Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. SCW Model
2.3. Experimental Design
2.4. Pain Behavior Assessment (Catwalk and Incapacitance Tests)
2.5. Isolation of the Knee Joints
2.6. Isolation of RNA (Synovium) and Subsequent qPCR
2.7. Association between Inflammation and Nociceptive Profile
2.8. Statistical Analyses
3. Results
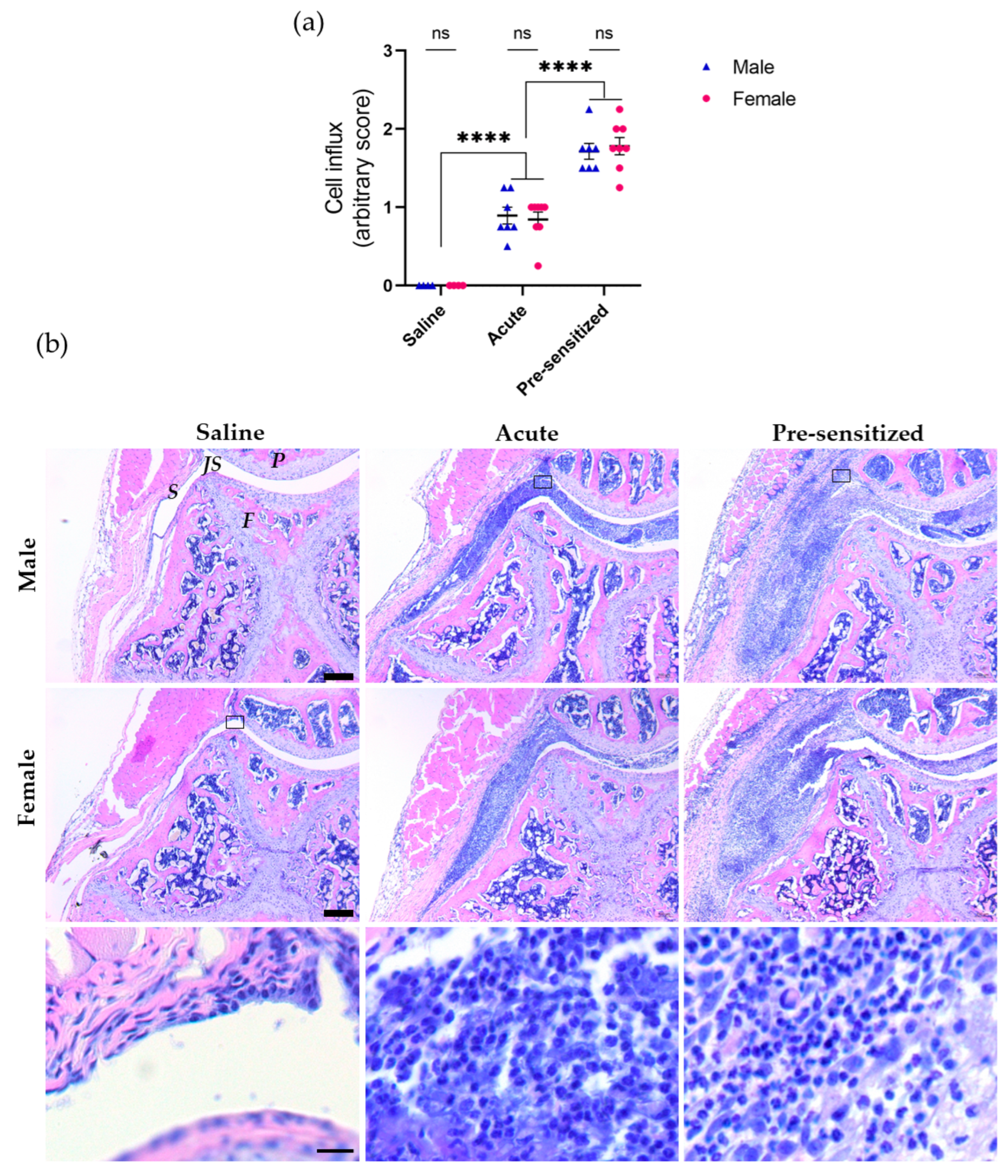
3.1. Joint Inflammation Induced by Serial Injections of SCW Assessed by Histology
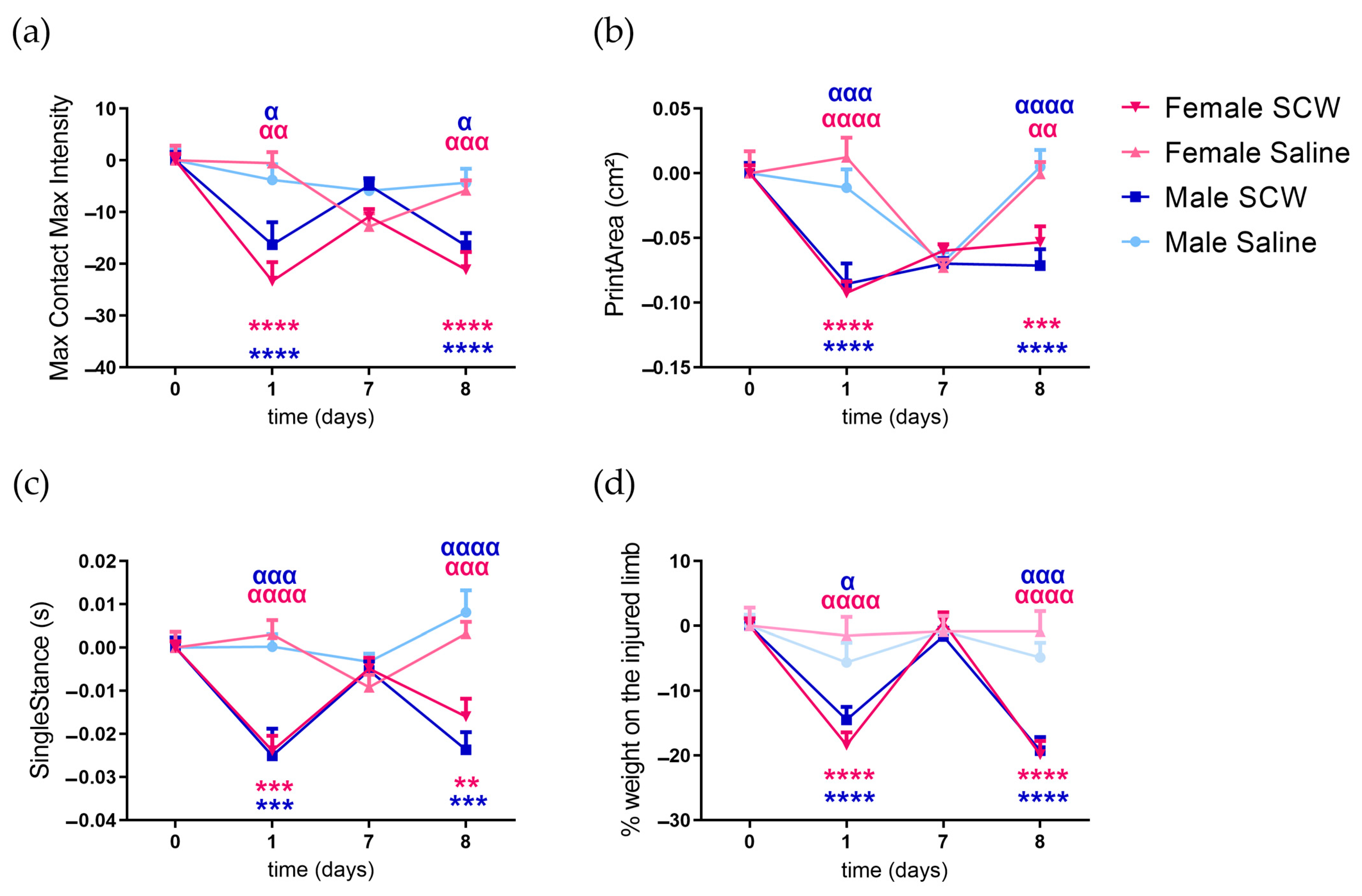
3.2. Pain Behavior of Mice with SCW-Induced Arthritis
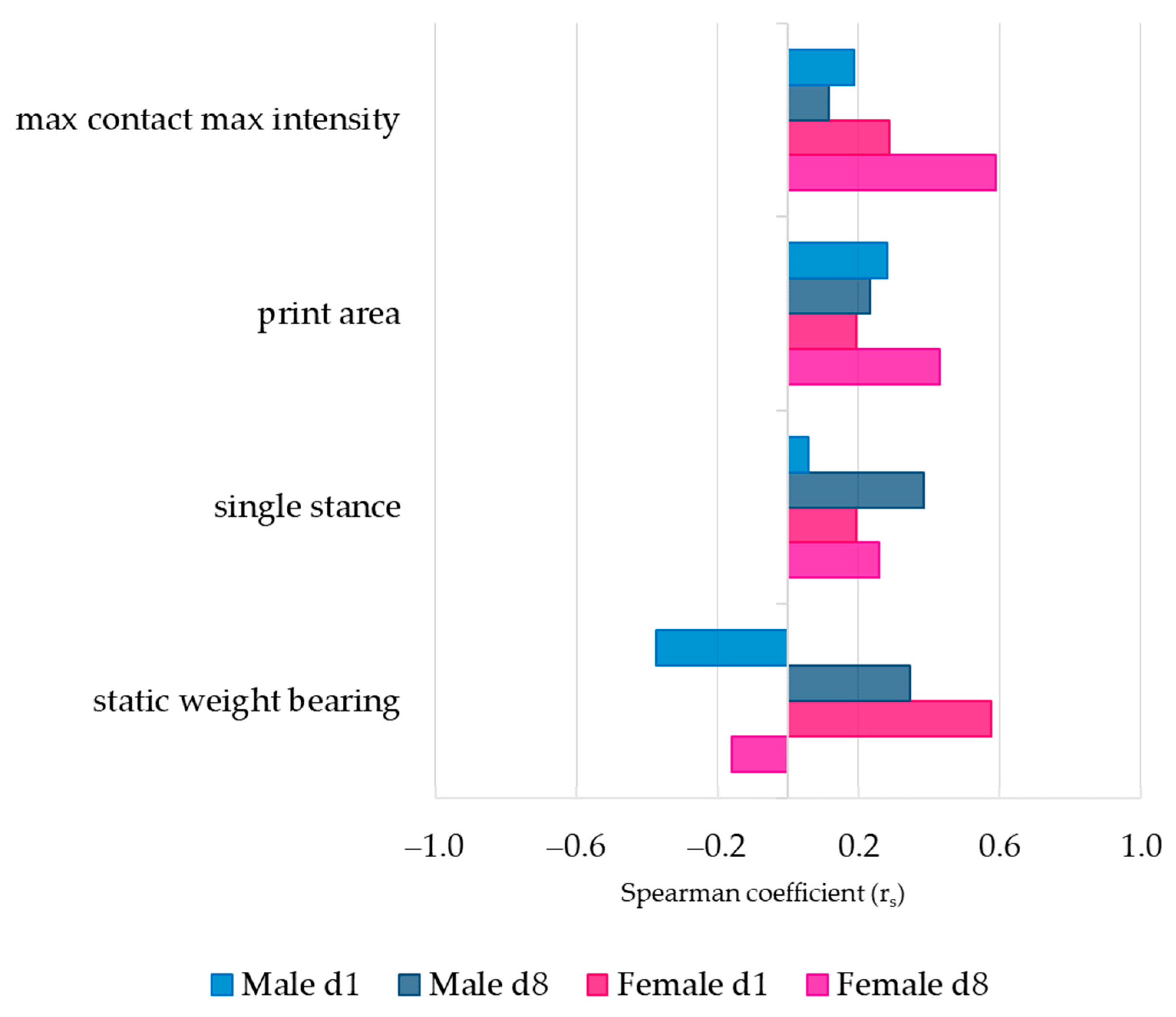
3.3. Association between Pain Behavior and SCW-Induced Joint Inflammation
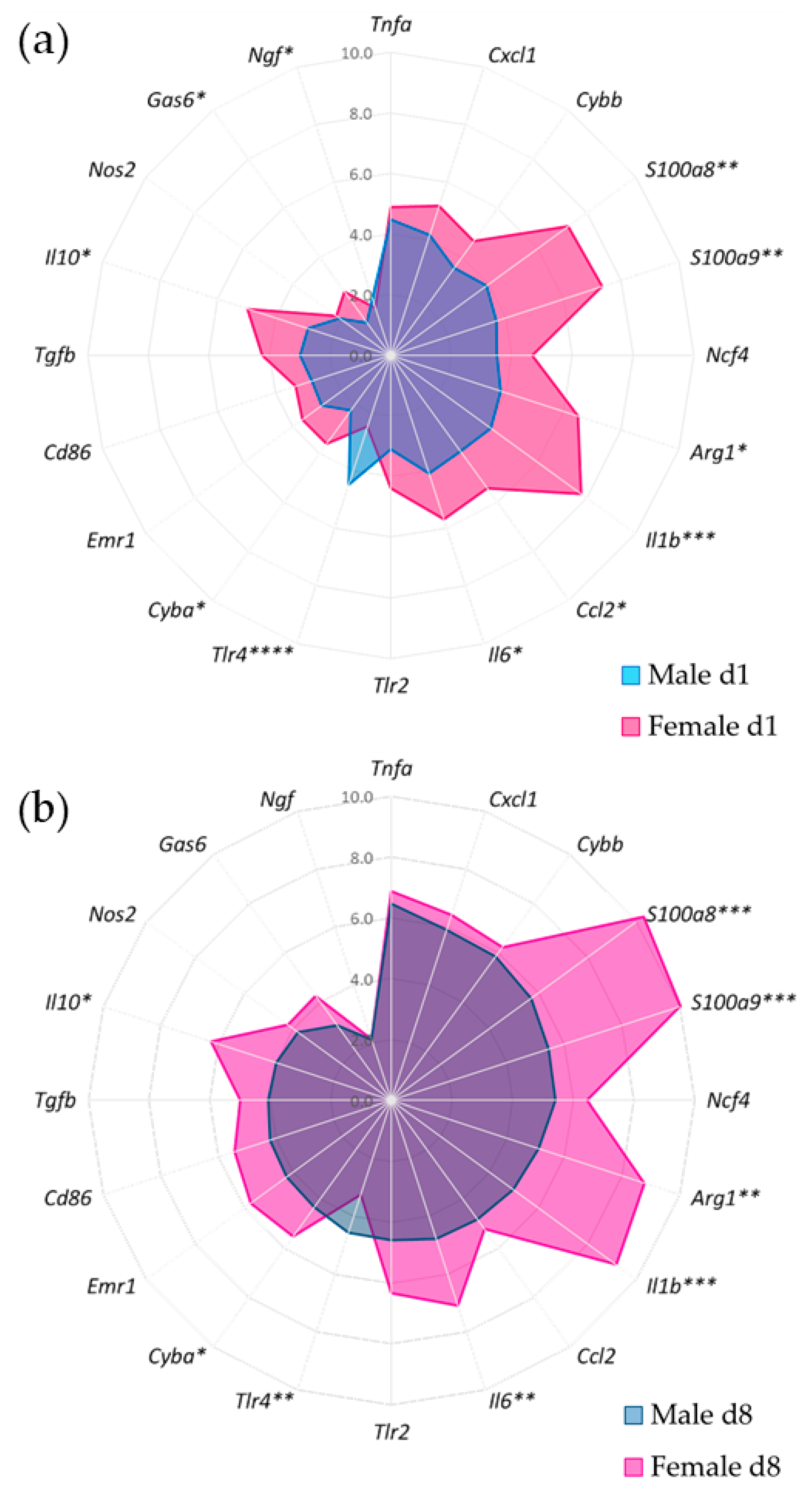
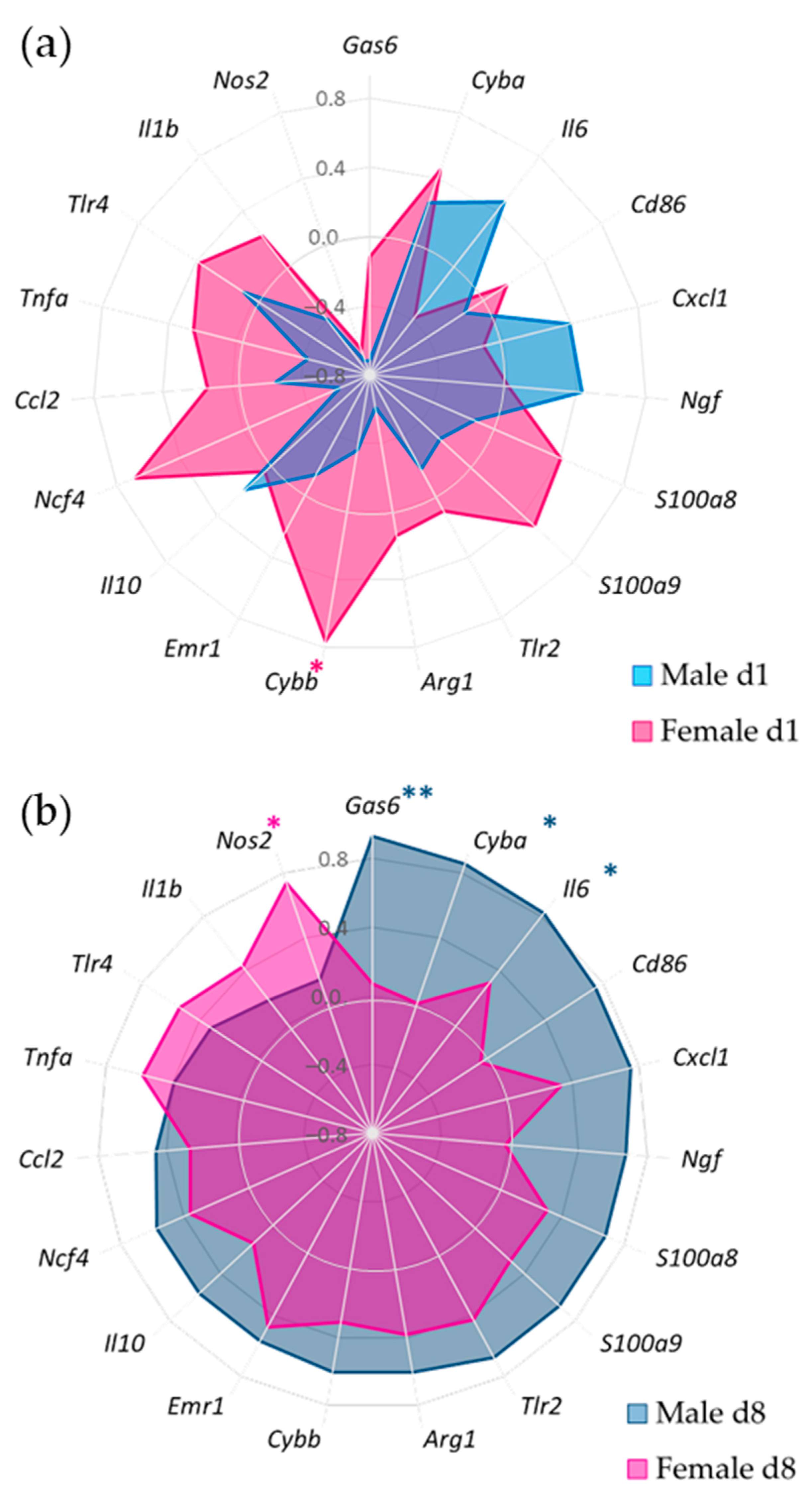
3.4. Gene Expression of Synovial Inflammatory Factors
3.5. Synovial Inflammatory Factors and Sex-Related Weight-Bearing Asymmetry in Acute SCW Arthritis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Glass, N.; Segal, N.A.; Sluka, K.A.; Torner, J.C.; Nevitt, M.C.; Felson, D.T.; Bradley, L.A.; Neogi, T.; Lewis, C.E.; Frey-Law, L.A. Examining sex differences in knee pain: The Multicenter Osteoarthritis Study. Osteoarthr. Cartil. 2014, 22, 1100–1106. [Google Scholar] [CrossRef]
- Barnabe, C.; Bessette, L.; Flanagan, C.; LeClercq, S.; Steiman, A.; Kalache, F.; Kung, T.; Pope, J.E.; Haraoui, B.; Hochman, J.; et al. Sex Differences in Pain Scores and Localization in Inflammatory Arthritis: A Systematic Review and Metaanalysis. J. Rheumatol. 2012, 39, 1221. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Bartley, E.J.; King, C.D.; Sibille, K.T.; Cruz-Almeida, Y.; Riley, J.L., 3rd; Glover, T.L.; Goodin, B.R.; Sotolongo, A.S.; Herbert, M.S.; Bulls, H.W.; et al. Enhanced Pain Sensitivity Among Individuals with Symptomatic Knee Osteoarthritis: Potential Sex Differences in Central Sensitization. Arthritis Care Res. 2016, 68, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lopez, E.; Coras, R.; Torres, A.; Lane, N.E.; Guma, M. Synovial inflammation in osteoarthritis progression. Nat. Rev. Rheumatol. 2022, 18, 258–275. [Google Scholar] [CrossRef]
