The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair
Abstract
1. Introduction
Skeletal Muscle Repair, Regeneration, and Adaptation
2. Muscle Stem Cells and Myogenesis
2.1. The Myogenic Program
2.2. The Role of Satellite Cells in Regeneration and Repair
2.3. Plasticity of Satellite Cells
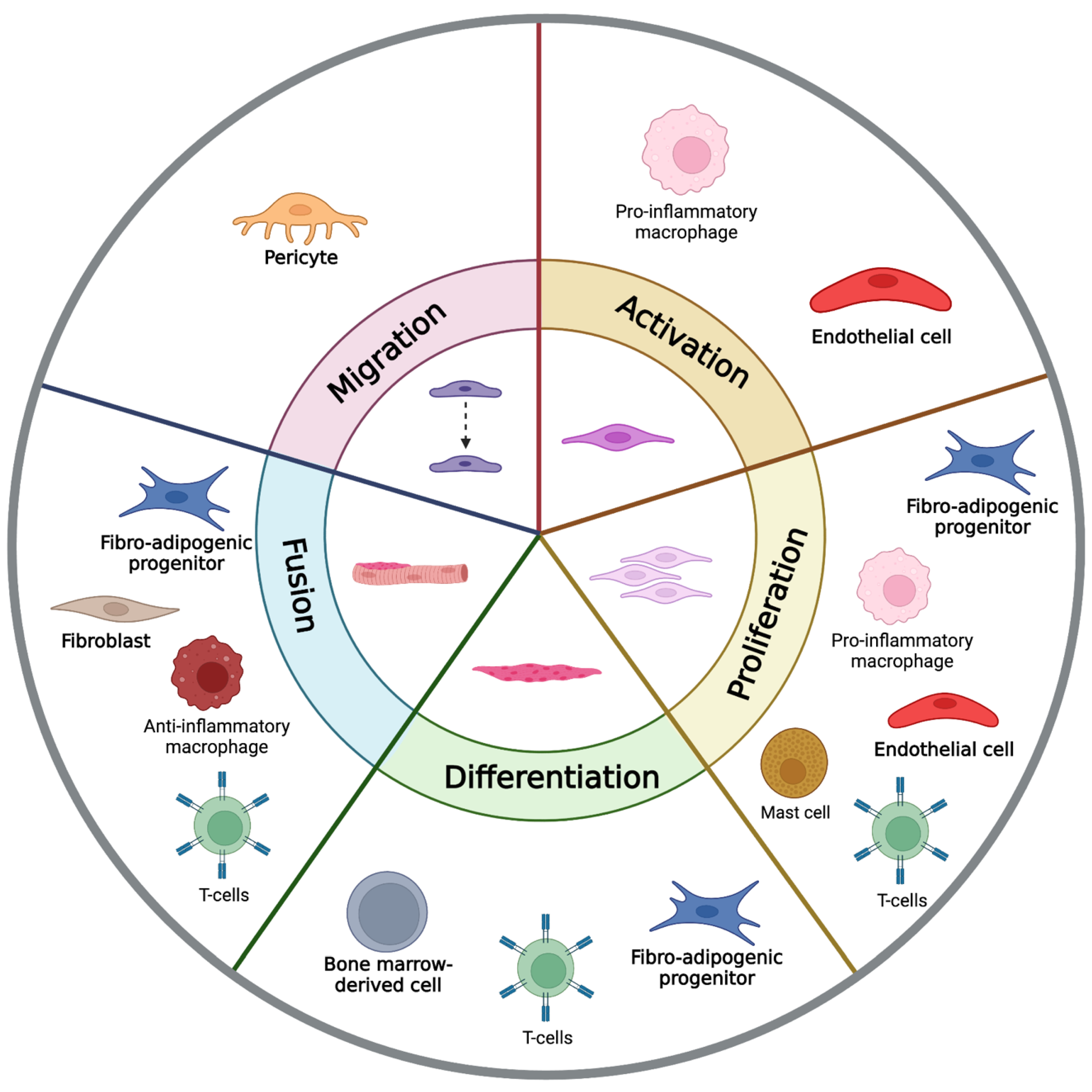
3. The Supporting Cells of Skeletal Muscle Regeneration
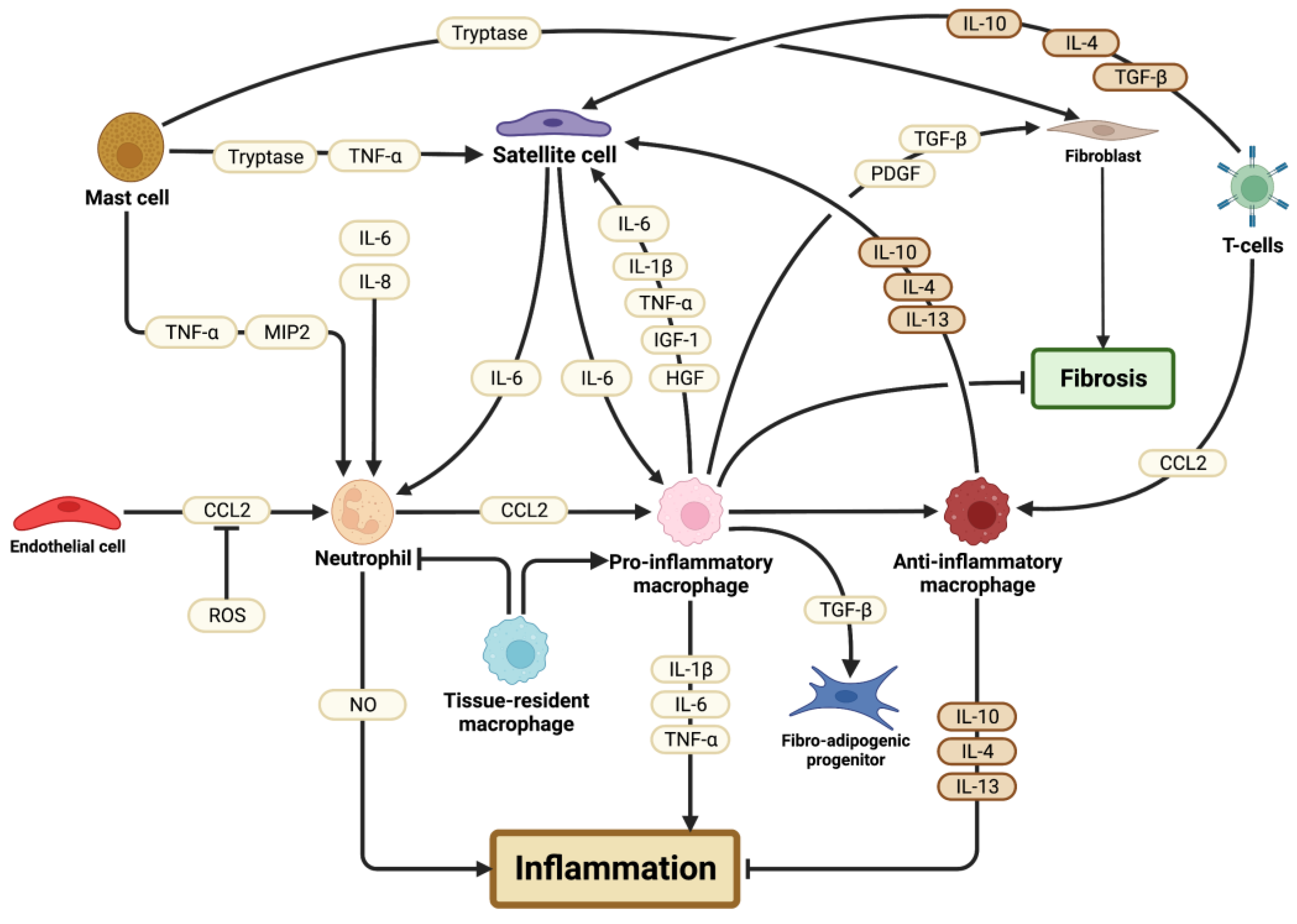
3.1. Immune Cells
3.1.1. Neutrophils
3.1.2. Mast Cells
3.1.3. Tissue-Resident Macrophages
3.1.4. Pro-Inflammatory Macrophages
3.1.5. Anti-Inflammatory Macrophages
3.1.6. T Lymphocytes
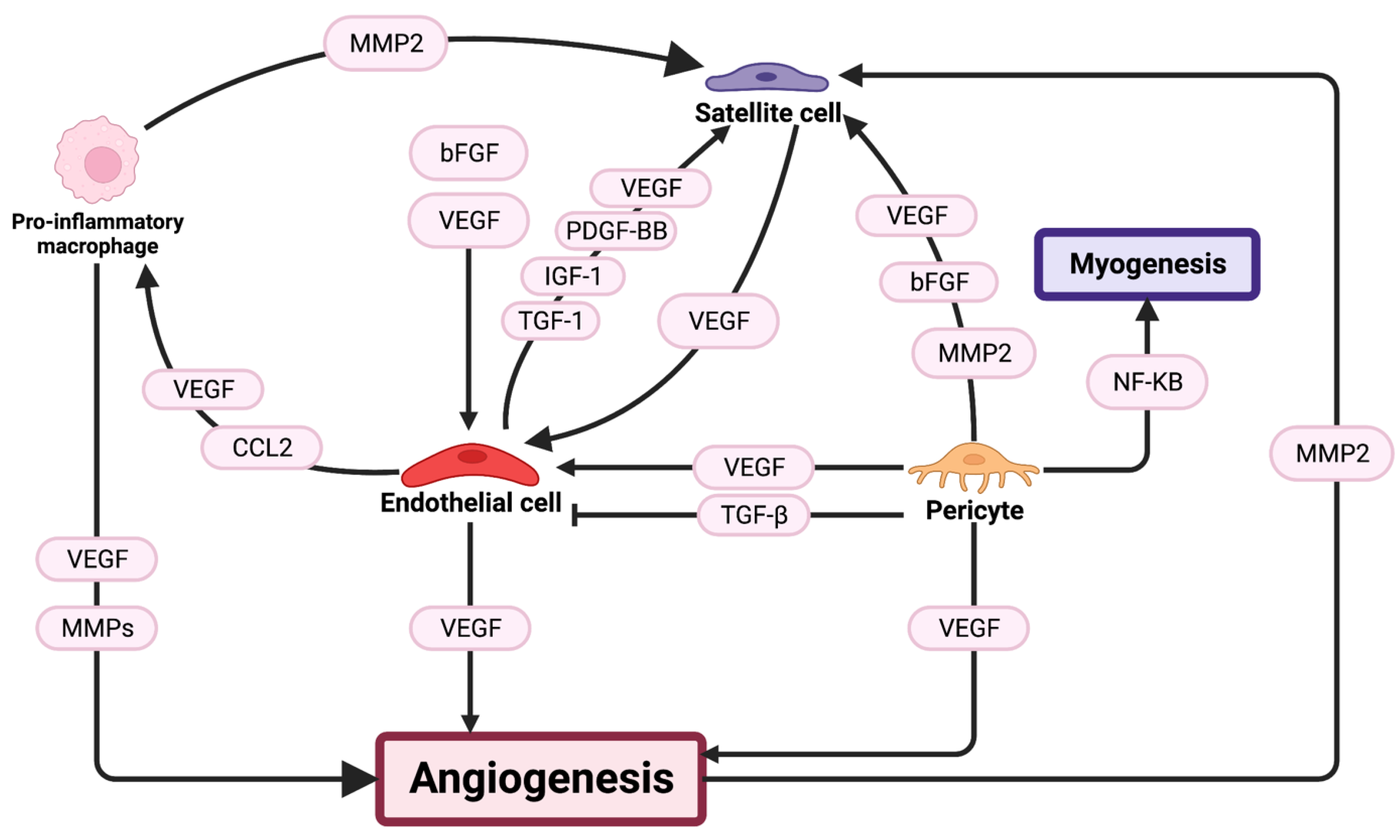
3.2. Angiogenesis
3.2.1. Capillaries
3.2.2. Pericytes
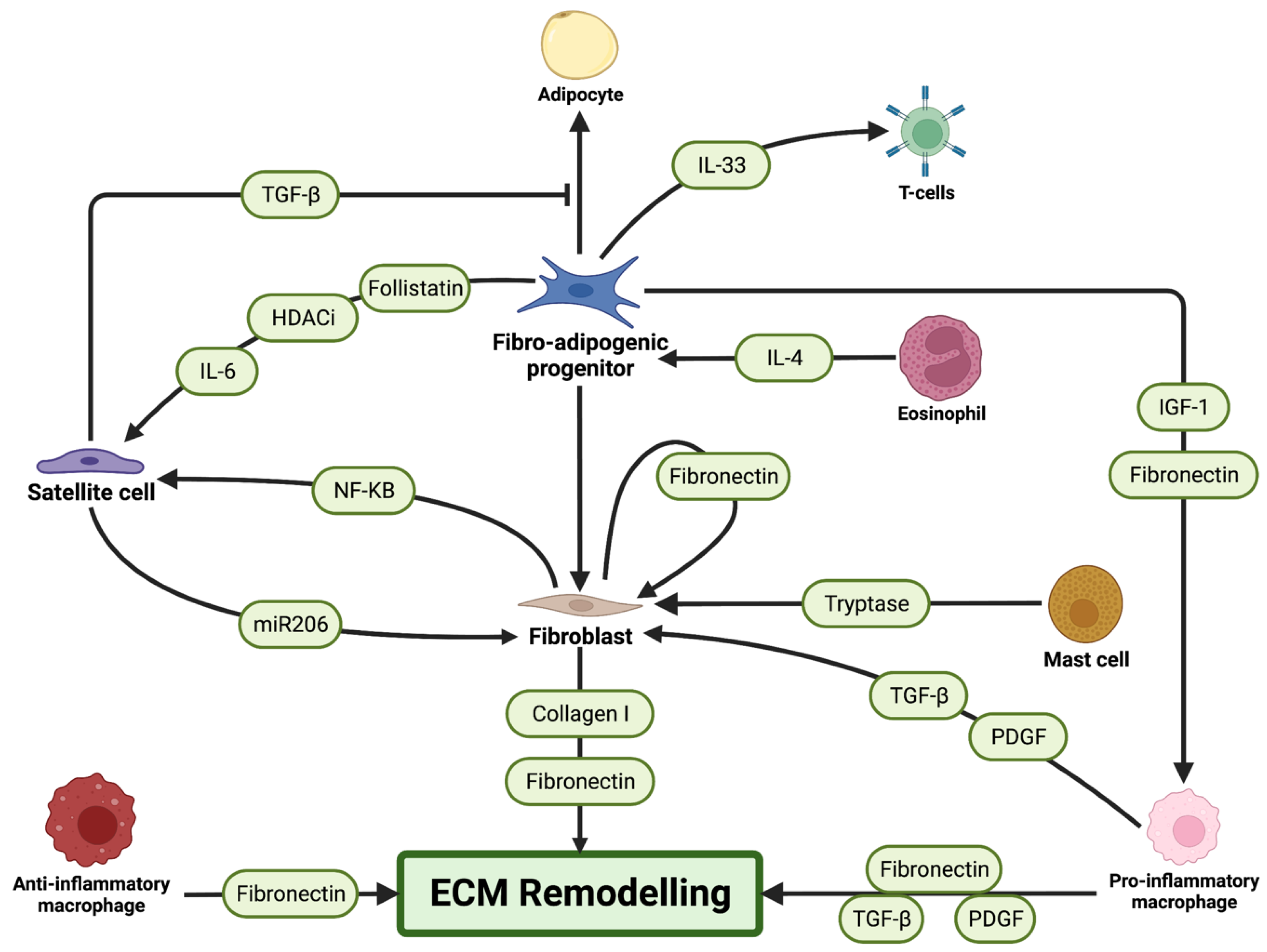
3.3. Fibrogenic Cells
3.3.1. Fibroblasts
3.3.2. Fibro-Adipogenic Progenitors
3.4. Bone-Marrow-Derived Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- Carlson, B.M. Regeneration of the Completely Excised Gastrocnemius Muscle in the Frog and Rat from Minced Muscle Fragments. J. Morphol. 1968, 125, 447–471. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem Cell Function, Self-Renewal, and Behavioral Heterogeneity of Cells from the Adult Muscle Satellite Cell Niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Mula, J.; Miyazaki, M.; Erfani, R.; Garrison, K.; Farooqui, A.B.; Srikuea, R.; Lawson, B.A.; Grimes, B.; Keller, C.; et al. Effective Fiber Hypertrophy in Satellite Cell-Depleted Skeletal Muscle. Development 2011, 138, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Khodabukus, A.; Rao, L.; Vandusen, K.; Abutaleb, N.; Bursac, N. Engineered Skeletal Muscles for Disease Modeling and Drug Discovery. Biomaterials 2019, 221, 119416. [Google Scholar] [CrossRef] [PubMed]
- Lund, D.K.; Mouly, V.; Cornelison, D. MMP-14 Is Necessary but Not Sufficient for Invasion of Three-Dimensional Collagen by Human Muscle Satellite Cells. Am. J. Physiol. Cell Physiol. 2014, 307, 140–149. [Google Scholar] [CrossRef]
- Contreras, O.; Rossi, F.M.; Brandan, E. Adherent Muscle Connective Tissue Fibroblasts Are Phenotypically and Biochemically Equivalent to Stromal Fibro/Adipogenic Progenitors. Matrix Biol. Plus 2019, 2, 100006. [Google Scholar] [CrossRef]
- Mathew, S.J.; Hansen, J.M.; Merrell, A.J.; Murphy, M.M.; Lawson, J.A.; Hutcheson, D.A.; Hansen, M.S.; Angus-Hill, M.; Kardon, G. Connective Tissue Fibroblasts and Tcf4 Regulate Myogenesis. Development 2011, 138, 371–384. [Google Scholar] [CrossRef]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and Adipogenesis Originate from a Common Mesenchymal Progenitor in Skeletal Muscle. J. Cell Sci. 2011, 124, 3654–3664. [Google Scholar] [CrossRef]
- Nassari, S.; Duprez, D.; Fournier-Thibault, C. Non-Myogenic Contribution to Muscle Development and Homeostasis: The Role of Connective Tissues. Front. Cell Dev. Biol. 2017, 5, 22. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pannérec, A.; Cadot, B.; Parlakian, A.; Besson, V.; Gomes, E.R.; Marazzi, G.; Sassoon, D.A. Identification and Characterization of a Non-Satellite Cell Muscle Resident Progenitor during Postnatal Development. Nat. Cell Biol. 2010, 12, 257–266. [Google Scholar] [CrossRef]
- Mitchell, P.O.; Mills, T.; O’Connor, R.S.; Graubert, T.; Dzierzak, E.; Pavlath, G.K. Sca-1 Negatively Regulates Proliferation and Differentiation of Muscle Cells. Dev. Biol. 2005, 283, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Mcgeachie, J.K.; Grounds, M.D.; Mcgeachie2, J.K.; Grounds’, M.D. The Exogenous Administration of Basic Fibroblast Growth Factor to Regenerating Skeletal Muscle in Mice Does Not Enhance the Process of Regeneration. Growth Factors 2009, 13, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Natarajan, A.; Le Grand, F.; So, L.; Wang, J.; Rudnicki, M.A.; Rossi, F.M.V. Muscle Injury Activates Resident Fibro/Adipogenic Progenitors That Facilitate Myogenesis. Nat. Cell Biol. 2010, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Mozzetta, C.; Consalvi, S.; Saccone, V.; Tierney, M.; Diamantini, A.; Mitchell, K.J.; Marazzi, G.; Borsellino, G.; Battistini, L.; Sassoon, D.; et al. Fibroadipogenic Progenitors Mediate the Ability of HDAC Inhibitors to Promote Regeneration in Dystrophic Muscles of Young, but Not Old Mdx Mice. EMBO Mol. Med. 2013, 5, 626–639. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.I.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal Progenitors Distinct from Satellite Cells Contribute to Ectopic Fat Cell Formation in Skeletal Muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming Growth Factor-Β1 Induces the Differentiation of Myogenic Cells into Fibrotic Cells in Injured Skeletal Muscle: A Key Event in Muscle Fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Dellavalle, A.; Maroli, G.; Covarello, D.; Azzoni, E.; Innocenzi, A.; Perani, L.; Antonini, S.; Sambasivan, R.; Brunelli, S.; Tajbakhsh, S.; et al. Pericytes Resident in Postnatal Skeletal Muscle Differentiate into Muscle Fibres and Generate Satellite Cells. Nat. Commun. 2011, 2, 499. [Google Scholar] [CrossRef]
