Estradiol through Endogenous Opioid Neurotransmission in Women. J. Neurosci. 2006, 26, 5777–5785. [Google Scholar] [CrossRef]
- Riley, J.L.; Robinson, M.E.; Wise, E.A.; Price, D. A meta-analytic review of pain perception across the menstrual cycle. Pain 1999, 81, 225–235. [Google Scholar] [CrossRef]
- Boerner, K.E.; Birnie, K.A.; Caes, L.; Schinkel, M.; Chambers, C.T. Sex differences in experimental pain among healthy children: A systematic review and meta-analysis. Pain 2014, 155, 983–993. [Google Scholar] [CrossRef]
- Naugle, K.M.; Cruz-Almeida, Y.; Vierck, C.J.; Mauderli, A.P.; Riley, J.L. Age-related differences in conditioned pain modulation of sensitizing and desensitizing trends during response dependent stimulation. Behav. Brain Res. 2015, 289, 61–68. [Google Scholar] [CrossRef]
- Yezierski, R.P. The Effects of Age on Pain Sensitivity: Preclinical Studies. Pain Med. 2012, 13, S27–S36. [Google Scholar] [CrossRef] [PubMed]
- Blankenburg, M.; Boekens, H.; Hechler, T.; Maier, C.; Krumova, E.; Scherens, A.; Magerl, W.; Aksu, F.; Zernikow, B. Reference values for quantitative sensory testing in children and adolescents: Developmental and gender differences of somatosensory perception. Pain 2010, 149, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Totsch, S.K. Sex differences in pain. J. Neurosci. Res. 2017, 95, 1271–1281. [Google Scholar] [PubMed]
- Cichon, J.; Sun, L.; Yang, G. Spared Nerve Injury Model of Neuropathic Pain in Mice. Bio-Protocol 2018, 8, e2777. [Google Scholar] [CrossRef] [PubMed]
- Inyang, K.E.; Szabo-Pardi, T.; Wentworth, E.; McDougal, T.A.; Dussor, G.; Burton, M.D.; Price, T.J. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019, 139, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.-S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.M.; Kim, H.K.; Chung, K. Segmental spinal nerve ligation model of neuropathic pain. In Pain Research: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2004; pp. 35–45. [Google Scholar]
- Lopes, D.M.; Malek, N.; Edye, M.; Jager, S.B.; McMurray, S.; McMahon, S.B.; Denk, F. Sex differences in peripheral not central immune responses to pain-inducing injury. Sci. Rep. 2017, 7, 16460. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Vacca, V.; Marinelli, S.; Pieroni, L.; Urbani, A.; Luvisetto, S.; Pavone, F. 17beta-estradiol counteracts neuropathic pain: A behavioural, immunohistochemical and proteomic investigation on sex-related differences in mice. Sci. Rep. 2016, 6, 18980. [Google Scholar] [CrossRef]
- Beutler, B.; Poltorak, A. The sole gateway to endotoxin response: How LPS was identified as Tlr4, and its role in innate immunity. Drug Metab. Dispos. 2001, 29, 474–478. [Google Scholar]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.-S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal Cord Toll-Like Receptor 4 Mediates Inflammatory and Neuropathic Hypersensitivity in Male but Not Female Mice. J. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Hanamsagar, R.; Alter, M.D.; Block, C.S.; Sullivan, H.; Bolton, J.L.; Bilbo, S.D. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 2017, 65, 1504–1520. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. Int. J. Mol. Sci. 2018, 19, 2793. [Google Scholar] [CrossRef]
- Castañeda-Corral, G.; Velázquez-Salazar, N.B.; Martínez-Martínez, A.; Taboada-Serrano, J.N.; Núñez-Aragón, P.N.; González-Palomares, L.; Acosta-González, R.I.; Petricevich, V.L.; Acevedo-Fernández, J.J.; Montes, S.; et al. Characterization of Mechanical Allodynia and Skin Innervation in a Mouse Model of Type-2 Diabetes Induced by Cafeteria-Style Diet and Low-Doses of Streptozotocin. Front. Pharmacol. 2021, 11, 628438. [Google Scholar] [CrossRef]
- Abraham, A.; Barnett, C.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Sex differences in neuropathic pain intensity in diabetes. J. Neurol. Sci. 2018, 388, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Alessio, N.; Belardo, C.; Trotta, M.C.; Paino, S.; Boccella, S.; Gargano, F.; Pieretti, G.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Vitamin D Deficiency Induces Chronic Pain and Microglial Phenotypic Changes in Mice. Int. J. Mol. Sci. 2021, 22, 3604. [Google Scholar] [CrossRef] [PubMed]
- Cabañero, D.; Villalba-Riquelme, E.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. ThermoTRP channels in pain sexual dimorphism: New insights for drug intervention. Pharmacol. Ther. 2022, 240, 108297. [Google Scholar] [CrossRef] [PubMed]
