Calcineurin B1 Deficiency Reduces Proliferation, Increases Apoptosis, and Alters Secretion in Enteric Glial Cells of Mouse Small Intestine in Culture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Primary Myenteric Culture
2.3. Proliferation Assay
2.4. Cell Death Assay
2.5. Purified EGC Culture
2.6. Western Blotting
2.7. ELISA
2.8. S100B Inhibition In Vivo
2.9. Hematoxylin-Eosin Staining of Cryosections
2.10. Statistical Analysis


3. Results
3.1. Reduced Proliferation of CNB1-Deficient EGCs
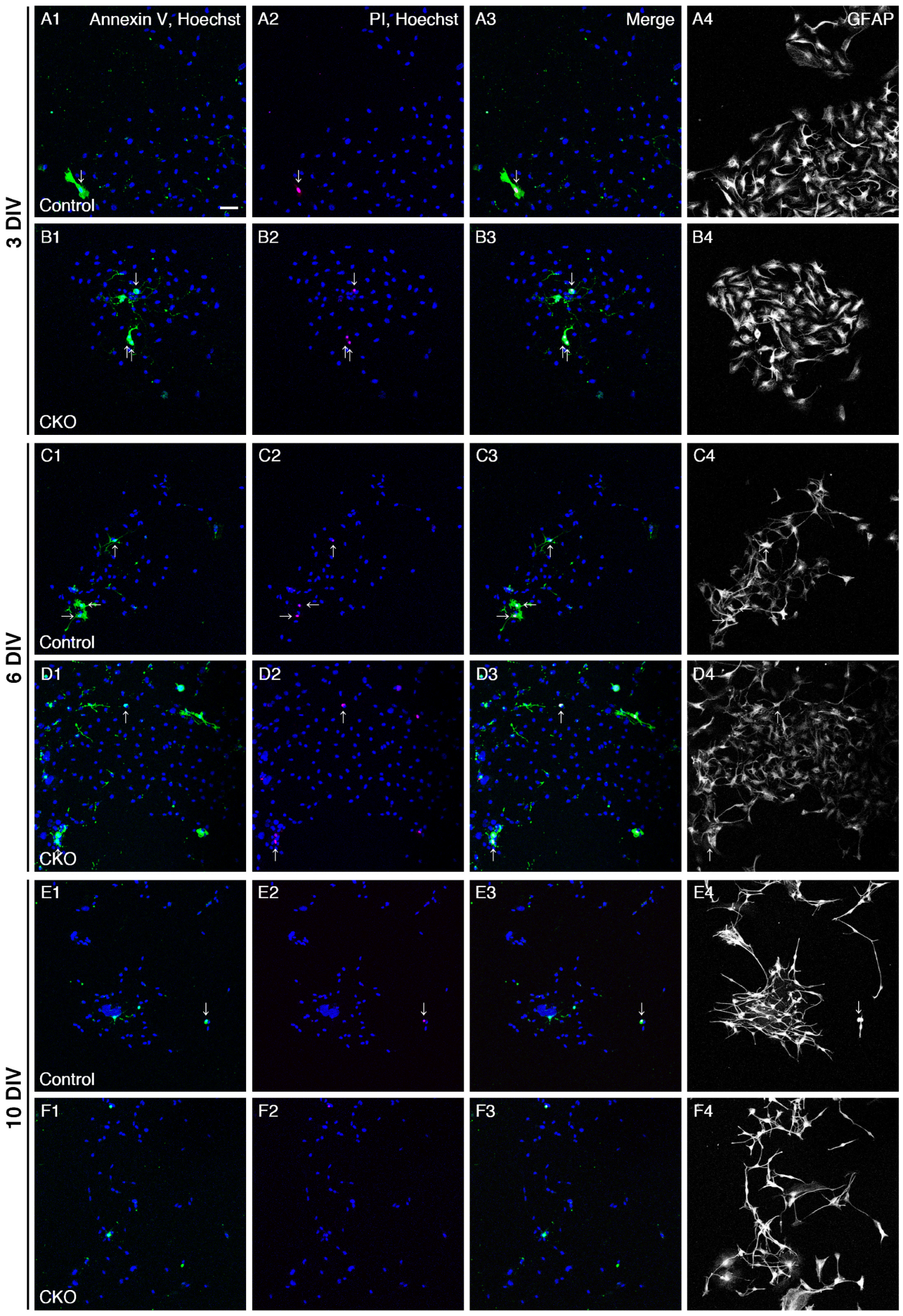
3.2. Increased Apoptosis of CNB1-Deficient EGCs
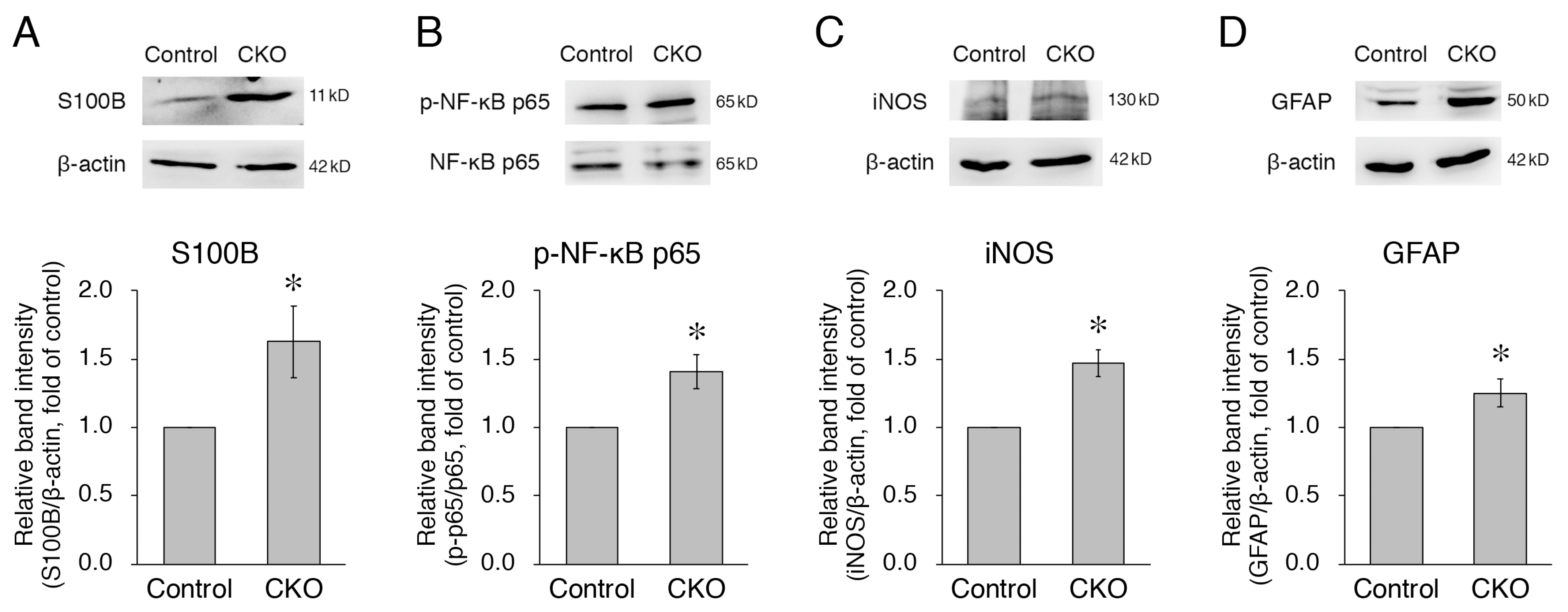
3.3. Increased Expression of S100B, iNOS, and GFAP and Phosphorylation of NF-κB p65 in CNB1-Deficient EGCs
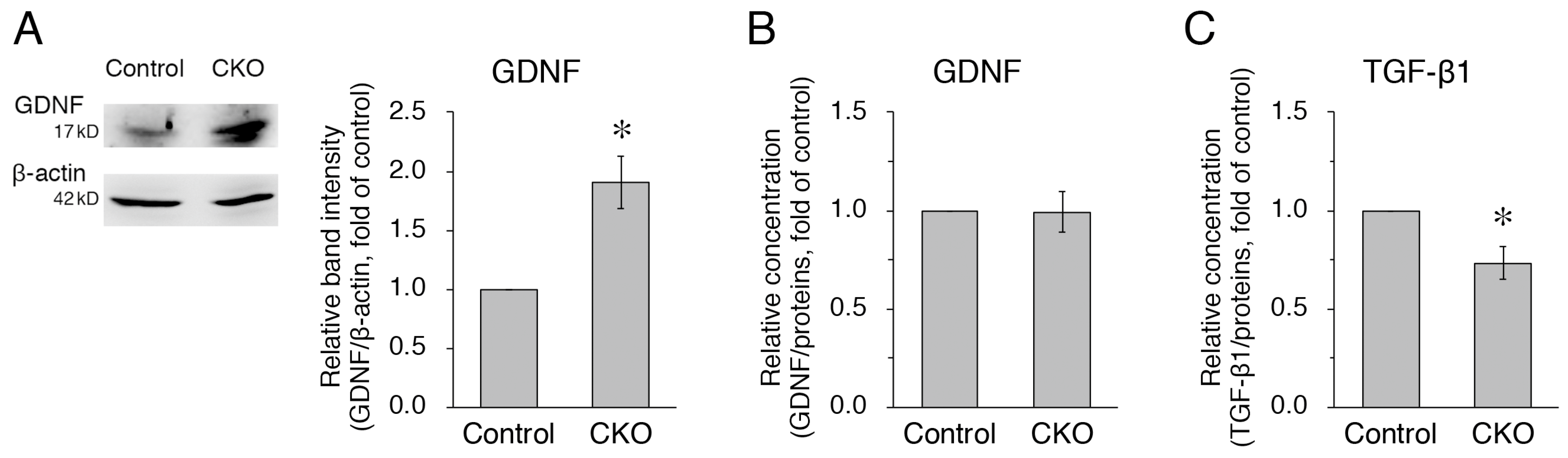
3.4. Increased Expression but Normal Secretion of GDNF and Reduced Secretion of TGF-β1 in CNB1-Deficient EGCs
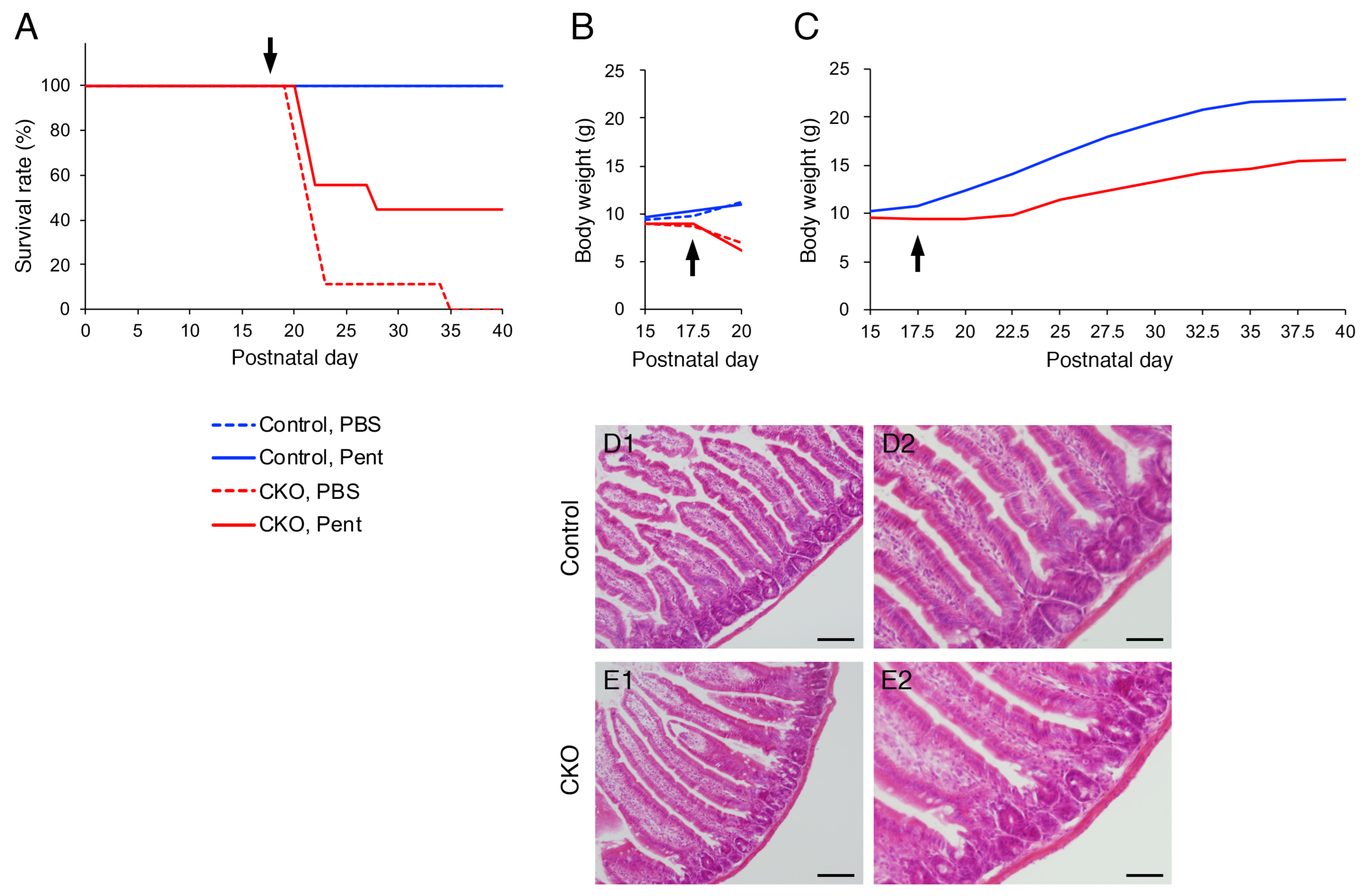
3.5. Effects of S100B Inhibition on CNB1-CKO Mice In Vivo
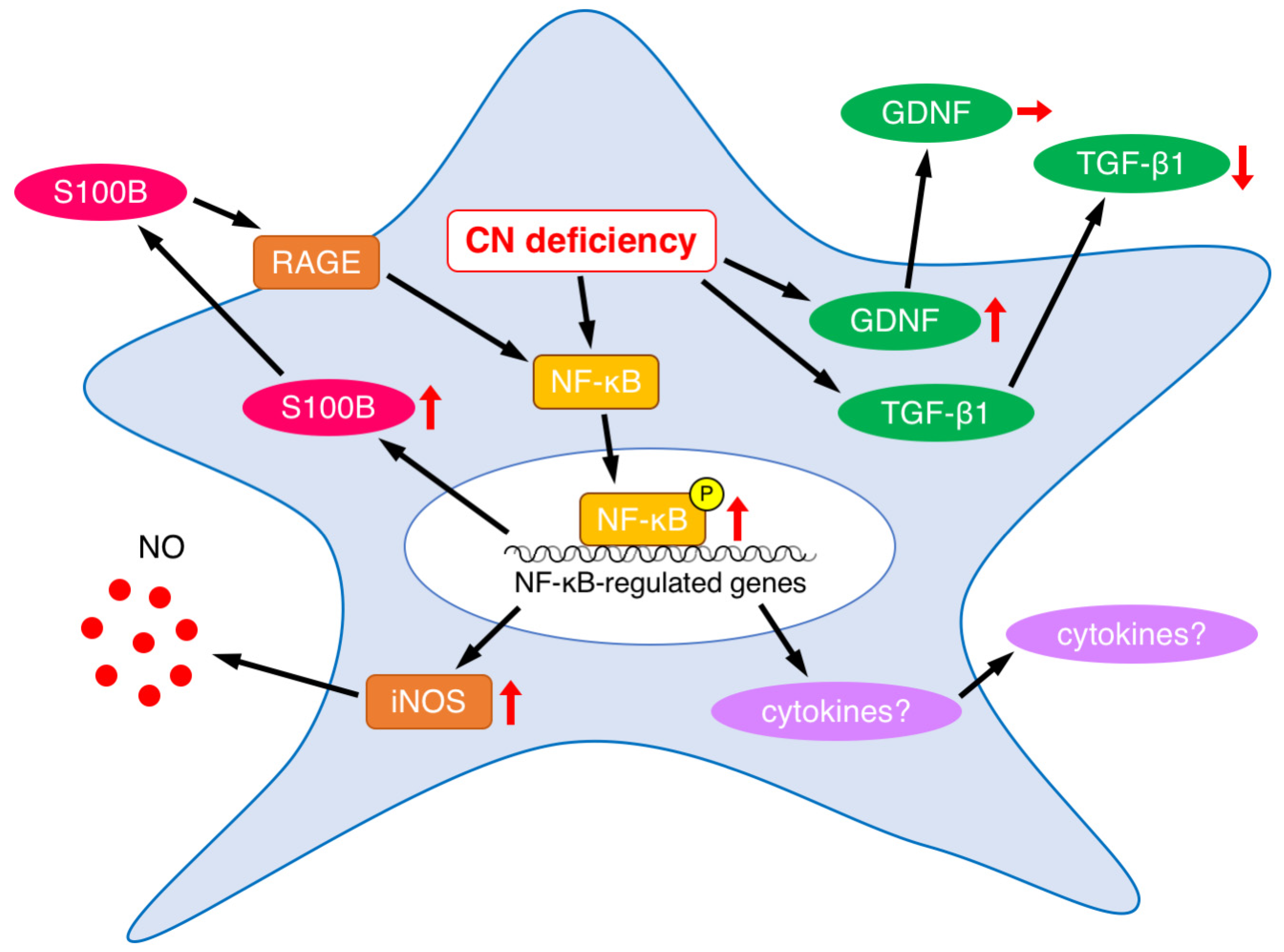
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rusnak, F.; Mertz, P. Calcineurin: Form and function. Physiol. Rev. 2000, 80, 1483–1521. [Google Scholar] [CrossRef]
- Tojima, T.; Hines, J.H.; Henley, J.R.; Kamiguchi, H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat. Rev. Neurosci. 2011, 12, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Baumgärtel, K.; Mansuy, I.M. Neural functions of calcineurin in synaptic plasticity and memory. Learn. Mem. 2012, 19, 375–384. [Google Scholar] [CrossRef] [Green Version]