- Dellavalle, A.; Sampaolesi, M.; Tonlorenzi, R.; Tagliafico, E.; Sacchetti, B.; Perani, L.; Innocenzi, A.; Galvez, B.G.; Messina, G.; Morosetti, R.; et al. Pericytes of Human Skeletal Muscle Are Myogenic Precursors Distinct from Satellite Cells. Nat. Cell Biol. 2007, 9, 255–267. [Google Scholar] [CrossRef]
- Park, T.S.; Gavina, M.; Chen, C.-W.; Sun, B.; Teng, P.-N.; Huard, J.; Deasy, B.M.; Zimmerlin, L.; Péault, B. Placental Perivascular Cells for Human Muscle Regeneration. Stem Cells Dev. 2011, 20, 451–463. [Google Scholar] [CrossRef]
- Dvoretskiy, S.; Garg, K.; Munroe, M.; Pincu, Y.; Mahmassani, Z.S.; Coombs, C.; Blackwell, B.; Garcia, G.; Waterstradt, G.; Lee, I.; et al. The Impact of Skeletal Muscle Contraction on CD146+Lin- Pericytes. Am. J. Physiol. Cell Physiol. 2019, 317, C1011–C1024. [Google Scholar] [CrossRef]
- Nehls, V.; Drenckhahn, D. Heterogeneity of Microvascular Pericytes for Smooth Muscle Type Alpha-Actin. J. Cell Biol. 1991, 113, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- De Micheli, A.J.; Laurilliard, E.J.; Heinke, C.L.; Ravichandran, H.; Fraczek, P.; Soueid-Baumgarten, S.; De Vlaminck, I.; Elemento, O.; Cosgrove, B.D. Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration. Cell Rep. 2020, 30, 3583–3595.e5. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.S.; Niccoli, S.; Hawdon, N.; Sarvas, J.L.; Frye, M.A. Pro-Inflammatory Mediation of Myoblast Proliferation. PLoS ONE 2014, 9, e92363. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M.; Faulkner, J.A. The Regeneration of Skeletal Muscle Fibers Following Injury: A Review. Med. Sci. Sports Exerc. 1983, 15, 187–198. [Google Scholar] [CrossRef]
- Kuang, S.; Kuroda, K.; Le Grand, F.; Rudnicki, M.A. Asymmetric Self-Renewal and Commitment of Satellite Stem Cells in Muscle. Cell 2007, 129, 999–1010. [Google Scholar] [CrossRef]
- Zammit, P.S.; Heslop, L.; Hudon, V.; Rosenblatt, J.D.; Tajbakhsh, S.; Buckingham, M.E.; Beauchamp, J.R.; Partridge, T.A. Kinetics of Myoblast Proliferation Show That Resident Satellite Cells Are Competent to Fully Regenerate Skeletal Muscle Fibers. Exp. Cell Res. 2002, 281, 39–49. [Google Scholar] [CrossRef]
- Kardon, G.; Harfe, B.D.; Tabin, C.J. A Tcf4-Positive Mesodermal Population Provides a Prepattern for Vertebrate Limb Muscle Patterning. Dev. Cell 2003, 5, 937–944. [Google Scholar] [CrossRef]
- Moss, F.P.; Leblond, C.P. Satellite Cells as the Source of Nuclei in Muscles of Growing Rats. Anat. Rec. 1971, 170, 421–435. [Google Scholar] [CrossRef]
- Knappe, S.; Zammit, P.S.; Knight, R.D. A Population of Pax7-Expressing Muscle Progenitor Cells Show Differential Responses to Muscle Injury Dependent on Developmental Stage and Injury Extent. Front. Aging Neurosci. 2015, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Carlson, B.M.; Gutmann, E. Regeneration in Free Grafts of Normal and Denervated Muscles in the Rat: Morphology and Histochemistry. Anat. Rec. 1975, 183, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Studitsky, A.N. Free Auto- and Homografts of Muscle Tissue in Experiments on Animals. Ann. N. Y. Acad. Sci. 2006, 120, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Lepper, C.; Partridge, T.A.; Fan, C.-M. An Absolute Requirement for Pax7-Positive Satellite Cells in Acute Injury-Induced Skeletal Muscle Regeneration. Development 2011, 138, 3639–3646. [Google Scholar] [CrossRef]
- Sambasivan, R.; Yao, R.; Kissenpfennig, A.; van Wittenberghe, L.; Paldi, A.; Gayraud-Morel, B.; Guenou, H.; Malissen, B.; Tajbakhsh, S.; Galy, A. Pax7-Expressing Satellite Cells Are Indispensable for Adult Skeletal Muscle Regeneration. Development 2011, 138, 3647–3656. [Google Scholar] [CrossRef]
- Shefer, G.; Wleklinski-Lee, M.; Yablonka-Reuveni, Z. Skeletal Muscle Satellite Cells Can Spontaneously Enter an Alternative Mesenchymal Pathway. J. Cell Sci. 2004, 117, 5393–5404. [Google Scholar] [CrossRef]
- Asakura, A.; Rudnicki, M.A.; Komaki, M. Muscle Satellite Cells Are Multipotential Stem Cells That Exhibit Myogenic, Osteogenic, and Adipogenic Differentiation. Differentiation 2001, 68, 245–253. [Google Scholar] [CrossRef]
- Wada, M.R.; Inagawa-Ogashiwa, M.; Shimizu, S.; Yasumoto, S.; Hashimoto, N. Generation of Different Fates from Multipotent Muscle Stem Cells. Development 2002, 129, 2987–2995. [Google Scholar] [CrossRef]