- Schmetzer, O.; Flörcken, A. Sex Differences in the Drug Therapy for Oncologic Diseases. In Sex and Gender Differences in Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 411–442. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Weerasinghe-Mudiyanselage, P.D.E.; Kim, J.; Choi, Y.; Moon, C. Ninjurin-1: A biomarker for reflecting the process of neuroinflammation after spinal cord injury. Neural Regen. Res. 2021, 16, 1331–1335. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Batti, L.; Sundukova, M.; Murana, E.; Pimpinella, S.; Reis, F.D.C.; Pagani, F.; Wang, H.; Pellegrino, E.; Perlas, E.; Di Angelantonio, S.; et al. TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell Rep. 2016, 15, 2608–2615. [Google Scholar] [CrossRef]
- Gu, N.; Eyo, U.B.; Murugan, M.; Peng, J.; Matta, S.; Dong, H.; Wu, L.-J. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav. Immun. 2016, 55, 82–92. [Google Scholar] [CrossRef]
- Jin, S.-X.; Zhuang, Z.-Y.; Woolf, C.J.; Ji, R.-R. p38 Mitogen-Activated Protein Kinase Is Activated after a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J. Neurosci. 2003, 23, 4017–4022. [Google Scholar] [CrossRef]
- Tsuda, M.; Shigemoto-Mogami, Y.; Koizumi, S.; Mizokoshi, A.; Kohsaka, S.; Salter, M.W.; Inoue, K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 2003, 424, 778–783. [Google Scholar] [CrossRef]
- Ghazisaeidi, S.; Muley, M.M.; Salter, M.W. Neuropathic pain: Mechanisms, sex differences, and potential therapies for a global problem. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 565–583. [Google Scholar] [PubMed]
- Masuda, T.; Ozono, Y.; Mikuriya, S.; Kohro, Y.; Tozaki-Saitoh, H.; Iwatsuki, K.; Uneyama, H.; Ichikawa, R.; Salter, M.W.; Tsuda, M.; et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. 2016, 7, 12529. [Google Scholar] [CrossRef] [PubMed]
- Mapplebeck, J.C.; Dalgarno, R.; Tu, Y.; Moriarty, O.; Beggs, S.; Kwok, C.H.; Halievski, K.; Assi, S.; Mogil, J.S.; Trang, T.; et al. Microglial P2X4R-evoked pain hypersensitivity is sexually dimorphic in rats. Pain 2018, 159, 1752–1763. [Google Scholar] [CrossRef]
- Taves, S.; Berta, T.; Liu, D.-L.; Gan, S.; Chen, G.; Kim, Y.H.; Van de Ven, T.; Laufer, S.; Ji, R.-R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav. Immun. 2016, 55, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Kuhn, J.A.; Wang, X.; Colquitt, B.; Solorzano, C.; Vaman, S.; Guan, A.K.; Evans-Reinsch, Z.; Braz, J.; Devor, M.; et al. Injured sensory neuron–derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat. Neurosci. 2016, 19, 94–101. [Google Scholar] [CrossRef]
- Kuhn, J.A.; Vainchtein, I.D.; Braz, J.; Hamel, K.; Bernstein, M.; Craik, V.; Dahlgren, M.W.; Ortiz-Carpena, J.; Molofsky, A.B.; Molofsky, A.V. Regulatory T-cells inhibit microglia-induced pain hypersensitivity in female mice. Elife 2021, 10, e69056. [Google Scholar]
- Coraggio, V.; Guida, F.; Boccella, S.; Scafuro, M.; Paino, S.; Romano, D.; Maione, S.; Luongo, L. Neuroimmune-Driven Neuropathic Pain Establishment: A Focus on Gender Differences. Int. J. Mol. Sci. 2018, 19, 281. [Google Scholar] [CrossRef]
- Mapplebeck, J.C.; Beggs, S.; Salter, M.W. Sex differences in pain: A tale of two immune cells. Pain 2016, 157, S2–S6. [Google Scholar] [CrossRef]
- Cao, L.; DeLeo, J.A. CNS-infiltrating CD4+ T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur. J. Immunol. 2008, 38, 448–458. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, D.-H.; Choi, J.-Y.; Kim, W.-J.; Kim, J.Y.; Senejani, A.G.; Hwang, S.S.; Kim, L.K.; Tobiasova, Z.; Lee, G.R.; et al. PPARγ Negatively Regulates T Cell Activation to Prevent Follicular Helper T Cells and Germinal Center Formation. PLoS ONE 2014, 9, e99127. [Google Scholar] [CrossRef]
- Zhang, M.A.; Rego, D.; Moshkova, M.; Kebir, H.; Chruscinski, A.; Nguyen, H.; Akkermann, R.; Stanczyk, F.Z.; Prat, A.; Steinman, L. Peroxisome proliferator-activated receptor (PPAR) α and-γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc. Natl. Acad. Sci. USA 2012, 109, 9505–9510. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, J.; You, B.; Oh, J.H.; Mok, H.J.; Kim, Y.S.; Yoon, B.; Kim, B.G.; Back, S.K.; Park, J.; et al. GT 1b functions as a novel endogenous agonist of toll-like receptor 2 inducing neuropathic pain. EMBO J. 2020, 39, e102214. [Google Scholar] [CrossRef]