- Perry, T.A.; Parkes, M.J.; Hodgson, R.J.; Felson, D.T.; Arden, N.K.; O’Neill, T.W. Association between Bone marrow lesions & synovitis and symptoms in symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2020, 28, 316–323. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Pope, R.M. The role of toll-like receptors in rheumatoid arthritis. Curr. Rheumatol. Rep. 2009, 11, 357–364. [Google Scholar] [CrossRef]
- Orlowsky, E.W.; Kraus, V.B. The role of innate immunity in osteoarthritis: When our first line of defense goes on the offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Edilova, M.I.; Akram, A.; Abdul-Sater, A.A. Innate immunity drives pathogenesis of rheumatoid arthritis. Biomed. J. 2021, 44, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57, iv43–iv50. [Google Scholar] [CrossRef]
- Syx, D.; Tran, P.B.; Miller, R.E.; Malfait, A.M. Peripheral Mechanisms Contributing to Osteoarthritis Pain. Curr. Rheumatol. Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. A general theory of sexual differentiation. J. Neurosci. Res. 2017, 95, 291–300. [Google Scholar] [CrossRef]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual Dimorphism in Innate Immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Sorge, R.E.; LaCroix-Fralish, M.L.; Tuttle, A.H.; Sotocinal, S.G.; Austin, J.S.; Ritchie, J.; Chanda, M.L.; Graham, A.C.; Topham, L.; Beggs, S.; et al. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 15450–15454. [Google Scholar] [CrossRef]
- Christianson, C.A.; Dumlao, D.S.; Stokes, J.A.; Dennis, E.A.; Svensson, C.I.; Corr, M.; Yaksh, T.L. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011, 152, 2881–2891. [Google Scholar] [CrossRef]
- Rudjito, R.; Agalave, N.M.; Farinotti, A.B.; Lundbäck, P.; Szabo-Pardi, T.A.; Price, T.J.; Harris, H.E.; Burton, M.D.; Svensson, C.I. Sex- and cell-dependent contribution of peripheral high mobility group box 1 and TLR4 in arthritis-induced pain. Pain 2021, 162, 459–470. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Sorge, R.E.; Totsch, S.K. Sex Differences in Pain. J. Neurosci. Res. 2017, 95, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.B.; Koenders, M.I.; Smeets, R.L.; Heuvelmans-Jacobs, M.; Helsen, M.M.A.; Takeda, K.; Akira, S.; Lubberts, E.; van de Loo, F.A.J.; van den Berg, W.B. Toll-like Receptor 2 Pathway Drives Streptococcal Cell Wall-Induced Joint Inflammation: Critical Role of Myeloid Differentiation Factor 88. J. Immunol. 2003, 171, 6145–6153. [Google Scholar] [CrossRef]