- Tarasova, E.O.; Gaydukov, A.E.; Balezina, O.P. Calcineurin and its role in synaptic transmission. Biochemistry 2018, 83, 674–689. [Google Scholar] [CrossRef]
- Furman, J.L.; Norris, C.M. Calcineurin and glial signaling: Neuroinflammation and beyond. J. Neuroinflamm. 2014, 11, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.; Tapella, L.; Dematteis, G.; Talmon, M.; Genazzani, A.A. Calcineurin signalling in astrocytes: From pathology to physiology and control of neuronal functions. Neurochem. Res. 2023, 48, 1077–1090. [Google Scholar] [CrossRef]
- Parpura, V.; Heneka, M.T.; Montana, V.; Oliet, S.H.R.; Schousboe, A.; Haydon, P.G.; Stout, R.F., Jr.; Spray, D.C.; Reichenbach, A.; Pannicke, T.; et al. Glial cells in (patho)physiology. J. Neurochem. 2012, 121, 4–27. [Google Scholar] [CrossRef] [Green Version]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Sood, A.; Preeti, K.; Fernandes, V.; Khatri, D.K.; Singh, S.B. Glia: A major player in glutamate-GABA dysregulation-mediated neurodegeneration. J. Neurosci. Res. 2020, 99, 3148–3169. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yagi, T.; Okura, U.; Tanaka, J.; Hirashima, N.; Tanaka, M. Calcineurin B1 deficiency in glial cells induces mucosal degeneration and inflammation in mouse small intestine. Biol. Pharm. Bull. 2018, 41, 786–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okura, U.; Hirashima, N.; Tanaka, M. Calcineurin B1 deficiency in glial cells reduces gastrointestinal motility and results in maldigestion and/or