Innate Immune Status of Glia Modulates Prion Propagation in Early Stage of Infection
Abstract
1. Introduction
2. Materials and Methods
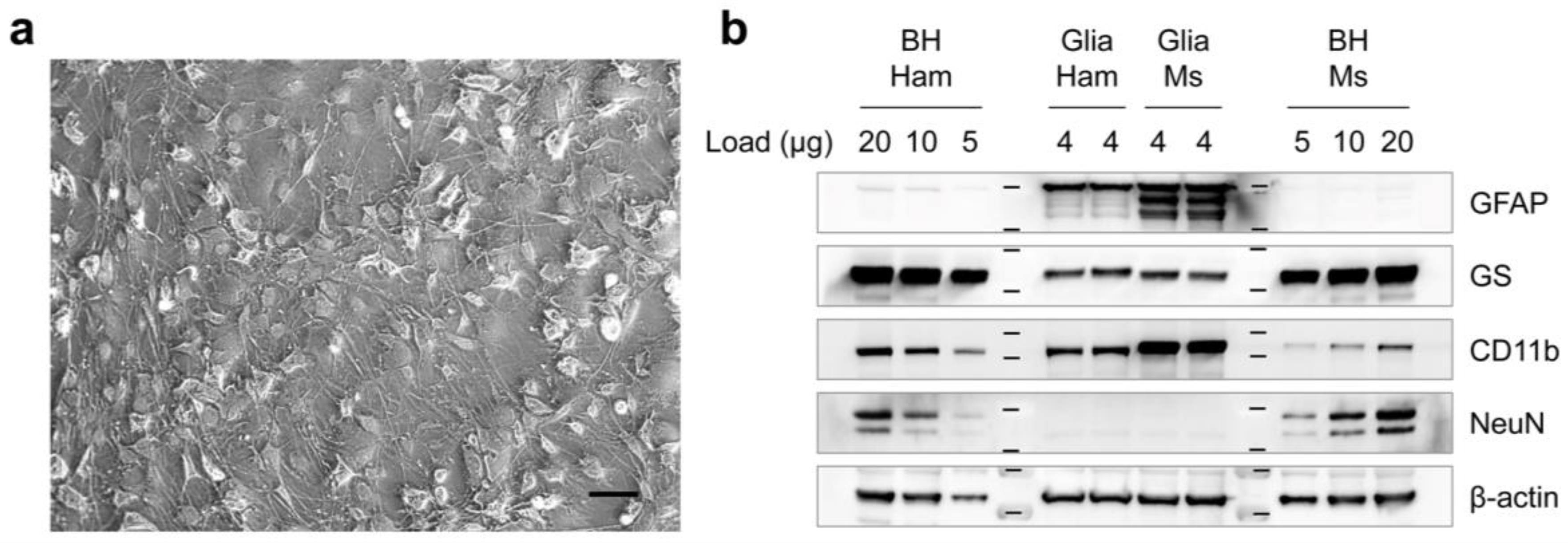
2.1. The Primary Cell Cultures and Prion Exposure
2.2. Prion Preparation (Prion-Affected Brain Homogenates)
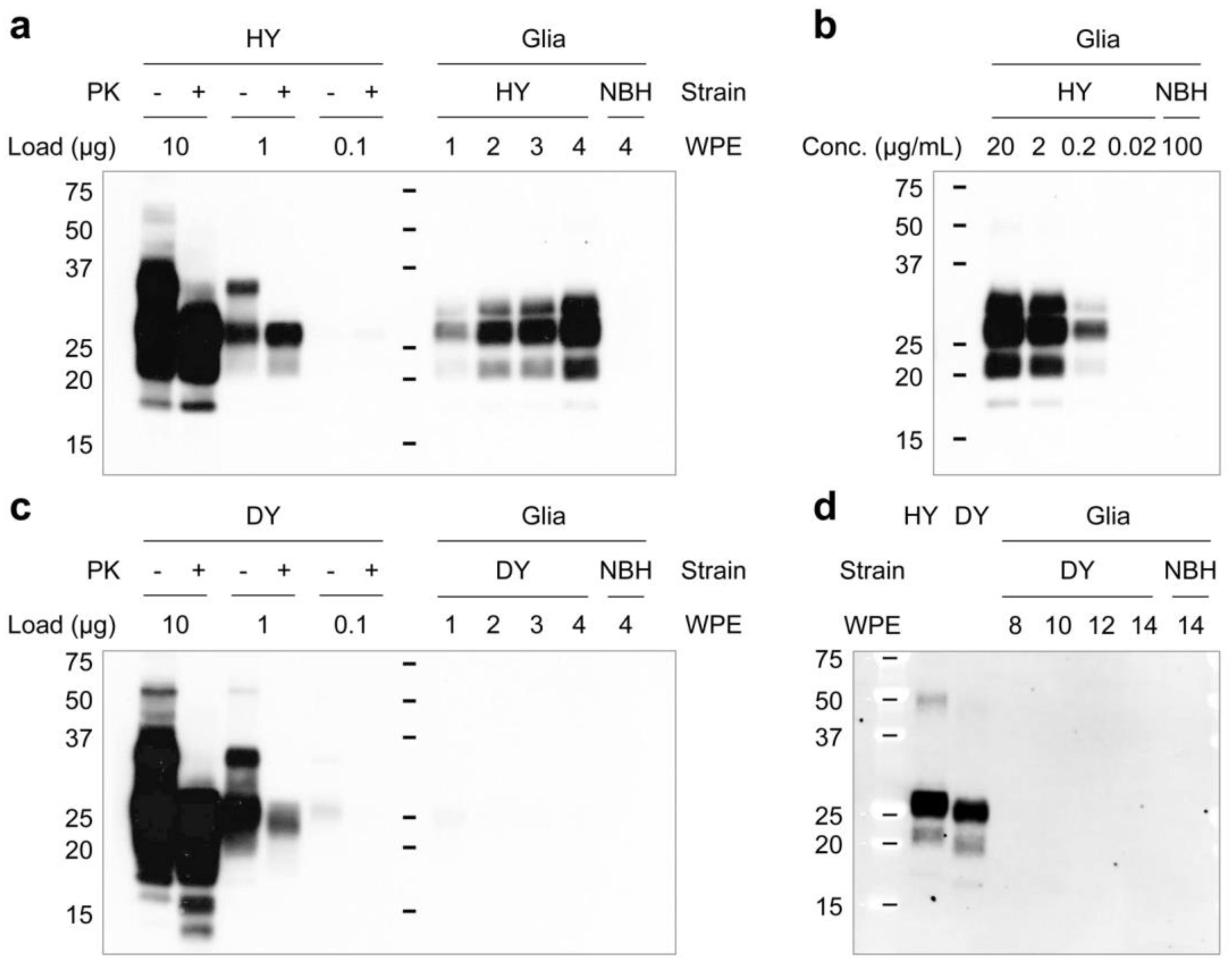
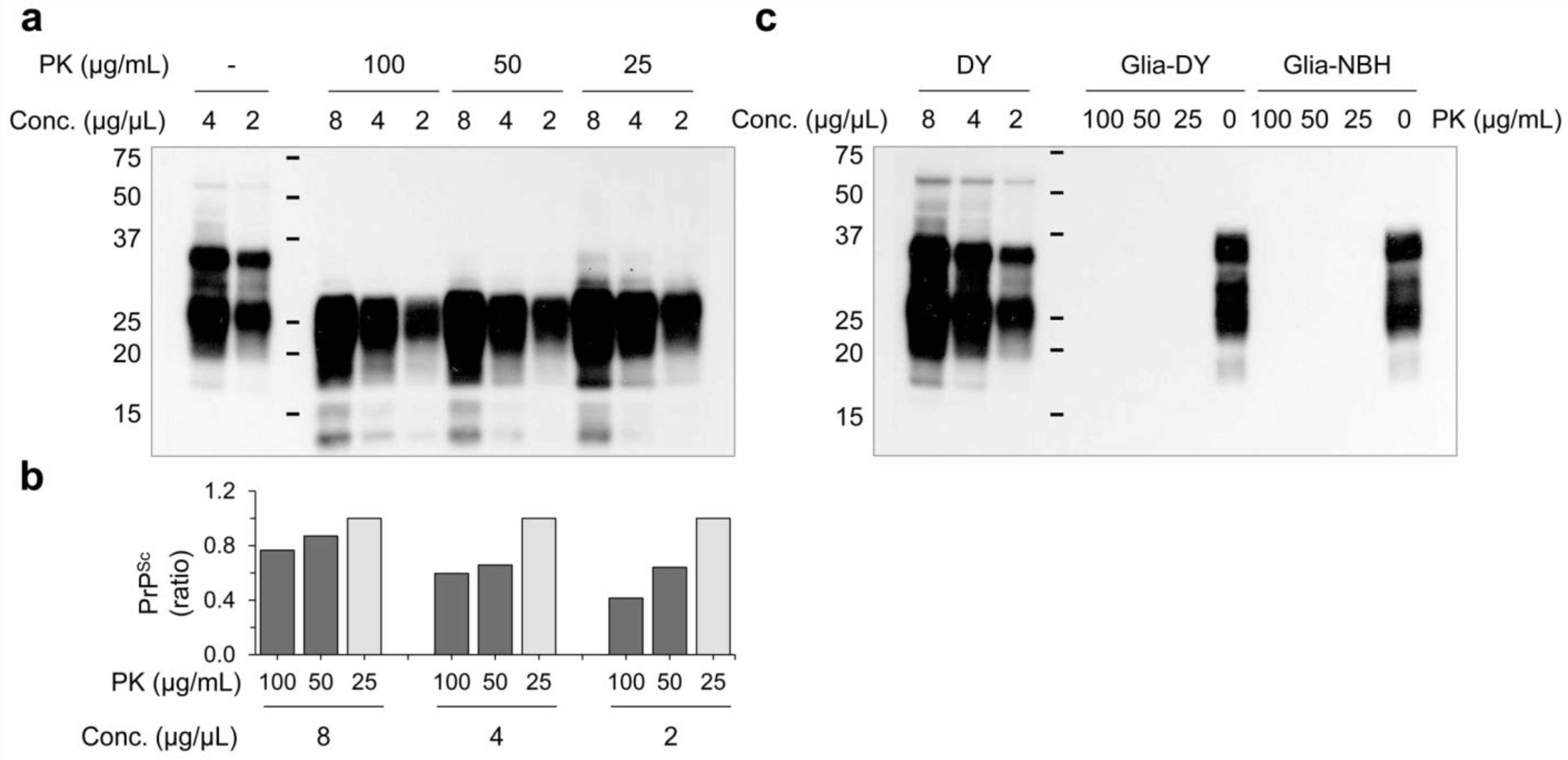
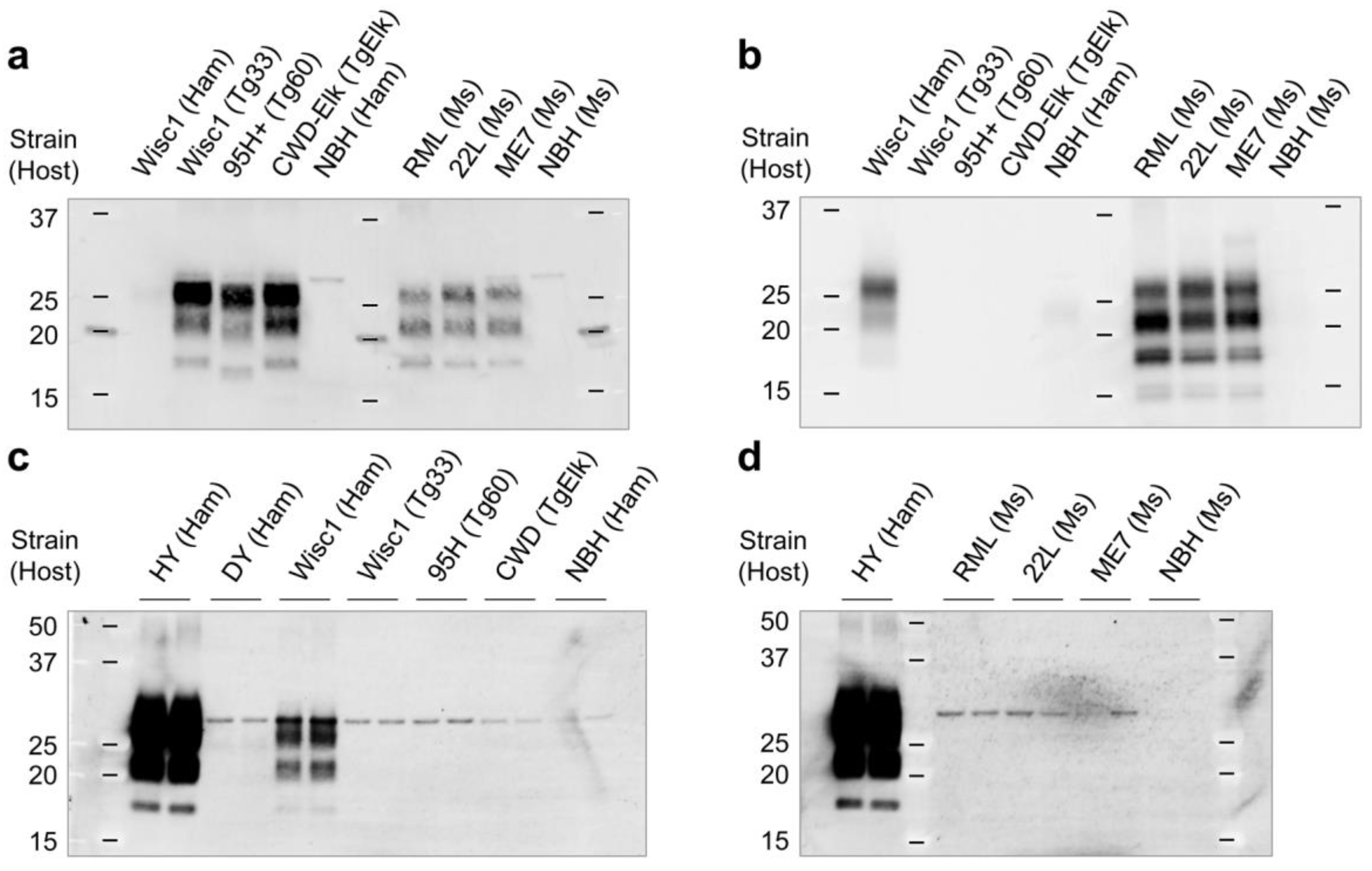
2.3. Western Blot
2.4. Immunocytochemistry
2.5. Cell Viability Assay
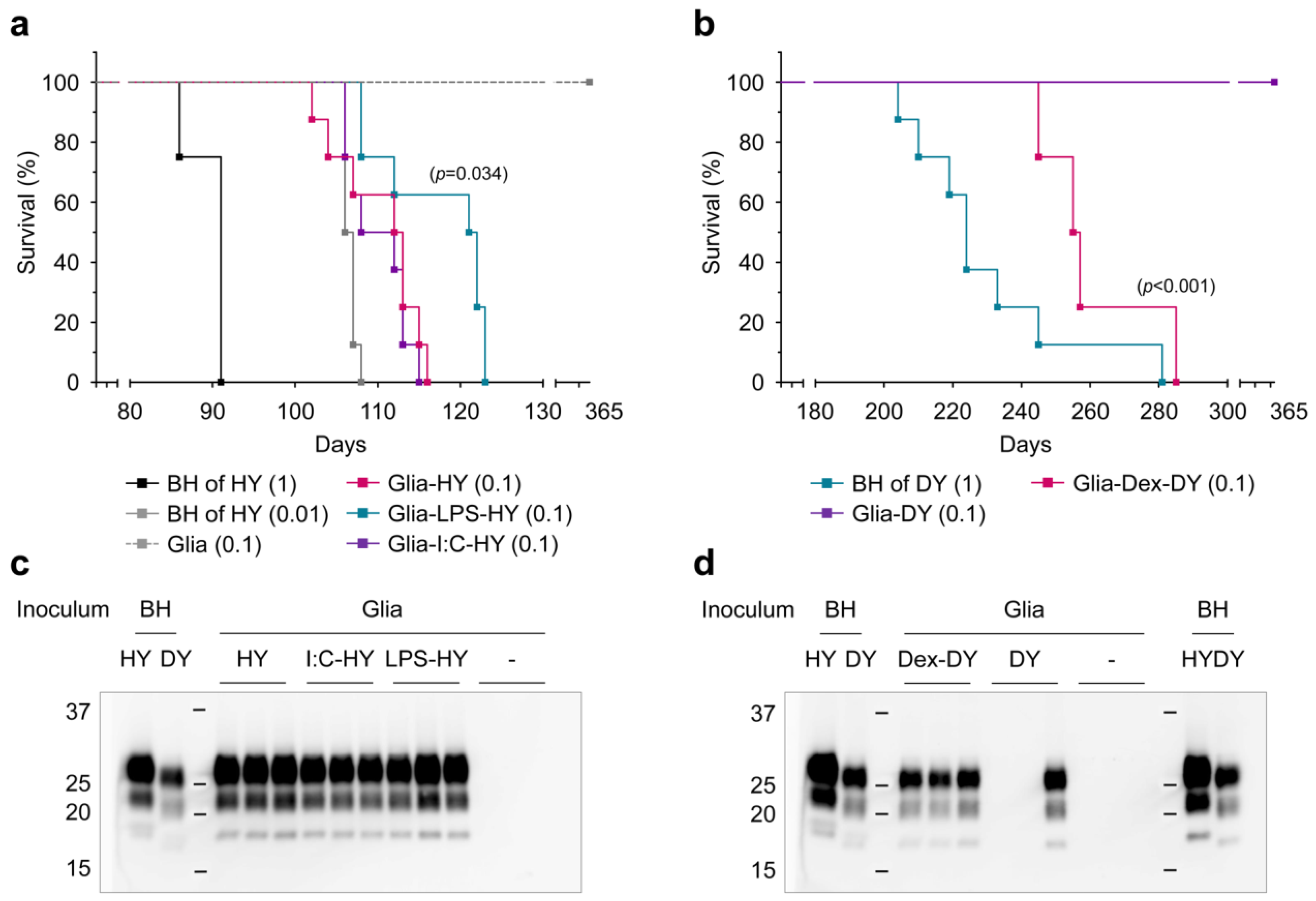
2.6. Animal Bioassay
2.7. Statistical Analysis
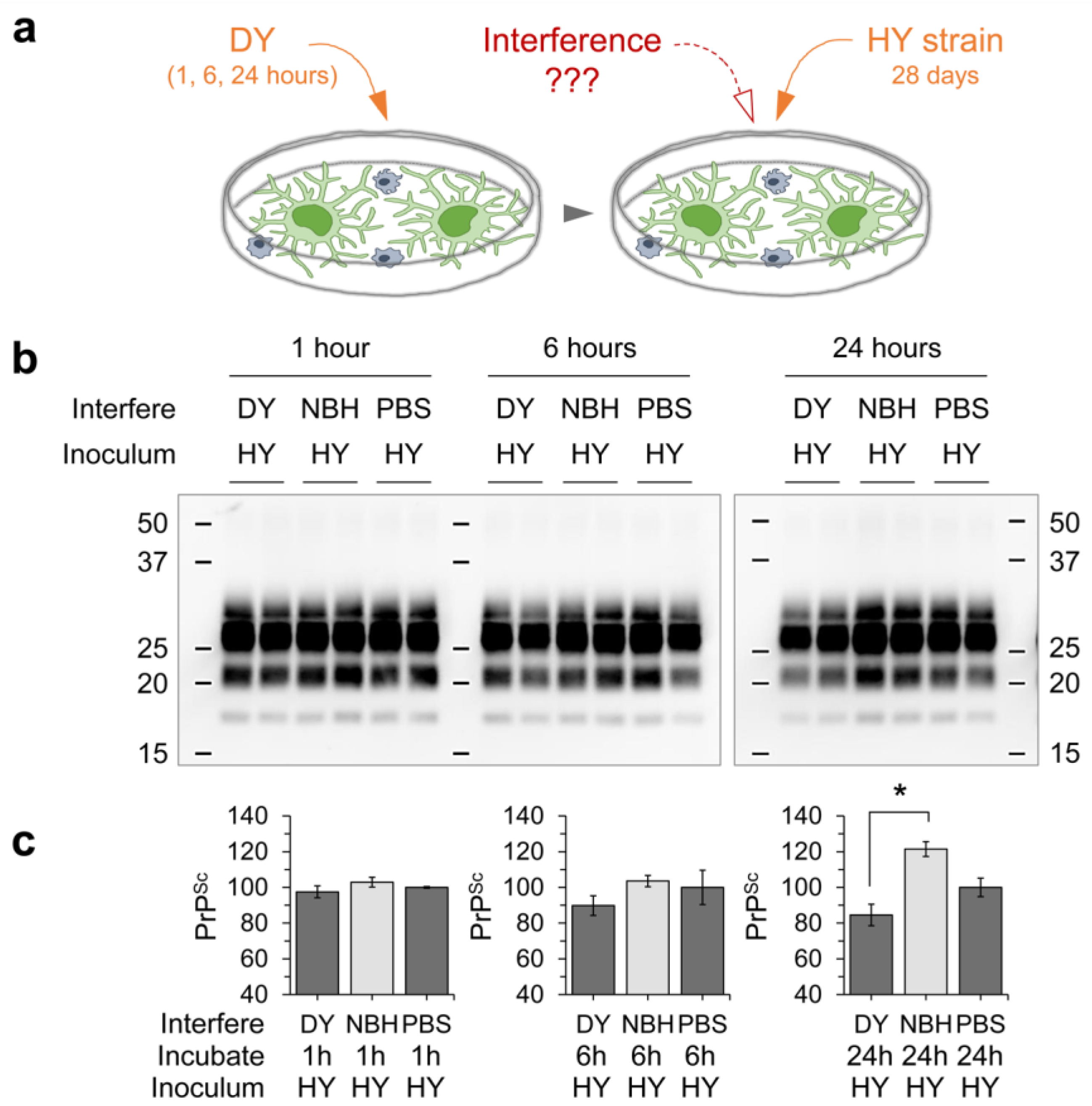
3. Results
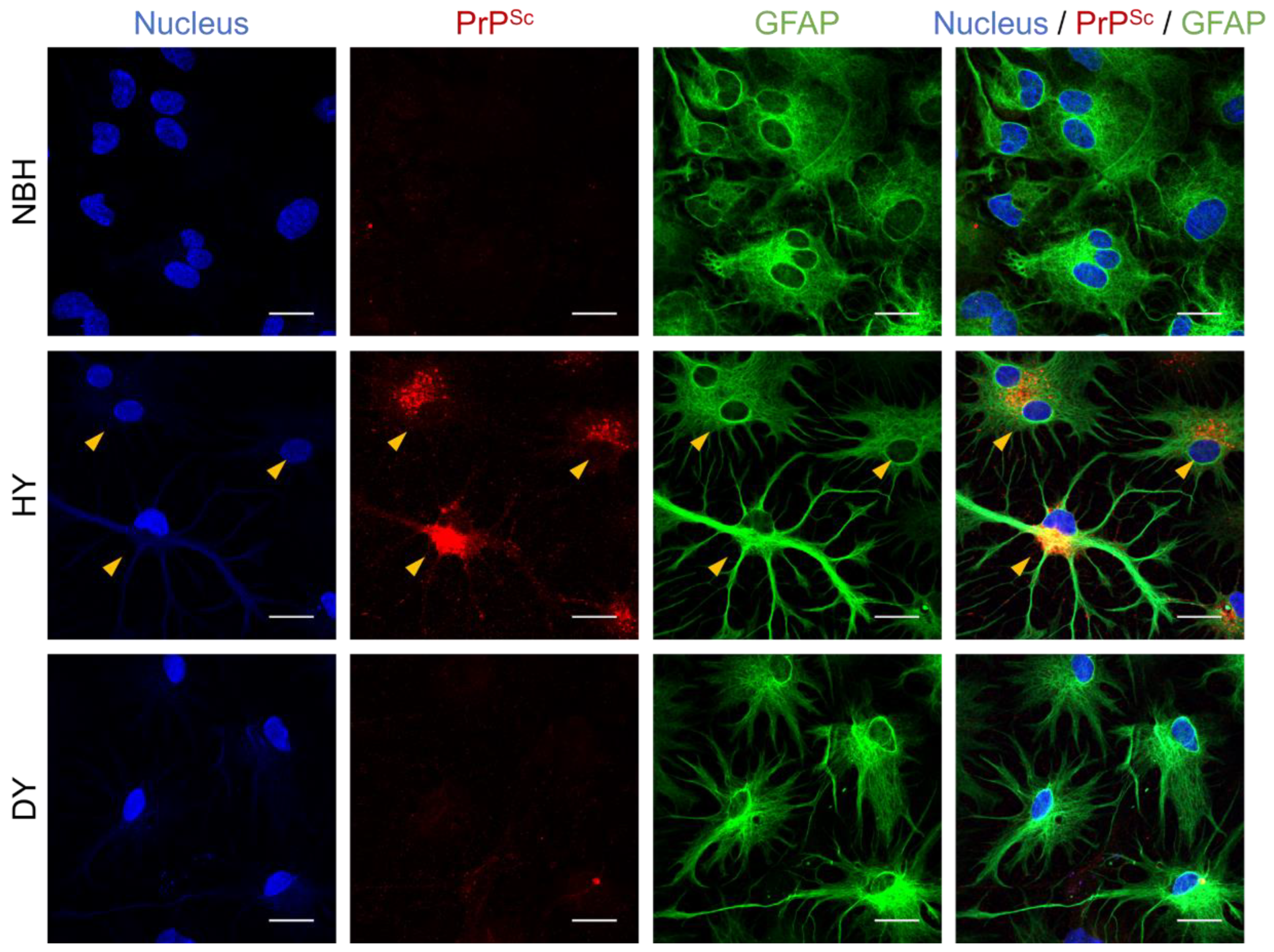
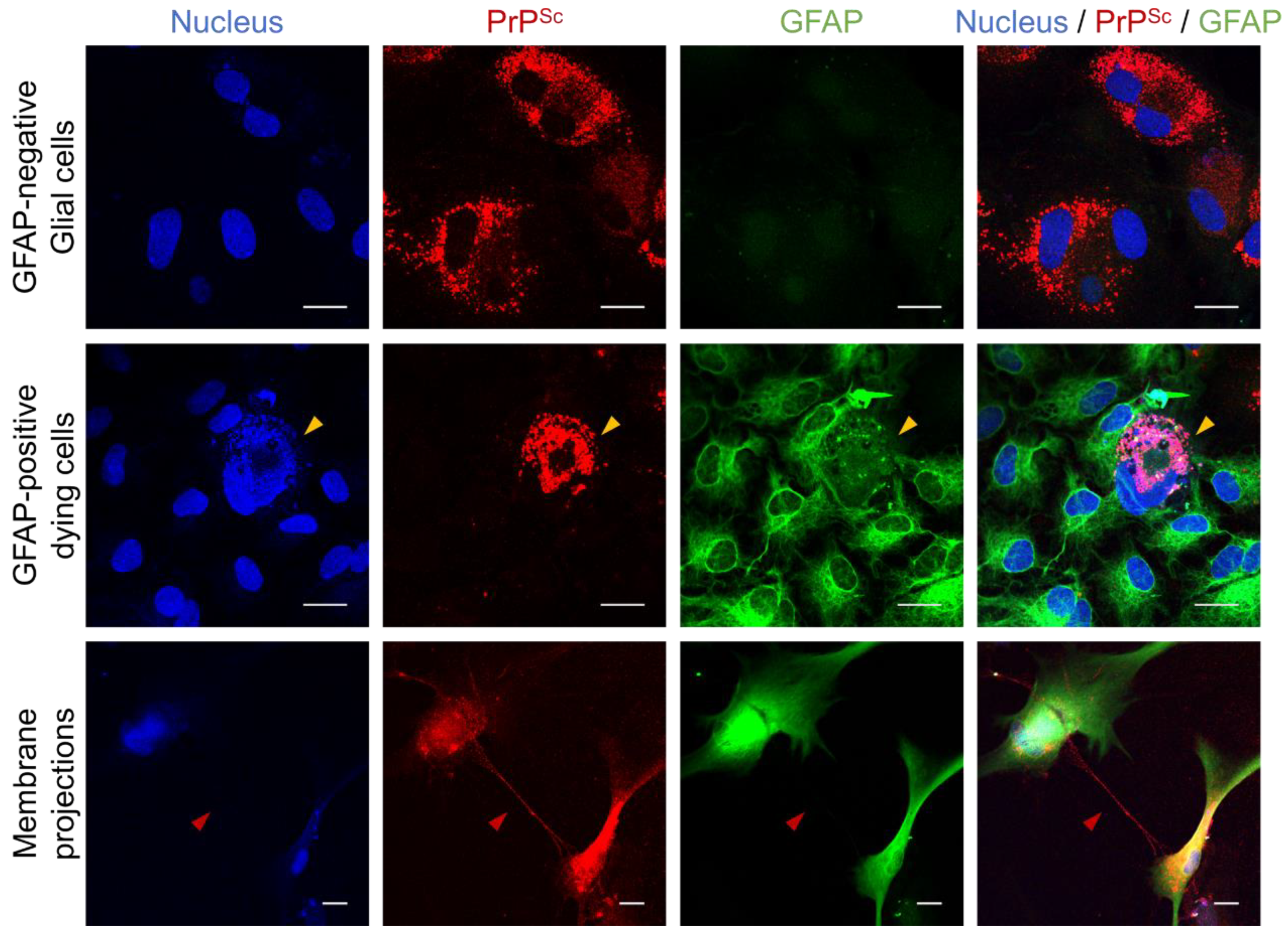
3.1. Differential Susceptibility of Primary Glial Cells to Prion Strains
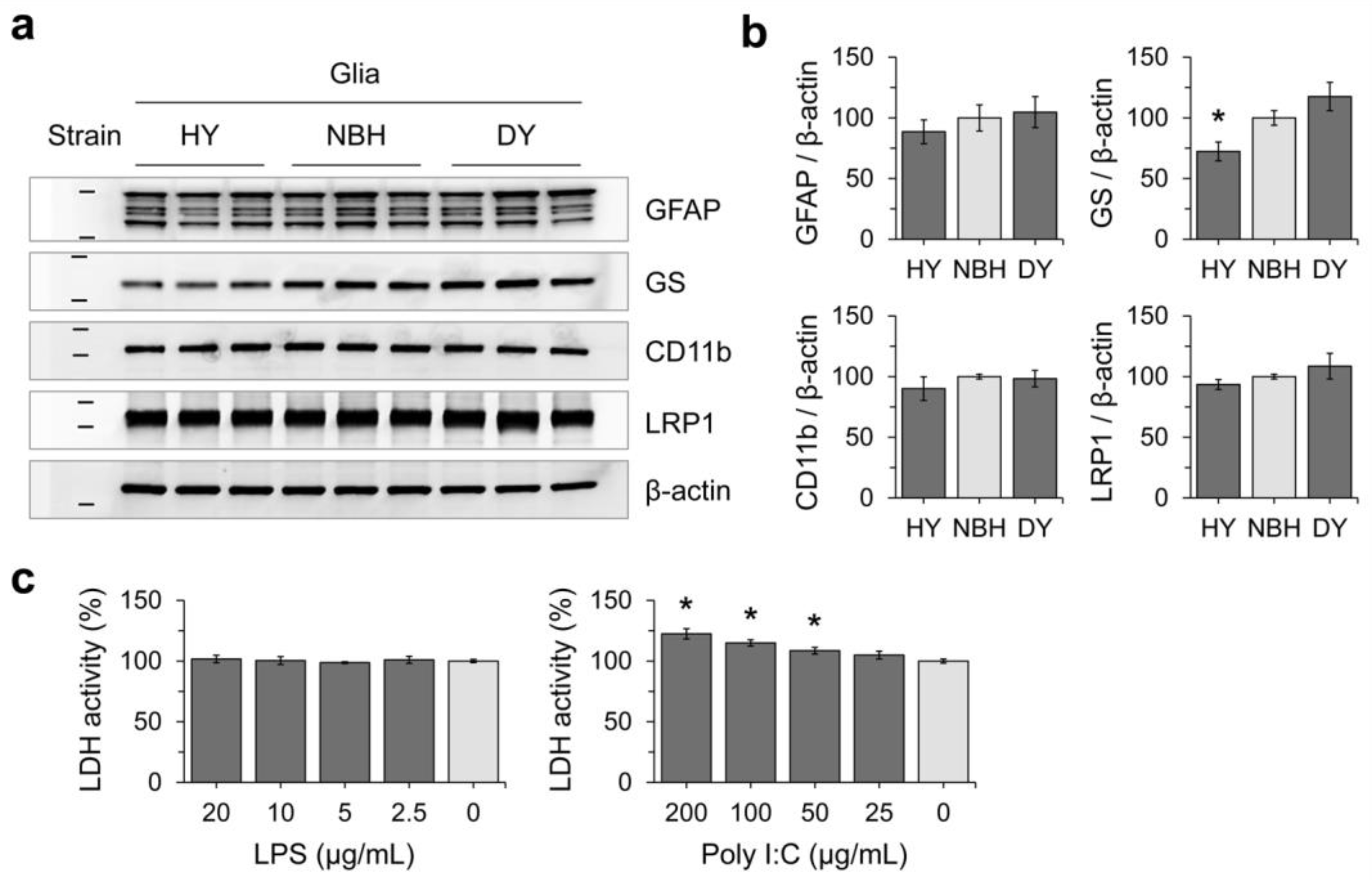
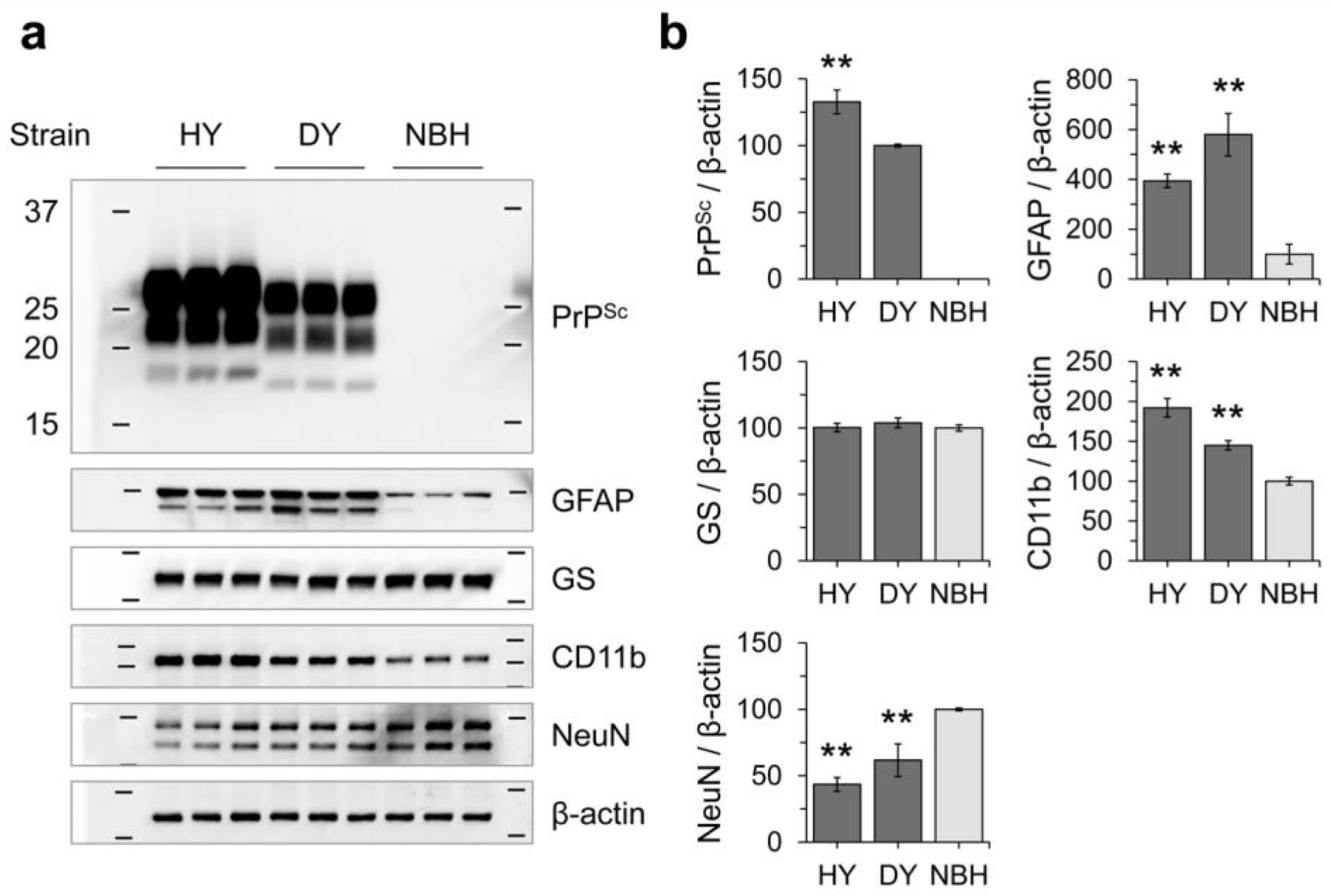
3.2. Effects of Prion Propagation on Glial Marker Expression
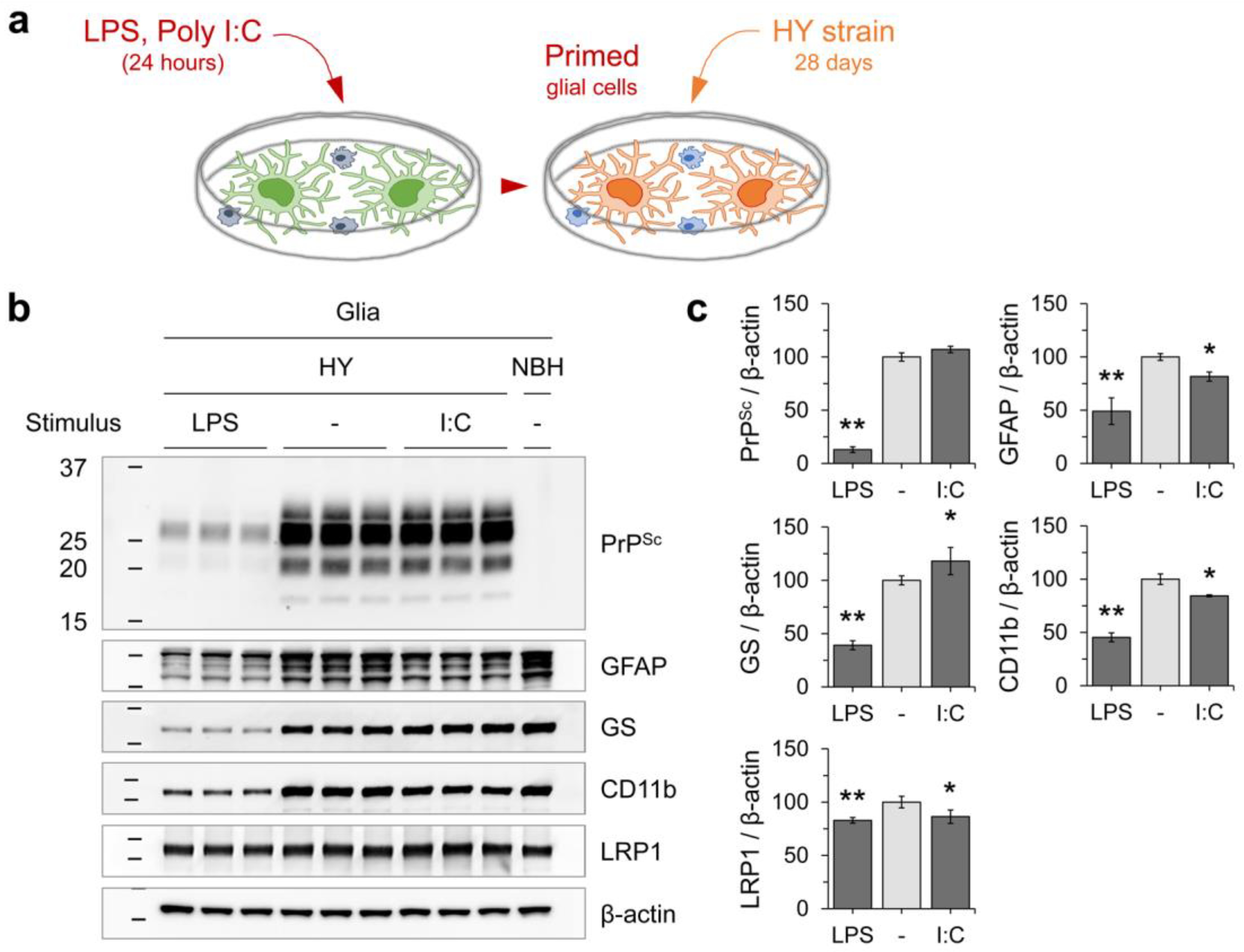
3.3. Innate Immune Status of Glial Cells Determines Prion Propagation
3.4. PrPSc Produced in Glial Cells Correlated with the Prion Infectivity
| Inoculum | BH HY | BH HY | BH DY | BH - | Glia LPS-HY | Glia I:C-HY | Glia HY | Glia - | Glia Dex-DY | Glia DY | PBS - |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (µg/µL) | 1 | 0.01 | 1 | 1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - |
| Attack rate | 4/4 | 8/8 | 8/8 | 0/4 | 8/8 | 8/8 | 8/8 | 0/4 | 4/4 | 1/3 | 0/3 |
| Latency (days) | 86 | 102 | 204 | - | 108 | 106 | 102 | - | 245 | 365 | - |
| 91 | 104 | 210 | 108 | 106 | 104 | 255 | |||||
