Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles
Abstract
1. Introduction
2. Materials and Methods
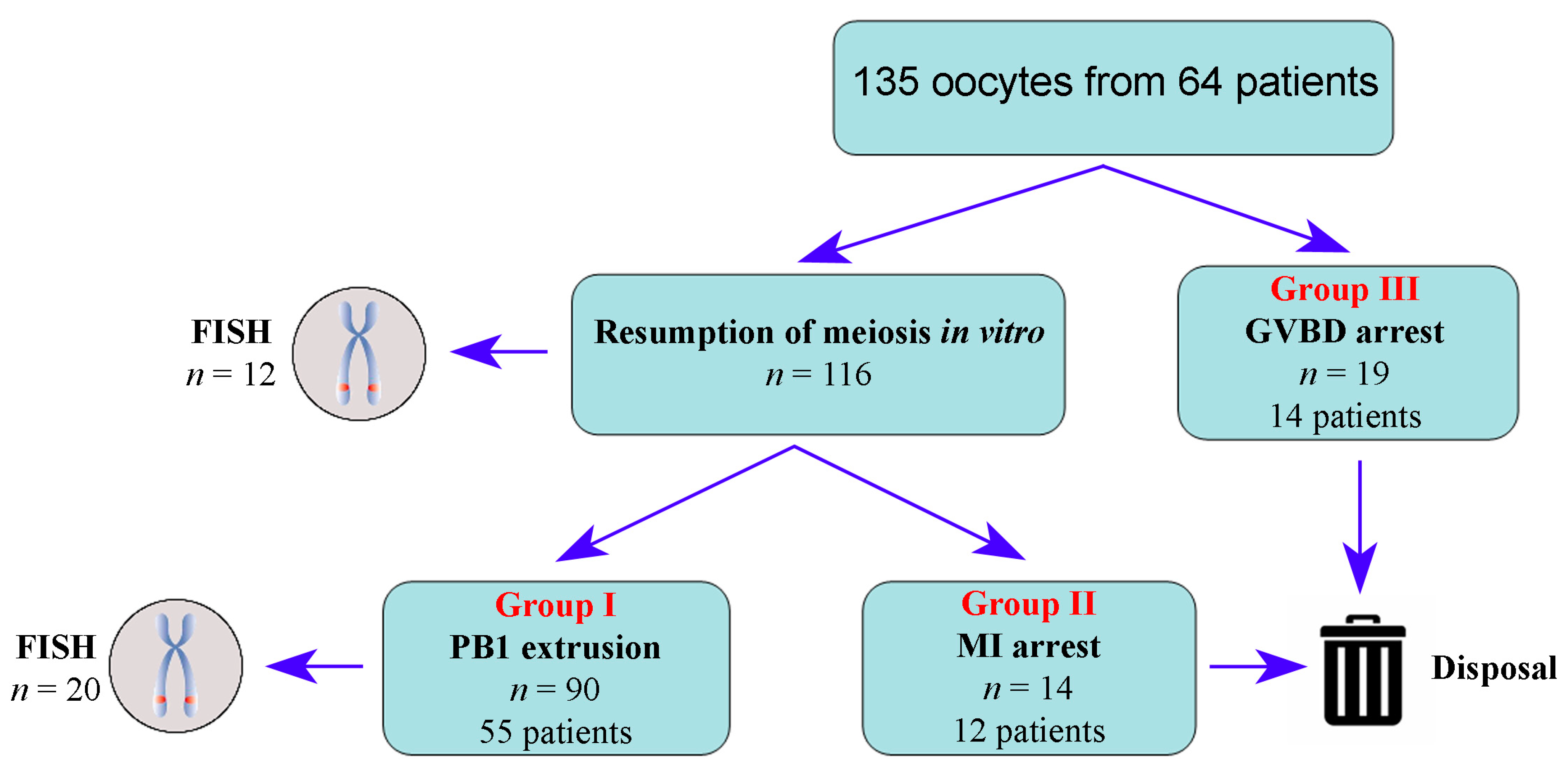
2.1. Study Design
2.2. Ovarian Stimulation, Oocyte Retrieval, and In Vitro Culture
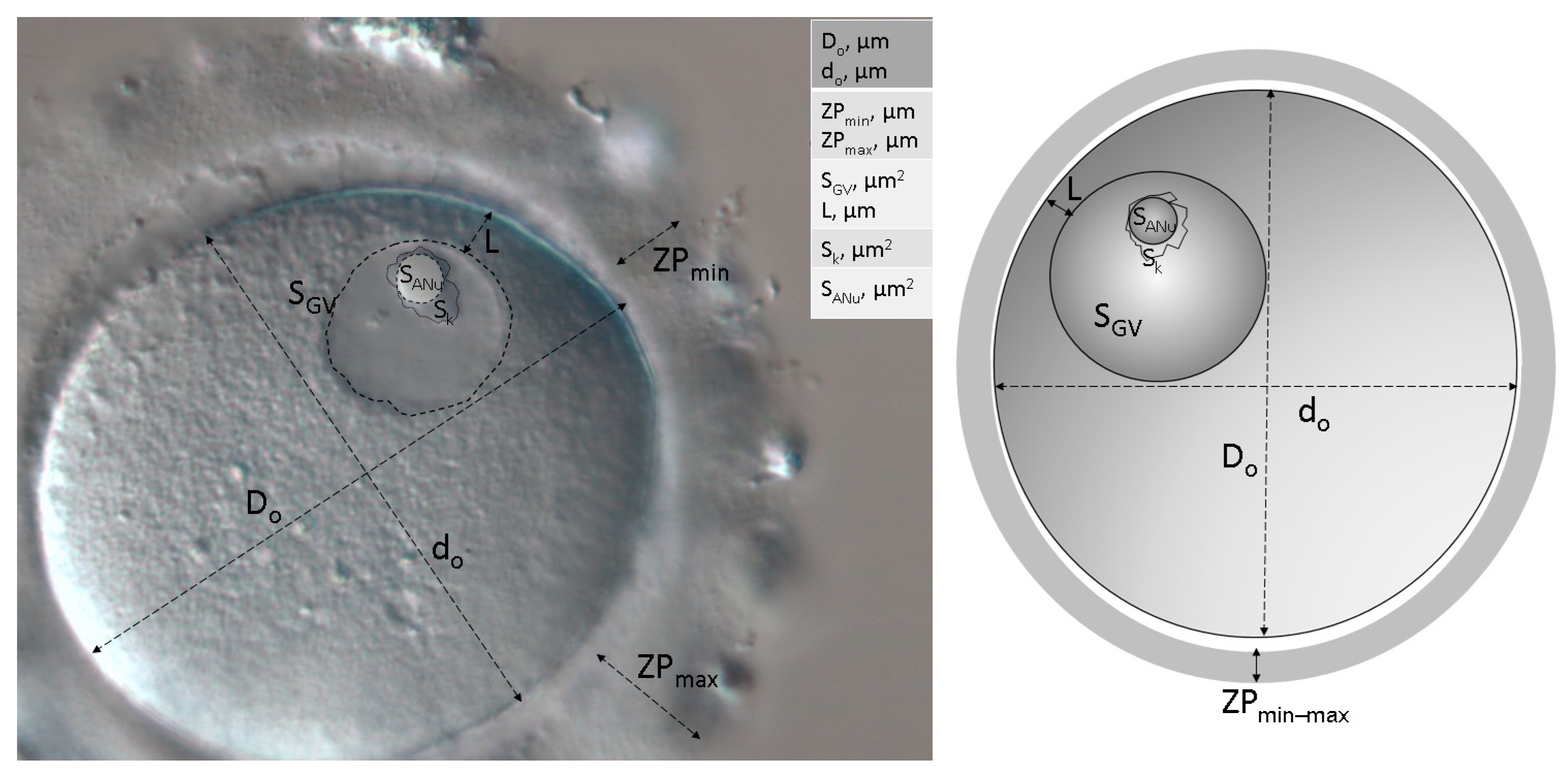
2.3. Morphological and Morphometric Assessment
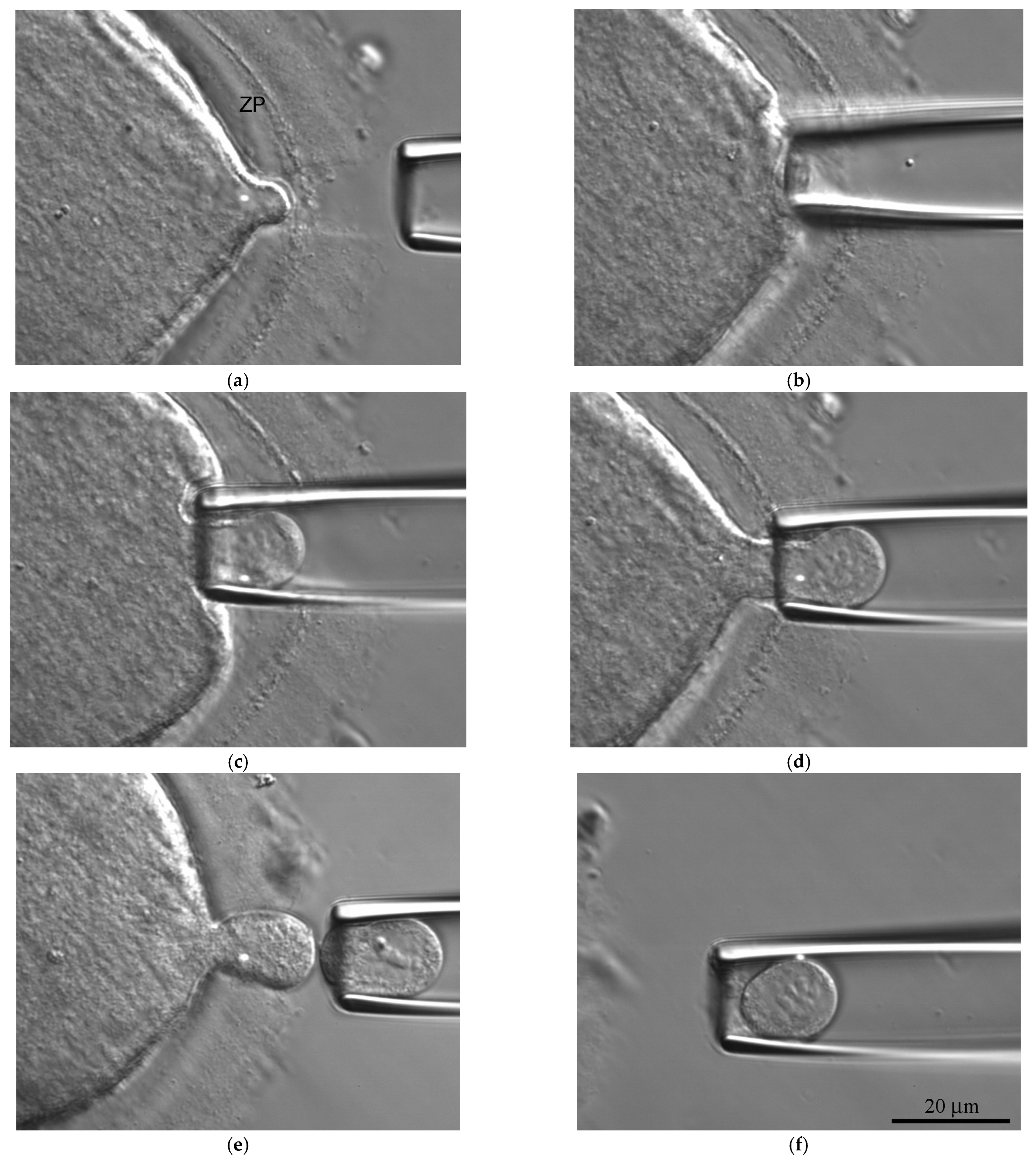
2.4. Morphodynamics
2.5. Biopsy and Fluorescent In Situ Hybridization (FISH)
2.6. Statistical Analysis
3. Results
3.1. Anamnestic Predictors
3.2. Morphological Predictors of GV Oocyte Maturation
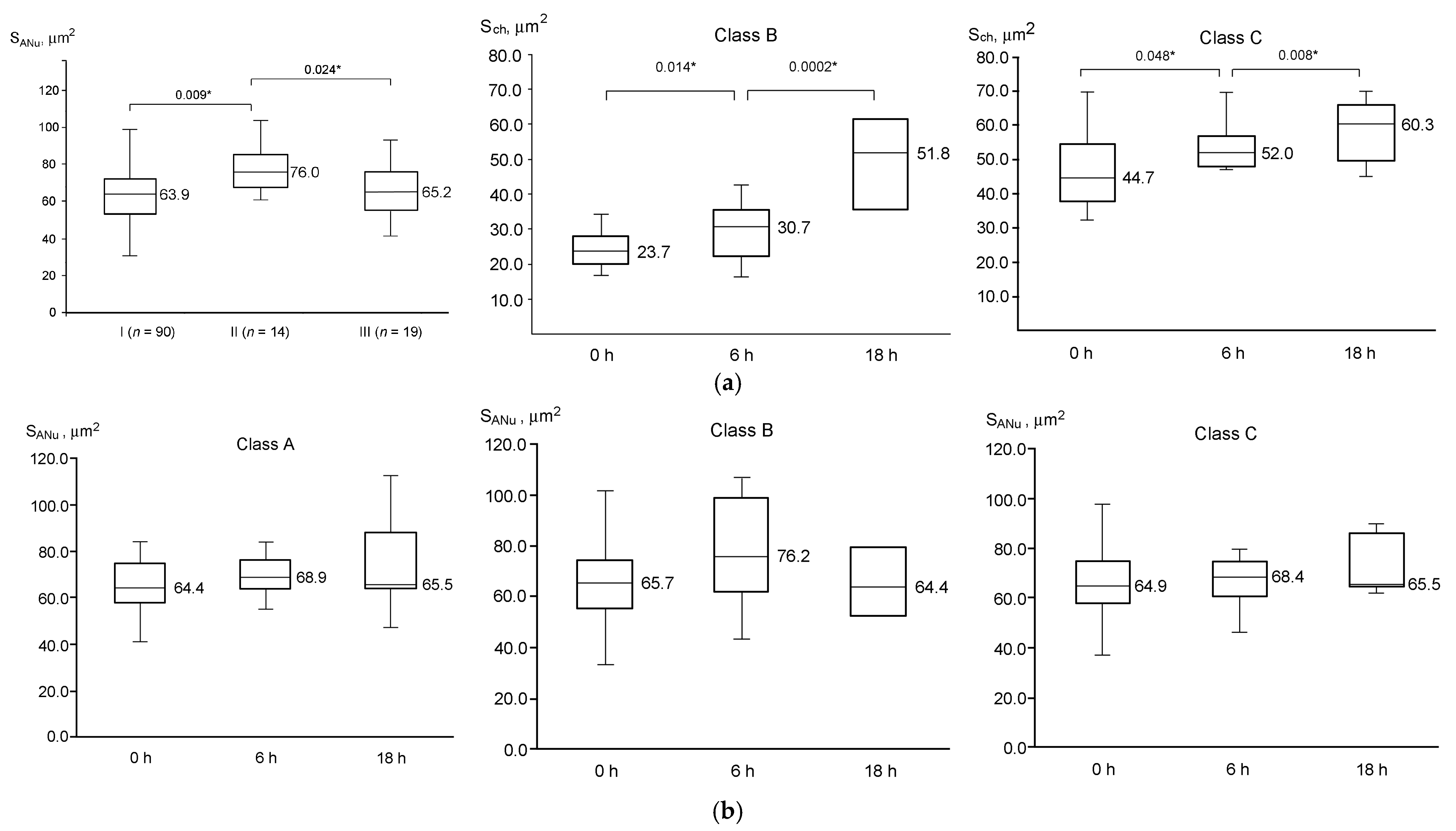
3.3. Dynamics of GV Oocyte Maturation
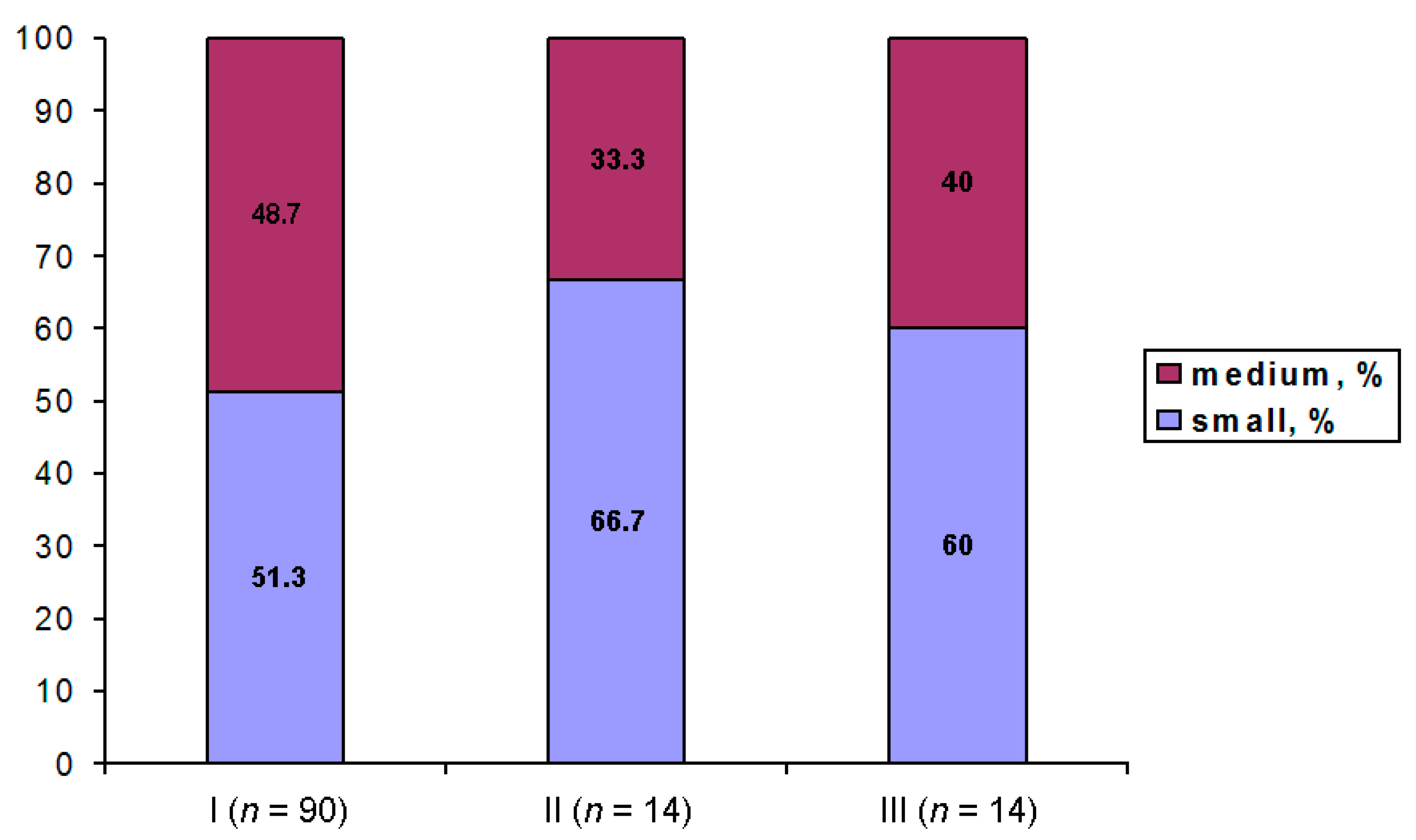
3.4. Configuration of Chromatin
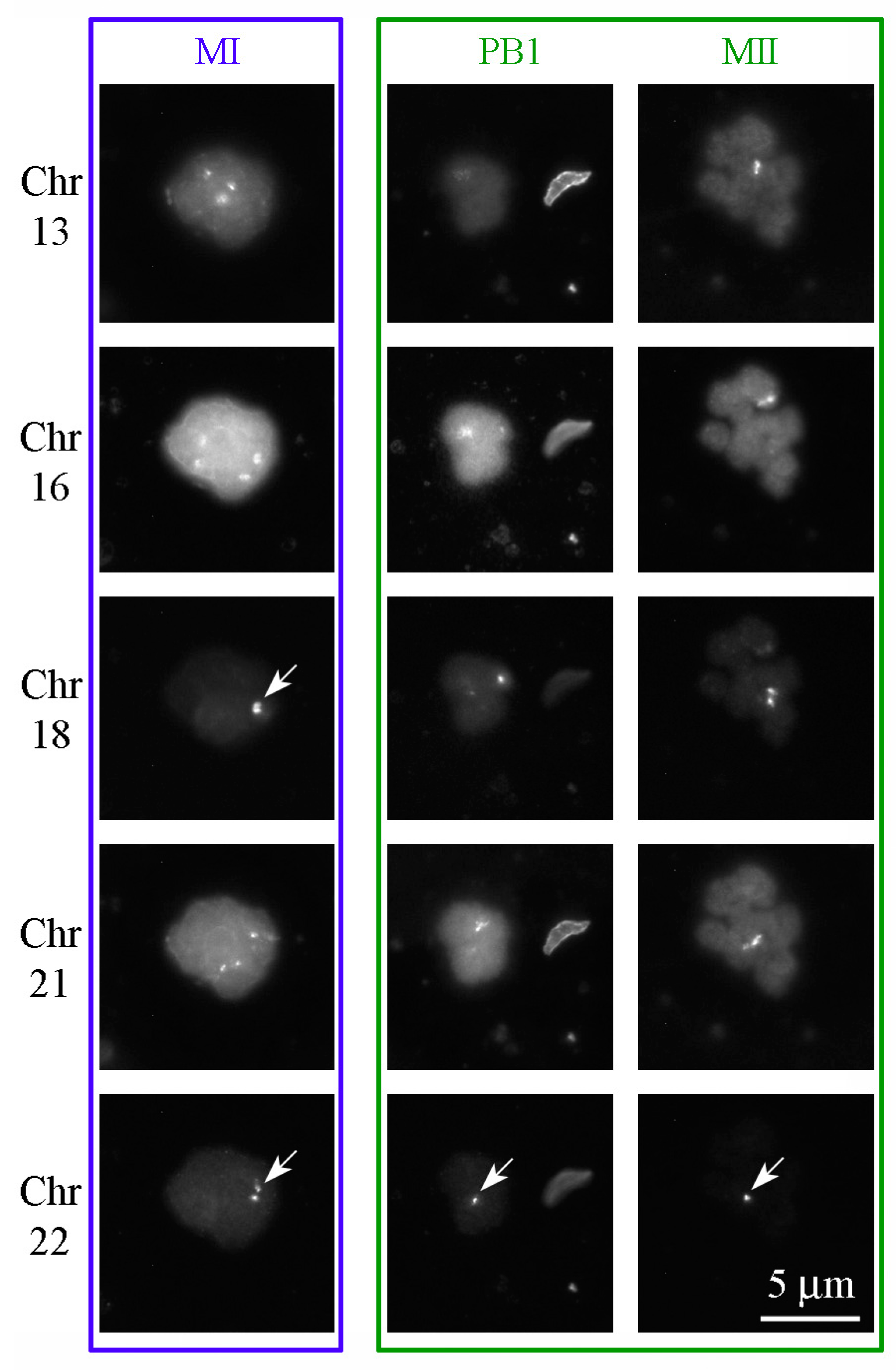
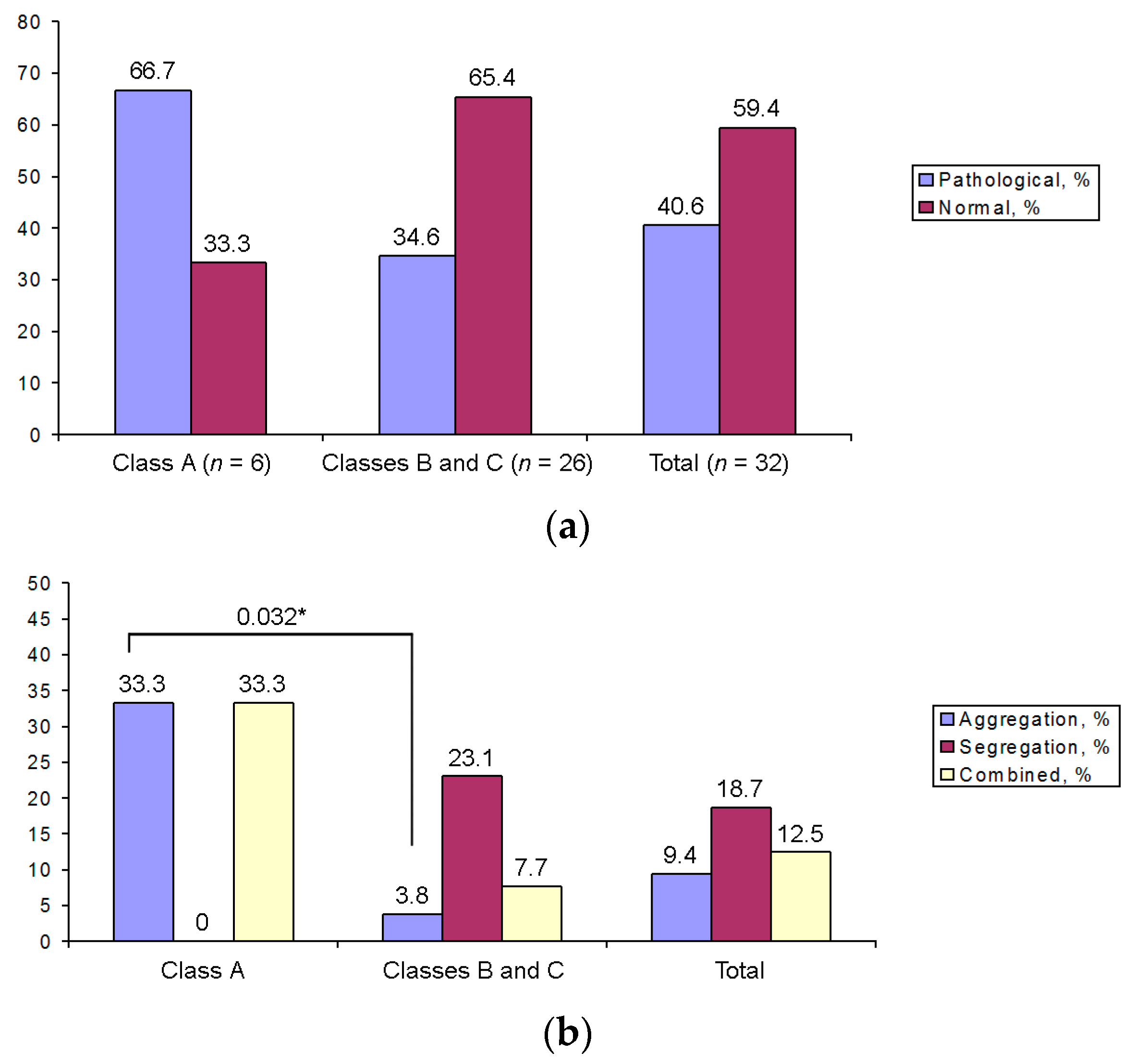
3.5. Aneuploidies in MI and MII Oocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Farini, D.; De Felici, M. The beginning of meiosis in mammalian female germ cells: A never-ending story of intrinsic and extrinsic factors. Int. J. Mol. Sci. 2022, 23, 12571. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Antonouli, S.; Macchiarelli, G.; Cecconi, S.; Bianchi, S.; Khalili, M.A.; Nottola, S.A. Ultrastructural evaluation of the human oocyte at the germinal vesicle stage during the application of assisted reproductive technologies. Cells 2022, 11, 1636. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; He, Y.; Pan, X.; Wang, P.; Yuan, X.; Ma, B. Advances in oocyte maturation in vivo and in vitro in mammals. Int. J. Mol. Sci. 2023, 24, 9059. [Google Scholar] [CrossRef] [PubMed]
- Strączyńska, P.; Papis, K.; Morawiec, E.; Czerwiński, M.; Gajewski, Z.; Olejek, A.; Bednarska-Czerwińska, A. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod. Biol. Endocrinol. 2022, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Franciosi, F. Acquisition of oocyte competence to develop as an embryo: Integrated nuclear and cytoplasmic events. Hum. Reprod. Update 2018, 24, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Dal Canto, M.; Renzini, M.M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Sirait, B.; Wiweko, B.; Jusuf, A.A.; Iftitah, D.; Muharam, R. Oocyte competence biomarkers associated with oocyte maturation: A review. Front. Cell Dev. Biol. 2021, 9, 710292. [Google Scholar] [CrossRef]
