Toll-like Receptors as Pro-Thrombotic Drivers in Viral Infections: A Narrative Review
Abstract
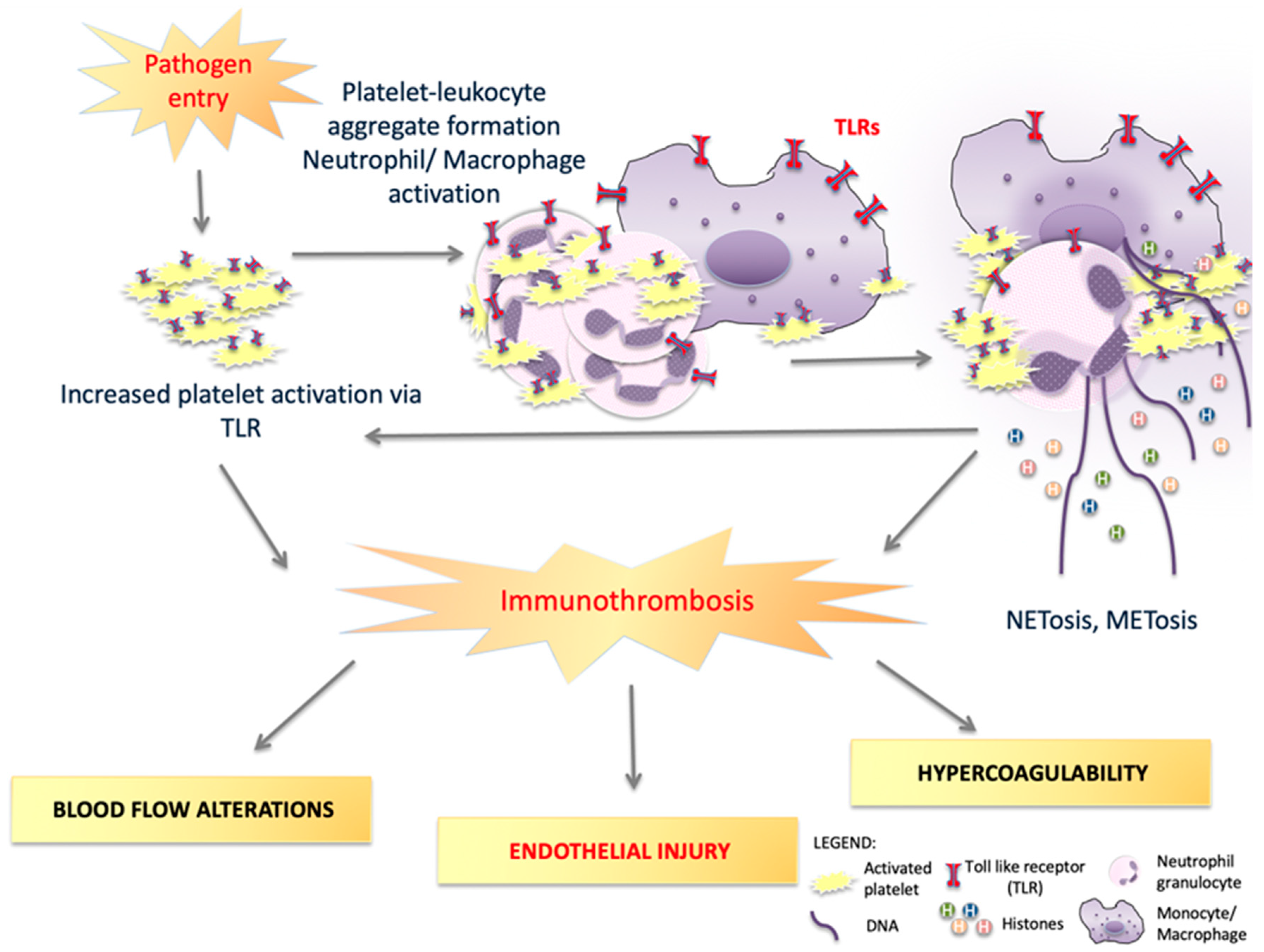
1. Toll-like Receptors’ Role in Inflammation and Thrombosis
2. TLR Signaling
3. TLRs and Diseases
3.1. TLR-1
3.2. TLR-2
3.3. TLR-3
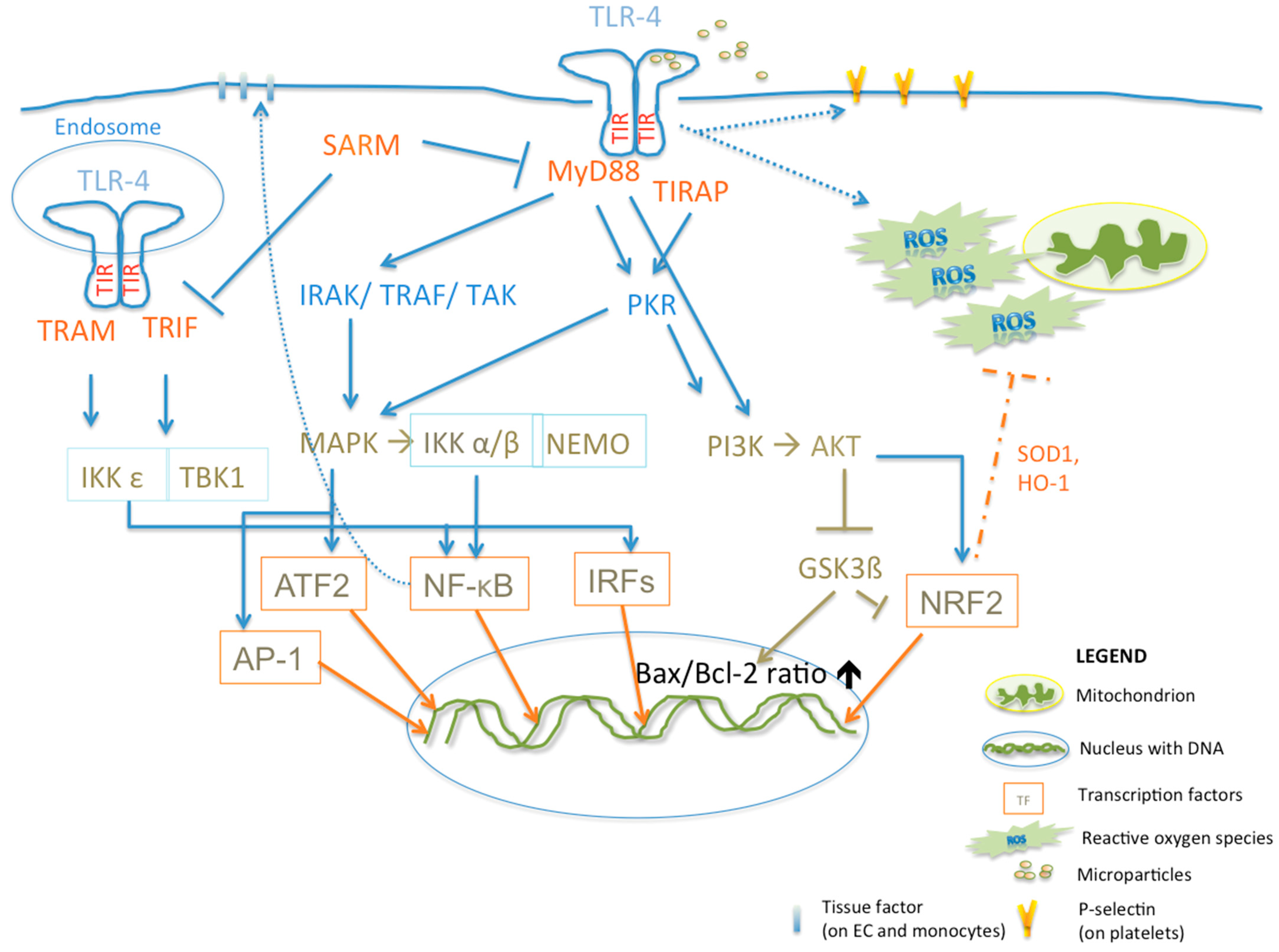
3.4. TLR-4
3.5. TLR-5
3.6. TLR-6
3.7. TLR-7 and -8
3.8. TLR-9
3.9. TLR-10
4. Discussion
| Diseases | TLRs Involved | References |
|---|---|---|
| Auto-immune | ||
| Graves’ Disease | 1, 2, 5, 7, 8 | [114,171] |
| Multiple Sclerosis | 9 | [204] |
| Rheumatoid Arthritis | 2, 8, 9 | [48,91,197,203] |
| Systemic Lupus Erythematosus | 3, 7, 8, 9 | [123,196] |
| Cardiovascular | ||
| Abdominal Aortic Aneurysm | 2, 4 | [95,98,99,104,105,106,108] |
| Acute Myocardial Infarction | 1, 2, 4 | [83,157,158] |
| Atherosclerosis | 1, 2, 4, 6 | [104,152,240] |
| Vasculitis | 4, 5 | [80] |
| Infectious | ||
| Chikungunya Virus | 1, 2, 3, 7, 8 | [15,192] |
| Cytomegalovirus | 2, 4, 7, 9 | [109,167,205] |
| Coronavirus Disease 2019 | 2, 4, 5, 6, 7, 8 | [42,72,74,75,168,169,175,250] |
| Dengue Virus | 4 | [159,160] |
| Epstein Barr Virus | 2, 7, 9 | [13,209] |
| Herpes Simplex Virus 1 and 2 | 9 | [207,208] |
| Hepatitis B | 2, 5, 7 | [13,112,173] |
| Hepatitis C | 2, 3, 4, 7 | [13,111,193] |
| Human Immunodeficiency Virus | 7, 8, 10 | [110,195,217] |
| Influenza | 2, 5, 6, 7, 9, 10 | [22,172,177,179,189,218] |
| Middle Eastern Respiratory Syndrome | 3, 7 | [16,190] |
| Respiratory Syncytial Virus | 4 | [166] |
| West Nile Virus | 3 | [126] |
| Varicella Zoster | 2 | [13] |
| Metabolic | ||
| Diabetes Mellitus Type 1 | 1, 2, 3, 4, 7, 9 | [82,84] |
| Diabetes Mellitus Type 2 | 1, 2, 4 | [81,82,113] |
| Non-Alcoholic Steatosis Hepatis | 9 | [202] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Zelechowska, P.; Kozlowska, E.; Brzezinska-Blaszczyk, E. Expression of surface and intracellular Toll-like receptors by mature mast cells. Cent. Eur. J. Immunol. 2016, 41, 333–338. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.E.; Cho, K.; Stickling, C.; Reynolds, J.M. Toll-like Receptor 2 in Autoimmune Inflammation. Immune Netw. 2021, 21, e18. [Google Scholar] [CrossRef] [PubMed]
- Reganon, E.; Vila, V.; Martinez-Sales, V.; Vaya, A.; Aznar, J. Inflammation, fibrinogen and thrombin generation in patients with previous myocardial infarction. Haematologica 2002, 87, 740–745, discussion 745. [Google Scholar] [PubMed]
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet Activation: The Mechanisms and Potential Biomarkers. BioMed Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef]
- Kaplan, R.C.; Frishman, W.H. Systemic inflammation as a cardiovascular disease risk factor and as a potential target for drug therapy. Heart Dis. 2001, 3, 326–332. [Google Scholar] [CrossRef]
- Vallance, T.M.; Zeuner, M.T.; Williams, H.F.; Widera, D.; Vaiyapuri, S. Toll-like Receptor 4 Signalling and Its Impact on Platelet Function, Thrombosis, and Haemostasis. Mediat. Inflamm. 2017, 2017, 9605894. [Google Scholar] [CrossRef]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Panzer, B.; Jozkowicz, A.; Kopp, C.W.; Gremmel, T.; Panzer, S.; Koppensteiner, R. Microvascular Thrombosis as a Critical Factor in Severe COVID-19. Int. J. Mol. Sci. 2023, 24, 2492. [Google Scholar] [CrossRef]