- Abdollahi-Roodsaz, S.; Joosten, L.A.; Helsen, M.M.; Walgreen, B.; van Lent, P.L.; van den Bersselaar, L.A.; Koenders, M.I.; van den Berg, W.B. Shift from toll-like receptor 2 (TLR-2) toward TLR-4 dependency in the erosive stage of chronic streptococcal cell wall arthritis coincident with TLR-4-mediated interleukin-17 production. Arthritis Rheum. 2008, 58, 3753–3764. [Google Scholar] [CrossRef] [PubMed]
- Joosten, L.A.; Abdollahi-Roodsaz, S.; Heuvelmans-Jacobs, M.; Helsen, M.M.; van den Bersselaar, L.A.; Oppers-Walgreen, B.; Koenders, M.I.; van den Berg, W.B. T cell dependence of chronic destructive murine arthritis induced by repeated local activation of Toll-like receptor-driven pathways: Crucial role of both interleukin-1beta and interleukin-17. Arthritis Rheum. 2008, 58, 98–108. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, M.F.; van den Berg, W.B.; van de Putte, L.B.; Severijnen, A.J. Streptococcal cell wall-induced arthritis and flare-up reaction in mice induced by homologous or heterologous cell walls. Am. J. Pathol. 1988, 133, 139–149. [Google Scholar] [PubMed]
- Blom, A.B.; van den Bosch, M.H.; Blaney Davidson, E.N.; Roth, J.; Vogl, T.; van de Loo, F.A.; Koenders, M.; van der Kraan, P.M.; Geven, E.J.; van Lent, P.L. The alarmins S100A8 and S100A9 mediate acute pain in experimental synovitis. Arthritis Res. Ther. 2020, 22, 199. [Google Scholar] [CrossRef]
- Vrinten, D.H.; Hamers, F.F. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain 2003, 102, 203–209. [Google Scholar] [CrossRef]
- Coulthard, P.; Pleuvry, B.J.; Brewster, M.; Wilson, K.L.; Macfarlane, T.V. Gait analysis as an objective measure in a chronic pain model. J. Neurosci. Methods 2002, 116, 197–213. [Google Scholar] [CrossRef]
- Coulthard, P.; Simjee, S.U.; Pleuvry, B.J. Gait analysis as a correlate of pain induced by carrageenan intraplantar injection. J. Neurosci. Methods 2003, 128, 95–102. [Google Scholar] [CrossRef]
- Van Meurs, J.B.J.; Van Lent, P.L.E.M.; Joosten, L.A.B.; Van der Kraan, P.M.; Van den Berg, W.B. Quantification of mRNA levels in joint capsule and articular cartilage of the murine knee joint by RT-PCR: Kinetics of stromelysin and IL-1 mRNA levels during arthritis. Rheumatol. Int. 1997, 16, 197–205. [Google Scholar] [CrossRef]
