Roles of Extracellular Vesicles on the Progression and Metastasis of Hepatocellular Carcinoma
Abstract
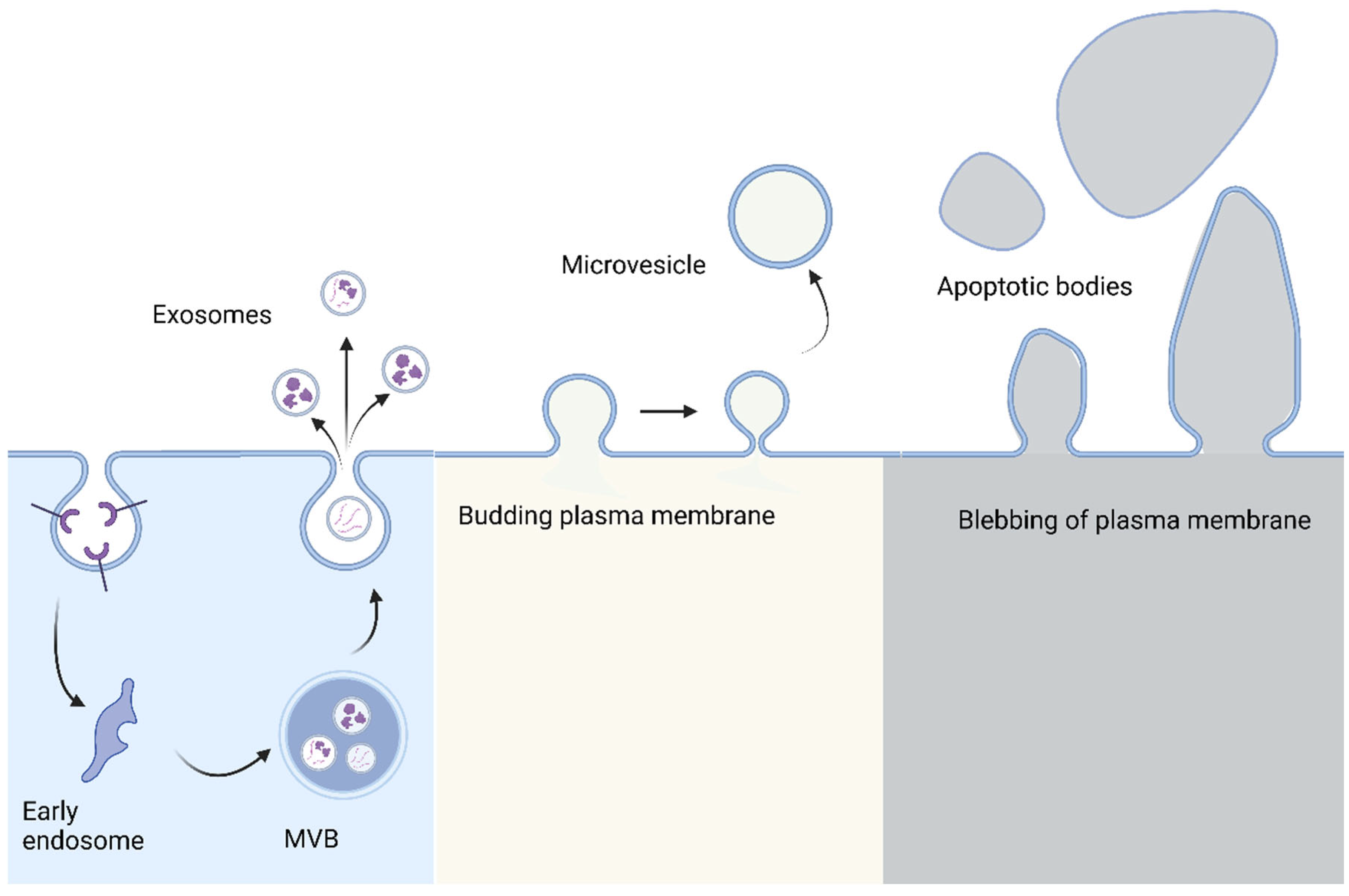
1. Introduction
2. Effect of Glycolytic Enzymes as EV Cargos on HCC Progression
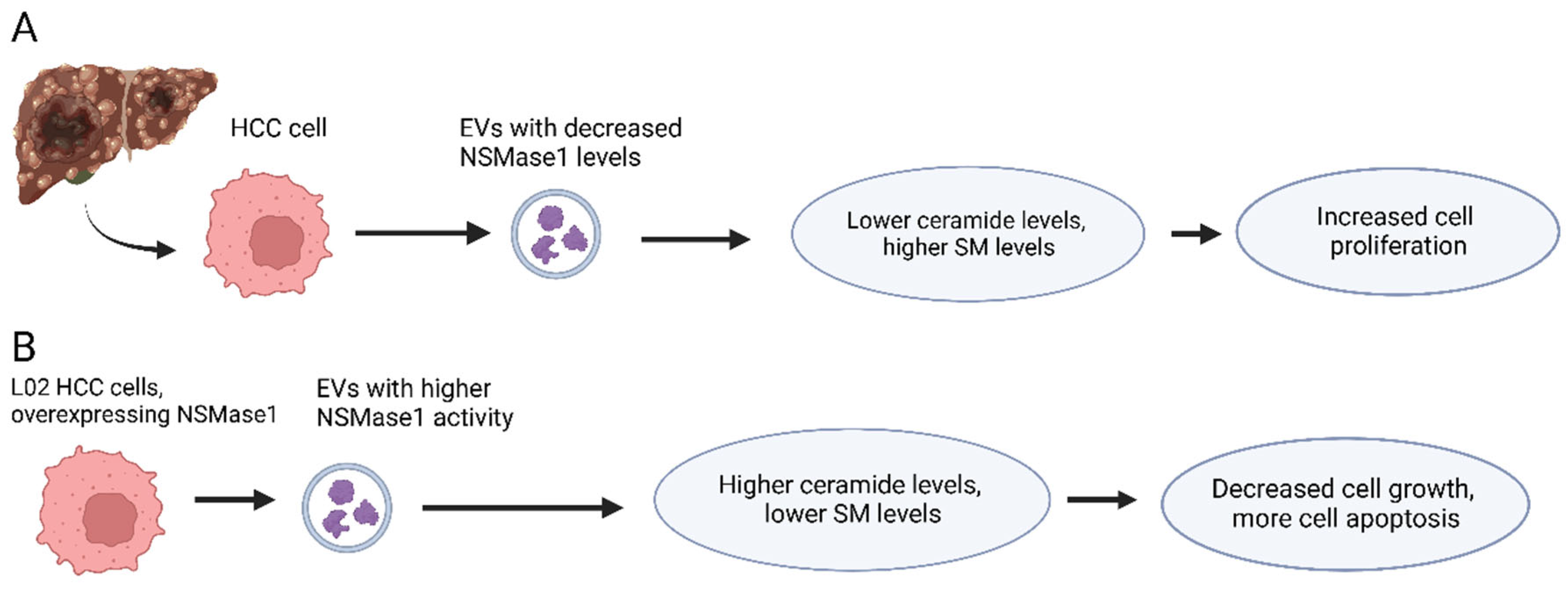
3. Caspase-3 Activators Incorporated into EVs Affect HCC Development
4. EV-Bound p120 Catenin Effects on HCC Proliferation and Metastasis
5. Upregulation of the 14-3-3ζ Protein Impairs Antitumor Functions
6. Impact of Lysyl Oxidase-Containing EVs on HCC Proliferation and Migration
7. Targeting the Complement System in HCC Progression
8. Encapsulated Circular RNAs Affect the Progression of HCC
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer, C.; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Kishta, S.; Tabll, A.; Omanovic Kolaric, T.; Smolic, R.; Smolic, M. Risk Factors Contributing to the Occurrence and Recurrence of Hepatocellular Carcinoma in Hepatitis C Virus Patients Treated with Direct-Acting Antivirals. Biomedicines 2020, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005 e1001. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef]
- Bonucci, E. Fine structure and histochemistry of “calcifying globules” in epiphyseal cartilage. Z. Zellforsch. Mikrosk. Anat. 1970, 103, 192–217. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; D’Asti, E.; Magnus, N.; Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles as mediators of intercellular communication in cancer—The emerging science of cellular ‘debris’. Semin. Immunopathol. 2011, 33, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Wurdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Sena-Esteves, M.; Curry, W.T., Jr.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Read, J.; Ingram, A.; Al Saleh, H.A.; Platko, K.; Gabriel, K.; Kapoor, A.; Pinthus, J.; Majeed, F.; Qureshi, T.; Al-Nedawi, K. Nuclear transportation of exogenous epidermal growth factor receptor and androgen receptor via extracellular vesicles. Eur. J. Cancer 2017, 70, 62–74. [Google Scholar] [CrossRef]
- Gabriel, K.; Ingram, A.; Austin, R.; Kapoor, A.; Tang, D.; Majeed, F.; Qureshi, T.; Al-Nedawi, K. Regulation of the tumor suppressor PTEN through exosomes: A diagnostic potential for prostate cancer. PLoS ONE 2013, 8, e70047. [Google Scholar] [CrossRef] [PubMed]
- Platko, K.; Haas-Neill, S.; Aziz, T.; Al-Nedawi, K. The role of circulating extracellular vesicles in breast cancer classification and molecular subtyping. Breast J. 2019, 25, 691–695. [Google Scholar] [CrossRef]
- Trevisan Franca de Lima, L.; Broszczak, D.; Zhang, X.; Bridle, K.; Crawford, D.; Punyadeera, C. The use of minimally invasive biomarkers for the diagnosis and prognosis of hepatocellular carcinoma. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188451. [Google Scholar] [CrossRef]