- Ozturk, S. Molecular determinants of the meiotic arrests in mammalian oocytes at different stages of maturation. Cell Cycle 2022, 21, 547–571. [Google Scholar] [CrossRef]
- Bogolyubova, I.; Bogolyubov, D. Heterochromatin morphodynamics in late oogenesis and early embryogenesis of mammals. Cells 2020, 9, 1497. [Google Scholar] [CrossRef]
- Fulka, J.J.; Benc, M.; Loi, P.; Langerova, A.; Fulka, H. Function of atypical mammalian oocyte/zygote nucleoli and its implications for reproductive biology and medicine. Int. J. Dev. Biol. 2019, 63, 105–112. [Google Scholar] [CrossRef]
- Parfenov, V.; Potchukalina, G.; Dudina, L.; Kostyuchek, D.; Gruzova, M. Human antral follicles: Oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data). Gamete Res. 1989, 22, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Bogolyubov, D.S. Karyosphere (karyosome): A peculiar structure of the oocyte nucleus. Int. Rev. Cell Mol. Biol. 2018, 337, 1–48. [Google Scholar]
- Combelles, C.M.H.; Cekleniak, N.A.; Racowsky, C.; Albertini, D.F. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum. Reprod. 2002, 17, 1006–1016. [Google Scholar] [CrossRef]
- Miyara, F.; Migne, C.; Dumont-Hassan, M.; Le Meur, A.; Cohen-Bacrie, P.; Aubriot, F.-X.; Glissant, A.; Nathan, C.; Douard, S.; Stanovici, A.; et al. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol. Reprod. Dev. 2003, 64, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Romero, S.; De Vos, M.; Verheyen, G.; Smitz, J. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum. Reprod. 2015, 30, 1396–1409. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lou, H.; Lou, Y.; Wang, N.; Jin, F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod. Biomed. Online 2014, 28, 284–299. [Google Scholar] [CrossRef]
- Yamochi, T.; Hashimoto, S.; Amo, A.; Goto, H.; Yamanaka, M.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Mitochondrial dynamics and their intracellular traffic in porcine oocytes. Zygote 2016, 24, 517–528. [Google Scholar] [CrossRef]