- Katagiri, T. Bone Morphogenetic Protein-2 Converts the Differentiation Pathway of C2C12 Myoblasts into the Osteoblast Lineage. J. Cell Biol. 1994, 127, 1755–1766, Erratum in: J. Cell Biol. 1995, 128, 13. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.; Choi, K.; Son, Y.; Lee, K.; Hwang, I.; Lee, H.; Chang, J.; Choi, I.; Lee, J.C. Satellite Cells Isolated from Adult Hanwoo Muscle Can Proliferate and Differentiate into Myoblasts and Adipose-like Cells. Mol. Cells 2006, 22, 239–245. [Google Scholar]
- De Coppi, P.; Milan, G.; Scarda, A.; Boldrin, L.; Centobene, C.; Piccoli, M.; Pozzobon, M.; Pilon, C.; Pagano, C.; Gamba, P.; et al. Rosiglitazone Modifies the Adipogenic Potential of Human Muscle Satellite Cells. Diabetologia 2006, 49, 1962–1973. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A Temporal Switch from Notch to Wnt Signaling in Muscle Stem Cells Is Necessary for Normal Adult Myogenesis. Cell Stem Cell 2008, 2, 50–59. [Google Scholar] [CrossRef]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt Signaling During Aging Alters Muscle Stem Cell Fate and Increases Fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Seale, P.; Girgis-Gabardo, A.; Rudnicki, M.A. Myogenic Specification of Side Population Cells in Skeletal Muscle. J. Cell Biol. 2002, 159, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute Phase Response in Exercise. III. Neutrophil and IL-1 Beta Accumulation in Skeletal Muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially Activated Macrophages Orchestrate Myogenic Precursor Cell Fate during Human Skeletal Muscle Regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef]
- McLennan, I.S. Degenerating and Regenerating Skeletal Muscles Contain Several Subpopulations of Macrophages with Distinct Spatial and Temporal Distributions. J. Anat. 1996, 188, 17–28. [Google Scholar]
- Bencze, M.; Negroni, E.; Vallese, D.; Yacoubyoussef, H.; Chaouch, S.; Wolff, A.; Aamiri, A.; Di Santo, J.P.; Chazaud, B.; Butler-Browne, G.; et al. Proinflammatory Macrophages Enhance the Regenerative Capacity of Human Myoblasts by Modifying Their Kinetics of Proliferation and Differentiation. Mol. Ther. 2012, 20, 2168–2179. [Google Scholar] [CrossRef]
- Burzyn, D.; Kuswanto, W.; Kolodin, D.; Shadrach, J.L.; Cerletti, M.; Jang, Y.; Sefik, E.; Tan, T.G.; Wagers, A.J.; Benoist, C.; et al. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 2013, 155, 1282–1295. [Google Scholar] [CrossRef]
- Kuswanto, W.; Burzyn, D.; Panduro, M.; Wang, K.K.; Jang, Y.C.; Wagers, A.J.; Benoist, C.; Mathis, D. Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 2016, 44, 355–367. [Google Scholar] [CrossRef]
- Parkin, J.; Cohen, B. An Overview of the Immune System. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Yatim, K.; Lakkis, F. A Brief Journey through the Immune System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory Interactions between Muscle and the Immune System during Muscle Regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages and Dendritic Cells. Science 2010, 327, 656. [Google Scholar] [CrossRef]
- Peake, J.M.; Suzuki, K.; Wilson, G.; Hordern, M.; Nosaka, K.; MacKinnon, L.; Coombes, J.S. Exercise-Induced Muscle Damage, Plasma Cytokines, and Markers of Neutrophil Activation. Med. Sci. Sports Exerc. 2005, 37, 737–745. [Google Scholar] [CrossRef]
- Nunes-Silva, A.; Bernardes, P.T.T.; Rezende, B.M.; Lopes, F.; Gomes, E.C.; Marques, P.E.; Lima, P.M.A.; Coimbra, C.C.; Menezes, G.B.; Teixeira, M.M.; et al. Treadmill Exercise Induces Neutrophil Recruitment into Muscle Tissue in a Reactive Oxygen Species-Dependent Manner. An Intravital Microscopy Study. PLoS ONE 2014, 9, e96464. [Google Scholar] [CrossRef]
- Toumi, H.; F’guyer, S.; Best, T.M. The Role of Neutrophils in Injury and Repair Following Muscle Stretch. J. Anat. 2006, 208, 459–470. [Google Scholar] [CrossRef]
- Tidball, J.G.; Wehling-Henricks, M. Macrophages Promote Muscle Membrane Repair and Muscle Fibre Growth and Regeneration during Modified Muscle Loading in Mice in vivo. J. Physiol. 2007, 578, 327–336. [Google Scholar] [CrossRef]
- Dumont, N.; Bouchard, P.; Frenette, J. Neutrophil-Induced Skeletal Muscle Damage: A Calculated and Controlled Response Following Hindlimb Unloading and Reloading. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1831–R1838. [Google Scholar] [CrossRef]
- Chen, S.-E.; Jin, B.; Li, Y.-P. TNF-α Regulates Myogenesis and Muscle Regeneration by Activating P38 MAPK. Am. J. Physiol. Cell Physiol. 2007, 292, C1660–C1671. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y. Role of Matrix Metalloproteinases in Skeletal Muscle. Cell Adh. Migr. 2009, 3, 337–341. [Google Scholar] [CrossRef]