- Sartorius, R.; Trovato, M.; Manco, R.; D’Apice, L.; De Berardinis, P. Exploiting viral sensing mediated by Toll-like receptors to design innovative vaccines. NPJ Vaccines 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Hally, K.; Fauteux-Daniel, S.; Hamzeh-Cognasse, H.; Larsen, P.; Cognasse, F. Revisiting Platelets and Toll-like Receptors (TLRs): At the Interface of Vascular Immunity and Thrombosis. Int. J. Mol. Sci. 2020, 21, 6150. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Chlichlia, K. Toll-like receptors and viruses: Induction of innate antiviral immune responses. Open Microbiol. J. 2008, 2, 49–59. [Google Scholar] [CrossRef]
- Lee, S.M.; Yip, T.F.; Yan, S.; Jin, D.Y.; Wei, H.L.; Guo, R.T.; Peiris, J.S.M. Recognition of Double-Stranded RNA and Regulation of Interferon Pathway by Toll-like Receptor 10. Front. Immunol. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Lopez, J.F.; Fernandez, G.J.; Urcuqui-Inchima, S. Synergistic Effects of Toll-like Receptor 1/2 and Toll-like Receptor 3 Signaling Triggering Interleukin 27 Gene Expression in Chikungunya Virus-Infected Macrophages. Front. Cell Dev. Biol. 2022, 10, 812110. [Google Scholar] [CrossRef] [PubMed]
- Totura, A.L.; Whitmore, A.; Agnihothram, S.; Schafer, A.; Katze, M.G.; Heise, M.T.; Baric, R.S. Toll-like Receptor 3 Signaling via TRIF Contributes to a Protective Innate Immune Response to Severe Acute Respiratory Syndrome Coronavirus Infection. mBio 2015, 6, e00638-15. [Google Scholar] [CrossRef]
- Ryan, T.A.J.; O’Neill, L.A.J. An Emerging Role for Type I Interferons as Critical Regulators of Blood Coagulation. Cells 2023, 12, 778. [Google Scholar] [CrossRef]
- Grandvaux, N.; Servant, M.J.; tenOever, B.; Sen, G.C.; Balachandran, S.; Barber, G.N.; Lin, R.; Hiscott, J. Transcriptional profiling of interferon regulatory factor 3 target genes: Direct involvement in the regulation of interferon-stimulated genes. J. Virol. 2002, 76, 5532–5539. [Google Scholar] [CrossRef]
- Vogel, S.; Bodenstein, R.; Chen, Q.; Feil, S.; Feil, R.; Rheinlaender, J.; Schaffer, T.E.; Bohn, E.; Frick, J.S.; Borst, O.; et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Investig. 2015, 125, 4638–4654. [Google Scholar] [CrossRef]
- Ruf, W. TRIF turns the switch for DIC in sepsis. Blood 2020, 135, 1073–1074. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-kappaB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Le Goffic, R.; Balloy, V.; Lagranderie, M.; Alexopoulou, L.; Escriou, N.; Flavell, R.; Chignard, M.; Si-Tahar, M. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2006, 2, e53. [Google Scholar] [CrossRef] [PubMed]
- Weithauser, A.; Bobbert, P.; Antoniak, S.; Bohm, A.; Rauch, B.H.; Klingel, K.; Savvatis, K.; Kroemer, H.K.; Tschope, C.; Stroux, A.; et al. Protease-activated receptor-2 regulates the innate immune response to viral infection in a coxsackievirus B3-induced myocarditis. J. Am. Coll. Cardiol. 2013, 62, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Khoufache, K.; LeBouder, F.; Morello, E.; Laurent, F.; Riffault, S.; Andrade-Gordon, P.; Boullier, S.; Rousset, P.; Vergnolle, N.; Riteau, B. Protective role for protease-activated receptor-2 against influenza virus pathogenesis via an IFN-gamma-dependent pathway. J. Immunol. 2009, 182, 7795–7802. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar]
- Dib, P.R.B.; Quirino-Teixeira, A.C.; Merij, L.B.; Pinheiro, M.B.M.; Rozini, S.V.; Andrade, F.B.; Hottz, E.D. Innate immune receptors in platelets and platelet-leukocyte interactions. J. Leukoc. Biol. 2020, 108, 1157–1182. [Google Scholar] [CrossRef]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Chien, P.; Indik, Z.K.; Schreiber, A.D. Human platelet FcgammaRIIA and phagocytes in immune-complex clearance. Mol. Immunol. 2011, 48, 691–696. [Google Scholar] [CrossRef]
- Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell. Mol. Life Sci. 2010, 67, 525–544. [Google Scholar] [CrossRef]
- Portier, I.; Campbell, R.A. Role of Platelets in Detection and Regulation of Infection. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 70–78. [Google Scholar] [CrossRef]
- Burkard, P.; Vogtle, T.; Nieswandt, B. Platelets in Thrombo-Inflammation: Concepts, Mechanisms, and Therapeutic Strategies for Ischemic Stroke. Hamostaseologie 2020, 40, 153–164. [Google Scholar] [CrossRef] [PubMed]
- von Hundelshausen, P.; Weber, C. Platelets as immune cells: Bridging inflammation and cardiovascular disease. Circ. Res. 2007, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef]
- Olsson, A.K.; Cedervall, J. The pro-inflammatory role of platelets in cancer. Platelets 2018, 29, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Kabelitz, D. Expression and function of Toll-like receptors in T lymphocytes. Curr. Opin. Immunol. 2007, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Dolganiuc, A.; Garcia, C.; Kodys, K.; Szabo, G. Distinct Toll-like receptor expression in monocytes and T cells in chronic HCV infection. World J. Gastroenterol. 2006, 12, 1198–1204. [Google Scholar] [CrossRef]
- Prince, L.R.; Whyte, M.K.; Sabroe, I.; Parker, L.C. The role of TLRs in neutrophil activation. Curr. Opin. Pharmacol. 2011, 11, 397–403. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Kumar, H.; Kawai, T.; Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011, 30, 16–34. [Google Scholar] [CrossRef]
- Lester, S.N.; Li, K. Toll-like receptors in antiviral innate immunity. J. Mol. Biol. 2014, 426, 1246–1264. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Rezaei, N. Role of Toll-like receptors in the pathogenesis of COVID-19. J. Med. Virol. 2021, 93, 2735–2739. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Pierce, S.K. How location governs toll-like receptor signaling. Traffic 2009, 10, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, T.; Kajita, E.; Miwa, S.; Defranco, A.L. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 2005, 280, 37107–37117. [Google Scholar] [CrossRef] [PubMed]
- Valencia Pacheco, G.J.; Pinzon Herrera, F.; Cruz Lopez, J.J.; Vera Gamboa Ldel, C.; Pavia Ruiz, N.; Santos Rivero, A.; Sanchez Lugo, S.; Puerto, F. Expression and activation of intracellular receptors TLR7, TLR8 and TLR9 in peripheral blood monocytes from HIV-infected patients. Colomb. Medica 2013, 44, 92–99. [Google Scholar] [CrossRef]
- Miyake, K. Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin. Immunol. 2007, 19, 3–10. [Google Scholar] [CrossRef]
- Fore, F.; Budipranama, M.; Destiawan, R.A. TLR10 and Its Role in Immunity. Handb. Exp. Pharmacol. 2022, 276, 161–174. [Google Scholar] [CrossRef]
- Shotorbani, S.S.; Su, Z.L.; Xu, H.X. Toll-like receptors are potential therapeutic targets in rheumatoid arthritis. World J. Biol. Chem. 2011, 2, 167–172. [Google Scholar] [CrossRef]
- Jin, M.S.; Lee, J.O. Structures of the toll-like receptor family and its ligand complexes. Immunity 2008, 29, 182–191. [Google Scholar] [CrossRef]
- Buwitt-Beckmann, U.; Heine, H.; Wiesmuller, K.H.; Jung, G.; Brock, R.; Akira, S.; Ulmer, A.J. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 2005, 35, 282–289. [Google Scholar] [CrossRef]
