Repeated Superovulation Accelerates Primordial Follicle Activation and Atresia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Collection of Oocytes, Granulosa Cells, Plasma, and Ovaries
2.3. ELISA
2.4. H & E Staining (Hematoxylin and Eosin Staining) and Follicle Counting
2.5. Total RNA Purification and Relative Quantitative Real-Time PCR (RT-qPCR)
2.6. Statistical Analysis
3. Results
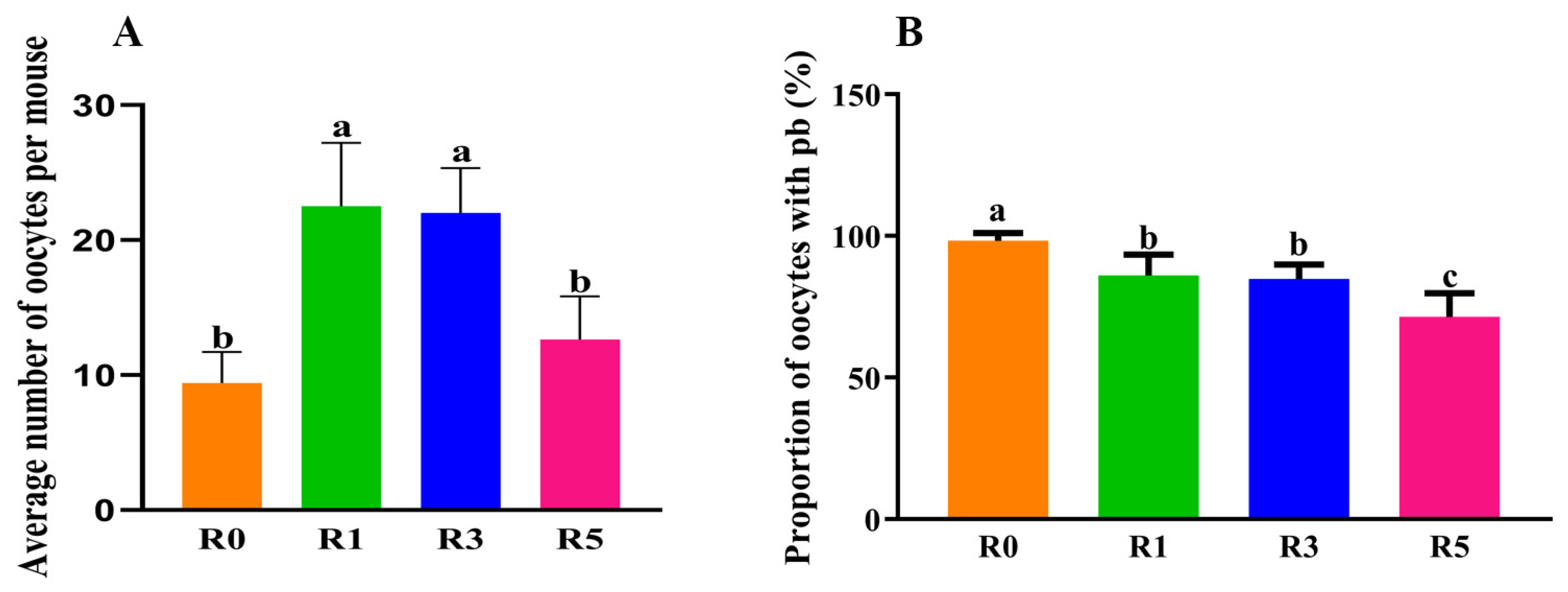
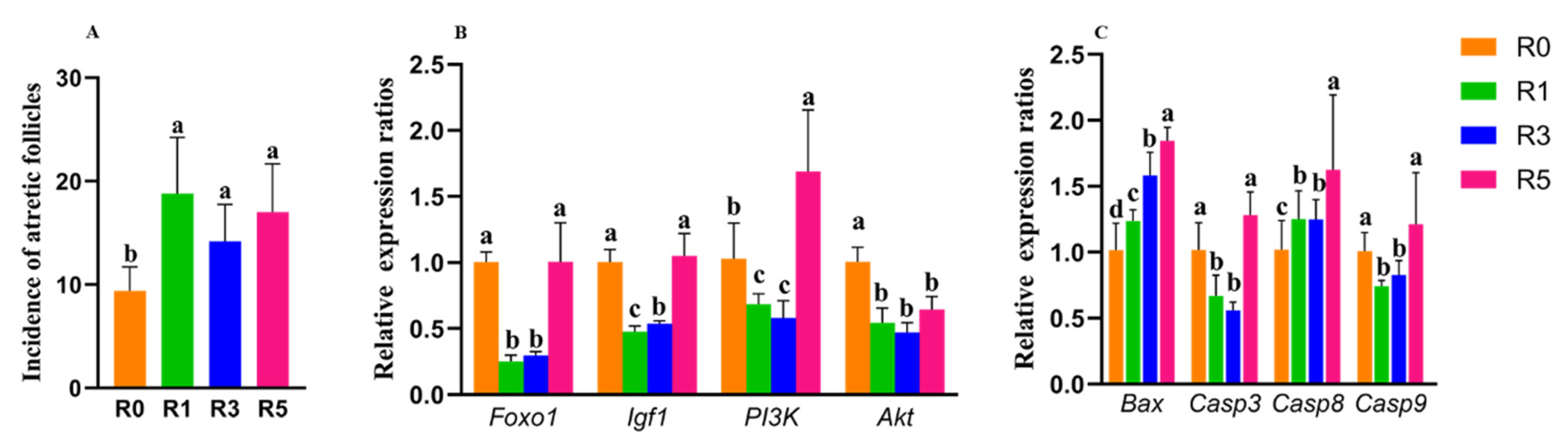
3.1. Repeated Superovulation Reduces the Oocyte Number
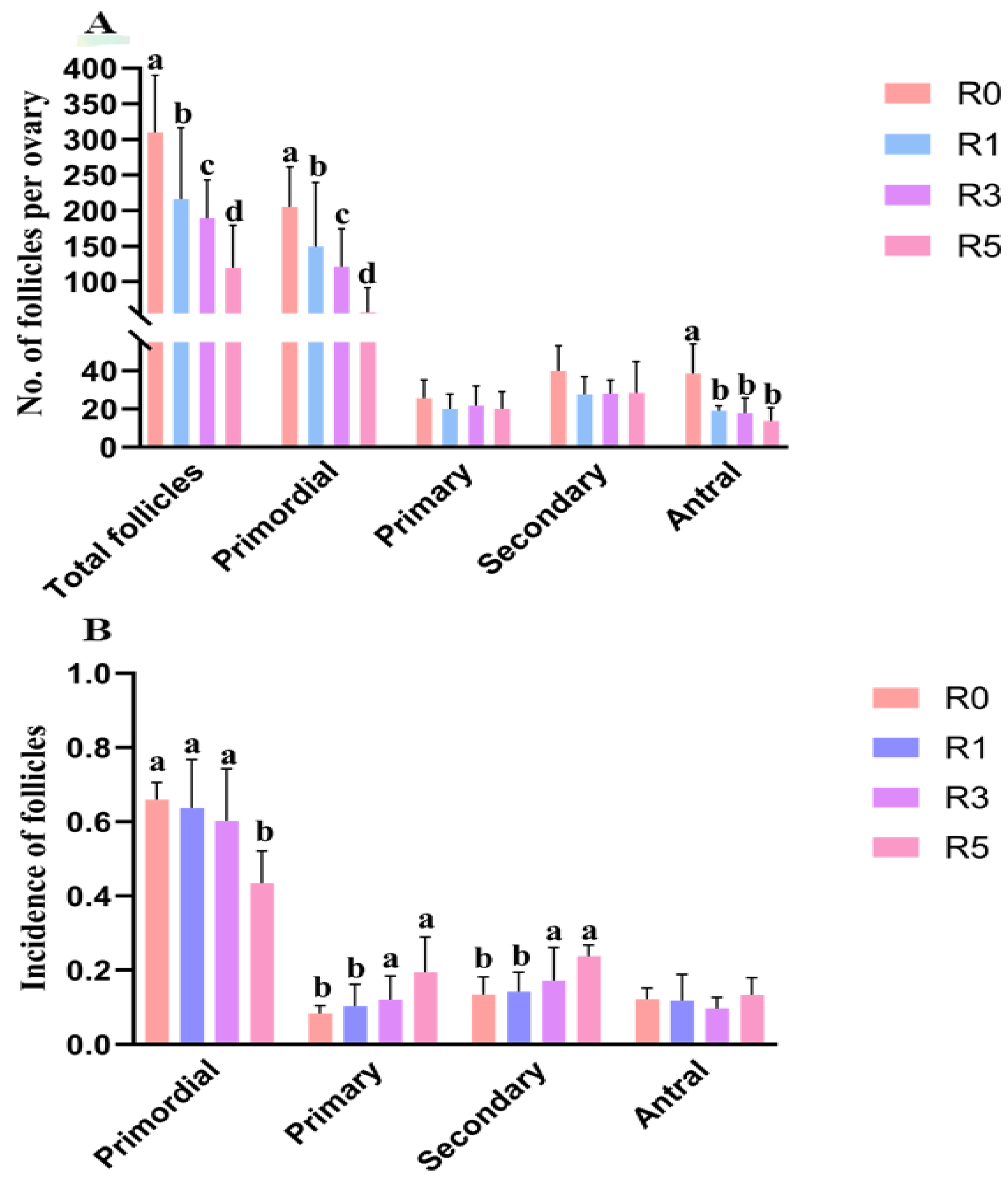
3.2. Follicular Development Is Impaired by Repeated Superovulation
3.3. Changes in Plasma Hormone Level May Be Involved in the Abnormality of Follicular Development
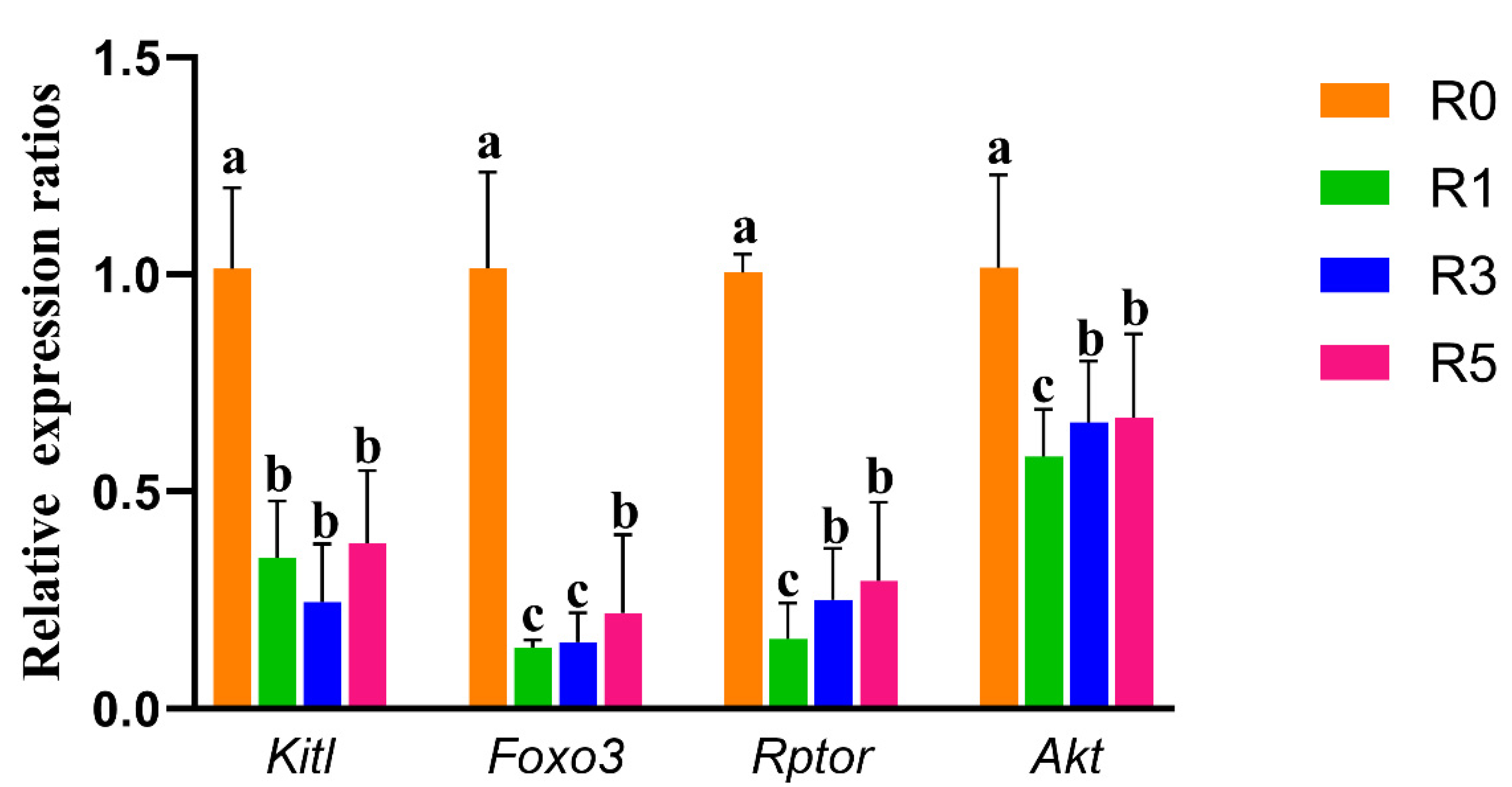
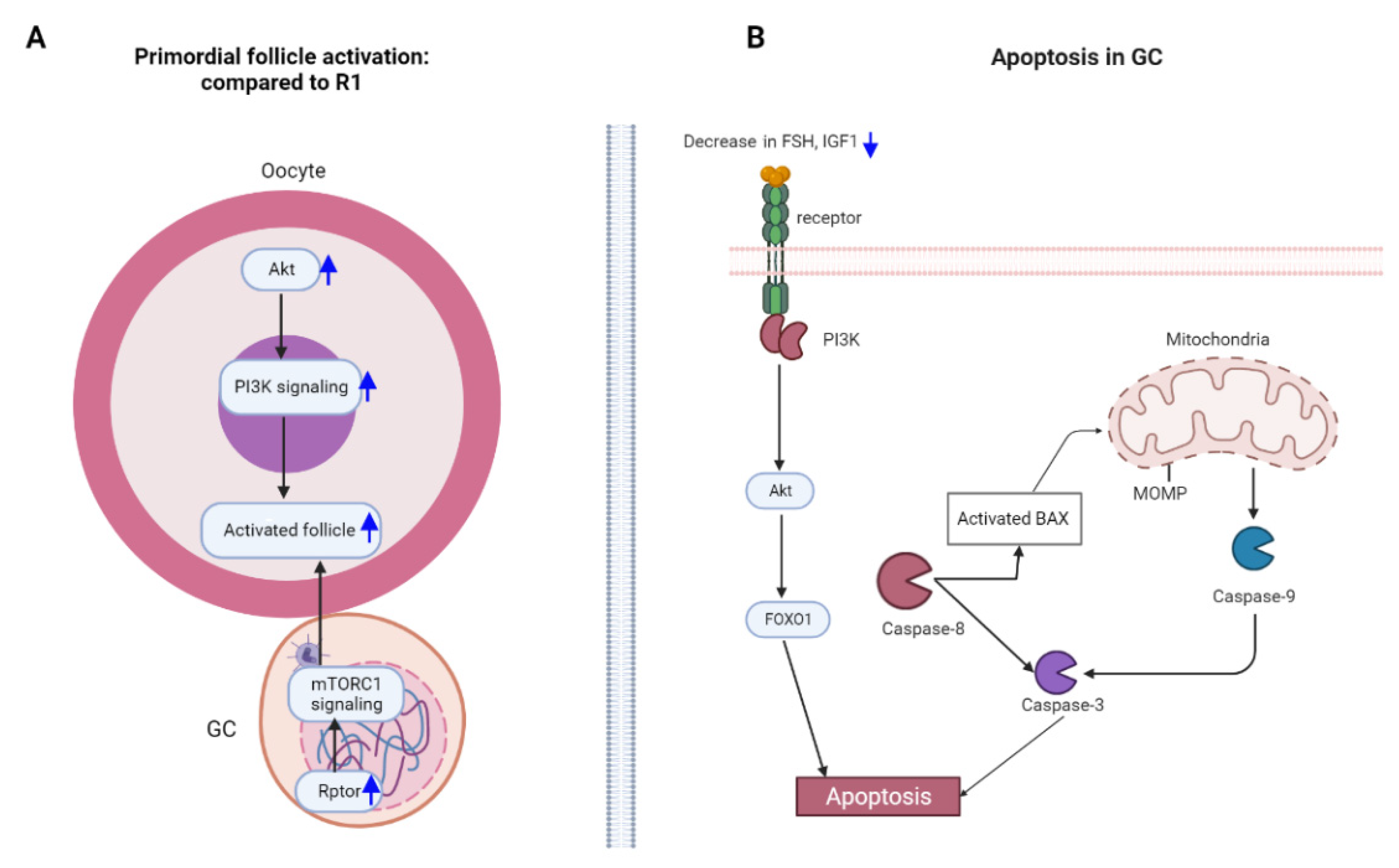
3.4. Repeated Superovulation May Accelerate Primordial Follicle Activation via PI3K-PTEN-AKT-FOXO3 and mTORC1 Signaling
3.5. Granulosa Cell Apoptosis Induced by Repeated Superovulation May Play a Role in the Abnormality of Follicular Development
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, G.E.; Ledger, W.L. In vitro fertilisation/intracytoplasmic sperm injection beyond 2020. BJOG 2019, 126, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Zhou, W.; Xi, B.; Ma, J.; Hu, J.; Fang, M.; Hu, K.; Qin, Y.; You, L.; Cao, Y.; et al. Increased risk of metabolic dysfunction in children conceived by assisted reproductive technology. Diabetologia 2020, 63, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, A.; Selman, H.; Valeri, C.; Napoletano, S.; Sbracia, M.; Antonini, G.; Biagiotti, G.; Pacchiarotti, A. Ovarian Stimulation Protocol in IVF: An Up-to-Date Review of the Literature. Curr. Pharm. Biotechnol. 