- Côťe, C.H.; Tremblay, M.H.; Duchesne, É.; Lapoite, B.M. Inflammation-Induced Leukocyte Accumulation in Injured Skeletal Muscle: Role of Mast Cells. Muscle Nerve 2008, 37, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Duchesne, E.; Tremblay, M.-H.; Côté, C.H. Mast Cell Tryptase Stimulates Myoblast Proliferation; a Mechanism Relying on Protease-Activated Receptor-2 and Cyclooxygenase-2. BMC Musculoskelet. Disord. 2011, 12, 235. [Google Scholar] [CrossRef]
- Cairns, J.A.; Walls, A.F. Mast Cell Tryptase Is a Mitogen for Epithelial Cells. Stimulation of IL-8 Production and Intercellular Adhesion Molecule-1 Expression. J. Immunol. 1996, 156, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.S.; Black, J.L.; Poronnik, P.; Johnson, P.R.A. Functional Effects of Protease-Activated Receptor-2 Stimulation on Human Airway Smooth Muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L1369–L1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thorlacius, H. Mast Cell-Derived Tumour Necrosis Factor-α Mediates Macrophage Inflammatory Protein-2-Induced Recruitment of Neutrophils in Mice. Br. J. Pharmacol. 2005, 145, 1062–1068. [Google Scholar] [CrossRef]
- Abe, M.; Kurosawa, M.; Ishikawa, O.; Miyachi, Y. Effect of Mast Cell–Derived Mediators and Mast Cell–Related Neutral Proteases on Human Dermal Fibroblast Proliferation and Type I Collagen Production. J. Allergy Clin. Immunol. 2000, 106, S78–S84. [Google Scholar] [CrossRef]
- Heredia, J.E.; Mukundan, L.; Chen, F.M.; Mueller, A.A.; Deo, R.C.; Locksley, R.M.; Rando, T.A.; Chawla, A. Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration. Cell 2013, 153, 376. [Google Scholar] [CrossRef]
- Hao, D.; Becker, N.; Mückter, E.; Müller, A.; Pishnamaz, M.; Bollheimer, L.C.; Hildebrand, F.; Nourbakhsh, M. In Vitro Model of Human Skeletal Muscle Tissue for the Study of Resident Macrophages and Stem Cells. Biology 2022, 11, 936. [Google Scholar] [CrossRef]
- Uderhardt, S.; Martins, A.J.; Tsang, J.S.; Lämmermann, T.; Germain, R.N. Resident macrophages cloak tissue microlesions to prevent neutrophil-driven inflammatory damage. Cell 2019, 177, 541. [Google Scholar] [CrossRef] [PubMed]
- Babaeijandaghi, F.; Cheng, R.; Kajabadi, N.; Soliman, H.; Chang, C.K.; Smandych, J.; Tung, L.W.; Long, R.; Ghassemi, A.; Rossi, F.M.V. Metabolic Reprogramming of Skeletal Muscle by Resident Macrophages Points to CSF1R Inhibitors as Muscular Dystrophy Therapeutics. Sci. Transl. Med. 2022, 14, eabg7504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sathe, A.A.; Smith, G.R.; Ruf-Zamojski, F.; Nair, V.; Lavine, K.J.; Xing, C.; Sealfon, S.C.; Zhou, L. Heterogeneous Origins and Functions of Mouse Skeletal Muscle-Resident Macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 20729–20740. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, O.; Sun, D.; Reyes-Reyna, S.M.; Waite, L.L.; Michalek, J.E.; McManus, L.M.; Shireman, P.K. Delayed Angiogenesis and VEGF Production in CCR2−/− Mice during Impaired Skeletal Muscle Regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R651–R661. [Google Scholar] [CrossRef]
- Lu, H.; Huang, D.; Ransohoff, R.M.; Zhou, L. Acute Skeletal Muscle Injury: CCL2 Expression by Both Monocytes and Injured Muscle Is Required for Repair. FASEB J. 2011, 25, 3344–3355. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. Interleukin-10 Triggers Changes in Macrophage Phenotype That Promote Muscle Growth and Regeneration. J. Immunol. 2012, 189, 3669. [Google Scholar] [CrossRef]
- Runyan, C.E.; Welch, L.C.; Lecuona, E.; Shigemura, M.; Amarelle, L.; Abdala-Valencia, H.; Joshi, N.; Lu, Z.; Nam, K.; Markov, N.S.; et al. Impaired Phagocytic Function in CX3CR1+ Tissue-Resident Skeletal Muscle Macrophages Prevents Muscle Recovery after Influenza A Virus-Induced Pneumonia in Old Mice. Aging Cell 2020, 19, e13180. [Google Scholar] [CrossRef]
- Ahmadi, M.; Karlsen, A.; Mehling, J.; Soendenbroe, C.; Mackey, A.L.; Hyldahl, R.D. Aging Is Associated with an Altered Macrophage Response during Human Skeletal Muscle Regeneration. Exp. Gerontol. 2022, 169, 111974. [Google Scholar] [CrossRef]
- Chazaud, B.; Sonnet, C.; Lafuste, P.; Bassez, G.; Rimaniol, A.C.; Poron, F.; Authier, F.J.; Dreyfus, P.A.; Gherardi, R.K. Satellite Cells Attract Monocytes and Use Macrophages as a Support to Escape Apoptosis and Enhance Muscle Growth. J. Cell Biol. 2003, 163, 1133. [Google Scholar] [CrossRef]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered Macrophage Phenotype Transition Impairs Skeletal Muscle Regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef]