malabsorption in mice. Biol. Pharm. Bull. 2019, 42, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric glial cells: A new frontier in neurogastroenterology and clinical target for inflammatory bowel diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubišić, V.; Verkhratsky, A.; Zorec, R.; Parpura, V. Enteric glia regulate gut motility in health and disease. Brain Res. Bull. 2018, 136, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Seguella, L.; Gulbransen, B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 571–587. [Google Scholar] [CrossRef]
- Rosenberg, H.J.; Rao, M. Enteric glia in homeostasis and disease: From fundamental biology to human pathology. iScience 2021, 24, 102863. [Google Scholar] [CrossRef]
- Sharkey, K.A.; Mawe, G.M. The enteric nervous system. Physiol. Rev. 2023, 103, 1487–1564. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Hirashima, N.; Tanaka, M. A simple method for purified primary culture of enteric glial cells from mouse small intestine. Biol. Pharm. Bull. 2022, 45, 547–551. [Google Scholar] [CrossRef]
- Costa, D.V.S.; Moura-Neto, V.; Bolick, D.T.; Guerrant, R.L.; Fawad, J.A.; Shin, J.H.; Medeiros, P.H.Q.S.; Ledwaba, S.E.; Kolling, G.L.; Martins, C.S.; et al. S100B inihibition attenuates intestinal damage and diarrhea severity during Clostridioides difficile infection by modulating inflammatory response. Front. Cell. Infect. Microbiol. 2021, 11, 739874. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Grosso, M.; Petruzzelli, R.; Izzo, P.; Calí, G.; D’armiento, F.P.; Rocco, A.; Nardone, G.; et al. Inceased mucosal nitric oxide production in ulcerative colitis is mediated in part by the enteroglial-derived S100B protein. Neurogastroenterol. Motil. 2009, 21, 1209-e112. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Esposito, G.; Turco, F.; Steardo, T.; Cuomo, R. S100B protein in the gut: The evidence for enteroglial-sustained intestinal inflammation. World J. Gastroenterol. 2011, 17, 1261–1266. [Google Scholar] [CrossRef]
- Rao, M.; Nelms, B.D.; Dong, L.; Salinas-Rios, V.; Rutlin, M.; Gershon, M.D.; Corfas, G. Enteric glia express proteolipid protein 1 and are a transcriptinally unique population of glia in the mammalian nervous system. Glia 2015, 63, 2040–2057. [Google Scholar] [CrossRef]
- Cirillo, C.; Sarnelli, G.; Turco, F.; Mango, A.; Grosso, M.; Aprea, G.; Masone, S.; Cuomo, R. Proinflammatory stimuli activates human-derived enteroglial cells and induces autocrine nitric oxide production. Neurogastroenterol. Motil. 2011, 23, e372–e382. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, amd futire directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.K.; He, F.Q.; Li, T.K.; Pang, X.H.; Cui, D.J.; Xie, Q.; Huang, X.L.; Gan, H.T. Glial-derived neurotrophic factor regulates intestinal epithelial barrier function and inflammation and is therapeutic for murine colitis. J. Pathol. 2010, 222, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Meir, M.; Flemming, S.; Burkard, N.; Bergauer, L.; Metzger, M.; Germer, C.