2016, 17, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Lamas, S.; Carvalheira, J.; Gartner, F.; Amorim, I. C57BL/6J mouse superovulation: Schedule and age optimization to increase oocyte yield and reduce animal use. Zygote 2021, 29, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.B.; Yang, L.L.; Zhang, T.T.; Wang, Q.; Yin, S.; Luo, S.M.; Shen, W.; Ge, Z.J.; Sun, Q.Y. Multiple superovulations alter histone modifications in mouse early embryos. Reproduction 2019, 157, 511–523. [Google Scholar] [CrossRef]
- Liang, X.W.; Cui, X.S.; Sun, S.C.; Jin, Y.X.; Heo, Y.T.; Namgoong, S.; Kim, N.H. Superovulation induces defective methylation in line-1 retrotransposon elements in blastocyst. Reprod. Biol. Endocrinol. 2013, 11, 69. [Google Scholar] [CrossRef]
- Xiao, P.; Nie, J.; Wang, X.; Lu, K.; Lu, S.; Liang, X. Melatonin alleviates the deterioration of oocytes from mice subjected to repeated superovulation. J. Cell. Physiol. 2019, 234, 13413–13422. [Google Scholar] [CrossRef]
- Xie, J.K.; Wang, Q.; Zhang, T.T.; Yin, S.; Zhang, C.L.; Ge, Z.J. Repeated superovulation may affect mitochondrial functions of cumulus cells in mice. Sci. Rep. 2016, 6, 31368. [Google Scholar] [CrossRef]
- Dong, G.; Guo, Y.; Cao, H.; Zhou, T.; Zhou, Z.; Sha, J.; Guo, X.; Zhu, H. Long-term effects of repeated superovulation on ovarian structure and function in rhesus monkeys. Fertil. Steril. 2014, 102, 1452–1457.e1. [Google Scholar] [CrossRef]
- VandeVoort, C.A.; Tarantal, A.F. Recombinant human gonadotropins for macaque superovulation: Repeated stimulations and post-treatment pregnancies. J. Med. Primatol. 2001, 30, 304–307. [Google Scholar] [CrossRef]