| 91 | 106 | 219 | 112 | 107 | 107 | 257 | |||||
| 91 | 106 | 224 | 121 | 108 | 112 | 285 | |||||
| 107 | 224 | 122 | 112 | 113 | |||||||
| 107 | 233 | 122 | 113 | 113 | |||||||
| 107 | 245 | 123 | 113 | 115 | |||||||
| 108 | 281 | 123 | 115 | 116 | |||||||
| Mean ± SD | 90 ± 3 | 106 ± 2 | 230 ± 24 | >365 | 117 ± 7 * | 110 ± 4 | 110 ± 5 | >365 | 261 ± 17 *** | >365 | >365 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Colby, D.W.; Prusiner, S.B. Prions. Cold Spring Harb. Perspect. Biol. 2011, 3, a006833. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Sigurdson, C.; Heikenwaelder, M. Molecular mechanisms of prion pathogenesis. Annu. Rev. Pathol. 2008, 3, 11–40. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Calella, A.M. Prions: Protein aggregation and infectious diseases. Physiol. Rev. 2009, 89, 1105–1152. [Google Scholar] [CrossRef] [PubMed]
- Aguzzi, A.; Nuvolone, M.; Zhu, C. The immunobiology of prion diseases. Nat. Rev. Immunol. 2013, 13, 888–902. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Jiang, S.; Maphis, N.M.; Binder, J.; Chisholm, D.; Weston, L.; Duran, W.; Peterson, C.; Zimmerman, A.; Mandell, M.A.; Jett, S.D.; et al. Proteopathic tau primes and activates interleukin-1beta via myeloid-cell-specific MyD88- and NLRP3-ASC-inflammasome pathway. Cell Rep. 2021, 36, 109720. [Google Scholar] [CrossRef]
- Stancu, I.C.; Cremers, N.; Vanrusselt, H.; Couturier, J.; Vanoosthuyse, A.; Kessels, S.; Lodder, C.; Brone, B.; Huaux, F.; Octave, J.N.; et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019, 137, 599–617. [Google Scholar] [CrossRef]