- Raoof, R.; Martin Gil, C.; Lafeber, F.; de Visser, H.; Prado, J.; Versteeg, S.; Pascha, M.N.; Heinemans, A.L.P.; Adolfs, Y.; Pasterkamp, J.; et al. Dorsal Root Ganglia Macrophages Maintain Osteoarthritis Pain. J. Neurosci. Off. J. Soc. Neurosci. 2021, 41, 8249–8261. [Google Scholar] [CrossRef] [PubMed]
- Nishizuka, M.; Katoh-Semba, R.; Eto, K.; Arai, Y.; Iizuka, R.; Kato, K. Age- and sex-related differences in the nerve growth factor distribution in the rat brain. Brain Res. Bull. 1991, 27, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.L.; Beck, C.; Perez-Polo, J.R. Sex differences in nerve growth factor levels in superior cervical ganglia and pineals. Int. J. Dev. Neurosci. 1987, 5, 383–390. [Google Scholar] [CrossRef]
- Iannitelli, A.; Tirassa, P.; Fiore, M.; Pacitti, F.; Quartini, A.; Rosso, P.; Fico, E.; Garavini, A.; Pompili, A.; Vitali, M.; et al. Gender differences in ultradian serum levels of NGF and BDNF correlate with psychophysical traits in healthy humans. Riv. Psichiatr. 2021, 56, 314–320. [Google Scholar] [CrossRef]
- Kassem, M.; Harris, S.A.; Spelsberg, T.C.; Riggs, B.L. Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. J. Bone Miner. Res. 1996, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Galien, R.; Garcia, T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. 1997, 25, 2424–2429. [Google Scholar] [CrossRef]
- Mun, C.J.; Letzen, J.E.; Nance, S.; Smith, M.T.; Khanuja, H.S.; Sterling, R.S.; Bicket, M.C.; Haythornthwaite, J.A.; Jamison, R.N.; Edwards, R.R.; et al. Sex Differences in Interleukin-6 Responses over Time Following Laboratory Pain Testing among Patients with Knee Osteoarthritis. J. Pain Off. J. Am. Pain Soc. 2020, 21, 731–741. [Google Scholar] [CrossRef]
- Perruccio, A.V.; Badley, E.M.; Power, J.D.; Canizares, M.; Kapoor, M.; Rockel, J.; Chandran, V.; Gandhi, R.; Mahomed, N.M.; Davey, J.R.; et al. Sex differences in the relationship between individual systemic markers of inflammation and pain in knee osteoarthritis. Osteoarthr. Cartil. Open 2019, 1, 100004. [Google Scholar] [CrossRef]
- Clauser, S.; Peyrard, S.V.; Gaussem, P.; Crespin, M.; Emmerich, J.; Aiach, M.; Borgel, D. Development of a Novel Immunoassay for the Assessment of Plasma Gas6 Concentrations and Their Variation with Hormonal Status. Clin. Chem. 2007, 53, 1808–1813. [Google Scholar] [CrossRef]