- Magarey, G.M.; Rodger, J.C.; Buist, J.M.; Mate, K.E. Effects of repeated superovulation and surgical oocyte collection on ovarian response and natural breeding ability of the tammar wallaby (Macropus eugenii). Reproduction 2003, 125, 701–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, B.; Wang, J.L.; Wang, X.M.; Zhang, C.; Dai, J.G.; Huang, X.M.; Gao, J.M. Reparative effects of lycium barbarum polysaccharide on mouse ovarian injuries induced by repeated superovulation. Theriogenology 2020, 145, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Dai, Y.; Zheng, Y.; Bao, D.; Chen, Q.; Yin, Y.; Fu, H.; Hou, D. Establishment of a Mouse Model of Premature Ovarian Failure Using Consecutive Superovulation. Cell. Physiol. Biochem. 2018, 51, 2341–2358. [Google Scholar] [CrossRef] [PubMed]
- Salha, O.; Abusheikha, N.; Sharma, V. Dynamics of human follicular growth and in-vitro oocyte maturation. Hum. Reprod. Updat. 1998, 4, 816–832. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.T.; Lee, S.Y.; Lee, H.M.; Liao, T.L.; Wei, Y.H.; Kao, S.H. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann. N. Y. Acad. Sci. 2005, 1042, 148–156. [Google Scholar] [CrossRef]
- Writing Group, M.; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e60. [Google Scholar] [CrossRef]
- Kaplan, J.R.; Manuck, S.B. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause 2008, 15, 768–776. [Google Scholar] [CrossRef]
- Meczekalski, B.; Podfigurna-Stopa, A.; Genazzani, A.R. Hypoestrogenism in young women and its influence on bone mass density. Gynecol. Endocrinol. 2010, 26, 652–657. [Google Scholar] [CrossRef]
- Suh, B.Y.; Liu, J.H.; Berga, S.L.; Quigley, M.E.; Laughlin, G.A.; Yen, S.S. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J. Clin. Endocrinol. Metab. 1988, 66, 733–739. [Google Scholar] [CrossRef]