- Bellone, M.; Zuccotti, M.; Redi, C.A.; Garagna, S. The position of the germinal vesicle and the chromatin organization together provide a marker of the developmental competence of mouse antral oocytes. Reproduction 2009, 138, 639–643. [Google Scholar] [CrossRef]
- Levi, M.; Ghetler, Y.; Shulman, A.; Shalgi, R. Morphological and molecular markers are correlated with maturation-competence of human oocytes. Hum. Reprod. 2013, 28, 2482–2489. [Google Scholar] [CrossRef]
- Almonacid, M.; Ahmed, W.W.; Bussonnier, M.; Mailly, P.; Betz, T.; Voituriez, R.; Gov, N.S.; Verlhac, M.-H. Active diffusion positions the nucleus in mouse oocytes. Nat. Cell Biol. 2015, 17, 470–479. [Google Scholar] [CrossRef]
- ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. The Vienna consensus: Report of an expert meeting on the development of ART laboratory performance indicators. Reprod. Biomed. Online 2017, 35, 494–510. [Google Scholar] [CrossRef]
- Escrich, L.; Grau, N.; Mercader, A.; Rubio, C.; Pellicer, A.; Escribá, M.-J. Spontaneous in vitro maturation and artificial activation of human germinal vesicle oocytes recovered from stimulated cycles. J. Assist. Reprod. Genet. 2011, 28, 111–117. [Google Scholar] [CrossRef]
- Escrich, L.; Galiana, Y.; Grau, N.; Insua, F.; Soler, N.; Pellicer, A.; Escribá, M. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod. Biomed. Online 2018, 37, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Gremeau, A.-S.; Andreadis, N.; Fatum, M.; Craig, J.; Turner, K.; McVeigh, E.; Child, T. In vitro maturation or in vitro fertilization for women with polycystic ovaries? A case–control study of 194 treatment cycles. Fertil. Steril. 2012, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Son, W.-Y.; Buckett, W.; Tulandi, T.; Holzer, H. In-vitro maturation versus IVF with GnRH antagonist for women with polycystic ovary syndrome: Treatment outcome and rates of ovarian hyperstimulation syndrome. Reprod. Biomed. Online 2014, 29, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Lee, M.G.; Lin, T.; Shin, H.Y.; Lee, J.E.; Kang, J.W.; Jin, D.-I. Effect of oocyte chromatin status in porcine follicles on the embryo development in vitro. Asian-Australas. J. Anim. Biosci. 2019, 32, 956–965. [Google Scholar] [CrossRef]