- Wang, C.; Fan, L.; Khawaja, R.R.; Liu, B.; Zhan, L.; Kodama, L.; Chin, M.; Li, Y.; Le, D.; Zhou, Y.; et al. Microglial NF-kappaB drives tau spreading and toxicity in a mouse model of tauopathy. Nat. Commun. 2022, 13, 1969. [Google Scholar] [CrossRef]
- Lee, H.J.; Bae, E.J.; Lee, S.J. Extracellular alpha--synuclein-a novel and crucial factor in Lewy body diseases. Nat. Rev. Neurol. 2014, 10, 92–98. [Google Scholar] [CrossRef]
- Rocha, N.P.; Charron, O.; Latham, L.B.; Colpo, G.D.; Zanotti-Fregonara, P.; Yu, M.; Freeman, L.; Furr Stimming, E.; Teixeira, A.L. Microglia Activation in Basal Ganglia Is a Late Event in Huntington Disease Pathophysiology. Neurol.-Neuroimmunol. Neuroinflam. 2021, 8, e984. [Google Scholar] [CrossRef]
- Lakkaraju, A.K.K.; Sorce, S.; Senatore, A.; Nuvolone, M.; Guo, J.; Schwarz, P.; Moos, R.; Pelczar, P.; Aguzzi, A. Glial activation in prion diseases is selectively triggered by neuronal PrP(Sc). Brain Pathol. 2022, 32, e13056. [Google Scholar] [CrossRef]
- Kushwaha, R.; Sinha, A.; Makarava, N.; Molesworth, K.; Baskakov, I.V. Non-cell autonomous astrocyte-mediated neuronal toxicity in prion diseases. Acta Neuropathol. Commun. 2021, 9, 22. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Zhu, C.; Herrmann, U.S.; Falsig, J.; Abakumova, I.; Nuvolone, M.; Schwarz, P.; Frauenknecht, K.; Rushing, E.J.; Aguzzi, A. A neuroprotective role for microglia in prion diseases. J. Exp. Med. 2016, 213, 1047–1059. [Google Scholar] [CrossRef]