- Kasemkijwattana, C.; Bosch, P.; Menetrey, J.; Somogyi, G.; Moreland, M.S.; Fu, F.H.; Buranapanitkit, B.; Watkins, S.S.; Huard, J. Use of Growth Factors to Improve Muscle Healing after Strain Injury. Clin. Orthop. Relat. Res. 2000, 370, 272–285. [Google Scholar] [CrossRef]
- Massimino, M.L.; Rapizzi, E.; Cantini, M.; Libera, L.D.; Mazzoleni, F.; Arslan, P.; Carraro, U. ED2+ Macrophages Increase Selectively Myoblast Proliferation in Muscle Cultures. Biochem. Biophys. Res. Commun. 1997, 235, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Merly, F.; Lescaudron, L.; Rouaud, T.; Crossin, F.; Gardahaut, M.F. Macrophages Enhance Muscle Satellite Cell Proliferation and Delay Their Differentiation. Muscle Nerve 1999, 22, 724–732. [Google Scholar] [CrossRef]
- Robertson, T.A.; Maley, M.A.L.; Grounds, M.D.; Papadimitriou, J.M. The Role of Macrophages in Skeletal Muscle Regeneration with Particular Reference to Chemotaxis. Exp. Cell Res. 1993, 207, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- McKay, B.R.; De Lisio, M.; Johnston, A.P.W.; O’Reilly, C.E.; Phillips, S.M.; Tarnopolsky, M.A.; Parise, G. Association of Interleukin-6 Signalling with the Muscle Stem Cell Response Following Muscle-Lengthening Contractions in Humans. PLoS ONE 2009, 4, e6027. [Google Scholar] [CrossRef]
- Cantini, M.; Massimino, M.L.; Rapizzi, E.; Rossini, K.; Catani, C.; Libera, L.D.; Carraro, U. Human Satellite Cell-Proliferation in vitro Is Regulated by Autocrine Secretion of IL-6 Stimulated by a Soluble Factor(s) Released by Activated Monocytes. Biochem. Biophys. Res. Commun. 1995, 216, 49–53. [Google Scholar] [CrossRef]
- Cantini, M.; Giurisato, E.; Radu, C.; Tiozzo, S.; Pampinella, F.; Senigaglia, D.; Zaniolo, G.; Mazzoleni, F.; Vitiello, L. Macrophage-Secreted Myogenic Factors: A Promising Tool for Greatly Enhancing the Proliferative Capacity of Myoblasts in vitro and in vivo. Neurol. Sci. 2002, 23, 189–194. [Google Scholar] [CrossRef]
- Allen, R.E.; Boxhorn, L.K. Regulation of Skeletal Muscle Satellite Cell Proliferation and Differentiation by Transforming Growth Factor-Beta, Insulin-like Growth Factor I, and Fibroblast Growth Factor. J. Cell Physiol. 1989, 138, 311–315. [Google Scholar] [CrossRef]
- Allen, R.E.; Sheehan, S.M.; Taylor, R.G.; Kendall, T.L.; Rice, G.M. Hepatocyte Growth Factor Activates Quiescent Skeletal Muscle Satellite Cells in vitro. J. Cell Physiol. 1995, 165, 307–312. [Google Scholar] [CrossRef]
- Hayashi, S.; Aso, H.; Watanabe, K.; Nara, H.; Rose, M.T.; Ohwada, S.; Yamaguchi, T. Sequence of IGF-I, IGF-II, and HGF Expression in Regenerating Skeletal Muscle. Histochem. Cell Biol. 2000, 122, 427–434. [Google Scholar] [CrossRef]
- Imaoka, Y.; Kawai, M.; Mori, F.; Miyata, H. Effect of Eccentric Contraction on Satellite Cell Activation in Human Vastus Lateralis Muscle. J. Physiol. Sci. 2015, 65, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Florini, J.R.; Magri, K.A. Effects of Growth Factors on Myogenic Differentiation. Am. J. Physiol. Physiol. 1989, 256, C701–C711. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A.; Joanisse, S.; Moore, D.R.; Bellamy, L.M.; Mitchell, C.J.; Phillips, S.M.; Parise, G. IGF-1 Colocalizes with Muscle Satellite Cells Following Acute Exercise in Humans. Appl. Physiol. Nutr. Metab. 2013, 39, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Lavin, K.M.; Perkins, R.K.; Jemiolo, B.; Raue, U.; Trappe, S.W.; Trappe, T.A. Effects of Aging and Lifelong Aerobic Exercise on Basal and Exercise-Induced Inflammation. J. Appl. Physiol. 2020, 128, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Cornelison, D.D.W. Context Matters: In Vivo and in Vitro Influences on Muscle Satellite Cell Activity. J. Cell Biochem. 2008, 105, 663–669. [Google Scholar] [CrossRef]
- Lemos, D.R.; Babaeijandaghi, F.; Low, M.; Chang, C.K.; Lee, S.T.; Fiore, D.; Zhang, R.H.; Natarajan, A.; Nedospasov, S.A.; Rossi, F.M.V. Nilotinib Reduces Muscle Fibrosis in Chronic Muscle Injury by Promoting TNF-Mediated Apoptosis of Fibro/Adipogenic Progenitors. Nat. Med. 2015, 21, 786–794. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; Van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory Monocytes Recruited after Skeletal Muscle Injury Switch into Antiinflammatory Macrophages to Support Myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef]
- Horsley, V.; Jansen, K.M.; Mills, S.T.; Pavlath, G.K. IL-4 Acts as a Myoblast Recruitment Factor during Mammalian Muscle Growth. Cell 2003, 113, 483–494. [Google Scholar] [CrossRef]