- Ve, T.; Williams, S.J.; Kobe, B. Structure and function of Toll/interleukin-1 receptor/resistance protein (TIR) domains. Apoptosis 2015, 20, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Carty, M.; Goodbody, R.; Schroder, M.; Stack, J.; Moynagh, P.N.; Bowie, A.G. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 2006, 7, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Schilling, D.; Thomas, K.; Nixdorff, K.; Vogel, S.N.; Fenton, M.J. Toll-like receptor 4 and Toll-IL-1 receptor domain-containing adapter protein (TIRAP)/myeloid differentiation protein 88 adapter-like (Mal) contribute to maximal IL-6 expression in macrophages. J. Immunol. 2002, 169, 5874–5880. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. DisSARMing Toll-like receptor signaling. Nat. Immunol. 2006, 7, 1023–1025. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, W.; Zhou, A.L.; Zhao, M.; Jiang, D.R. Inhibitory effect of oxymatrine on hepatocyte apoptosis via TLR4/PI3K/Akt/GSK-3beta signaling pathway. World J. Gastroenterol. 2017, 23, 3839–3849. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Jiang, W.; Zhao, M.; Gan, J. Role of TLR4-Mediated PI3K/AKT/GSK-3beta Signaling Pathway in Apoptosis of Rat Hepatocytes. BioMed Res. Int. 2015, 2015, 631326. [Google Scholar] [CrossRef]
- Ernst, O.; Vayttaden, S.J.; Fraser, I.D.C. Measurement of NF-kappaB Activation in TLR-Activated Macrophages. Methods Mol. Biol. 2018, 1714, 67–78. [Google Scholar] [CrossRef]
- Rubio, D.; Xu, R.H.; Remakus, S.; Krouse, T.E.; Truckenmiller, M.E.; Thapa, R.J.; Balachandran, S.; Alcami, A.; Norbury, C.C.; Sigal, L.J. Crosstalk between the type 1 interferon and nuclear factor kappa B pathways confers resistance to a lethal virus infection. Cell Host Microbe 2013, 13, 701–710. [Google Scholar] [CrossRef]
- Lima, B.H.F.; Marques, P.E.; Gomides, L.F.; Mattos, M.S.; Kraemer, L.; Queiroz-Junior, C.M.; Lennon, M.; Hirsch, E.; Russo, R.C.; Menezes, G.B.; et al. Converging TLR9 and PI3Kgamma signaling induces sterile inflammation and organ damage. Sci. Rep. 2019, 9, 19085. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like receptors activation, signaling, and targeting: An overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Teixeira, H.S.; Zhao, J.; Kazmierski, E.; Kinane, D.F.; Benakanakere, M.R. TLR3-Dependent Activation of TLR2 Endogenous Ligands via the MyD88 Signaling Pathway Augments the Innate Immune Response. Cells 2020, 9, 1910. [Google Scholar] [CrossRef] [PubMed]
- Van Quickelberghe, E.; De Sutter, D.; van Loo, G.; Eyckerman, S.; Gevaert, K. A protein-protein interaction map of the TNF-induced NF-kappaB signal transduction pathway. Sci. Data 2018, 5, 180289. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Weikert, C.; Pultar, J.; Lee, S.; Eichelberger, B.; Koppensteiner, R.; Lang, I.M.; Panzer, S.; Gremmel, T. Ticagrelor Inhibits Toll-like and Protease-Activated Receptor Mediated Platelet Activation in Acute Coronary Syndromes. Cardiovasc. Drugs Ther. 2020, 34, 53–63. [Google Scholar] [CrossRef]
- Cognasse, F.; Nguyen, K.A.; Damien, P.; McNicol, A.; Pozzetto, B.; Hamzeh-Cognasse, H.; Garraud, O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front. Immunol. 2015, 6, 83. [Google Scholar] [CrossRef]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Damien, P.; Cognasse, F.; Payrastre, B.; Spinelli, S.L.; Blumberg, N.; Arthaud, C.A.; Eyraud, M.A.; Phipps, R.P.; McNicol, A.; Pozzetto, B.; et al. NF-kappaB Links TLR2 and PAR1 to Soluble Immunomodulator Factor Secretion in Human Platelets. Front. Immunol. 2017, 8, 85. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. Inflammation and thrombosis in COVID-19 pathophysiology: Proteinase-activated and purinergic receptors as drivers and candidate therapeutic targets. Physiol. Rev. 2021, 101, 545–567. [Google Scholar] [CrossRef]