- Von Felden, J.; Garcia-Lezana, T.; Schulze, K.; Losic, B.; Villanueva, A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut 2020, 69, 2025–2034. [Google Scholar] [CrossRef]
- Mann, J.; Reeves, H.L.; Feldstein, A.E. Liquid biopsy for liver diseases. Gut 2018, 67, 2204–2212. [Google Scholar] [CrossRef]
- Liu, B.H.M.; Tey, S.K.; Mao, X.; Ma, A.P.Y.; Yeung, C.L.S.; Wong, S.W.K.; Ng, T.H.; Xu, Y.; Yao, Y.; Fung, E.Y.M.; et al. TPI1-reduced extracellular vesicles mediated by Rab20 downregulation promotes aerobic glycolysis to drive hepatocarcinogenesis. J. Extracell. Vesicles 2021, 10, e12135. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, P.; He, Y.; Chen, Z.; Chen, L.; Luo, Y.; Qi, L.; Liu, Y.; Wu, Q.; Cui, Y.; et al. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.P.; Luo, L.J.; Chen, H.Z.; Chen, Q.T.; Bian, X.L.; Wu, S.F.; Zhou, J.X.; Zhao, W.X.; Liu, J.M.; Wang, X.M.; et al. Ectosomal PKM2 Promotes HCC by Inducing Macrophage Differentiation and Remodeling the Tumor Microenvironment. Mol. Cell 2020, 78, 1192–1206 e1110. [Google Scholar] [CrossRef] [PubMed]
- Didiasova, M.; Schaefer, L.; Wygrecka, M. When Place Matters: Shuttling of Enolase-1 Across Cellular Compartments. Front. Cell Dev. Biol. 2019, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, H.; Yu, Y.; Chen, J.; Chen, X.; Ren, F.; Ren, Z.; Cui, G. Enolase-1 serves as a biomarker of diagnosis and prognosis in hepatocellular carcinoma patients. Cancer Manag. Res. 2018, 10, 5735–5745. [Google Scholar] [CrossRef]
- Jiang, K.; Dong, C.; Yin, Z.; Li, R.; Mao, J.; Wang, C.; Zhang, J.; Gao, Z.; Liang, R.; Wang, Q.; et al. Exosome-derived ENO1 regulates integrin alpha6beta4 expression and promotes hepatocellular carcinoma growth and metastasis. Cell Death Dis. 2020, 11, 972. [Google Scholar] [CrossRef]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Haimovitz-Friedman, A.; Kolesnick, R.N.; Fuks, Z. Ceramide signaling in apoptosis. Br. Med. Bull. 1997, 53, 539–553. [Google Scholar] [CrossRef]
- Krautbauer, S.; Meier, E.M.; Rein-Fischboeck, L.; Pohl, R.; Weiss, T.S.; Sigruener, A.; Aslanidis, C.; Liebisch, G.; Buechler, C. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1767–1774. [Google Scholar] [CrossRef]
- Lin, M.; Liao, W.; Dong, M.; Zhu, R.; Xiao, J.; Sun, T.; Chen, Z.; Wu, B.; Jin, J. Exosomal neutral sphingomyelinase 1 suppresses hepatocellular carcinoma via decreasing the ratio of sphingomyelin/ceramide. FEBS J. 2018, 285, 3835–3848. [Google Scholar] [CrossRef] [PubMed]