- Hung, Y.-J.; Lee, C.-H.; Shieh, Y.-S.; Hsiao, F.-C.; Lin, F.-H.; Hsieh, C.-H. Gender differences in plasma growth arrest-specific protein 6 levels in adult subjects. Clin. Chim. Acta 2015, 441, 1–5. [Google Scholar] [CrossRef]
- Woitowich, N.C.; Beery, A.; Woodruff, T. A 10-year follow-up study of sex inclusion in the biological sciences. Elife 2020, 9, e56344. [Google Scholar] [CrossRef] [PubMed]
- Dakin, P.; DiMartino, S.J.; Gao, H.; Maloney, J.; Kivitz, A.J.; Schnitzer, T.J.; Stahl, N.; Yancopoulos, G.D.; Geba, G.P. The Efficacy, Tolerability, and Joint Safety of Fasinumab in Osteoarthritis Pain: A Phase IIb/III Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Arthritis Rheumatol. 2019, 71, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Shoji, S.; Suzuki, A.; Gaitonde, P.; Cai, C.-H.; Marshall, S. Population pharmacokinetics of tanezumab following intravenous or subcutaneous administration to patients with osteoarthritis or chronic low back pain. Br. J. Clin. Pharmacol. 2022, 88, 3321–3334. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C.; Tive, L.A.; Abramson, S.B.; Vignon, E.; Verburg, K.M.; West, C.R.; Smith, M.D.; Hungerford, D.S. When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016, 68, 382–391. [Google Scholar] [CrossRef]
- Golden, T.N.; Venosa, A.; Gow, A.J. Cell Origin and iNOS Function Are Critical to Macrophage Activation Following Acute Lung Injury. Front. Pharmacol. 2021, 12, 761496. [Google Scholar] [CrossRef]
- Vago, J.P.; Amaral, F.A.; van de Loo, F.A.J. Resolving inflammation by TAM receptor activation. Pharmacol. Ther. 2021, 227, 107893. [Google Scholar] [CrossRef] [PubMed]
- Scotland, R.S.; Stables, M.J.; Madalli, S.; Watson, P.; Gilroy, D.W. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 2011, 118, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Owlett, L.D.; Karaahmet, B.; Le, L.; Belcher, E.K.; Dionisio-Santos, D.; Olschowka, J.A.; Elliott, M.R.; O’Banion, M.K. Gas6 induces inflammation and reduces plaque burden but worsens behavior in a sex-dependent manner in the APP/PS1 model of Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 38. [Google Scholar] [CrossRef]
