The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms
Abstract
1. Introduction
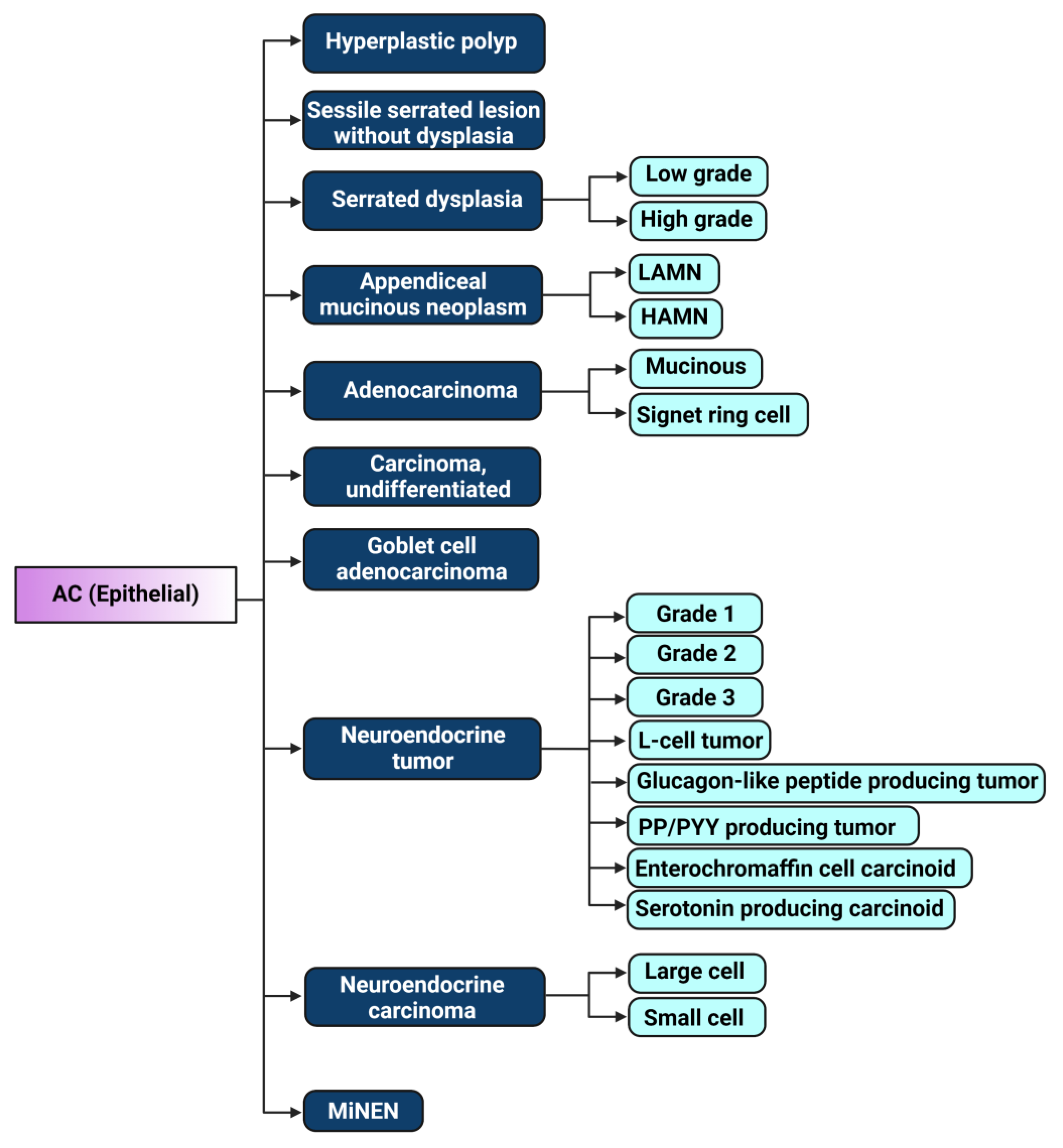
2. The Evolution of Appendiceal Cancer Classification
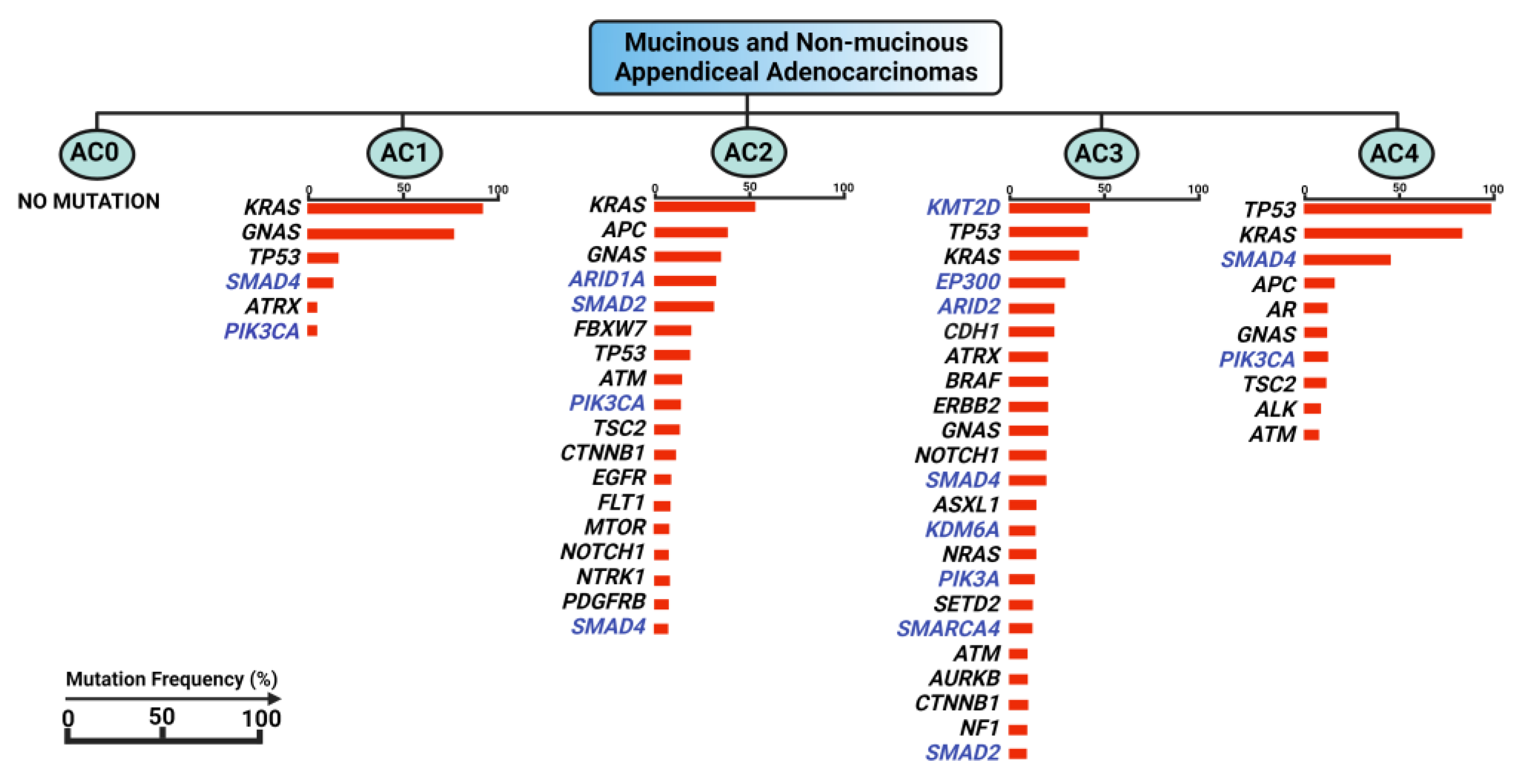
3. Genomic Landscape of Appendiceal Cancers vs. Colorectal Cancer
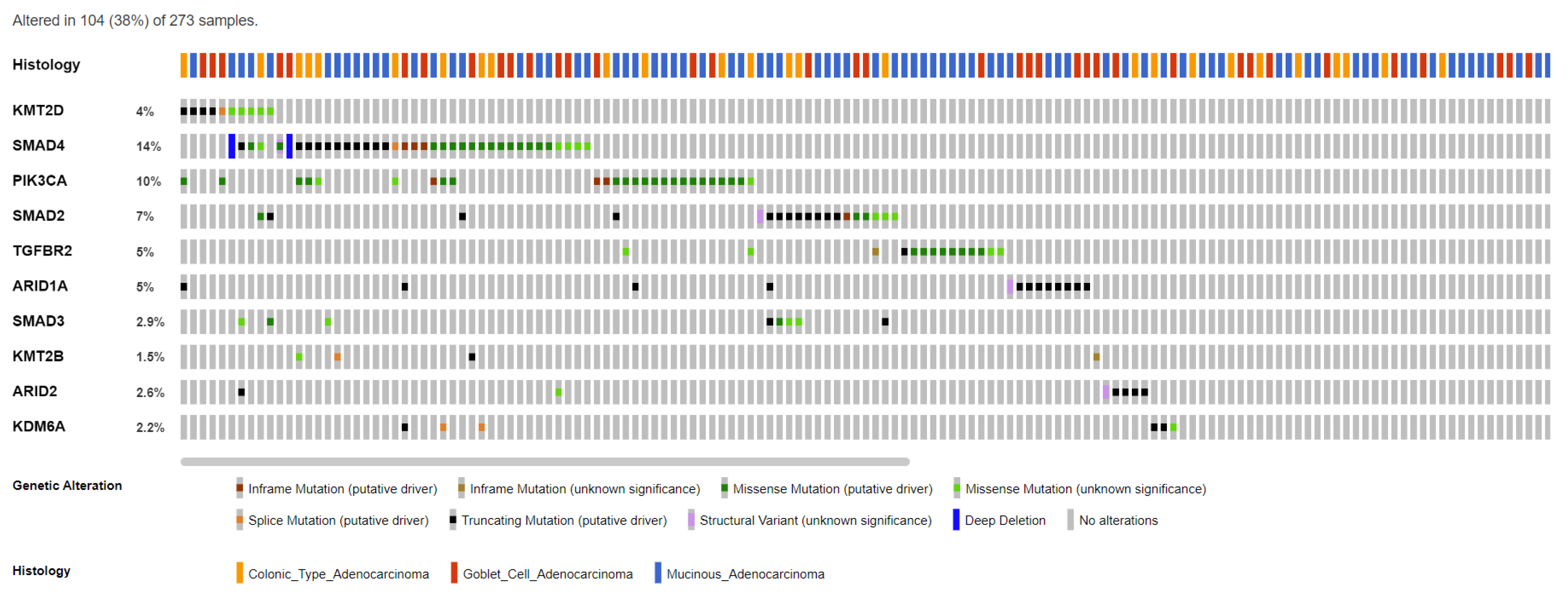
4. The Epigenetic Landscape of Appendiceal Cancers
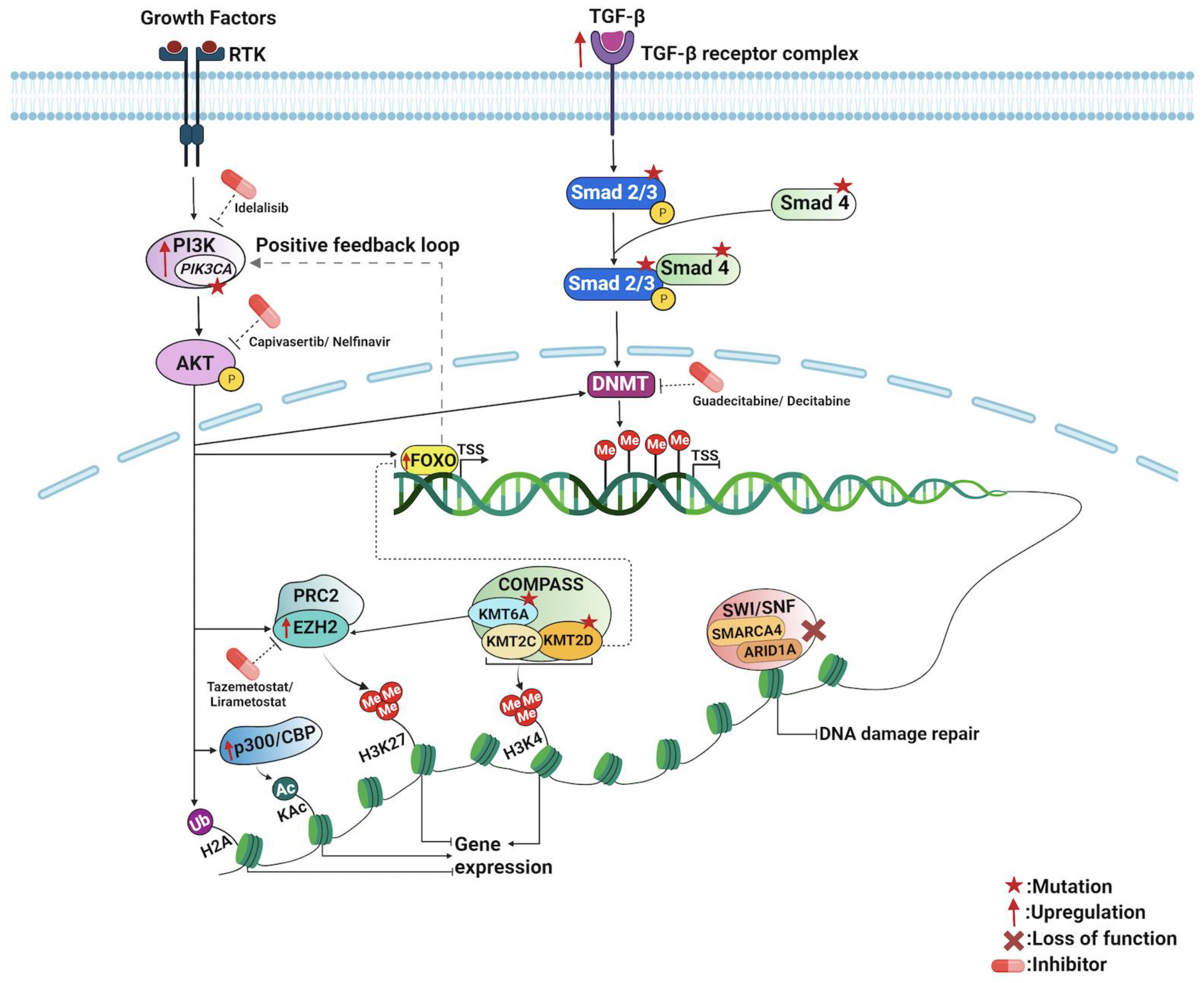
5. Epigenetic Mechanisms of PI3K/AKT Pathway in Appendiceal Cancers
6. Epigenetic Mechanisms of TGF-β/SMAD Pathway in Appendiceal Cancers
7. Chromatin Remodeling and Transcription in Appendiceal Cancers
7.1. SWI/SNF Chromatin Remodeling Complex
7.2. COMPASS Chromatin Remodeling Complex
7.3. The Forkhead Box O (FoxO) Transcription Factors
8. Exploration of Potential Epigenetics-Based Biomarkers for Novel Therapeutic Targets