- Monti, M.; Zanoni, M.; Calligaro, A.; Ko, M.S.H.; Mauri, P.; Redi, C.A. Developmental arrest and mouse antral not-surrounded nucleolus oocytes. Biol. Reprod. 2013, 8, 2. [Google Scholar] [CrossRef]
- Monti, M.; Calligaro, A.; Behr, B.; Pera, R.R.; Redi, C.A.; Wossidlo, M. Functional topography of the fully grown human oocyte. Eur. J. Histochem. 2017, 61, 2769. [Google Scholar] [CrossRef]
- Baumann, C.; Viveiros, M.M.; De La Fuente, R. Loss of maternal ATRX results in centromere instability and aneuploidy in the mammalian oocyte and pre-implantation embryo. PLoS Genet. 2010, 6, e1001137. [Google Scholar] [CrossRef]
- Escrich, L.; Grau, N.; Meseguer, M.; Pellicer, A.; Escribá, M.-J. Morphologic indicators predict the stage of chromatin condensation of human germinal vesicle oocytes recovered from stimulated cycles. Fertil. Steril. 2010, 93, 2557–2564. [Google Scholar] [CrossRef]
- Wada, T.; Ikegami, M.; Nagase, Y.; Yamamoto, Y.; Matsuda, Y.; Yonezawa, J.; Matsuura, T. Maturation rate and subsequent blastocyst development of human oocytes derived from various follicle size at the time of oocyte pick up. Fertil. Steril. 2014, 102 (Suppl. 3), e133. [Google Scholar] [CrossRef]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The oocyte. Hum. Reprod. 2012, 27, i2–i21. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Maro, B. Germinal vesicle position and meiotic maturation in mouse oocyte. Reproduction 2007, 133, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Buratini, J.; Dellaqua, T.T.; Dal Canto, M.; La Marca, A.; Carone, D.; Renzini, M.M.; Webb, R. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: From fertility prognosis to mechanisms underlying age-related subfertility. Hum. Reprod. Update 2022, 28, 232–254. [Google Scholar] [CrossRef]
- Macer, M.L. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet. Gynecol. Clin. N. Am. 2012, 39, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.; Zhao, M.; Alqawasmeh, O.A.M.; Chan, C.P.S.; Lee, T.L.; Li, T.; Chan, D.Y.L. In vitro rescue immature oocytes—A literature review. Hum. Fertil. 2022, 25, 640–650. [Google Scholar] [CrossRef]