- Pauli, S.A.; Berga, S.L. Athletic amenorrhea: Energy deficit or psychogenic challenge? Ann. N. Y. Acad. Sci. 2010, 1205, 33–38. [Google Scholar] [CrossRef]
- Tang, G.Y.; Meng, X.; Gan, R.Y.; Zhao, C.N.; Liu, Q.; Feng, Y.B.; Li, S.; Wei, X.L.; Atanasov, A.G.; Corke, H.; et al. Health Functions and Related Molecular Mechanisms of Tea Components: An Update Review. Int. J. Mol. Sci. 2019, 20, 6196. [Google Scholar] [CrossRef]
- Ge, Z.J.; Luo, S.M.; Lin, F.; Liang, Q.X.; Huang, L.; Wei, Y.C.; Hou, Y.; Han, Z.M.; Schatten, H.; Sun, Q.Y. DNA methylation in oocytes and liver of female mice and their offspring: Effects of high-fat-diet-induced obesity. Environ. Health Perspect. 2014, 122, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Lin, F.; Zhang, J.; Tang, Y.; Chen, W.K.; Liu, H. Involvement of the up-regulated FoxO1 expression in follicular granulosa cell apoptosis induced by oxidative stress. J. Biol. Chem. 2012, 287, 25727–25740. [Google Scholar] [CrossRef]
- Tilly, J.L. Ovarian follicle counts--not as simple as 1, 2, 3. Reprod. Biol. Endocrinol. 2003, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Britt, K.L.; Wreford, N.G.; Ebling, F.J.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Mamo, S.; Gal, A.B.; Bodo, S.; Dinnyes, A. Quantitative evaluation and selection of reference genes in mouse oocytes and embryos cultured in vivo and in vitro. BMC Dev. Biol. 2007, 7, 14. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Mitteer, D.R.; Greer, B.D.; Fisher, W.W.; Cohrs, V.L. Teaching behavior technicians to create publication-quality, single-case design graphs in graphpad prism 7. J. Appl. Behav. Anal. 2018, 51, 998–1010. [Google Scholar] [CrossRef]
- Peters, H. The development of the mouse ovary from birth to maturity. Acta Endocrinol. (Copenh.) 1969, 62, 98–116. [Google Scholar] [CrossRef]