-T.; Schlegel, N. Glial cell line-derived neurotrophic factor promotes barrier maturation and wound healing in intestinal epithelial cells in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G613–G624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meir, M.; Burkard, N.; Ungewiß, H.; Diefenbacher, M.; Flemming, S.; Kannapin, F.; Germer, C.-T.; Schweinlin, M.; Metzger, M.; Waschke, J.; et al. Neurotrophic factor GDNF regulates intestinal barrier function in inflammatory bowel disease. J. Clin. Investig. 2019, 129, 2824–2840. [Google Scholar] [CrossRef] [Green Version]
- Beck, P.L.; Rosenberg, I.M.; Xavier, R.J.; Koh, T.; Wong, J.F.; Podolsky, D.K. Transforming growth factor-β mediates intestinal helaing and susceptibility to injury in vitro and in vivo through epithelial cells. Am. J. Pathol. 2003, 162, 597–608. [Google Scholar] [CrossRef]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-β regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Neunlist, M.; Aubert, P.; Bonnaud, S.; Van Landeghem, L.; Coron, E.; Wedel, T.; Naveilhan, P.; Ruhl, A.; Lardeux, B.; Savidge, T.; et al. Enteric glia inhibit intestinal epithelial cell proliferation partly through a TGF-β1-dependent pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G231–G241. [Google Scholar] [CrossRef] [Green Version]
- Steinkamp, M.; Geerling, I.; Seufferlein, T.; von Boyen, G.; Egger, B.; Grossmann, J.; Ludwig, L.; Adler, G.; Reinshagen, M. Glial-derived neurotrophic factor regulates apoptosis in colonic epithelial cells. Gastroenterology 2003, 124, 1748–1757. [Google Scholar] [CrossRef]