9. Epigenetics-Based Biomarkers for Monitoring, Surveillance, and Prognostication of Appendiceal Cancers
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salazar, M.C.; Canavan, M.E.; Chilakamarry, S.; Boffa, D.J.; Schuster, K.M. Appendiceal Cancer in the National Cancer Database: Increasing Frequency, Decreasing Age, and Shifting Histology. J. Am. Coll. Surg. 2022, 234, 1082–1089. [Google Scholar] [CrossRef]
- Marmor, S.; Portschy, P.R.; Tuttle, T.M.; Virnig, B.A. The rise in appendiceal cancer incidence: 2000–2009. J. Gastrointest. Surg. 2015, 19, 743–750. [Google Scholar] [CrossRef]
- Yilmaz, S.; Bolukbasi, H. Appendiceal neoplasms: Suspected findings and reports of 14 cases. Indian J. Cancer 2022. [Google Scholar]
- O’Donnell, M.E.; Badger, S.A.; Beattie, G.C.; Carson, J.; Garstin, W.I. Malignant neoplasms of the appendix. Int. J. Colorectal. Dis. 2007, 22, 1239–1248. [Google Scholar] [CrossRef]
- McGory, M.L.; Maggard, M.A.; Kang, H.; O’Connell, J.B.; Ko, C.Y. Malignancies of the appendix: Beyond case series reports. Dis. Colon Rectum 2005, 48, 2264–2271. [Google Scholar] [CrossRef]
- McCusker, M.E.; Coté, T.R.; Clegg, L.X.; Sobin, L.H. Primary malignant neoplasms of the appendix: A population-based study from the surveillance, epidemiology and end-results program, 1973–1998. Cancer 2002, 94, 3307–3312. [Google Scholar] [CrossRef]
- Hatch, Q.M.; Gilbert, E.W. Appendiceal Neoplasms. Clin. Colon Rectal Surg. 2018, 31, 278–287. [Google Scholar] [CrossRef]
- American Cancer Society, National Cancer Institute, and the National Organization for Rare Disorders. Appendix Cancer: Statistics. 2022. Available online: https://www.cancer.net/cancer-types/appendix-cancer/statistics (accessed on 30 March 2023).