- Pankhurst, M.W. A putative role for anti-Mullerian hormone (AMH) in optimising ovarian reserve expenditure. J. Endocrinol. 2017, 233, R1–R13. [Google Scholar] [CrossRef]
- Durlinger, A.L.; Kramer, P.; Karels, B.; de Jong, F.H.; Uilenbroek, J.T.; Grootegoed, J.A.; Themmen, A.P. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 1999, 140, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.; Kushnir, V.; Ma, X.; Biswas, A.; Prizant, H.; Gleicher, N.; Sen, A. Intra-cellular mechanism of Anti-Mullerian hormone (AMH) in regulation of follicular development. Mol. Cell. Endocrinol. 2016, 433, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, R.; Sekii, K.; Morohaku, K.; Li, J.; Pepin, D.; Obata, Y. Blocking estrogen-induced AMH expression is crucial for normal follicle formation. Development 2021, 148. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, V.P.; Ghosh, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. A Gatekeeping Role of ESR2 to Maintain the Primordial Follicle Reserve. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Adhikari, D.; Liu, K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009, 30, 438–464. [Google Scholar] [CrossRef]
- Zhang, H.; Risal, S.; Gorre, N.; Busayavalasa, K.; Li, X.; Shen, Y.; Bosbach, B.; Brannstrom, M.; Liu, K. Somatic cells initiate primordial follicle activation and govern the development of dormant oocytes in mice. Curr. Biol. 2014, 24, 2501–2508. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K. Cellular and molecular regulation of the activation of mammalian primordial follicles: Somatic cells initiate follicle activation in adulthood. Hum. Reprod. Update 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Macklon, N.S.; Fauser, B.C. Follicle-stimulating hormone and advanced follicle development in the human. Arch. Med. Res. 2001, 32, 595–600. [Google Scholar] [CrossRef]
