Intratumoral PD1+CD38+Tim3+ CD8+ T Cells in Pre-BCG Tumor Tissues Are Associated with Poor Responsiveness to BCG Immunotherapy in Patients with Non-Muscle Invasive Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Tumor Tissues
2.2. Inclusion and Exclusion Criteria for the Patients
2.3. Single Cell Preparation from Tumor Tissue
2.4. Isolation of PBMC Peripheral Blood
2.5. Flow Cytometry-Based Analysis of Immune Cell Subsets
| Antibodies | Source | Clone#, Cat No# |
|---|---|---|
| Anti-Human CD3 BV711 | Biolegend | OKT3, 317328 |
| Anti-Human CD4-BUV395 | BD Bioscience | RPA-T4, 564724 |
| Anti-Human CD4-Percp cy5.5 | Biolegend | RPA-T4, 300530 |
| Anti-Human CD8-A700 | Biolgend | HIT8a, 300920 |
| Anti-Human CD8-FITC | Biolegend | HIT8a, 300916 |
| Anti-Human PD1-PECY7 | Thermo Fisher Scientific | EBioJ105, 25-2799-42 |
| Anti-Human CD38-APC-CY7 | Thermo Fisher Scientific | HIT2, 47-0389-42 |
| Anti-Human TIM3-BV421 | Biolegend BD Bioscience | F38-2E2, 345008 7D3, 565562 |
| Anti-Human IFNγ-APC | Invitrogen | 4S.B3, 17-7319-82 |
| Anti-Human GZMB-PE | Biolegend Invitrogen | QA16A02, 372208 GB12, MHGB04 |
| LIVE DEAD Fixable Yellow Fluorescent Dye | Invitrogen | L34968A |
2.6. BCG Administration Timeline
2.7. Statistical Analysis
3. Results
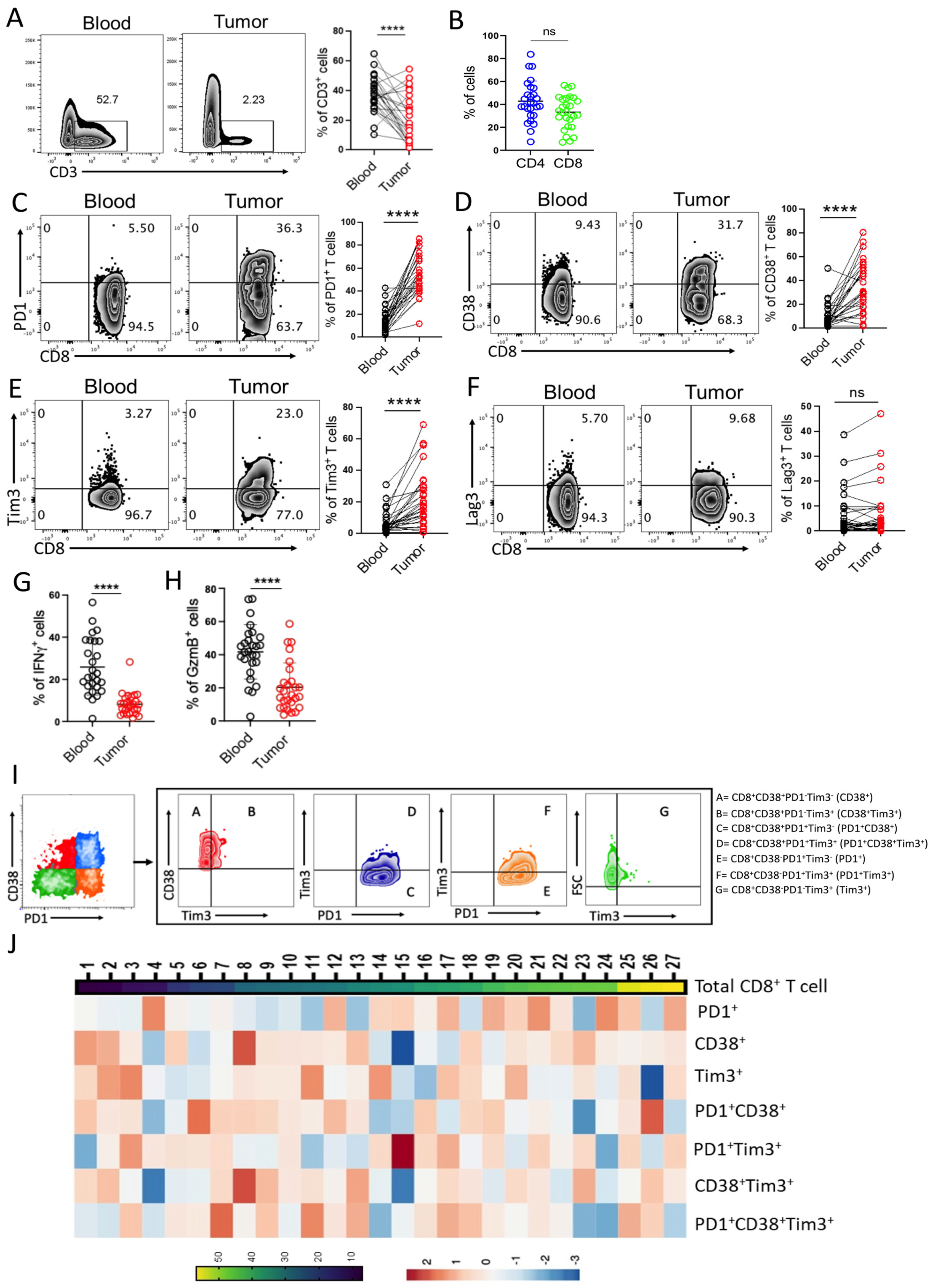
3.1. CD8+ T Cells in Bladder Tumor Tissues Exhibit Heterogeneity in Their Exhaustion States
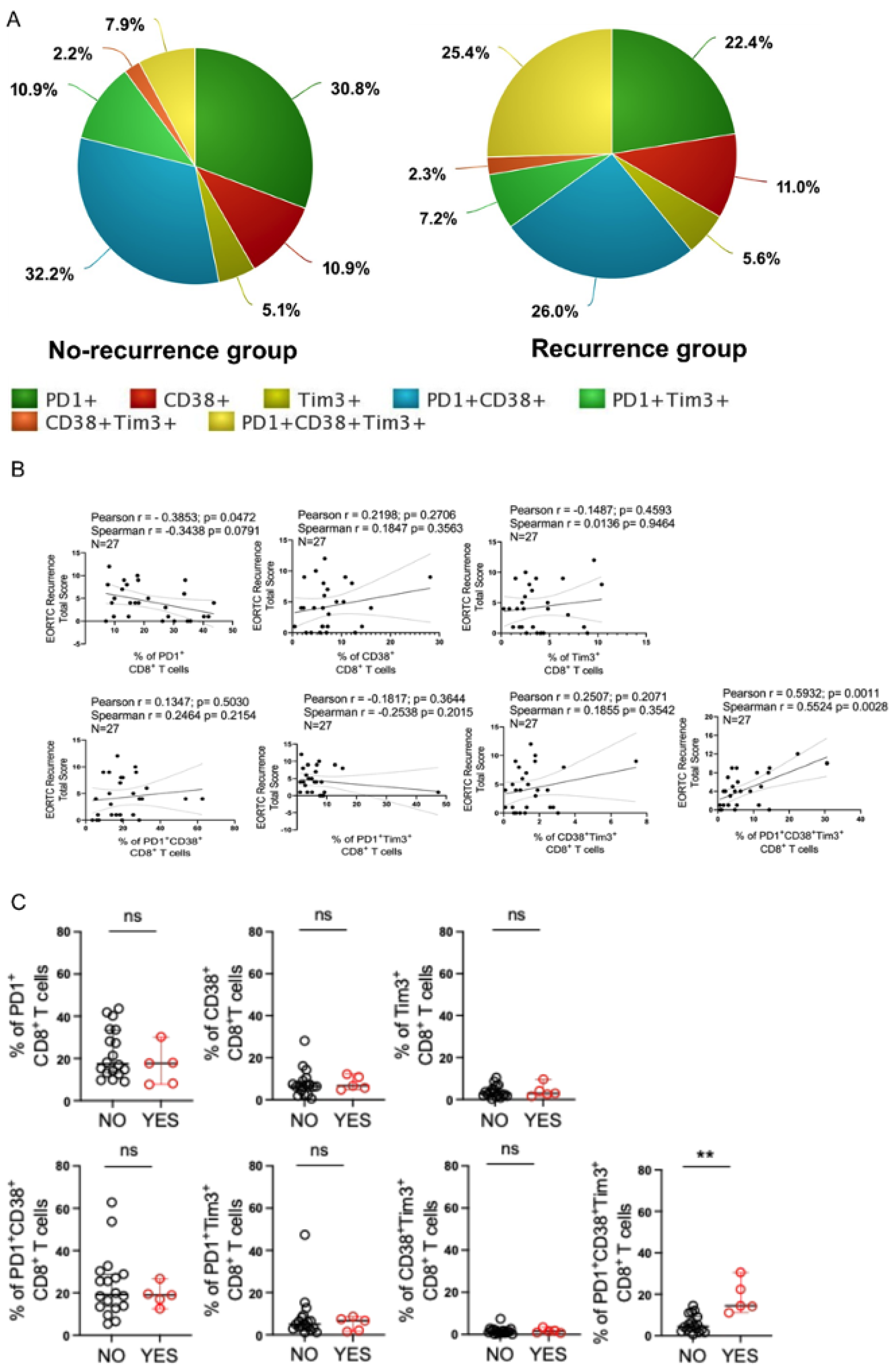
3.2. Intratumoral PD1+CD38+Tim3+ CD8+ T Cell Subset Is Positively Associated with Increased Tumor Burden in Patients with NMIBC
3.3. Intratumoral PD1+CD38+Tim3+ CD8+ T Cells Could Predict Disease Recurrence in Bladder Cancer Patients
4. Discussion
5. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; Rodríguez, O.; Hernández, V.; Turturica, D.; Bauerová, L.; Bruins, H.M.; Bründl, J.; van der Kwast, T.H.; Brisuda, A.; Rubio-Briones, J.; et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021, 79, 480–488. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, M.A.; Lamm, D.L. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A. Management of BCG failures in superficial bladder cancer: A review. Eur. Urol. 2006, 49, 790–797. [Google Scholar] [CrossRef]