- Ni, S.; Li, D.; Wei, H.; Miao, K.-S.; Zhuang, C. PPARγ Attenuates Interleukin-1β-Induced Cell Apoptosis by Inhibiting NOX2/ROS/p38MAPK Activation in Osteoarthritis Chondrocytes. Oxidative Med. Cell. Longev. 2021, 2021, 5551338. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yu, H.H.; Lau, Y.L.; Chan, K.W.; Chiang, B.L. A new autosomal recessive, heterozygous pair of mutations of CYBA in a patient with chronic granulomatous disease. Ann. Allergy Asthma. Immunol. 2010, 105, 183–185. [Google Scholar] [CrossRef]
- Nunoi, H.; Nakamura, H.; Nishimura, T.; Matsukura, M. Recent topics and advanced therapies in chronic granulomatous disease. Human Cell 2023, 36, 515–527. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; Starkey Lewis, P.J.; Pellicoro, A.; Aucott, R.L.; Man, J.; O’Duibhir, E.; Mok, S.E.; Ferreira-Gonzalez, S.; Livingstone, E.; Greenhalgh, S.N.; et al. The STAT3-IL-10-IL-6 Pathway Is a Novel Regulator of Macrophage Efferocytosis and Phenotypic Conversion in Sterile Liver Injury. J. Immunol. 2018, 200, 1169–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Choksi, S.; Qu, J.; Jang, J.; Choe, M.; Banfi, B.; Engelhardt, J.F.; Liu, Z.G. NADPH Oxidases Are Essential for Macrophage Differentiation. J. Biol. Chem. 2016, 291, 20030–20041. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Diaz, M.; Song, M.; Heller, N. Androgen and Androgen Receptors as Regulators of Monocyte and Macrophage Biology in the Healthy and Diseased Lung. Front. Immunol. 2020, 11, 1698. [Google Scholar] [CrossRef]
- Martinon, F. Signaling by ROS drives inflammasome activation. Eur. J. Immunol. 2010, 40, 616–619. [Google Scholar] [CrossRef]
- Kou, X.X.; Li, C.S.; He, D.Q.; Wang, X.D.; Hao, T.; Meng, Z.; Zhou, Y.H.; Gan, Y.H. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. J. Immunol. 2015, 194, 2810–2818. [Google Scholar] [CrossRef]
| Gene | Full Name | Forward Primer | Reverse Primer |
|---|---|---|---|
| Tlr2 | Toll-like receptor 2 | 5′-aacctcagacaaagcgtcaaatc-3′ | 5′-accaagatccagaagagccaaa-3′ |
| Tlr4 | Toll-like receptor 4 | 5′-ttccttcttcaaccaagaacatagatc-3′ | 5′-ttgtttcaatttcacacctggataa-3′ |
| S100a8 | S100 calcium-binding protein A8 | 5′-tgtcctcagtttgtgcagaatataaat-3′ | 5′-tttatcaccatcgcaaggaactc-3′ |
| S100a9 | S100 calcium-binding protein A9 | 5′-ggcaaaggctgtgggaagt-3′ | 5′-ccattgagtaagccattcccttta-3′ |
| Ccl2 (MCP-1) | C-C motif chemokine 2 | 5′-ttggctcagccagatgca-3′ | 5′-cctactcattgggatcatcttgct-3′ |
| Cxcl1 (KC) | C-x-C motif chemokine ligand 1 | 5′-tggctgggattcacctcaa-3′ | 5′-gagtgtggctatgacttcggttt-3′ |