- Carroll, J.A.; Race, B.; Williams, K.; Striebel, J.; Chesebro, B. Microglia Are Critical in Host Defense against Prion Disease. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Hartmann, K.; Sepulveda-Falla, D.; Rose, I.V.L.; Madore, C.; Muth, C.; Matschke, J.; Butovsky, O.; Liddelow, S.; Glatzel, M.; Krasemann, S. Complement 3(+)-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol. Commun. 2019, 7, 83. [Google Scholar] [CrossRef]
- Makarava, N.; Mychko, O.; Chang, J.C.; Molesworth, K.; Baskakov, I.V. The degree of astrocyte activation is predictive of the incubation time to prion disease. Acta Neuropathol. Commun. 2021, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Scheckel, C.; Imeri, M.; Schwarz, P.; Aguzzi, A. Ribosomal profiling during prion disease uncovers progressive translational derangement in glia but not in neurons. Elife 2020, 9, e62911. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Yamasaki, T.; Suzuki, A.; Hasebe, R.; Horiuchi, M. Flow Cytometric Detection of PrP(Sc) in Neurons and Glial Cells from Prion-Infected Mouse Brains. J. Virol. 2018, 92, e01457-17. [Google Scholar] [CrossRef]
- Carroll, J.A.; Striebel, J.F.; Rangel, A.; Woods, T.; Phillips, K.; Peterson, K.E.; Race, B.; Chesebro, B. Prion Strain Differences in Accumulation of PrPSc on Neurons and Glia Are Associated with Similar Expression Profiles of Neuroinflammatory Genes: Comparison of Three Prion Strains. PLoS Pathog. 2016, 12, e1005551. [Google Scholar] [CrossRef] [PubMed]