- Brake, M.; Loertzer, H.; Horsch, R.; Keller, H. Recurrence and progression of stage T1, grade 3 transitional cell carcinoma of the bladder following intravesical immunotherapy with bacillus Calmette-Guerin. J. Urol. 2000, 163, 1697–1701. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Zitvogel, L.; Sautes-Fridman, C.; Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017, 14, 717–734. [Google Scholar] [CrossRef]
- Ratliff, T.L.; Ritchey, J.K.; Yuan, J.J.; Andriole, G.L.; Catalona, W.J. T-cell subsets required for intravesical BCG immunotherapy for bladder cancer. J. Urol. 1993, 150, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Biot, C.; Rentsch, C.A.; Gsponer, J.R.; Birkhäuser, F.D.; Jusforgues-Saklani, H.; Lemaître, F.; Auriau, C.; Bachmann, A.; Bousso, P.; Demangel, C.; et al. Preexisting BCG-specific T cells improve intravesical immunotherapy for bladder cancer. Sci. Transl. Med. 2012, 4, 137ra72. [Google Scholar] [CrossRef]
- Prescott, S.; James, K.; Hargreave, T.B.; Chisholm, G.D.; Smyth, J.F. Intravesical Evans strain BCG therapy: Quantitative immunohistochemical analysis of the immune response within the bladder wall. J. Urol. 1992, 147, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Kates, M.; Nirschl, T.; Sopko, N.A.; Matsui, H.; Kochel, C.M.; Reis, L.O.; Netto, G.J.; Hoque, M.O.; Hahn, N.M.; McConkey, D.J.; et al. Intravesical BCG Induces CD4(+) T-Cell Expansion in an Immune Competent Model of Bladder Cancer. Cancer Immunol. Res. 2017, 5, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.J.; Nguyen, P.H.D.; Wasser, M.; Kumar, P.; Lee, Y.H.; Nasir, N.J.M.; Chua, C.; Lai, L.; Hazirah, S.N.; Loh, J.J.H.; et al. Immunological Hallmarks for Clinical Response to BCG in Bladder Cancer. Front. Immunol. 2020, 11, 615091. [Google Scholar] [CrossRef]
- Hashizume, A.; Umemoto, S.; Yokose, T.; Nakamura, Y.; Yoshihara, M.; Shoji, K.; Wada, S.; Miyagi, Y.; Kishida, T.; Sasada, T. Enhanced expression of PD-L1 in non-muscle-invasive bladder cancer after treatment with Bacillus Calmette-Guerin. Oncotarget 2018, 9, 34066–34078. [Google Scholar] [CrossRef] [PubMed]
- Castellano, E.; Samba, C.; Esteso, G.; Simpson, L.; Vendrame, E.; García-Cuesta, E.M.; López-Cobo, S.; Álvarez-Maestro, M.; Linares, A.; Leibar, A.; et al. CyTOF analysis identifies unusual immune cells in urine of BCG-treated bladder cancer patients. Front. Immunol. 2022, 13, 970931. [Google Scholar] [CrossRef]