- Sheahan, T.; Morrison, T.E.; Funkhouser, W.; Uematsu, S.; Akira, S.; Baric, R.S.; Heise, M.T. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008, 4, e1000240. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.; Perlman, S. Autocrine interferon priming in macrophages but not dendritic cells results in enhanced cytokine and chemokine production after coronavirus infection. mBio 2010, 1, e00219-10. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.D. TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Vercellotti, G.M. Effects of viral activation of the vessel wall on inflammation and thrombosis. Blood Coagul. Fibrinolysis 1998, 9 (Suppl. 2), S3–S6. [Google Scholar] [PubMed]
- Sariol, A.; Perlman, S. SARS-CoV-2 takes its Toll. Nat. Immunol. 2021, 22, 801–802. [Google Scholar] [CrossRef] [PubMed]
- Manik, M.; Singh, R.K. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 2022, 94, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Tosato, G.; Jones, K.D. Interleukin-1 induces interleukin-6 production in peripheral blood monocytes. Blood 1990, 75, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Piechota-Polanczyk, A.; Andreas, M.; Kopp, C.W. Cardiovascular Disease Management in the Context of Global Crisis. Int. J. Environ. Res. Public Health 2022, 20, 689. [Google Scholar] [CrossRef]
- Carnevale, R.; Cammisotto, V.; Bartimoccia, S.; Nocella, C.; Castellani, V.; Bufano, M.; Loffredo, L.; Sciarretta, S.; Frati, G.; Coluccia, A.; et al. Toll-like Receptor 4-Dependent Platelet-Related Thrombosis in SARS-CoV-2 Infection. Circ. Res. 2023, 132, 290–305. [Google Scholar] [CrossRef]
- Deng, J.; Ma-Krupa, W.; Gewirtz, A.T.; Younge, B.R.; Goronzy, J.J.; Weyand, C.M. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ. Res. 2009, 104, 488–495. [Google Scholar] [CrossRef]
- Badr, R.E.; Salama, M.I.; Abd-Elmaogood, A.K.; Eldeib, A.E.M. Toll-like receptor 2 expression on monocytes and microvascular complications in type 2 diabetic patients. Diabetes Metab. Syndr. 2019, 13, 1299–1302. [Google Scholar] [CrossRef]
- Jialal, I.; Kaur, H. The Role of Toll-like Receptors in Diabetes-Induced Inflammation: Implications for Vascular Complications. Curr. Diabetes Rep. 2012, 12, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Hally, K.E.; La Flamme, A.C.; Larsen, P.D.; Harding, S.A. Platelet Toll-like receptor (TLR) expression and TLR-mediated platelet activation in acute myocardial infarction. Thromb. Res. 2017, 158, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Lien, E.; Zipris, D. The role of Toll-like receptor pathways in the mechanism of type 1 diabetes. Curr. Mol. Med. 2009, 9, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Picard, F.; Adjedj, J.; Varenne, O. Diabetes Mellitus, a prothrombotic disease. Ann. Cardiol. Angeiol. 2017, 66, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Schwameis, M.; Buchtele, N.; Wadowski, P.P.; Schoergenhofer, C.; Jilma, B. Chikungunya vaccines in development. Hum. Vaccines Immunother. 2016, 12, 716–731. [Google Scholar] [CrossRef]
- Hassan, T.; Abdel Rahman, D.; Raafat, N.; Fathy, M.; Shehab, M.; Hosny, A.; Fawzy, R.; Zakaria, M. Contribution of interleukin 27 serum level to pathogenesis and prognosis in children with immune thrombocytopenia. Medicine 2022, 101, e29504. [Google Scholar] [CrossRef]
- Lannoy, V.; Cote-Biron, A.; Asselin, C.; Rivard, N. TIRAP, TRAM, and Toll-like Receptors: The Untold Story. Mediat. Inflamm. 2023, 2023, 2899271. [Google Scholar] [CrossRef]
- Stack, J.; Doyle, S.L.; Connolly, D.J.; Reinert, L.S.; O’Keeffe, K.M.; McLoughlin, R.M.; Paludan, S.R.; Bowie, A.G. TRAM is required for TLR2 endosomal signaling to type I IFN induction. J. Immunol. 2014, 193, 6090–6102. [Google Scholar] [CrossRef]