- Tahir, W.; Thapa, S.; Schatzl, H.M. Astrocyte in prion disease: A double-edged sword. Neural Regen. Res. 2022, 17, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Diedrich, J.F.; Bendheim, P.E.; Kim, Y.S.; Carp, R.I.; Haase, A.T. Scrapie-associated prion protein accumulates in astrocytes during scrapie infection. Proc. Natl. Acad. Sci. USA 1991, 88, 375–379. [Google Scholar] [CrossRef]
- Kang, S.G.; Kim, C.; Cortez, L.M.; Carmen Garza, M.; Yang, J.; Wille, H.; Sim, V.L.; Westaway, D.; McKenzie, D.; Aiken, J. Toll-like receptor-mediated immune response inhibits prion propagation. Glia 2016, 64, 937–951. [Google Scholar] [CrossRef]
- Choi, J.K.; Park, S.J.; Jun, Y.C.; Oh, J.M.; Jeong, B.H.; Lee, H.P.; Park, S.N.; Carp, R.I.; Kim, Y.S. Generation of monoclonal antibody recognized by the GXXXG motif (glycine zipper) of prion protein. Hybridoma 2006, 25, 271–277. [Google Scholar] [CrossRef]
- Ayers, J.I.; Kincaid, A.E.; Bartz, J.C. Prion strain targeting independent of strain-specific neuronal tropism. J. Virol. 2009, 83, 81–87. [Google Scholar] [CrossRef]
- Hu, P.P.; Morales, R.; Duran-Aniotz, C.; Moreno-Gonzalez, I.; Khan, U.; Soto, C. Role of Prion Replication in the Strain-dependent Brain Regional Distribution of Prions. J. Biol. Chem. 2016, 291, 12880–12887. [Google Scholar] [CrossRef]
- Iniguez, V.; McKenzie, D.; Mirwald, J.; Aiken, J. Strain-specific propagation of PrP(Sc) properties into baculovirus-expressed hamster PrP(C). J. Gen. Virol. 2000, 81, 2565–2571. [Google Scholar] [CrossRef]
- Makarava, N.; Chang, J.C.; Molesworth, K.; Baskakov, I.V. Region-specific glial homeostatic signature in prion diseases is replaced by a uniform neuroinflammation signature, common for brain regions and prion strains with different cell tropism. Neurobiol. Dis. 2020, 137, 104783. [Google Scholar] [CrossRef] [PubMed]