- Martínez, R.; Tapia, G.; De Muga, S.; Hernández, A.; Cao, M.G.; Teixidó, C.; Urrea, V.; García, E.; Pedreño-López, S.; Ibarz, L.; et al. Combined assessment of peritumoral Th1/Th2 polarization and peripheral immunity as a new biomarker in the prediction of BCG response in patients with high-risk NMIBC. Oncoimmunology 2019, 8, 1602460. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, N.; Svatek, R.S.; Mansour, A.M. Role of immunotherapy in bacillus Calmette-Guerin-unresponsive non-muscle invasive bladder cancer. Urol. Oncol. 2018, 36, 103–108. [Google Scholar] [CrossRef]
- Beltra, J.-C.; Manne, S.; Abdel-Hakeem, M.S.; Kurachi, M.; Giles, J.R.; Chen, Z.; Casella, V.; Ngiow, S.F.; Khan, O.; Huang, Y.J.; et al. Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 2020, 52, 825–841.e8. [Google Scholar] [CrossRef]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Philip, M.; Fairchild, L.; Sun, L.; Horste, E.; Camara, S.; Shakiba, M.; Scott, A.; Viale, A.; Lauer, P.; Merghoub, T.; et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452–456. [Google Scholar] [CrossRef]
- Siddiqui, I.; Schaeuble, K.; Chennupati, V.; Marraco, S.A.F.; Calderon-Copete, S.; Ferreira, D.P.; Carmona, S.J.; Scarpellino, L.; Gfeller, D.; Pradervand, S.; et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 2019, 50, 195–211.e10. [Google Scholar] [CrossRef] [PubMed]
- St Paul, M.; Ohashi, P.S. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef]
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 2016, 537, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Q.; Wang, Z.; Zhang, H.; Zeng, H.; Huang, Q.; Chen, Y.; Jiang, W.; Lin, Z.; Qu, Y.; et al. Intratumoral TIGIT(+) CD8(+) T-cell infiltration determines poor prognosis and immune evasion in patients with muscle-invasive bladder cancer. J. Immunother. Cancer. 2020, 8, e000978. [Google Scholar] [CrossRef] [PubMed]
- Sponaas, A.-M.; Yang, R.; Rustad, E.H.; Standal, T.; Thoresen, A.S.; Vo, C.D.; Waage, A.; Slørdahl, T.S.; Børset, M.; Sundan, A. PD1 is expressed on exhausted T cells as well as virus specific memory CD8+ T cells in the bone marrow of myeloma patients. Oncotarget 2018, 9, 32024–32035. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Wherry, E.J.; Jin, B.; Xu, B.; Zou, Z.; Zhang, S.; Li, B.; Wang, H.; Wu, H.; et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology 2008, 134, 1938–1949. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakeem, M.S.; Manne, S.; Beltra, J.-C.; Stelekati, E.; Chen, Z.; Nzingha, K.; Ali, M.-A.; Johnson, J.L.; Giles, J.R.; Mathew, D.; et al. Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation. Nat. Immunol. 2021, 22, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Yates, K.B.; Tonnerre, P.; Martin, G.E.; Gerdemann, U.; Al Abosy, R.; Comstock, D.E.; Weiss, S.A.; Wolski, D.; Tully, D.C.; Chung, R.T.; et al. Epigenetic scars of CD8(+) T cell exhaustion persist after cure of chronic infection in humans. Nat. Immunol. 2021, 22, 1020–1029. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basak, D.; Mondal, S.; Srivastava, S.K.; Sarkar, D.; Sarkar, I.; Basu, S.; Bhoumik, A.; Chowdhury, S.; Pal, D.K.; Chatterjee, S. Intratumoral PD1+CD38+Tim3+ CD8+ T Cells in Pre-BCG Tumor Tissues Are Associated with Poor Responsiveness to BCG Immunotherapy in Patients with Non-Muscle Invasive Bladder Cancer. Cells 2023, 12, 1939. https://doi.org/10.3390/cells12151939
Basak D, Mondal S, Srivastava SK, Sarkar D, Sarkar I, Basu S, Bhoumik A, Chowdhury S, Pal DK, Chatterjee S. Intratumoral PD1+CD38+Tim3+ CD8+ T Cells in Pre-BCG Tumor Tissues Are Associated with Poor Responsiveness to BCG Immunotherapy in Patients with Non-Muscle Invasive Bladder Cancer. Cells. 2023; 12(15):1939. https://doi.org/10.3390/cells12151939
Chicago/Turabian StyleBasak, Debashree, Soumya Mondal, Swadeep Kumar Srivastava, Deborpita Sarkar, Ishita Sarkar, Sukanya Basu, Arpita Bhoumik, Snehanshu Chowdhury, Dilip Kumar Pal, and Shilpak Chatterjee. 2023. "Intratumoral PD1+CD38+Tim3+ CD8+ T Cells in Pre-BCG Tumor Tissues Are Associated with Poor Responsiveness to BCG Immunotherapy in Patients with Non-Muscle Invasive Bladder Cancer" Cells 12, no. 15: 1939. https://doi.org/10.3390/cells12151939
APA StyleBasak, D., Mondal, S., Srivastava, S. K., Sarkar, D., Sarkar, I., Basu, S., Bhoumik, A., Chowdhury, S., Pal, D. K., & Chatterjee, S. (2023). Intratumoral PD1+CD38+Tim3+ CD8+ T Cells in Pre-BCG Tumor Tissues Are Associated with Poor Responsiveness to BCG Immunotherapy in Patients with Non-Muscle Invasive Bladder Cancer. Cells, 12(15), 1939. https://doi.org/10.3390/cells12151939