| Il1b | Interleukin-1 beta | 5′-ggacagaatatcaaccaacaagtgata-3′ | 5′-gtgtgccgtctttcattacacag-3′ |
| Tnfa | Tumor necrosis factor alfa | 5′-cagaccctcacactcagatcatct-3′ | 5′-cctccacttggtggtttgcta-3′ |
| Il6 | Interleukin-6 | 5′-caagtcggaggcttaattacacatg-3′ | 5′-attgccattgcacaactcttttct-3′ |
| Il10 | Interleukin-10 | 5′-atttgaattccctgggtgagaa-3′ | 5′-acaccttggtcttggagcttattaa-3′ |
| Cyba | Cytochrome b-245, alpha polypeptide | 5′-gaggcaccatcaagcaacca-3′ | 5′-caccctcactcggcttcttt-3′ |
| Cybb (NOX2) | Cytochrome b-245, beta polypeptide | 5′-accctcctatgacttggaaatgg-3′ | 5′-cgaaccaacctctcacaaaggt-3′ |
| Ncf4 | Neutrophil cytosolic factor 4 | 5′-ccaactggctacgatgctactt-3′ | 5′-tctctggaactcacgcctcatg-3′ |
| Nos2 | Nitric oxide synthase, inducible | 5′-cgtttcgggatctgaatgtga-3′ | 5′-gggcagcctgtgagacctt-3′ |
| Ngf | Nerve growth factor | 5′-tgcggccagtatagaaagct-3′ | 5′-ggggagcgcatcgagtttt-3′ |
| Emr1(F4/80) | EGF module-containing mucin-like receptor | 5′-actgtggaaagcaccatgttag-3′ | 5′-gctgccaagttaatggactca-3′ |
| Cd86 | Cluster of Differentiation 86 | 5′-agtgatcgccaacttcagtgaac-3′ | 5′-gcaggtcaaatttatgccagaat-3′ |
| Arginase1 | Arginase1 | 5′-gaaagttcccagatgtaccaggat-3′ | 5′-cgatgtctttggcagatatgca-3′ |
| Gas6 | Growth arrest-specific protein 6 | 5′-cctgccagaagtatcggtgatt-3′ | 5′-gtccaggattttcccgtttacc-3′ |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | 5′-cctgccagaagtatcggtgatt-3′ | 5′-gtccaggattttcccgtttacc-3′ |
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| First Response | Second Response | 1st × 2nd | First Response | Second Response | 1st × 2nd | ||
| Receptors | |||||||
| Tlr2 | 3.1 ± 0.9 * | 4.6 ± 1.5 *** | ns | 4.4 ± 1.1 *** | 6.3 ± 2.2 **** | ns | |
| Tlr4 | 4.5 ± 0.4 **** | 4.6 ± 0.8 **** | ns | 2.5 ± 0.8 **** | 3.3 ± 0.3 **** | ns | |
| Alarmins | |||||||
| S100a8 | 3.9 ± 1.4 * | 5.7 ± 2.1 *** | ns | 7.2 ± 1.5 **** | 10.3 ± 2.2 **** | ** | |
| S100a9 | 3.7 ± 1.4 * | 5.5 ± 2.2 *** | * | 7.3 ± 1.5 **** | 10.0 ± 1.9 **** | * | |
| Chemokines | |||||||
| Ccl2 (MCP-1) | 3.9 ± 1.1 *** | 4.8 ± 0.9 **** | ns | 5.4 ± 0.9 **** | 5.2 ± 0.8 **** | ns | |
| Cxcl1 (KC) | 4.2 ± 1.5 *** | 5.9 ± 1.5 **** | ns | 5.2 ± 1.4 **** | 6.4 ± 0.9 **** | ns | |
| Cytokines | |||||||
| Il1b | 4.1 ± 1.3 ** | 5.0 ± 2.3 *** | ns | 7.8 ± 1.6 **** | 9.2 ± 1.2 **** | ns | |