- Dignass, A.U.; Podolsky, D.K. Cytokine modulation of intestinal epithelial cell restitution: Central role of transforming growth factor-β. Gastroenterology 1993, 105, 1323–1332. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, T.-H.; Kim, Y.-H.; Noh, J.-R.; Choi, D.-H.; Kim, K.-S.; Lee, E.-Y.; Kim, B.-C.; Kim, M.H.; Kim, H.; et al. Gadd45b promotes regeneration after injury through TGFβ-dependent restitution in experimental colitis. Exp. Mol. Med. 2019, 51, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markowitz, J.; Chen, I.; Gitti, R.; Baldisseri, D.M.; Pan, Y.; Udan, R.; Carrier, F.; MacKerell, A.D., Jr.; Weber, D.J. Identification and characterization of small molecule inhibitors of the calcium-dependent S100B-p53 tumor suppressor interaction. J. Med. Chem. 2004, 47, 5085–5093. [Google Scholar] [CrossRef]
- Charpentier, T.H.; Wilder, P.T.; Liriano, M.A.; Varney, K.M.; Pozharski, E.; MacKerell, A.D., Jr.; Coop, A.; Toth, E.A.; Weber, D.J. Divalent metal ion complexes of S100B in the absence and presence of pentamidine. J. Mol. BIol. 2008, 382, 56–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, G.; Capoccia, E.; Sarnelli, G.; Scuderi, C.; Cirillo, C.; Cuomo, R.; Steardo, L. The antiprotozoal drug pentamidine ameliorates experimentally induced acute colitis in mice. J. Neuroinflamm. 2012, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Costa, D.V.S.; Bon-Frauches, A.C.; Silva, A.M.H.P.; Lima-Júnior, R.C.P.; Martins, C.S.; Leitão, R.F.C.; Freitas, G.B.; Castelucci, P.; Bolick, D.T.; Guerrant, R.L.; et al. 5-Fluorouracil induces enteric neuron death and glial activation during intestinal mucositis via a S100B-RAGE-NFκB-dependent pathway. Sci. Rep. 2019, 9, 665. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Zhang, L.; Liu, Z.; Zhao, S.; Xu, D.; Li, L.; Peng, Q.; Ai, Y. Pentamidine protects mice from cecal ligation and puncture-induced brain damage via inhibiting S100B/RAGE/NF-κB. Biochem. Biophy. Res. Commun. 2019, 517, 221–226. [Google Scholar] [CrossRef]
- Zhao, Y.; Tozawa, Y.; Iseki, R.; Mukai, M.; Iwata, M. Calcineurin activation protects T cells from glucocorticoid-induced apoptosis. J. Immunol. 1995, 154, 6346–6354. [Google Scholar] [CrossRef]
- Shibasaki, F.; Hallin, U.; Uchino, H. Calcineurin as a multifunctional regulator. J. Biochem. 2002, 131, 1–15. [Google Scholar] [CrossRef] [PubMed]
- von Boyen, G.B.T.; Schulte, N.; Pflüger, C.; Spaniol, U.; Hartmann, C.; Steinkamp, M. Distribution of enteric glia and GDNF during gut inflammation. BMC Gastroenterol. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, I.J.; Aizenman, E. Pentamidine is an N-methyl-D-aspartate receptor antagonist and is neuroprotective in vitro. J. Neurosci. 1992, 12, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Arima, T.; Sato, T.; Nakamura, J.; Nomura, Y. Inhibitory effects of pentamidine on N-methyl-D-aspartate (NMDA) receptor/channels in the rat brain. Biol. Pharm. Bull. 1995, 18, 234–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartman, K.G.; McKnight, L.M.; Liriano, M.A.; Weber, D.J. The