- Shikiya, R.A.; Langenfeld, K.A.; Eckland, T.E.; Trinh, J.; Holec, S.A.M.; Mathiason, C.K.; Kincaid, A.E.; Bartz, J.C. PrPSc formation and clearance as determinants of prion tropism. PLoS Pathog. 2017, 13, e1006298. [Google Scholar] [CrossRef] [PubMed]
- Gousset, K.; Schiff, E.; Langevin, C.; Marijanovic, Z.; Caputo, A.; Browman, D.T.; Chenouard, N.; de Chaumont, F.; Martino, A.; Enninga, J.; et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat. Cell Biol. 2009, 11, 328–336. [Google Scholar] [CrossRef]
- Jen, A.; Parkyn, C.J.; Mootoosamy, R.C.; Ford, M.J.; Warley, A.; Liu, Q.; Bu, G.; Baskakov, I.V.; Moestrup, S.; McGuinness, L.; et al. Neuronal low-density lipoprotein receptor-related protein 1 binds and endocytoses prion fibrils via receptor cluster 4. J. Cell Sci. 2010, 123, 246–255. [Google Scholar] [CrossRef]
- Rauch, J.N.; Luna, G.; Guzman, E.; Audouard, M.; Challis, C.; Sibih, Y.E.; Leshuk, C.; Hernandez, I.; Wegmann, S.; Hyman, B.T.; et al. LRP1 is a master regulator of tau uptake and spread. Nature 2020, 580, 381–385. [Google Scholar] [CrossRef]
- Chen, K.; Martens, Y.A.; Meneses, A.; Ryu, D.H.; Lu, W.; Raulin, A.C.; Li, F.; Zhao, J.; Chen, Y.; Jin, Y.; et al. LRP1 is a neuronal receptor for alpha-synuclein uptake and spread. Mol. Neurodegener. 2022, 17, 57. [Google Scholar] [CrossRef]
- Ishibashi, D.; Atarashi, R.; Nishida, N. Protective role of MyD88-independent innate immune responses against prion infection. Prion 2012, 6, 443–446. [Google Scholar] [CrossRef]