- Van Hengel, J.; van Roy, F. Diverse functions of p120ctn in tumors. Biochim. Biophys. Acta 2007, 1773, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, M.A.; Reynolds, A.B. Altered expression of the catenin p120 in human cancer: Implications for tumor progression. Differentiation 2002, 70, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bonnomet, A.; Polette, M.; Strumane, K.; Gilles, C.; Dalstein, V.; Kileztky, C.; Berx, G.; van Roy, F.; Birembaut, P.; Nawrocki-Raby, B. The E-cadherin-repressed hNanos1 gene induces tumor cell invasion by upregulating MT1-MMP expression. Oncogene 2008, 27, 3692–3699. [Google Scholar] [CrossRef]
- Berx, G.; van Roy, F. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a003129. [Google Scholar] [CrossRef]
- Pieters, T.; van Roy, F.; van Hengel, J. Functions of p120ctn isoforms in cell-cell adhesion and intracellular signaling. Front. Biosci. 2012, 17, 1669–1694. [Google Scholar] [CrossRef]
- Cheng, Z.; Lei, Z.; Yang, P.; Si, A.; Xiang, D.; Tang, X.; Guo, G.; Zhou, J.; Huser, N. Exosome-transmitted p120-catenin suppresses hepatocellular carcinoma progression via STAT3 pathways. Mol. Carcinog. 2019, 58, 1389–1399. [Google Scholar] [CrossRef]
- Han, Q.; Lv, L.; Wei, J.; Lei, X.; Lin, H.; Li, G.; Cao, J.; Xie, J.; Yang, W.; Wu, S.; et al. Vps4A mediates the localization and exosome release of beta-catenin to inhibit epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2019, 457, 47–59. [Google Scholar] [CrossRef]
- Wei, J.X.; Lv, L.H.; Wan, Y.L.; Cao, Y.; Li, G.L.; Lin, H.M.; Zhou, R.; Shang, C.Z.; Cao, J.; He, H.; et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology 2015, 61, 1284–1294. [Google Scholar] [CrossRef]
- Wang, X.; Shen, H.; Zhangyuan, G.; Huang, R.; Zhang, W.; He, Q.; Jin, K.; Zhuo, H.; Zhang, Z.; Wang, J.; et al. 14-3-3zeta delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018, 9, 159. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wen, Y.Y.; Chen, C.H.; Lozano, G.; Lee, M.H. 14-3-3 sigma positively regulates p53 and suppresses tumor growth. Mol. Cell Biol. 2003, 23, 7096–7107. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liu, S.; Li, N.; Guo, W.; Shi, J.; Yu, H.; Zhang, L.; Wang, K.; Liu, S.; Cheng, S. 14-3-3ζ promotes hepatocellular carcinoma venous metastasis by modulating hypoxia-inducible factor-1α. Oncotarget 2016, 7, 15854–15867. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Y.; Wang, N.; Zhang, C.; Chan, Y.T.; Yuen, M.F.; Feng, Y. Lysyl Oxidase-Like 4 Fosters an Immunosuppressive Microenvironment During Hepatocarcinogenesis. Hepatology 2021, 73, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, Y.; Zhang, X.; Feng, M.; Ma, J.; Li, J.; Yang, X.; Fang, F.; Xia, Q.; Zhang, Z.; et al. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol. Cancer 2019, 18, 18. [Google Scholar] [CrossRef]