evolution of S100B inihibitors for the treatment of malignant melanoma. Future Med. Chem. 2013, 5, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirillo, C.; Capoccia, E.; Iuvone, T.; Cuomo, R.; Sarnelli, G.; Steardo, L.; Esposito, G. S100B inhibitor pentamidine attenuates reactive gliosis and reduces neuronal loss in a mouse model of Alzheimer’s disease. BioMed. Res. Int. 2015, 2015, 508342. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teramoto, H.; Hirashima, N.; Tanaka, M. Calcineurin B1 Deficiency Reduces Proliferation, Increases Apoptosis, and Alters Secretion in Enteric Glial Cells of Mouse Small Intestine in Culture. Cells 2023, 12, 1867. https://doi.org/10.3390/cells12141867
Teramoto H, Hirashima N, Tanaka M. Calcineurin B1 Deficiency Reduces Proliferation, Increases Apoptosis, and Alters Secretion in Enteric Glial Cells of Mouse Small Intestine in Culture. Cells. 2023; 12(14):1867. https://doi.org/10.3390/cells12141867
Chicago/Turabian StyleTeramoto, Hikaru, Naohide Hirashima, and Masahiko Tanaka. 2023. "Calcineurin B1 Deficiency Reduces Proliferation, Increases Apoptosis, and Alters Secretion in Enteric Glial Cells of Mouse Small Intestine in Culture" Cells 12, no. 14: 1867. https://doi.org/10.3390/cells12141867
APA StyleTeramoto, H., Hirashima, N., & Tanaka, M. (2023). Calcineurin B1 Deficiency Reduces Proliferation, Increases Apoptosis, and Alters Secretion in Enteric Glial Cells of Mouse Small Intestine in Culture. Cells, 12(14), 1867. https://doi.org/10.3390/cells12141867