- Ziemkiewicz, N.; Hilliard, G.; Pullen, N.A.; Garg, K. The Role of Innate and Adaptive Immune Cells in Skeletal Muscle Regeneration. Int. J. Mol. Sci. 2021, 22, 3265. [Google Scholar] [CrossRef]
- Villalta, S.A.; Rosenthal, W.; Martinez, L.; Kaur, A.; Sparwasser, T.; Tidball, J.G.; Margeta, M.; Spencer, M.J.; Bluestone, J.A. Regulatory T Cells Suppress Muscle Inflammation and Injury in Muscular Dystrophy. Sci. Transl. Med. 2014, 6, 258ra142. [Google Scholar] [CrossRef]
- Castiglioni, A.; Corna, G.; Rigamonti, E.; Basso, V.; Vezzoli, M.; Monno, A.; Almada, A.E.; Mondino, A.; Wagers, A.J.; Manfredi, A.A.; et al. FOXP3+ T Cells Recruited to Sites of Sterile Skeletal Muscle Injury Regulate the Fate of Satellite Cells and Guide Effective Tissue Regeneration. PLoS ONE 2015, 10, e0128094. [Google Scholar] [CrossRef]
- Christov, C.; Chrétien, F.; Abou-Khalil, R.; Bassez, G.; Vallet, G.; me Authier, F.-J.; Bassaglia, Y.; Shinin, V.; Tajbakhsh, S.; Chazaud, B.; et al. Muscle Satellite Cells and Endothelial Cells: Close Neighbors and Privileged Partners. Mol. Biol. Cell 2007, 18, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Hendrickse, P.; Degens, H. The Role of the Microcirculation in Muscle Function and Plasticity. J. Muscle Res. Cell Motil. 2019, 40, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.L.; Morton, A.B.; Segal, S.S. Angiogenesis Precedes Myogenesis during Regeneration Following Biopsy Injury of Skeletal Muscle. Skelet. Muscle 2023, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.L.; Norton, C.E.; Shaw, R.L.; Cornelison, D.D.W.; Segal, S.S. Myofibre injury induces capillary disruption and regeneration of disorganized microvascular networks. J. Physiol. 2022, 600, 41–60. [Google Scholar] [CrossRef]
- Nederveen, J.; Betz, M.; Snijders, T.; Parise, G. The Importance of Muscle Capillarization for Optimizing Satellite Cell Plasticity. Exerc. Sport. Sci. Rev. 2021, 49, 284–290. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Joanisse, S.; Snijders, T.; Thomas, A.C.Q.; Kumbhare, D.; Parise, G. The Influence of Capillarization on Satellite Cell Pool Expansion and Activation Following Exercise-Induced Muscle Damage in Healthy Young Men. J. Physiol. 2018, 596, 1063–1078. [Google Scholar] [CrossRef]
- Nederveen, J.P.; Joanisse, S.; Snijders, T.; Ivankovic, V.; Baker, S.K.; Phillips, S.M.; Parise, G. Skeletal Muscle Satellite Cells Are Located at a Closer Proximity to Capillaries in Healthy Young Compared with Older Men. J. Cachexia Sarcopenia Muscle 2016, 7, 547. [Google Scholar] [CrossRef]
- Baker, J.M.; Nederveen, J.P.; Parise, G. Aerobic Exercise in Humans Mobilizes HSCs in an Intensity-Dependent Manner. J. Appl. Physiol. 2017, 122, 182–190. [Google Scholar] [CrossRef]
- Verma, M.; Asakura, Y.; Murakonda, B.S.R.; Pengo, T.; Latroche, C.; Chazaud, B.; McLoon, L.K.; Asakura, A. Muscle Satellite Cell Cross-Talk with a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling. Cell Stem Cell 2018, 23, 530. [Google Scholar] [CrossRef]
- Hoier, B.; Hellsten, Y. Exercise-Induced Capillary Growth in Human Skeletal Muscle and the Dynamics of VEGF. Microcirculation 2014, 21, 301–314. [Google Scholar] [CrossRef]
- Dar, A.; Domev, H.; Ben-Yosef, O.; Tzukerman, M.; Zeevi-Levin, N.; Novak, A.; Germanguz, I.; Amit, M.; Itskovitz-Eldor, J. Multipotent Vasculogenic Pericytes from Human Pluripotent Stem Cells Promote Recovery of Murine Ischemic Limb. Circulation 2012, 125, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gianni-Barrera, R.; Butschkau, A.; Uccelli, A.; Certelli, A.; Valente, P.; Bartolomeo, M.; Groppa, E.; Burger, M.G.; Hlushchuk, R.; Heberer, M.; et al. PDGF-BB Regulates Splitting Angiogenesis in Skeletal Muscle by Limiting VEGF-Induced Endothelial Proliferation. Angiogenesis 2018, 21, 883–900. [Google Scholar] [CrossRef]
- Bellamy, L.M.; Johnston, A.P.W.; De Lisio, M.; Parise, G. Skeletal Muscle-Endothelial Cell Cross Talk through Angiotensin II. Am. J. Physiol. Cell Physiol. 2010, 299, C1402–C1408. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.P.W.; Baker, J.; De Lisio, M.; Parise, G. Skeletal Muscle Myoblasts Possess a Stretch-Responsive Local Angiotensin Signalling System. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Schwartz, L.M.; Clarkson, P.M. NF-KB Activity Functions in Primary Pericytes in a Cell- and Non-Cell-Autonomous Manner to Affect Myotube Formation. Muscle Nerve 2013, 47, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Milkiewicz, M.; Davis, S.J.; Zhou, A.L.; Egginton, S.; Brown, M.D.; Madri, J.A.; Hudlicka, A.O.; Hudlicka, O. Matrix Metalloproteinase Activity Is Required for Activity-Induced Angiogenesis in Rat Skeletal Muscle. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H1540-7. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Mintz, A.; Delbono, O. Type-1 Pericytes Participate in Fibrous Tissue Deposition in Aged Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2013, 305, C1098–C1113. [Google Scholar] [CrossRef]