- Janeway, C.A.J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Mao, X.; Zhou, L.; Tey, S.K.; Ma, A.P.Y.; Yeung, C.L.S.; Ng, T.H.; Wong, S.W.K.; Liu, B.H.M.; Fung, Y.M.E.; Patz, E.F., Jr.; et al. Tumour extracellular vesicle-derived Complement Factor H promotes tumorigenesis and metastasis by inhibiting complement-dependent cytotoxicity of tumour cells. J. Extracell. Vesicles 2020, 10, e12031. [Google Scholar] [CrossRef]
- Laskowski, J.; Renner, B.; Pickering, M.C.; Serkova, N.J.; Smith-Jones, P.M.; Clambey, E.T.; Nemenoff, R.A.; Thurman, J.M. Complement factor H–deficient mice develop spontaneous hepatic tumors. J. Clin. Investig. 2020, 130, 4039–4054. [Google Scholar] [CrossRef]
- Szabo, L.; Salzman, J. Detecting circular RNAs: Bioinformatic and experimental challenges. Nat. Rev. Genet. 2016, 17, 679–692. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21. [Google Scholar] [CrossRef]
- Vromman, M.; Vandesompele, J.; Volders, P.J. Closing the circle: Current state and perspectives of circular RNA databases. Brief. Bioinform. 2021, 22, 288–297. [Google Scholar] [CrossRef]
- Xue, C.; Gu, X.; Bao, Z.; Su, Y.; Lu, J.; Li, L. The mechanism underlying the ncRNA dysregulation pattern in hepatocellular carcinoma and its tumor microenvironment. Front. Immunol. 2022, 13, 847728. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, G.; Zhao, Y.; Gao, H.; Li, L.; Yin, Y.; Jiang, J.; Wang, L.; Mang, Y.; Gao, Y.; et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol. Cancer 2023, 22, 55. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, W.; Zhuo, H.; Zhu, D.; Rong, D.; Sun, J.; Song, J. Cancer-associated fibroblast exosomes promote chemoresistance to cisplatin in hepatocellular carcinoma through circZFR targeting signal transducers and activators of transcription (STAT3)/ nuclear factor -kappa B (NF-κB) pathway. Bioengineered 2022, 13, 4786–4797. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. An Effective Peptide-Based Platform for Efficient Exosomal Loading and Cellular Delivery of a microRNA. ACS Appl. Mater. Interfaces 2023, 15, 3851–3866. [Google Scholar] [CrossRef] [PubMed]


| Protein | Source | Target | Cell Model/Line | Animal Model | Clinical Samples | Reference |
|---|---|---|---|---|---|---|
| Rab20 | HCC cells | Secretory pathway | MIHA cell line | BALB/C nude mice | Obtained from Queen Mary Hospital, Hong Kong | [21] |
| PKM2 | HCC cells | Tumor microenvironment | Derived from C57BL/6 mice bone marrow, blood, and liver | C57BL/6 mice | Obtained from Zhongshan Hospital, Xiamen University | [23] |
| ENO1 | HCC cells | Integrin α6β4 and the FAK/Src-p38MAPK pathway | HepG2, LO2, MHCC97L, MHCC97H, and HCCLM3 | BALB/c-nu/nu nude mice | Not used | [26] |