- Lee, J.; Chung, S.; Hwang, M.; Kwon, Y.; Han, S.H.; Lee, S.J. Estrogen Mediates the Sexual Dimorphism of GT1b-Induced Central Pain Sensitization. Cells 2023, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Sutterwala, F.S.; Ogura, Y.; Szczepanik, M.; Lara-Tejero, M.; Lichtenberger, G.S.; Grant, E.P.; Bertin, J.; Coyle, A.J.; Galán, J.E.; Askenase, P.W. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity 2006, 24, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Monack, D.M. Molecular mechanisms of inflammasome activation during microbial infections. Immunol. Rev. 2011, 243, 174–190. [Google Scholar] [CrossRef]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 73–118. [Google Scholar]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals 2016, 9, 72. [Google Scholar] [CrossRef]
- Mohandass, A.; Krishnan, V.; Gribkova, E.D.; Asuthkar, S.; Baskaran, P.; Nersesyan, Y.; Hussain, Z.; Wise, L.M.; George, R.E.; Stokes, N.; et al. TRPM8 as the rapid testosterone signaling receptor: Implications in the regulation of dimorphic sexual and social behaviors. FASEB J. 2020, 34, 10887–10906. [Google Scholar] [CrossRef]
- Gkika, D.; Lolignier, S.; Grolez, G.P.; Bavencoffe, A.; Shapovalov, G.; Gordienko, D.; Kondratskyi, A.; Meleine, M.; Prival, L.; Chapuy, E.; et al. Testosterone-androgen receptor: The steroid link inhibiting TRPM8-mediated cold sensitivity. FASEB J. 2020, 34, 7483–7499. [Google Scholar] [CrossRef]
- Artero-Morales, M.; González-Rodríguez, S.; Ferrer-Montiel, A. TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain. Front. Mol. Biosci. 2018, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Bennett, H.L.; Gustafsson, J.; Keast, J.R. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton. Neurosci. 2003, 105, 90–100. [Google Scholar] [CrossRef]
- Payrits, M.; Sághy, É.; Csekő, K.; Pohóczky, K.; Bölcskei, K.; Ernszt, D.; Barabás, K.; Szolcsányi, J.; Ábrahám, I.M.; Helyes, Z.; et al. Estradiol Sensitizes the Transient Receptor Potential Vanilloid 1 Receptor in Pain Responses. Endocrinology 2017, 158, 3249–3258. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chen, C.-W.; Wang, S.-Y.; Wu, F.-S. 17β-Estradiol Mediates the Sex Difference in Capsaicin-Induced Nociception in Rats. J. Pharmacol. Exp. Ther. 2009, 331, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alarcón, D.; Cabañero, D.; de Andrés-López, J.; Nikolaeva-Koleva, M.; Giorgi, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 2022, 13, 6304. [Google Scholar] [CrossRef]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cotman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflammation 2012, 9, 264. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Gelosa, P.; Castiglioni, L.; Cimino, M.; Rizzi, N.; Pepe, G.; Lolli, F.; Marcello, E.; Sironi, L.; Vegeto, E.; et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep. 2018, 23, 3501–3511. [Google Scholar] [CrossRef]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflammation 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, H.Y.; Ju, B.-G.; Yune, T.Y. Estrogen alleviates neuropathic pain induced after spinal cord injury by inhibiting microglia and astrocyte activation. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2472–2480. [Google Scholar] [CrossRef]
- Vaccari, J.P.D.R.; Bramlett, H.M.; Perez-Pinzon, M.A.; Raval, A.P. Estrogen preconditioning: A promising strategy to reduce inflammation in the ischemic brain. Cond. Med. 2019, 2, 106–113. [Google Scholar]
- Thakkar, R.; Wang, R.; Sareddy, G.; Wang, J.; Thiruvaiyaru, D.; Vadlamudi, R.; Zhang, Q.; Brann, D. NLRP3 Inflammasome Activation in the Brain after Global Cerebral Ischemia and Regulation by 17β-Estradiol. Oxidative Med. Cell. Longev. 2016, 2016, 8309031. [Google Scholar] [CrossRef] [PubMed]
- Averitt, D.L.; Hornung, R.S.; Murphy, A.Z. Role of sex hormones on pain. Oxf. Res. Encycl. Neurosci. 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcelon, E.; Chung, S.; Lee, J.; Lee, S.J. Sexual Dimorphism in the Mechanism of Pain Central Sensitization. Cells 2023, 12, 2028. https://doi.org/10.3390/cells12162028
Barcelon E, Chung S, Lee J, Lee SJ. Sexual Dimorphism in the Mechanism of Pain Central Sensitization. Cells. 2023; 12(16):2028. https://doi.org/10.3390/cells12162028
Chicago/Turabian StyleBarcelon, Ellane, Seohyun Chung, Jaesung Lee, and Sung Joong Lee. 2023. "Sexual Dimorphism in the Mechanism of Pain Central Sensitization" Cells 12, no. 16: 2028. https://doi.org/10.3390/cells12162028
APA StyleBarcelon, E., Chung, S., Lee, J., & Lee, S. J. (2023). Sexual Dimorphism in the Mechanism of Pain Central Sensitization. Cells, 12(16), 2028. https://doi.org/10.3390/cells12162028