- Hillier, S.G. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum. Reprod. 1994, 9, 188–191. [Google Scholar] [CrossRef]
- Filatov, M.; Khramova, Y.; Parshina, E.; Bagaeva, T.; Semenova, M. Influence of gonadotropins on ovarian follicle growth and development in vivo and in vitro. Zygote 2017, 25, 235–243. [Google Scholar] [CrossRef]
- Liu, Y.X.; Hsueh, A.J. Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: Studies on the two-cell, two-gonadotropin hypothesis using steroid antisera. Biol. Reprod. 1986, 35, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Y.; Cheung, C.K.; Wang, Y.; Tsang, B.K. Regulation of cell death and cell survival gene expression during ovarian follicular development and atresia. Front. Biosci. 2003, 8, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Fortune, J.E.; Armstrong, D.T. Androgen production by theca and granulosa isolated from proestrous rat follicles. Endocrinology 1977, 100, 1341–1347. [Google Scholar] [CrossRef]
- Erickson, G.F.; Hsueh, A.J. Stimulation of aromatase activity by follicle stimulating hormone in rat granulosa cells in vivo and in vitro. Endocrinology 1978, 102, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Mihm, M.; Bleach, E.C. Endocrine regulation of ovarian antral follicle development in cattle. Anim. Reprod. Sci. 2003, 78, 217–237. [Google Scholar] [CrossRef]
- Gieske, M.C.; Kim, H.J.; Legan, S.J.; Koo, Y.; Krust, A.; Chambon, P.; Ko, C. Pituitary gonadotroph estrogen receptor-alpha is necessary for fertility in females. Endocrinology 2008, 149, 20–27. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Li, C.; Zhou, J.; Liu, Z.; Zhou, J.; Yao, W.; Tao, J.; Shen, M.; Liu, H. FSH prevents porcine granulosa cells from hypoxia-induced apoptosis via activating mitophagy through the HIF-1alpha-PINK1-Parkin pathway. FASEB J. 2020, 34, 3631–3645. [Google Scholar] [CrossRef]