- Martin-Palomino Olid, N.; García, D.; Rodríguez, A.; Vassena, R. Could fertility clinics offer a sizable improvement of live birth rates by maturing post-GVBD oocytes in vitro? J. Assist. Reprod. Genet. 2019, 36, 1927–1934. [Google Scholar] [CrossRef]
- Otsuki, J.; Momma, Y.; Takahashi, K.; Miyakura, S.; Nagai, Y. Timed IVM followed by ICSI in a patient with immature ovarian oocytes. Reprod. Biomed. Online 2006, 13, 101–103. [Google Scholar] [CrossRef]
- Holubcová, Z.; Blayney, M.; Elder, K.; Schuh, M. Error-prone chromosome-mediated spindle assembly favors chromosome segregation defects in human oocytes. Science 2015, 348, 1143–1147. [Google Scholar] [CrossRef]
- Bennabi, I.; Terret, M.-E.; Verlhac, M.-H. Meiotic spindle assembly and chromosome segregation in oocytes. J. Cell Biol. 2016, 215, 611–619. [Google Scholar] [CrossRef]
- Delhanty, J.D.A.; SenGupta, S.B.; Ghevaria, H. How common is germinal mosaicism that leads to premeiotic aneuploidy in the female? J. Assist. Reprod. Genet. 2019, 36, 2403–2418. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Wilton, L. Aneuploidy of chromosomes 11, 17, 16 and 22 in mature and immature human oocytes. Fertil. Steril. 2000, 74 (Suppl. 1), S18. [Google Scholar] [CrossRef]
- Daina, G.; Ramos, L.; Rius, M.; Obradors, A.; Del Rey, J.; Giralt, M.; Campillo, M.; Velilla, E.; Pujol, A.; Martinez-Pasarell, O.; et al. Non-meiotic chromosome instability in human immature oocytes. Eur. J. Hum. Genet. 2014, 22, 202–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tesařík, J.; Trávník, P.; Kopečný, V.; Kristek, F. Nucleolar transformations in the human oocyte after completion of growth. Gamete Res. 1983, 8, 266–277. [Google Scholar] [CrossRef]
- Tesařík, J.; Kopečny, V.; Dvořák, M.; Pilka, L.; Kurilo, L.F. Human nonovulatory oocyte-cumulus complexes: Ultrastructure, macromolecular synthesis, and developmental potential. Gamete Res. 1984, 9, 153–165. [Google Scholar] [CrossRef]
- Zatsepina, O.V.; Bouniol-Baly, C.; Amirand, C.; Debey, P. Functional and molecular reorganization of the nucleolar apparatus in maturing mouse oocytes. Dev. Biol. 2000, 223, 354–370. [Google Scholar] [CrossRef]