| NSMase1 | HCC cells | Sphingomyelin (SM) | SMMC7721 and LO2 | Not used | Obtained from Affiliated Hospital of Guilin Medical University | [31] |
| p120ctn | Liver cancer cells | STAT3 pathway | CSQT-2, HCCLM3 (LM3), 7701, and 7702 | Male nude mice | Obtained from Eastern Hepatobiliary Surgery Hospital | [37] |
| Vps4A | HCC cells | CHMP4B and β-catenin | SMMC-7721, MHCC97H, and Huh-7 | Not used | Obtained from Sun Yatsen Memorial Hospital, Sun Yat-sen University | [38,39] |
| 14-3-3ζ protein | HCC cells | Tumor-infiltrating T lymphocytes (TILs) | Human PBTC cells | Male C57BL/6 mice | Obtained from First Affiliated Hospital of Nanjing Medical University | [40] |
| LOXL4 | HCC cells | FAK/Src pathway | SK-Hep1, SUN-423, Hep3B, Huh7, SMMC-7721, MHCC-97 L, and MHCC-LM3 | Male BALB/c-nu/nu mice | Obtained from Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University | [45] |
| CFH | HCC cells | Complement and immune system | Hep3B, Huh7 PLC/PRF/5, human 293FT, murine Hepa1-6, HLE, MHCC97L, and MHCCLM3 | Male BALB/cAnN-nu mice, BALB/c nude mice, and C57BL/6N mice | Not used | [47] |
| circRNAs | HCC cells | miRNAs | LO2, Huh7, and MHCC97L | Not used | Obtained from First People’s Hospital of Kunming City | [51,52] |
| Topics | Relevant References |
|---|---|
| Effect of glycolytic enzymes as EV cargos on HCC progression | [21,22,23,24,25,26] |
| Caspase-3 activators incorporated into EVs affect HCC development | [27,28,29,30,31] |
| EV-bound p120 catenin effects on HCC proliferation and metastasis | [32,33,34,35,36,37,38,39] |
| Upregulation of the 14-3-3ζ protein impairs antitumor functions | [40,41,42] |
| Impact of lysyl oxidase-containing EVs on HCC proliferation and migration | [43,44,45] |
| Targeting the complement system in HCC progression | [46,47,48] |
| Encapsulated circular RNAs affect the progression of HCC | [49,50,51,52,53,54,55,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seay, T.W.; Suo, Z. Roles of Extracellular Vesicles on the Progression and Metastasis of Hepatocellular Carcinoma. Cells 2023, 12, 1879. https://doi.org/10.3390/cells12141879
Seay TW, Suo Z. Roles of Extracellular Vesicles on the Progression and Metastasis of Hepatocellular Carcinoma. Cells. 2023; 12(14):1879. https://doi.org/10.3390/cells12141879
Chicago/Turabian StyleSeay, Turner W., and Zucai Suo. 2023. "Roles of Extracellular Vesicles on the Progression and Metastasis of Hepatocellular Carcinoma" Cells 12, no. 14: 1879. https://doi.org/10.3390/cells12141879
APA StyleSeay, T. W., & Suo, Z. (2023). Roles of Extracellular Vesicles on the Progression and Metastasis of Hepatocellular Carcinoma. Cells, 12(14), 1879. https://doi.org/10.3390/cells12141879