- Alajääski, J.; Lietzén, E.; Grönroos, J.M.; Mecklin, J.P.; Leppäniemi, A.; Nordström, P.; Rautio, T.; Rantanen, T.; Sand, J.; Paajanen, H.; et al. The association between appendicitis severity and patient age with appendiceal neoplasm histology-a population-based study. Int. J. Colorectal. Dis. 2022, 37, 1173–1180. [Google Scholar] [CrossRef]
- Skendelas, J.P.; Alemany, V.S.; Au, V.; Rao, D.; McNelis, J.; Kim, P.K. Appendiceal adenocarcinoma found by surgery for acute appendicitis is associated with older age. BMC Surg. 2021, 21, 228. [Google Scholar] [CrossRef]
- van den Heuvel, M.G.; Lemmens, V.E.; Verhoeven, R.H.; de Hingh, I.H. The incidence of mucinous appendiceal malignancies: A population-based study. Int. J. Colorectal. Dis. 2013, 28, 1307–1310. [Google Scholar] [CrossRef]
- Singh, H.; Koomson, A.S.; Decker, K.M.; Park, J.; Demers, A.A. Continued increasing incidence of malignant appendiceal tumors in Canada and the United States: A population-based study. Cancer 2020, 126, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Van de Moortele, M.; De Hertogh, G.; Sagaert, X.; Van Cutsem, E. Appendiceal cancer: A review of the literature. Acta Gastroenterol. Belg. 2020, 83, 441–448. [Google Scholar] [PubMed]
- Flum, D.R.; Davidson, G.H.; Monsell, S.E.; Shapiro, N.I.; Odom, S.R.; Sanchez, S.E.; Drake, F.T.; Fischkoff, K.; Johnson, J.; Patton, J.H.; et al. A Randomized Trial Comparing Antibiotics with Appendectomy for Appendicitis. N. Engl. J. Med. 2020, 383, 1907–1919. [Google Scholar] [CrossRef]
- Sallinen, V.; Akl, E.A.; You, J.J.; Agarwal, A.; Shoucair, S.; Vandvik, P.O.; Agoritsas, T.; Heels-Ansdell, D.; Guyatt, G.H.; Tikkinen, K.A. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br. J. Surg. 2016, 103, 656–667. [Google Scholar] [CrossRef]
- Newdow, M. Management of Acute Appendicitis—Longer-Term Outcomes. N. Engl. J. Med. 2022, 386, 900. [Google Scholar] [CrossRef]
- Ang, C.S.; Shen, J.P.; Hardy-Abeloos, C.J.; Huang, J.K.; Ross, J.S.; Miller, V.A.; Jacobs, M.T.; Chen, I.L.; Xu, D.; Ali, S.M.; et al. Genomic Landscape of Appendiceal Neoplasms. JCO Precis. Oncol. 2018, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Raghav, K.P.S.; Loree, J.M.; Fournier, K.F.; Shaw, K.R.; Taggart, M.W.; Foo, W.C.; Matamoros, A.; Mehdizadeh, A.; Ahmed, S.U.; Guerra, J.L.; et al. Comprehensive genomic profiling of appendiceal adenocarcinoma. J. Clin. Oncol. 2018, 36 (Suppl. S4), 298. [Google Scholar] [CrossRef]
- Levine, E.A.; Votanopoulos, K.I.; Qasem, S.A.; Philip, J.; Cummins, K.A.; Chou, J.W.; Ruiz, J.; D’Agostino, R.; Shen, P.; Miller, L.D. Prognostic Molecular Subtypes of Low-Grade Cancer of the Appendix. J. Am. Coll. Surg. 2016, 222, 493–503. [Google Scholar] [CrossRef]
- Johncilla, M.; Stachler, M.; Misdraji, J.; Lisovsky, M.; Yozu, M.; Lindeman, N.; Lauwers, G.Y.; Odze, R.D.; Srivastava, A. Mutational landscape of goblet cell carcinoids and adenocarcinoma ex goblet cell carcinoids of the appendix is distinct from typical carcinoids and colorectal adenocarcinomas. Mod. Pathol. 2018, 31, 989–996. [Google Scholar] [CrossRef]
- Borazanci, E.; Millis, S.Z.; Kimbrough, J.; Doll, N.; Von Hoff, D.; Ramanathan, R.K. Potential actionable targets in appendiceal cancer detected by immunohistochemistry, fluorescent in situ hybridization, and mutational analysis. J. Gastrointest. Oncol. 2017, 8, 164–172. [Google Scholar] [CrossRef]
- Jung, G.; Hernández-Illán, E.; Moreira, L.; Balaguer, F.; Goel, A. Epigenetics of colorectal cancer: Biomarker and therapeutic potential. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 111–130. [Google Scholar] [CrossRef] [PubMed]
- Assarzadegan, N.; Montgomery, E. What is New in the 2019 World Health Organization (WHO) Classification of Tumors of the Digestive System: Review of Selected Updates on Neuroendocrine Neoplasms, Appendiceal Tumors, and Molecular Testing. Arch. Pathol. Lab. Med. 2021, 145, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Washington, M.K. Tumours of the appendix. In Digestive System Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019; pp. 135–156. [Google Scholar]