| Tnfa | 4.5 ± 0.9 *** | 6.5 ± 1.7 **** | ns | 4.9 ± 1.4 **** | 6.9 ± 1.2 **** | * | |
| Il6 | 4.1 ± 0.7 **** | 4.8 ± 1.2 **** | ns | 5.7 ± 1.0 **** | 7.1 ± 1.1 **** | * | |
| Il10 | 2.9 ± 1.2 * | 4.0 ± 1.0 ** | ns | 5.0 ± 1.6 **** | 6.3 ± 1.3 **** | ns | |
| Redox signaling | |||||||
| Cybb (NOX2) | 3.6 ± 1.3 ** | 5.9 ± 1.7 **** | * | 4.7 ± 1.1 **** | 6.2 ± 0.7 **** | ns | |
| Ncf4 | 3.5 ± 1.2 ** | 5.4 ± 1.7 **** | * | 4.7 ± 1.1 **** | 6.5 ± 0.5 **** | * | |
| Nos2 | 2.1 ± 1.0 ns | 3.8 ± 2.2 * | ns | 2.2 ± 1.3 ns | 4.2 ± 1.8 ** | ns | |
| Neurotropic factor | |||||||
| Ngf | 0.6 ± 0.6 ns | 2.1 ± 0.7 ** | ** | 1.7 ± 0.6 ** | 2.1 ± 1.0 *** | ns | |
| Immune cells | |||||||
| Emr1(F4/80) | 2.8 ± 0.9 ** | 4.3 ± 1.4 *** | ns | 3.6 ± 1.3 *** | 5.7 ± 1.2 **** | ** | |
| Cd86 | 2.7 ± 1.0 * | 4.2 ± 1.7 ** | ns | 3.3 ± 1.2 ** | 5.4 ± 1.9 *** | * | |
| Arginase1 | 3.8 ± 1.9 * | 5.1 ± 2.3 ** | ns | 6.5 ± 1.2 **** | 8.8 ± 1.3 **** | ns | |
| Pro-resolution | |||||||
| Gas6 | 1.3 ± 0.2 ns | 3.0 ± 1.4 *** | ** | 2.6 ± 0.9 *** | 4.3 ± 0.6 **** | ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdrighi, N.; Blom, A.B.; Vago, J.P.; van Beuningen, H.M.; Vitters, E.L.; Helsen, M.M.; Walgreen, B.; Arntz, O.J.; Koenders, M.I.; van der Kraan, P.M.; et al. Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis. Cells 2023, 12, 1913. https://doi.org/10.3390/cells12141913
Valdrighi N, Blom AB, Vago JP, van Beuningen HM, Vitters EL, Helsen MM, Walgreen B, Arntz OJ, Koenders MI, van der Kraan PM, et al. Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis. Cells. 2023; 12(14):1913. https://doi.org/10.3390/cells12141913
Chicago/Turabian StyleValdrighi, Natália, Arjen B. Blom, Juliana P. Vago, Henk M. van Beuningen, Elly L. Vitters, Monique M. Helsen, Birgitte Walgreen, Onno J. Arntz, Marije I. Koenders, Peter M. van der Kraan, and et al. 2023. "Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis" Cells 12, no. 14: 1913. https://doi.org/10.3390/cells12141913
APA StyleValdrighi, N., Blom, A. B., Vago, J. P., van Beuningen, H. M., Vitters, E. L., Helsen, M. M., Walgreen, B., Arntz, O. J., Koenders, M. I., van der Kraan, P. M., Blaney Davidson, E. N., & van de Loo, F. A. J. (2023). Innate Immunity and Sex: Distinct Inflammatory Profiles Associated with Murine Pain in Acute Synovitis. Cells, 12(14), 1913. https://doi.org/10.3390/cells12141913