- Petnicki-Ocwieja, T.; Chung, E.; Acosta, D.I.; Ramos, L.T.; Shin, O.S.; Ghosh, S.; Kobzik, L.; Li, X.; Hu, L.T. TRIF mediates Toll-like receptor 2-dependent inflammatory responses to Borrelia burgdorferi. Infect. Immun. 2013, 81, 402–410. [Google Scholar] [CrossRef]
- Thwaites, R.S.; Unterberger, S.; Chamberlain, G.; Gray, H.; Jordan, K.; Davies, K.A.; Harrison, N.A.; Sacre, S. Expression of sterile-alpha and armadillo motif containing protein (SARM) in rheumatoid arthritis monocytes correlates with TLR2-induced IL-1beta and disease activity. Rheumatology 2021, 60, 5843–5853. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Eichelberger, B.; Kopp, C.W.; Pultar, J.; Seidinger, D.; Koppensteiner, R.; Lang, I.M.; Panzer, S.; Gremmel, T. Disaggregation Following Agonist-Induced Platelet Activation in Patients on Dual Antiplatelet Therapy. J. Cardiovasc. Transl. Res. 2017, 10, 359–367. [Google Scholar] [CrossRef][Green Version]
- Panzer, B.; Wadowski, P.P.; Huber, K.; Panzer, S.; Gremmel, T. Protease-activated receptor-mediated platelet aggregation in patients with type 2 diabetes on potent P2Y(12) inhibitors. Diabet. Med. 2022, 39, e14868. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Pultar, J.; Weikert, C.; Eichelberger, B.; Panzer, B.; Huber, K.; Lang, I.M.; Koppensteiner, R.; Panzer, S.; Gremmel, T. Protease-activated receptor-mediated platelet aggregation in acute coronary syndrome patients on potent P2Y(12) inhibitors. Res. Pract. Thromb. Haemost. 2019, 3, 383–390. [Google Scholar] [CrossRef]
- Jablonska, A.; Zagrapan, B.; Neumayer, C.; Klinger, M.; Eilenberg, W.; Nanobachvili, J.; Paradowska, E.; Brostjan, C.; Huk, I. TLR2 2029C/T and TLR3 1377C/T and -7C/A Polymorphisms Are Associated with the Occurrence of Abdominal Aortic Aneurysm. J. Immunol. 2020, 204, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Haque, K.; Bhargava, P. Abdominal Aortic Aneurysm. Am. Fam. Physician 2022, 106, 165–172. [Google Scholar] [PubMed]
- Kessler, V.; Klopf, J.; Eilenberg, W.; Neumayer, C.; Brostjan, C. AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis. Biomedicines 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, A.; Neumayer, C.; Bolliger, M.; Gollackner, B.; Klinger, M.; Paradowska, E.; Nanobachvili, J.; Huk, I. Analysis of host Toll-like receptor 3 and RIG-I-like receptor gene expression in patients with abdominal aortic aneurysm. J. Vasc. Surg. 2018, 68, 39S–46S. [Google Scholar] [CrossRef]
- Jablonska, A.; Neumayer, C.; Bolliger, M.; Burghuber, C.; Klinger, M.; Demyanets, S.; Nanobachvili, J.; Huk, I. Insight into the expression of toll-like receptors 2 and 4 in patients with abdominal aortic aneurysm. Mol. Biol. Rep. 2020, 47, 2685–2692. [Google Scholar] [CrossRef]
- Treska, V.; Kocova, J.; Boudova, L.; Neprasova, P.; Topolcan, O.; Pecen, L.; Tonar, Z. Inflammation in the wall of abdominal aortic aneurysm and its role in the symptomatology of aneurysm. Cytokines Cell. Mol. Ther. 2002, 7, 91–97. [Google Scholar] [CrossRef]
- Klopf, J.; Brostjan, C.; Neumayer, C.; Eilenberg, W. Neutrophils as Regulators and Biomarkers of Cardiovascular Inflammation in the Context of Abdominal Aortic Aneurysms. Biomedicines 2021, 9, 1236. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.M.; Brophy, C.M.; Tilson, M.D. Characterization of an elastase from aneurysmal aorta which degrades intact aortic elastin. Ann. Vasc. Surg. 1992, 6, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Newman, K.M.; Malon, A.M.; Shin, R.D.; Scholes, J.V.; Ramey, W.G.; Tilson, M.D. Matrix metalloproteinases in abdominal aortic aneurysm: Characterization, purification, and their possible sources. Connect. Tissue Res. 1994, 30, 265–276. [Google Scholar] [CrossRef]