- Fair, T.; Hyttel, P.; Lonergan, P.; Boland, M.P. Immunolocalization of nucleolar proteins during bovine oocyte growth, meiotic maturation, and fertilization. Biol. Reprod. 2001, 64, 1516–1525. [Google Scholar] [CrossRef]
- Shishova, K.V.; Lavrentyeva, E.A.; Dobrucki, J.W.; Zatsepina, O.V. Nucleolus-like bodies of fully-grown mouse oocytes contain key nucleolar proteins but are impoverished for rRNA. Dev. Biol. 2015, 397, 267–281. [Google Scholar] [CrossRef]


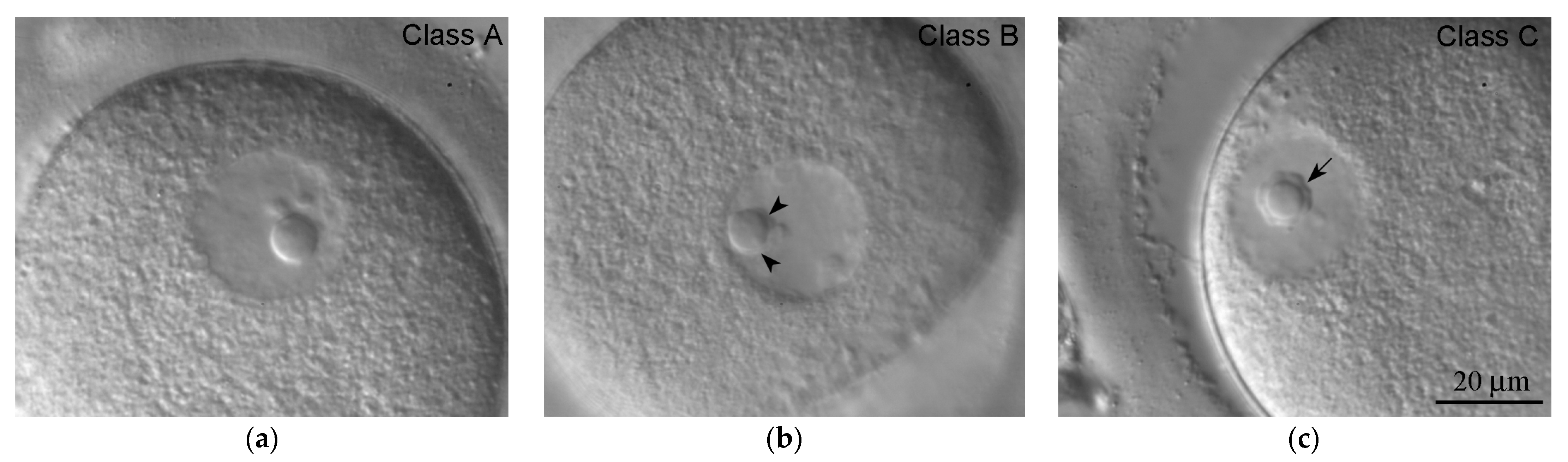


| Class | Description | State of the Karyosphere |
|---|---|---|
| A | Chromatin is diffuse; only single/scarce heterochromatin clumps are observed in the vicinity of the atypical nucleolus (ANu) | No karyosphere |
| B | All intermediate pictures between classes A and C; the ANu may be embedded into a chromatin mass, but much condensed chromatin is still distributed throughout the GV; the peri-ANu heterochromatin rim, if in existence, is often incomplete | Incomplete karyosphere in most cases |
| C | All the chromatin surrounds the ANu | Fully formed compact karyosphere |
| Probe Name | Chromosomal Region | Fluorophore | Wavelength (Excitation/Emission, λmax) |
|---|---|---|---|
| Vysis LSI 13 | 13q14.2 | Spectrum Red | 596/615 nm |
| Satellite II DNA Vysis CEP 16 | 16q11.2 | Spectrum Aqua | 434/481 nm |
| Alpha Satellite DNA CEP 18 | 18p11.1–q11.1 | Spectrum Blue | 405/449 nm |
| Vysis LSI 21 | 21q22.13–q22.2 | Spectrum Green | 499/522 nm |
| Vysis LSI 22 | 22q11.2 | Spectrum Gold | 537/559 nm |
| Parameter | Groups of Oocytes | p-Value | |||||
|---|---|---|---|---|---|---|---|
| I (n = 90) | II (n = 14) | III (n = 19) | I/III | II/III | I/II | ||
| Age, years a | 33.0 (29.0–36.0) | 31.0 (30.0–33.0) | 33.0 (32.0–37.0) | 0.394 | 0.046 * | 0.377 | |
| FSH, mIU/mL a | 6.9 (6.0–9.1) | 7.8 (6.3–9.6) | 7.1 (6.2–10.3) | 0.695 | 0.760 | 0.432 | |
| AMH, ng/mL a | 2.5 (1.6–4.4) | 3.5 (1.7–6.1) | 1.4 (0.4–2.4) | 0.020 * | 0.069 | 0.522 | |
| Number of antral follicles a | right ovary | 7.0 (5.0–9.0) | 6.0 (4.0–8.0) | 6.0 (5.0–9.0) | 0.683 | 0.843 | 0.621 |