- Shi, F.; Yang, L.; Kouadir, M.; Yang, Y.; Wang, J.; Zhou, X.; Yin, X.; Zhao, D. The NALP3 inflammasome is involved in neurotoxic prion peptide-induced microglial activation. J. Neuroinflam. 2012, 9, 73. [Google Scholar] [CrossRef]
- Sakai, K.; Hasebe, R.; Takahashi, Y.; Song, C.H.; Suzuki, A.; Yamasaki, T.; Horiuchi, M. Absence of CD14 delays progression of prion diseases accompanied by increased microglial activation. J. Virol. 2013, 87, 13433–13445. [Google Scholar] [CrossRef]
- Carroll, J.A.; Race, B.; Williams, K.; Striebel, J.; Chesebro, B. RNA-seq and network analysis reveal unique glial gene expression signatures during prion infection. Mol. Brain 2020, 13, 71. [Google Scholar] [CrossRef]
- Aguzzi, A.; Zhu, C. Microglia in prion diseases. J. Clin. Investig. 2017, 127, 3230–3239. [Google Scholar] [CrossRef] [PubMed]
- De Waard, D.M.; Bugiani, M. Astrocyte-Oligodendrocyte-Microglia Crosstalk in Astrocytopathies. Front. Cell. Neurosci. 2020, 14, 608073. [Google Scholar] [CrossRef] [PubMed]
- Bartz, J.C.; Dejoia, C.; Tucker, T.; Kincaid, A.E.; Bessen, R.A. Extraneural prion neuroinvasion without lymphoreticular system infection. J. Virol. 2005, 79, 11858–11863. [Google Scholar] [CrossRef]
- Bessen, R.A.; Martinka, S.; Kelly, J.; Gonzalez, D. Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J. Virol. 2009, 83, 6435–6445. [Google Scholar] [CrossRef]
- Schneider, D.A.; Harrington, R.D.; Zhuang, D.; Yan, H.; Truscott, T.C.; Dassanayake, R.P.; O’Rourke, K.I. Disease-associated prion protein in neural and lymphoid tissues of mink (Mustela vison) inoculated with transmissible mink encephalopathy. J. Comp. Pathol. 2012, 147, 508–521. [Google Scholar] [CrossRef]