- Mullick, A.E.; Tobias, P.S.; Curtiss, L.K. Modulation of atherosclerosis in mice by Toll-like receptor 2. J. Clin. Investig. 2005, 115, 3149–3156. [Google Scholar] [CrossRef]
- Aoyama, N.; Suzuki, J.; Ogawa, M.; Watanabe, R.; Kobayashi, N.; Hanatani, T.; Ashigaki, N.; Sekinishi, A.; Izumi, Y.; Isobe, M. Toll-like receptor-2 plays a fundamental role in periodontal bacteria-accelerated abdominal aortic aneurysms. Circ. J. 2013, 77, 1565–1573. [Google Scholar] [CrossRef]
- Yan, H.; Cui, B.; Zhang, X.; Fu, X.; Yan, J.; Wang, X.; Lv, X.; Chen, Z.; Hu, Z. Antagonism of toll-like receptor 2 attenuates the formation and progression of abdominal aortic aneurysm. Acta Pharm. Sin. B 2015, 5, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Wang, K.C.; Lee, F.T.; Tsai, H.W.; Ma, C.Y.; Cheng, T.L.; Chang, B.I.; Yang, Y.J.; Shi, G.Y.; Wu, H.L. Toll-like Receptor 4 Is Essential in the Development of Abdominal Aortic Aneurysm. PLoS ONE 2016, 11, e0146565. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Rateri, D.L.; Howatt, D.A.; Moore, K.J.; Tobias, P.S.; Curtiss, L.K.; Lu, H.; Cassis, L.A.; Daugherty, A. MyD88 deficiency attenuates angiotensin II-induced abdominal aortic aneurysm formation independent of signaling through Toll-like receptors 2 and 4. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2813–2819. [Google Scholar] [CrossRef]
- Jablonska, A.; Zagrapan, B.; Paradowska, E.; Neumayer, C.; Eilenberg, W.; Brostjan, C.; Klinger, M.; Nanobachvili, J.; Huk, I. Abdominal aortic aneurysm and virus infection: A potential causative role for cytomegalovirus infection? J. Med. Virol. 2021, 93, 5017–5024. [Google Scholar] [CrossRef]
- Hogh, J.; Pham, M.H.C.; Knudsen, A.D.; Thudium, R.F.; Gelpi, M.; Sigvardsen, P.E.; Fuchs, A.; Kuhl, J.T.; Afzal, S.; Nordestgaard, B.G.; et al. HIV infection is associated with thoracic and abdominal aortic aneurysms: A prospective matched cohort study. Eur. Heart J. 2021, 42, 2924–2931. [Google Scholar] [CrossRef]
- Shehata, M.A.; Abou El-Enein, A.; El-Sharnouby, G.A. Significance of toll-like receptors 2 and 4 mRNA expression in chronic hepatitis C virus infection. Egypt. J. Immunol. 2006, 13, 141–152. [Google Scholar]
- Li, Q.; Wang, J.; Islam, H.; Kirschning, C.; Lu, H.; Hoffmann, D.; Dittmer, U.; Lu, M. Hepatitis B virus particles activate B cells through the TLR2-MyD88-mTOR axis. Cell Death Dis. 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Sepehri, Z.; Kiani, Z.; Nasiri, A.A.; Kohan, F. Toll-like receptor 2 and type 2 diabetes. Cell. Mol. Biol. Lett. 2016, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Grywalska, E.; Klatka, J.; Rolinski, J.; Matyjaszek-Matuszek, B.; Klatka, M. Toll-like Receptors-2 and -4 in Graves’ Disease-Key Players or Bystanders? Int. J. Mol. Sci. 2019, 20, 4732. [Google Scholar] [CrossRef] [PubMed]
- Kariko, K.; Ni, H.; Capodici, J.; Lamphier, M.; Weissman, D. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 2004, 279, 12542–12550. [Google Scholar] [CrossRef]
- Cho, W.G.; Albuquerque, R.J.; Kleinman, M.E.; Tarallo, V.; Greco, A.; Nozaki, M.; Green, M.G.; Baffi, J.Z.; Ambati, B.K.; De Falco, M.; et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc. Natl. Acad. Sci. USA 2009, 106, 7137–7142. [Google Scholar] [CrossRef]
- D’Atri, L.P.; Etulain, J.; Rivadeneyra, L.; Lapponi, M.J.; Centurion, M.; Cheng, K.; Yin, H.; Schattner, M. Expression and functionality of Toll-like receptor 3 in the megakaryocytic lineage. J. Thromb. Haemost. 2015, 13, 839–850. [Google Scholar] [CrossRef]