| left ovary | 6.0 (4.0–8.0) | 7.0 (5.0–8.0) | 5.0 (2.0–8.0) | 0.264 | 0.226 | 0.601 | |
| Infertility, % b | right ovary | 36.7(33) | 64.3 (9) | 36.8 (7) | 0.988 | 0.119 | 0.050 |
| left ovary | 63.3(57) | 35.7 (5) | 63.2 (12) | 0.988 | 0.119 | 0.050 | |
| PCOS, % b | 23.3 (21) | 50.0 (7) | 36.8 (7) | 0.221 | 0.449 | 0.036 * | |
| POF, % b | 12.2 (11) | 21.4 (3) | 21.2 (4) | 0.310 | 0.979 | 0.348 | |
| Endometriosis, % b | 16.7 (15) | 14.3 (2) | 36.8 (7) | 0.046 * | 0.150 | 0.822 | |
| Ovarian resection, % b | 18.9 (17) | 7.1 (1) | 0.0 (0) | 0.039 * | 0.237 | 0.279 | |
| Parameter | Groups of Oocytes | p-Value | |||||
|---|---|---|---|---|---|---|---|
| I (n = 90) | II (n = 14) | III (n = 19) | I/III | II/III | I/II | ||
| GnRH agonist, % | long protocol | 32.2 (n = 29) | 50.0 (n = 7) | 47.4 (n = 9) | 0.208 | 0.881 | 0.193 |
| short protocol | 67.8 (n = 61) | 50.0 (n = 7) | 52.6 (n = 10) | 0.208 | 0.881 | 0.193 | |
| Trigger hCG, % | manufacturer 1 | 70.0 (n = 63) | 71.4 (n = 10) | 68.4 (n = 13) | 0.892 | 0.853 | 0.913 |
| manufacturer 2 | 22.2 (n = 20) | 21.4 (n = 3) | 21.1 (n = 4) | 0.911 | 0.979 | 0.947 | |
| Trigger hCG, % | manufacturer 1 | 70.0 (n = 63) | 71.4 (n = 10) | 68.4 (n = 13) | 0.892 | 0.853 | 0.913 |
| manufacturer 2 | 22.2 (n = 20) | 21.4 (n = 3) | 21.1 (n = 4) | 0.911 | 0.979 | 0.947 | |
| Category | Groups of Oocytes | p-Value | ||||
|---|---|---|---|---|---|---|
| I | II | III | I | II | III | |
| E2, pg/mL * | 6522.0 (3804.0–11,680.0) | 8031.0 (5964.0–11,255.0) | 9161.5 (3461.0–12,708.0) | 0.773 | 0.694 | 0.233 |
| Number of follicles * | 14.0 (10.0–18.0) | 15.5 (14.0–16.0) | 16.0 (12.0–17.0) | 0.478 | 0.872 | 0.436 |
| Total number of oocytes * | 12.0 (8.0–17.0) | 14.0 (12.0–16.0) | 14.0 (12.0–16.0) | 0.453 | 0.733 | 0.562 |
| GV, % * | 27.3 (14.3–40.0) | 27.2 (16.7–35.7) | 28.6 (25.0–35.7) | 0.264 | 0.439 | 0.966 |
| MII, % * | 52.9 (40.0–64.7) | 52.8 (35.3–58.3) | 42.9 (31.3–58.3) | 0.170 | 0.627 | 0.627 |
| Good quality embryos D3, % * | 50.0 (40.0–80.0) | 80.0 (50.0–100.0) | 50.0 (40.0–80.0) | 0.821 | 0.163 | 0.05 |
| Good quality blastocysts D5, % * | 42.8 (30.0–63.6) | 40.0 (33.3–50.0) | 40.0 (33.3–63.1) | 0.805 | 1.000 | 0.717 |