- Carr, N.J.; Bibeau, F.; Bradley, R.F.; Dartigues, P.; Feakins, R.M.; Geisinger, K.R.; Gui, X.; Isaac, S.; Milione, M.; Misdraji, J.; et al. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology 2017, 71, 847–858. [Google Scholar] [CrossRef]
- Ko, Y.H.; Jung, C.K.; Oh, S.N.; Kim, T.H.; Won, H.S.; Kang, J.H.; Kim, H.J.; Kang, W.K.; Oh, S.T.; Hong, Y.S. Primary signet ring cell carcinoma of the appendix: A rare case report and our 18-year experience. World J. Gastroenterol. 2008, 14, 5763–5768. [Google Scholar] [CrossRef]
- Kelly, K.J. Management of Appendix Cancer. Clin. Colon Rectal Surg. 2015, 28, 247–255. [Google Scholar] [CrossRef]
- Feely, M.; Gonzalez, R.S. LAMN and HAMN. Available online: https://www.pathologyoutlines.com/topic/appendixmucinousneoplasm.html (accessed on 29 March 2023).
- Palmer, K.; Weerasuriya, S.; Chandrakumaran, K.; Rous, B.; White, B.E.; Paisey, S.; Srirajaskanthan, R.; Ramage, J.K. Goblet Cell Adenocarcinoma of the Appendix: A Systematic Review and Incidence and Survival of 1225 Cases from an English Cancer Registry. Front. Oncol. 2022, 12, 915028. [Google Scholar] [CrossRef]
- Pape, U.F.; Perren, A.; Niederle, B.; Gross, D.; Gress, T.; Costa, F.; Arnold, R.; Denecke, T.; Plöckinger, U.; Salazar, R.; et al. ENETS Consensus Guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 2012, 95, 135–156. [Google Scholar] [CrossRef]
- Wen, K.W.; Grenert, J.P.; Joseph, N.M.; Shafizadeh, N.; Huang, A.; Hosseini, M.; Kakar, S. Genomic profile of appendiceal goblet cell carcinoid is distinct compared to appendiceal neuroendocrine tumor and conventional adenocarcinoma. Hum. Pathol. 2018, 77, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Baca, Y.; Battaglin, F.; Kawanishi, N.; Wang, J.; Soni, S.; Zhang, W.; Millstein, J.; Johnston, C.; Goldberg, R.M.; et al. Molecular Characterization of Appendiceal Goblet Cell Carcinoid. Mol. Cancer Ther. 2020, 19, 2634–2640. [Google Scholar] [CrossRef]
- Shaib, W.; Krishna, K.; Kim, S.; Goodman, M.; Rock, J.; Chen, Z.; Brutcher, E.; Staley, C.I.; Maithel, S.K.; Abdel-Missih, S.; et al. Appendiceal Neuroendocrine, Goblet and Signet-Ring Cell Tumors: A Spectrum of Diseases with Different Patterns of Presentation and Outcome. Cancer Res. Treat. 2016, 48, 596–604. [Google Scholar] [CrossRef]
- Foote, M.B.; Walch, H.; Chatila, W.; Vakiani, E.; Chandler, C.; Steinruecke, F.; Nash, G.M.; Stadler, Z.; Chung, S.; Yaeger, R.; et al. Molecular Classification of Appendiceal Adenocarcinoma. J. Clin. Oncol. 2023, 41, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Garland-Kledzik, M.; Scholer, A.; Ensenyat-Mendez, M.; Orozco, J.I.J.; Khader, A.; Santamaria-Barria, J.; Fischer, T.; Pigazzi, A.; Marzese, D.M. Establishing Novel Molecular Subtypes of Appendiceal Cancer. Ann. Surg. Oncol. 2022, 29, 2118–2125. [Google Scholar] [CrossRef]
- Raghav, K.; Shen, J.P.; Jácome, A.A.; Guerra, J.L.; Scally, C.P.; Taggart, M.W.; Foo, W.C.; Matamoros, A.; Shaw, K.R.; Fournier, K.; et al. Integrated clinico-molecular profiling of appendiceal adenocarcinoma reveals a unique grade-driven entity distinct from colorectal cancer. Br. J. Cancer 2020, 123, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Strong, E.; Clark Gamblin, T.; Clarke, C.; Tsai, S.; Thomas, J.; George, B.; Mogal, H. Molecular and Genetic Markers in Appendiceal Mucinous Tumors: A Systematic Review. Ann. Surg. Oncol. 2020, 27, 85–97. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- LaFramboise, W.A.; Pai, R.K.; Petrosko, P.; Belsky, M.A.; Dhir, A.; Howard, P.G.; Becich, M.J.; Holtzman, M.P.; Ahrendt, S.A.; Pingpank, J.F.; et al. Discrimination of low- and high-grade appendiceal mucinous neoplasms by targeted sequencing of cancer-related variants. Mod. Pathol. 2019, 32, 1197–1209. [Google Scholar] [CrossRef]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Fardi, M.; Solali, S.; Farshdousti Hagh, M. Epigenetic mechanisms as a new approach in cancer treatment: An updated review. Genes Dis. 2018, 5, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef]