- Frey, S.P.; Jansen, H.; Raschke, M.J.; Meffert, R.H.; Ochman, S. VEGF Improves Skeletal Muscle Regeneration after Acute Trauma and Reconstruction of the Limb in a Rabbit Model. Clin. Orthop. Relat. Res. 2012, 470, 3607–3614. [Google Scholar] [CrossRef]
- Uezumi, A.; Ikemoto-Uezumi, M.; Zhou, H.; Kurosawa, T.; Yoshimoto, Y.; Nakatani, M.; Hitachi, K.; Yamaguchi, H.; Wakatsuki, S.; Araki, T.; et al. Mesenchymal Bmp3b Expression Maintains Skeletal Muscle Integrity and Decreases in Age-Related Sarcopenia. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Motohashi, N.; Uezumi, A.; Yada, E.; Fukada, S.I.; Fukushima, K.; Imaizumi, K.; Miyagoe-Suzuki, Y.; Takeda, S. Muscle CD31(−) CD45(−) Side Population Cells Promote Muscle Regeneration by Stimulating Proliferation and Migration of Myoblasts. Am. J. Pathol. 2008, 173, 781–791. [Google Scholar] [CrossRef]
- Hughes, S.M.; Blau, H.M. Migration of Myoblasts across Basal Lamina during Skeletal Muscle Development. Nature 1990, 345, 350–353. [Google Scholar] [CrossRef]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Mohamad Azhar, N.I.F.; Lefebvre, O.; Zammit, P.S.; Borycki, A.-G. Basal Lamina Remodeling at the Skeletal Muscle Stem Cell Niche Mediates Stem Cell Self-Renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Cao, B.; Crisan, M.; Sun, B.; Li, G.; Logar, A.; Yap, S.; Pollett, J.B.; Drowley, L.; Cassino, T.; et al. Prospective Identification of Myogenic Endothelial Cells in Human Skeletal Muscle. Nat. Biotechnol. 2007, 25, 1025–1034. [Google Scholar] [CrossRef]
- Valero, M.C.; Huntsman, H.D.; Liu, J.; Zou, K.; Boppart, M.D. Eccentric Exercise Facilitates Mesenchymal Stem Cell Appearance in Skeletal Muscle. PLoS ONE 2012, 7, e29760. [Google Scholar] [CrossRef]
- De Angelis, L.; Berghella, L.; Coletta, M.; Lattanzi, L.; Zanchi, M.; Cusella-De Angelis, M.G.; Ponzetto, C.; Cossu, G. Skeletal Myogenic Progenitors Originating from Embryonic Dorsal Aorta Coexpress Endothelial and Myogenic Markers and Contribute to Postnatal Muscle Growth and Regeneration. J. Cell Biol. 1999, 147, 869–878. [Google Scholar] [CrossRef]
- Murphy, M.M.; Lawson, J.A.; Mathew, S.J.; Hutcheson, D.A.; Kardon, G. Satellite Cells, Connective Tissue Fibroblasts and Their Interactions Are Crucial for Muscle Regeneration. Development 2011, 138, 3625–3637. [Google Scholar] [CrossRef]
- Dahlman, J.M.; Bakkar, N.; He, W.; Guttridge, D.C. NF-ΚB Functions in Stromal Fibroblasts to Regulate Early Postnatal Muscle Development. J. Biol. Chem. 2010, 285, 5479–5487. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.S.; Kirby, T.J.; Kosmac, K.; McCarthy, J.J.; Peterson, C.A. Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy. Cell Stem Cell 2017, 20, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Vumbaca, S.; Giuliani, G.; Fiorentini, V.; Tortolici, F.; Cerquone Perpetuini, A.; Riccio, F.; Sennato, S.; Gargioli, C.; Fuoco, C.; Castagnoli, L.; et al. Characterization of the Skeletal Muscle Secretome Reveals a Role for Extracellular Vesicles and IL1α/IL1β in Restricting Fibro/Adipogenic Progenitor Adipogenesis. Biomolecules 2021, 11, 1171. [Google Scholar] [CrossRef]
- Fiore, D.; Judson, R.N.; Low, M.; Lee, S.; Zhang, E.; Hopkins, C.; Xu, P.; Lenzi, A.; Rossi, F.M.V.; Lemos, D.R. Pharmacological Blockage of Fibro/Adipogenic Progenitor Expansion and Suppression of Regenerative Fibrogenesis Is Associated with Impaired Skeletal Muscle Regeneration. Stem Cell Res. 2016, 17, 161–169. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Konishi, C.T.; Perez Carbajal, E.E.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035.e5. [Google Scholar] [CrossRef]
- Lemos, D.R.; Paylor, B.; Chang, C.; Sampaio, A.; Underhill, T.M.; Rossi, F.M.V. Functionally Convergent White Adipogenic Progenitors of Different Lineages Participate in a Diffused System Supporting Tissue Regeneration. Stem Cells 2012, 30, 1152–1162. [Google Scholar] [CrossRef]
- Wallner, C.; Rausch, A.; Drysch, M.; Dadras, M.; Wagner, J.M.; Becerikli, M.; Lehnhardt, M.; Behr, B. Regulatory Aspects of Myogenic Factors GDF-8 and Follistatin on the Intake of Combined Oral Contraceptives. Gynecol. Endocrinol. 2020, 36, 406–412. [Google Scholar] [CrossRef]