| CPR, % ** | 41.1 | 28.6 | 47.4 | 0.616 | 0.275 | 0.372 |
| Parameter | Groups of Oocytes | p-Value | ||||
|---|---|---|---|---|---|---|
| I | II | III | I/III | II/III | I/II | |
| Do, µm | 114.0 (112–116) | 116.0 (113–119) | 112.0 (108–116) | 0.104 | 0.042 * | 0.216 |
| do, µm | 108.5 (105–112) | 111.0 (106–114) | 109.0 (105–112) | 0.620 | 0.461 | 0.199 |
| So, µm2 | 9671.5 (9324–10,203) | 10,117.2 (9676–10,423) | 9675.8 (8991–10,116) | 0.660 | 0.106 | 0.082 |
| D, µm | 111.0 (109–114) | 113.5 (111–115.5) | 111.0 (107–114) | 0.582 | 0.114 | 0.089 |
| ZPmin, µm | 13.0 (12–16) | 14.0 (10–15) | 14.0 (12–16) | 0.707 | 0.653 | 0.803 |
| ZPmax, µm | 18.0 (16–20) | 16.5 (15–21) | 19.0 (16–20) | 0.877 | 0.843 | 0.696 |
| SGV, µm2 | 699.6 (635–737) | 711.6 (624–766) | 784.6 (697–840) | 0.0001 * | 0.065 | 0.425 |
| DGV, µm | 29.8 (28.4–30.6) | 30.1 (28.2–31.2) | 31.6 (29.8–33) | 0.0001 * | 0.065 | 0.425 |
| Parameter | Groups of Oocytes | p-Value | ||||
|---|---|---|---|---|---|---|
| I | II | III | I/III | II/III | I/II | |
| TGVBD, min | 220.5 (106–454) | 1478.0 (551–3415) | N/A | N/A | N/A | 0.00001 * |
| TPB1ex, min | 995.0 (930–1077) | N/A | N/A | N/A | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salimov, D.; Lisovskaya, T.; Otsuki, J.; Gzgzyan, A.; Bogolyubova, I.; Bogolyubov, D. Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles. Cells 2023, 12, 1976. https://doi.org/10.3390/cells12151976
Salimov D, Lisovskaya T, Otsuki J, Gzgzyan A, Bogolyubova I, Bogolyubov D. Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles. Cells. 2023; 12(15):1976. https://doi.org/10.3390/cells12151976
Chicago/Turabian StyleSalimov, Daniil, Tatiana Lisovskaya, Junko Otsuki, Alexandre Gzgzyan, Irina Bogolyubova, and Dmitry Bogolyubov. 2023. "Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles" Cells 12, no. 15: 1976. https://doi.org/10.3390/cells12151976
APA StyleSalimov, D., Lisovskaya, T., Otsuki, J., Gzgzyan, A., Bogolyubova, I., & Bogolyubov, D. (2023). Chromatin Morphology in Human Germinal Vesicle Oocytes and Their Competence to Mature in Stimulated Cycles. Cells, 12(15), 1976. https://doi.org/10.3390/cells12151976