- Chhoda, A.; Sharma, A.; Sailo, B.; Tang, H.; Ruzgar, N.; Tan, W.Y.; Ying, L.; Khatri, R.; Narayanan, A.; Mane, S.; et al. Utility of promoter hypermethylation in malignant risk stratification of intraductal papillary mucinous neoplasms. Clin. Epigenet. 2023, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Sharma, A.; Chhoda, A.; Ruzgar, N.; Hasan, N.; Kwak, R.; Wolfgang, C.L.; Wang, T.H.; Kunstman, J.W.; Salem, R.R.; et al. Methylation-based Cell-free DNA Signature for Early Detection of Pancreatic Cancer. Pancreas 2021, 50, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.A.L.; Lerner, L.; Abdelfatah, E.; Shankar, N.; Canner, J.K.; Hasan, N.M.; Yaghoobi, V.; Huang, B.; Kerner, Z.; Takaesu, F.; et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin. Epigenet. 2019, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.M.; Guzzetta, A.A.; Bailey, V.J.; Downing, S.R.; Van Neste, L.; Chiappinelli, K.B.; Keeley, B.P.; Stark, A.; Herrera, A.; Wolfgang, C.; et al. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin. Cancer Res. 2013, 19, 6544–6555. [Google Scholar] [CrossRef]
- Tepus, M.; Yau, T.O. Non-Invasive Colorectal Cancer Screening: An Overview. Gastrointest. Tumors 2020, 7, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Lin, D.L.; Wang, L.L.; Zhao, P.; Ran, W.W.; Wang, W.; Zhang, L.X.; Han, M.; Bao, H.; Liu, K.; Wu, X.; et al. Gastrointestinal Goblet Cell Adenocarcinomas Harbor Distinctive Clinicopathological, Immune, and Genomic Landscape. Front. Oncol. 2021, 11, 758643. [Google Scholar] [CrossRef]
- Spangle, J.M.; Roberts, T.M.; Zhao, J.J. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 123–131. [Google Scholar] [CrossRef]
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Chen, C.C. Akt phosphorylation of p300 at Ser-1834 is essential for its histone acetyltransferase and transcriptional activity. Mol. Cell. Biol. 2005, 25, 6592–6602. [Google Scholar] [CrossRef] [PubMed]
- Benard, A.; Goossens-Beumer, I.J.; van Hoesel, A.Q.; de Graaf, W.; Horati, H.; Putter, H.; Zeestraten, E.C.; van de Velde, C.J.; Kuppen, P.J. Histone trimethylation at H3K4, H3K9 and H4K20 correlates with patient survival and tumor recurrence in early-stage colon cancer. BMC Cancer 2014, 14, 531. [Google Scholar] [CrossRef]
- Trelford, C.B.; Dagnino, L.; Di Guglielmo, G.M. Transforming growth factor-β in tumour development. Front. Mol. Biosci. 2022, 9, 991612. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.; Vieth, E.; Lee, J.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics 2014, 9, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, L.; Li, H.; Li, Y.; Ruan, Y.; Lin, D.; Yang, M.; Jin, X.; Guo, Y.; Zhang, X.; et al. SMAD2 Inactivation Inhibits CLDN6 Methylation to Suppress Migration and Invasion of Breast Cancer Cells. Int. J. Mol. Sci. 2017, 18, 1863. [Google Scholar] [CrossRef]
- Yeh, K.T.; Chen, T.H.; Yang, H.W.; Chou, J.L.; Chen, L.Y.; Yeh, C.M.; Chen, Y.H.; Lin, R.I.; Su, H.Y.; Chen, G.C.; et al. Aberrant TGFβ/SMAD4 signaling contributes to epigenetic silencing of a putative tumor suppressor, RunX1T1 in ovarian cancer. Epigenetics 2011, 6, 727–739. [Google Scholar] [CrossRef]
- Mittal, P.; Roberts, C.W.M. The SWI/SNF complex in cancer—Biology, biomarkers and therapy. Nat. Rev. Clin. Oncol. 2020, 17, 435–448. [Google Scholar] [CrossRef]
- Jones, C.A.; Tansey, W.P.; Weissmiller, A.M. Emerging Themes in Mechanisms of Tumorigenesis by SWI/SNF Subunit Mutation. Epigenet. Insights 2022, 15, 25168657221115656. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, J.; Tang, Y. Advances in the role of SWI/SNF complexes in tumours. J. Cell. Mol. Med. 2023, 27, 1023–1031. [Google Scholar] [CrossRef]
- Qi, W.; Wang, R.; Chen, H.; Wang, X.; Xiao, T.; Boldogh, I.; Ba, X.; Han, L.; Zeng, X. BRG1 promotes the repair of DNA double-strand breaks by facilitating the replacement of RPA with RAD51. J. Cell Sci. 2015, 128, 317–330. [Google Scholar] [CrossRef]