- Shikiya, R.A.; Ayers, J.I.; Schutt, C.R.; Kincaid, A.E.; Bartz, J.C. Coinfecting prion strains compete for a limiting cellular resource. J. Virol. 2010, 84, 5706–5714. [Google Scholar] [CrossRef]
- Labzin, L.I.; Heneka, M.T.; Latz, E. Innate Immunity and Neurodegeneration. Annu. Rev. Med. 2018, 69, 437–449. [Google Scholar] [CrossRef]
- Bartz, J.C.; Kramer, M.L.; Sheehan, M.H.; Hutter, J.A.; Ayers, J.I.; Bessen, R.A.; Kincaid, A.E. Prion interference is due to a reduction in strain-specific PrPSc levels. J. Virol. 2007, 81, 689–697. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-G.; Kim, C.; Aiken, J.; McKenzie, D. Innate Immune Status of Glia Modulates Prion Propagation in Early Stage of Infection. Cells 2023, 12, 1878. https://doi.org/10.3390/cells12141878
Kang S-G, Kim C, Aiken J, McKenzie D. Innate Immune Status of Glia Modulates Prion Propagation in Early Stage of Infection. Cells. 2023; 12(14):1878. https://doi.org/10.3390/cells12141878
Chicago/Turabian StyleKang, Sang-Gyun, Chiye Kim, Judd Aiken, and Debbie McKenzie. 2023. "Innate Immune Status of Glia Modulates Prion Propagation in Early Stage of Infection" Cells 12, no. 14: 1878. https://doi.org/10.3390/cells12141878
APA StyleKang, S.-G., Kim, C., Aiken, J., & McKenzie, D. (2023). Innate Immune Status of Glia Modulates Prion Propagation in Early Stage of Infection. Cells, 12(14), 1878. https://doi.org/10.3390/cells12141878