- Zhou, G.; Hu, R.K.; Xia, G.C.; Yan, S.H.; Ren, Q.L.; Zhao, J.; Wang, F.H.; Huang, C.C.; Yao, Q.; Tan, Y.; et al. Tyrosine nitrations impaired intracellular trafficking of FSHR to the cell surface and FSH-induced Akt-FoxO3a signaling in human granulosa cells. Aging 2019, 11, 3094–3116. [Google Scholar] [CrossRef]
- Kosaka, N.; Sudo, N.; Miyamoto, A.; Shimizu, T. Vascular endothelial growth factor (VEGF) suppresses ovarian granulosa cell apoptosis in vitro. Biochem. Biophys. Res. Commun. 2007, 363, 733–737. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, S.; Fan, P.; Zhu, H.; Li, Y.; Huang, W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod. Biol. Endocrinol. 2020, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Guo, X.; Jia, K.; Jing, J.; Dang, W.; Li, Y.; Qin, X.; Li, P.; Ren, Y.; Liu, W.; et al. Effects of FOXO1 on the proliferation and cell cycle-, apoptosis- and steroidogenesis-related genes expression in sheep granulosa cells. Anim. Reprod. Sci. 2020, 221, 106604. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation mechanism of selective atresia in porcine follicles: Regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [PubMed]
- Orimoto, A.M.; Dumaresq-Doiron, K.; Jiang, J.Y.; Tanphaichitr, N.; Tsang, B.K.; Carmona, E. Mammalian hyaluronidase induces ovarian granulosa cell apoptosis and is involved in follicular atresia. Endocrinology 2008, 149, 5835–5847. [Google Scholar] [CrossRef]

| Genes | Sequence |
|---|---|
| Casp9-F | TCCTGGTACATCGAGACCTTG |
| Casp9-R | AAGTCCCTTTCGCAGAAACAG |