- Biferali, B.; Proietti, D.; Mozzetta, C.; Madaro, L. Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network. Front. Physiol. 2019, 10, 1074. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, S.; Di Padova, M.; Serra, C.; Caretti, G.; Simone, C.; Maklan, E.; Minetti, G.; Zhao, P.; Hoffman, E.P.; Puri, P.L.; et al. Deacetylase Inhibitors Increase Muscle Cell Size by Promoting Myoblast Recruitment and Fusion through Induction of Follistatin. Dev. Cell 2004, 6, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Minetti, G.C.; Colussi, C.; Adami, R.; Serra, C.; Mozzetta, C.; Parente, V.; Fortuni, S.; Straino, S.; Sampaolesi, M.; Di Padova, M.; et al. Functional and Morphological Recovery of Dystrophic Muscles in Mice Treated with Deacetylase Inhibitors. Nat. Med. 2006, 12, 1147–1150. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, Y.-S.; Zimmers, T.A.; Soleimani, A.; Matzuk, M.M.; Tsuchida, K.; Cohn, R.D.; Barton, E.R. Regulation of Muscle Mass by Follistatin and Activins. Mol. Endocrinol. 2010, 24, 1998–2008. [Google Scholar] [CrossRef] [PubMed]
- Renzini, A.; D’Onghia, M.; Coletti, D.; Moresi, V. Histone Deacetylases as Modulators of the Crosstalk Between Skeletal Muscle and Other Organs. Front. Physiol. 2022, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Doyonnas, R.; LaBarge, M.A.; Sacco, A.; Charlton, C.; Blau, H.M. Hematopoietic Contribution to Skeletal Muscle Regeneration by Myelomonocytic Precursors. Proc. Natl. Acad. Sci. USA 2004, 101, 13507–13512. [Google Scholar] [CrossRef]
- Labarge, M.A.; Blau, H.M. Biological Progression from Adult Bone Marrow to Mononucleate Muscle Stem Cell to Multinucleate Muscle Fiber in Response to Injury. Cell 2002, 111, 589–601. [Google Scholar] [CrossRef]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a Novel Population of Muscle Stem Cells in Mice. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Ojima, K.; Uezumi, A.; Miyoshi, H.; Masuda, S.; Morita, Y.; Fukase, A.; Hattori, A.; Nakauchi, H.; Miyagoe-Suzuki, Y.; Takeda, S. Mac-1low Early Myeloid Cells in the Bone Marrow-Derived SP Fraction Migrate into Injured Skeletal Muscle and Participate in Muscle Regeneration. Biochem. Biophys. Res. Commun. 2004, 321, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Torrente, Y.; Belicchi, M.; Sampaolesi, M.; Pisati, F.; Meregalli, M.; D’Antona, G.; Tonlorenzi, R.; Porretti, L.; Gavina, M.; Mamchaoui, K.; et al. Human Circulating AC133+ Stem Cells Restore Dystrophin Expression and Ameliorate Function in Dystrophic Skeletal Muscle. J. Clin. Investig. 2004, 114, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Lin, J.; Sun, Y.; Wang, C.; Chen, J. Bone Marrow Stromal Cell-Derived Exosomes Promote Muscle Healing Following Contusion Through Macrophage Polarization. Stem Cells Dev. 2021, 30, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Bittner, R.E.; Schöfer, C.; Weipoltshammer, K.; Ivanova, S.; Streubel, B.; Hauser, E.; Freilinger, M.; Höger, H.; Elbe-Bürger, A.; Wachtler, F. Recruitment of Bone-Marrow-Derived Cells by Skeletal and Cardiac Muscle in Adult Dystrophic Mdx Mice. Anat. Embryol. 1999, 199, 391–396. [Google Scholar] [CrossRef]
- Sherwood, R.I.; Christensen, J.L.; Weissman, I.L.; Wagers, A.J. Determinants of Skeletal Muscle Contributions from Circulating Cells, Bone Marrow Cells, and Hematopoietic Stem Cells. Stem Cells 2004, 22, 1292–1304. [Google Scholar] [CrossRef]
- Gussoni, E.; Soneoka, Y.; Strickland, C.D.; Buzney, E.A.; Khan, M.K.; Flint, A.F.; Kunkel, L.M.; Mulligan, R.C. Dystrophin Expression in the Mdx Mouse Restored by Stem Cell Transplantation. Nature 1999, 401, 390–394. [Google Scholar] [CrossRef]
- Ferrari, G.; Cusella–, G.; Angelis, D.; Coletta, M.; Paolucci, E.; Stornaiuolo, A.; Cossu, G.; Mavilio, F. Muscle Regeneration by Bone Marrow-Derived Myogenic Progenitors. Science 1998, 279, 1528–1530. [Google Scholar] [CrossRef]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn, R.F.; Otsuru, S.; Lovering, R.M. Exosomes Isolated From Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function after Muscle Injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, A.L.; Kamal, M.; Parise, G. The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair. Cells 2023, 12, 1968. https://doi.org/10.3390/cells12151968
Johnson AL, Kamal M, Parise G. The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair. Cells. 2023; 12(15):1968. https://doi.org/10.3390/cells12151968
Chicago/Turabian StyleJohnson, Amanda L., Michael Kamal, and Gianni Parise. 2023. "The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair" Cells 12, no. 15: 1968. https://doi.org/10.3390/cells12151968
APA StyleJohnson, A. L., Kamal, M., & Parise, G. (2023). The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair. Cells, 12(15), 1968. https://doi.org/10.3390/cells12151968