- Watanabe, R.; Ui, A.; Kanno, S.; Ogiwara, H.; Nagase, T.; Kohno, T.; Yasui, A. SWI/SNF factors required for cellular resistance to DNA damage include ARID1A and ARID1B and show interdependent protein stability. Cancer Res. 2014, 74, 2465–2475. [Google Scholar] [CrossRef]
- Seton-Rogers, S. Pancreatic cancer: The COMPASS shows the way. Nat. Rev. Cancer 2018, 18, 373. [Google Scholar] [CrossRef]
- Revia, S.; Seretny, A.; Wendler, L.; Banito, A.; Eckert, C.; Breuer, K.; Mayakonda, A.; Lutsik, P.; Evert, M.; Ribback, S.; et al. Histone H3K27 demethylase KDM6A is an epigenetic gatekeeper of mTORC1 signalling in cancer. Gut 2022, 71, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Lavery, W.J.; Barski, A.; Wiley, S.; Schorry, E.K.; Lindsley, A.W. KMT2C/D COMPASS complex-associated diseases [K(CD)COM-ADs]: An emerging class of congenital regulopathies. Clin. Epigenet. 2020, 12, 10. [Google Scholar] [CrossRef]
- Lv, S.; Wen, H.; Shan, X.; Li, J.; Wu, Y.; Yu, X.; Huang, W.; Wei, Q. Loss of KMT2D induces prostate cancer ROS-mediated DNA damage by suppressing the enhancer activity and DNA binding of antioxidant transcription factor FOXO3. Epigenetics 2019, 14, 1194–1208. [Google Scholar] [CrossRef] [PubMed]
- Schulz, W.A.; Lang, A.; Koch, J.; Greife, A. The histone demethylase UTX/KDM6A in cancer: Progress and puzzles. Int. J. Cancer 2019, 145, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yadav, R.K.; Chauhan, A.S.; Zhuang, L.; Gan, B. FoxO transcription factors in cancer metabolism. Semin. Cancer Biol. 2018, 50, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Gupta, A.K.; Li, B.; Cerniglia, G.J.; Ahmed, M.S.; Hahn, S.M.; Maity, A. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia 2007, 9, 271–278. [Google Scholar] [CrossRef]
- Lin, A.; Piao, H.L.; Zhuang, L.; dos Sarbassov, D.; Ma, L.; Gan, B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacologic inhibition of the PI3K-AKT pathway. Cancer Res. 2014, 74, 1682–1693. [Google Scholar] [CrossRef]
- Gulati, N.; Béguelin, W.; Giulino-Roth, L. Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk. Lymphoma 2018, 59, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022, 13, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, Y.; Li, Z.M.; Li, L.; Su, H.; Jin, Z.; Zuo, X.; Wu, J.; Zhou, H.; Li, K.; et al. SHR2554, an EZH2 inhibitor, in relapsed or refractory mature lymphoid neoplasms: A first-in-human, dose-escalation, dose-expansion, and clinical expansion phase 1 trial. Lancet Haematol. 2022, 9, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Eich, M.L.; Athar, M.; Ferguson, J.E., 3rd; Varambally, S. EZH2-Targeted Therapies in Cancer: Hype or a Reality. Cancer Res. 2020, 80, 5449–5458. [Google Scholar] [CrossRef]
- Kuser-Abali, G.; Gong, L.; Yan, J.; Liu, Q.; Zeng, W.; Williamson, A.; Lim, C.B.; Molloy, M.E.; Little, J.B.; Huang, L.; et al. An EZH2-mediated epigenetic mechanism behind p53-dependent tissue sensitivity to DNA damage. Proc. Natl. Acad. Sci. USA 2018, 115, 3452–3457. [Google Scholar] [CrossRef]
- Black, J.C.; Kutateladze, T.G. Atypical histone targets of PHD fingers. J. Biol. Chem. 2023, 299, 104601. [Google Scholar] [CrossRef]
- Bhushan, B.; Erdmann, A.; Zhang, Y.; Belle, R.; Johannson, C.; Oppermann, U.; Hopkinson, R.J.; Schofield, C.J.; Kawamura, A. Investigations on small molecule inhibitors targeting the histone H3K4 tri-methyllysine binding PHD-finger of JmjC histone demethylases. Bioorg. Med. Chem. 2018, 26, 2984–2991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Yang, H.; Ortiz, G.; Trnka, M.J.; Petronikolou, N.; Burlingame, A.L.; DeGrado, W.F.; Fujimori, D.G. Covalent labeling of a chromatin reader domain using proximity-reactive cyclic peptides. Chem. Sci. 2022, 13, 6599–6609. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.R.; Park, T.; Saridakis, A.; Golshan, M.; Greenup, R.A.; Ahuja, N. Immunotherapy Treatment for Triple Negative Breast Cancer. Pharmaceuticals 2021, 14, 763. [Google Scholar] [CrossRef]