| Casp8-F | TGCTTGGACTACATCCCACAC |
| Casp8-R | TGCAGTCTAGGAAGTTGACCA |
| Casp3-F | TGGTGATGAAGGGGTCATTTATG |
| Casp3-R | TTCGGCTTTCCAGTCAGACTC |
| Bax-F | TGAAGACAGGGGCCTTTTTG |
| Bax-R | AATTCGCCGGAGACACTCG |
| Igf1-F | GTGAGCCAAAGACACACCCA |
| Igf1-R | ACCTCTGATTTTCCGAGTTGC |
| PI3k-F | ACACCACGGTTTGGACTATGG |
| PI3k-R | GGCTACAGTAGTGGGCTTGG |
| Akt-F | AGAAGAGACGATGGACTTCCG |
| Akt-R | TCAAACTCGTTCATGGTCACAC |
| Foxo1-F | GGGTCCCACAGCAACGATG |
| Foxo1-R | CACCAGGGAATGCACGTCC |
| Foxo3-F | CTGGGGGAACCTGTCCTATG |
| Foxo3-R | TCATTCTGAACGCGCATGAAG |
| Kitl-F | GAATCTCCGAAGAGGCCAGAA |
| Kitl-R | GCTGCAACAGGGGGTAACAT |
| Rptor-F | TTTGTCTACGACTGTTCCAATGC |
| Rptor-R | GCTACCTCTAGTTCCTGCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhao, S.-X.; He, J.-N.; Zhao, H.; Gu, B.-X.; Xie, J.-K.; Zhao, Y.-J.; Zhang, C.-L.; Ge, Z.-J. Repeated Superovulation Accelerates Primordial Follicle Activation and Atresia. Cells 2023, 12, 92. https://doi.org/10.3390/cells12010092
Wang Q, Zhao S-X, He J-N, Zhao H, Gu B-X, Xie J-K, Zhao Y-J, Zhang C-L, Ge Z-J. Repeated Superovulation Accelerates Primordial Follicle Activation and Atresia. Cells. 2023; 12(1):92. https://doi.org/10.3390/cells12010092
Chicago/Turabian StyleWang, Qian, Shu-Xian Zhao, Jian-Ning He, Hua Zhao, Bao-Xia Gu, Juan-Ke Xie, Yi-Jun Zhao, Cui-Lian Zhang, and Zhao-Jia Ge. 2023. "Repeated Superovulation Accelerates Primordial Follicle Activation and Atresia" Cells 12, no. 1: 92. https://doi.org/10.3390/cells12010092
APA StyleWang, Q., Zhao, S.-X., He, J.-N., Zhao, H., Gu, B.-X., Xie, J.-K., Zhao, Y.-J., Zhang, C.-L., & Ge, Z.-J. (2023). Repeated Superovulation Accelerates Primordial Follicle Activation and Atresia. Cells, 12(1), 92. https://doi.org/10.3390/cells12010092