- Chiappinelli, K.B.; Zahnow, C.A.; Ahuja, N.; Baylin, S.B. Combining Epigenetic and Immunotherapy to Combat Cancer. Cancer Res. 2016, 76, 1683–1689. [Google Scholar] [CrossRef]
- Soares, K.C.; Zheng, L.; Ahuja, N. Overcoming immune system evasion by personalized immunotherapy. Pers. Med. 2014, 11, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, L.; Álvarez-Errico, D.; Esteller, M. The Contribution of Epigenetics to Cancer Immunotherapy. Trends Immunol. 2020, 41, 676–691. [Google Scholar] [CrossRef]
- Levine, E.A.; Blazer, D.G., 3rd; Kim, M.K.; Shen, P.; Stewart, J.H.T.; Guy, C.; Hsu, D.S. Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J. Am. Coll. Surg. 2012, 214, 599–606, discussion 606–597. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.Y.; Sharma, A.; Das, P.; Ahuja, N. Early Detection of Cancers in the Era of Precision Oncology. Curr. Opin. Oncol. 2023, 35, 115–124. [Google Scholar] [CrossRef]
- Riviere, P.; Fanta, P.T.; Ikeda, S.; Baumgartner, J.; Heestand, G.M.; Kurzrock, R. The Mutational Landscape of Gastrointestinal Malignancies as Reflected by Circulating Tumor DNA. Mol. Cancer Ther. 2018, 17, 297–305. [Google Scholar] [CrossRef]
- Pourali, G.; Khalili-Tanha, G.; Nazari, E.; Maftooh, M.; Nassiri, M.; Hassanian, S.M.; Mobarhan, M.G.; Khazaei, M.; Ferns, G.A.; Avan, A. Circulating tumor cells and cell-free nucleic acids as biomarkers in colorectal cancer. Curr. Pharm. Des. 2023, 29, 748–765. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, F.; Mei, L.; Huang, D.; Shen, X.; Zhang, H.; She, X.; Ma, Z. The Potential Use of Dynamics Changes of ctDNA and cfDNA in the Perioperative Period to Predict the Recurrence Risk in Early NSCLC. Front. Oncol. 2021, 11, 671963. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.L.; Zakka, K.; Staley, C., 3rd; Roberts, A.; Akce, M.; Wu, C.; Alese, O.B.; El-Rayes, B.F. Blood-Based Next-Generation Sequencing Analysis of Appendiceal Cancers. Oncologist 2020, 25, 414–421. [Google Scholar] [CrossRef] [PubMed]
| Goblet Cell Adenocarcinoma (GCA) N = 53 | Appendiceal Neuroendocrine Tumor (ANET) N = 14 | Appendiceal Adenocarcinoma (AA) N = 428 | |||
|---|---|---|---|---|---|
| Genes | Percent Mutation (%) | Genes | Percent Mutation (%) | Genes | Percent Mutation (%) |
| TP53 | 24 | KRAS | 28.6 | KRAS | 60.4 |
| ARID1A | 15.4 | APC | 28.6 | TP53 | 37.0 |
| SMAD4 | 9.4 | TP53 | 14.3 | GNAS | 34.4 |
| KRAS | 7.5 | CDH1 | 7.7 | ARID1A | 20.0 |
| BRAF | 3.8 | BRAF | 7.7 | SMAD4 | 18.3 |
| FBXW7 | 3.8 | BCOR | 7.7 | APC | 11.7 |
| CDH1 | 3.8 | BRCA2 | 7.1 | PI3KCA | 7.0 |
| KDM6A | 2.7 | FANCA | 7.1 | RNF43 | 5.9 |
| APC | 1.9 | ERBB2 | 7.1 | ATM | 5.0 |
| PIK3CA | 1.9 | - | - | BRAF | 4.0 |
| ATM | 1.9 | - | - | FBXW7 | 3.6 |
| Genes | Percentage of Gene Mutations (%) | ||
|---|---|---|---|
| Mucinous Adenocarcinoma N = 164 | Goblet Cell Adenocarcinoma N = 72 | Appendiceal Adenocarcinoma (Non-Mucinous) N = 37 | |
| KMT2D | 3.0 | 4.2 | 5.4 |
| KMT2B | 0.9 | 4.5 | 5.9 |
| KDM6A | 0.6 | 2.8 | 8.1 |
| SMAD2 | 6.7 | 4.2 | 10.8 |
| SMAD3 | 3.0 | - | 8.1 |
| SMAD4 | 11.0 | 12.5 | 21.6 |
| PIK3CA | 7.3 | 5.6 | 27.0 |
| TGFBR2 | 7.3 | 1.4 | 2.7 |
| ARID1A | 3.0 | 8.3 | 2.7 |
| ARID2 | 1.8 | 2.8 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladel, L.; Tan, W.Y.; Jeyakanthan, T.; Sailo, B.; Sharma, A.; Ahuja, N. The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms. Cells 2023, 12, 1962. https://doi.org/10.3390/cells12151962
Ladel L, Tan WY, Jeyakanthan T, Sailo B, Sharma A, Ahuja N. The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms. Cells. 2023; 12(15):1962. https://doi.org/10.3390/cells12151962
Chicago/Turabian StyleLadel, Luisa, Wan Ying Tan, Thanushiya Jeyakanthan, Bethsebie Sailo, Anup Sharma, and Nita Ahuja. 2023. "The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms" Cells 12, no. 15: 1962. https://doi.org/10.3390/cells12151962
APA StyleLadel, L., Tan, W. Y., Jeyakanthan, T., Sailo, B., Sharma, A., & Ahuja, N. (2023). The Promise of Epigenetics Research in the Treatment of Appendiceal Neoplasms. Cells, 12(15), 1962. https://doi.org/10.3390/cells12151962