- Najem, M.; Rys, R.; Laurance, S.; Couturaud, F.; Blostein, M.D.; Lemarié, C.A. TLR3 promotes venous thrombosis through neutrophil recruitment. Rev. Mal. Respir. 2021, 38, 580. [Google Scholar] [CrossRef]
- Shibamiya, A.; Hersemeyer, K.; Schmidt Woll, T.; Sedding, D.; Daniel, J.M.; Bauer, S.; Koyama, T.; Preissner, K.T.; Kanse, S.M. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood 2009, 113, 714–722. [Google Scholar] [CrossRef]
- Posma, J.J.; Grover, S.P.; Hisada, Y.; Owens, A.P., 3rd; Antoniak, S.; Spronk, H.M.; Mackman, N. Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 13–24. [Google Scholar] [CrossRef]
- Antoniak, S.; Mackman, N. Multiple roles of the coagulation protease cascade during virus infection. Blood 2014, 123, 2605–2613. [Google Scholar] [CrossRef]
- Tatsumi, K.; Schmedes, C.M.; Houston, E.R.; Butler, E.; Mackman, N.; Antoniak, S. Protease-activated receptor 4 protects mice from Coxsackievirus B3 and H1N1 influenza A virus infection. Cell. Immunol. 2019, 344, 103949. [Google Scholar] [CrossRef]
- Laska, M.J.; Troldborg, A.; Hansen, B.; Stengaard-Pedersen, K.; Junker, P.; Nexo, B.A.; Voss, A. Polymorphisms within Toll-like receptors are associated with systemic lupus erythematosus in a cohort of Danish females. Rheumatology 2014, 53, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Homood, I.A. Thrombosis in systemic lupus erythematosus: A review article. ISRN Rheumatol. 2012, 2012, 428269. [Google Scholar] [CrossRef]
- Hewson, C.A.; Jardine, A.; Edwards, M.R.; Laza-Stanca, V.; Johnston, S.L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 2005, 79, 12273–12279. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Mayhan, W.G. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002, 927, 144–152. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Endogenous ligands of Toll-like receptors. J. Leukoc. Biol. 2004, 76, 514–519. [Google Scholar] [CrossRef]
- Midwood, K.; Sacre, S.; Piccinini, A.M.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Malara, A.; Gruppi, C.; Abbonante, V.; Cattaneo, D.; De Marco, L.; Massa, M.; Iurlo, A.; Gianelli, U.; Balduini, C.L.; Tira, M.E.; et al. EDA fibronectin-TLR4 axis sustains megakaryocyte expansion and inflammation in bone marrow fibrosis. J. Exp. Med. 2019, 216, 587–604. [Google Scholar] [CrossRef]
- Roberts, A.L.; Mavlyutov, T.A.; Perlmutter, T.E.; Curry, S.M.; Harris, S.L.; Chauhan, A.K.; McDowell, C.M. Fibronectin extra domain A (FN-EDA) elevates intraocular pressure through Toll-like receptor 4 signaling. Sci. Rep. 2020, 10, 9815. [Google Scholar] [CrossRef]
- Qiang, X.; Yang, W.L.; Wu, R.; Zhou, M.; Jacob, A.; Dong, W.; Kuncewitch, M.; Ji, Y.; Yang, H.; Wang, H.; et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 2013, 19, 1489–1495. [Google Scholar] [CrossRef]
- Lee, Y.; Reilly, B.; Tan, C.; Wang, P.; Aziz, M. Extracellular CIRP Induces Macrophage Extracellular Trap Formation Via Gasdermin D Activation. Front. Immunol. 2021, 12, 780210. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Horng, T.; Barton, G.M.; Medzhitov, R. TIRAP: An adapter molecule in the Toll signaling pathway. Nat. Immunol. 2001, 2, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Shih, R.H.; Lin, C.C.; Chen, J.T.; Yang, C.M. Role of TLR4/NADPH oxidase/ROS-activated p38 MAPK in VCAM-1 expression induced by lipopolysaccharide in human renal mesangial cells. Cell Commun. Signal 2012, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Liu, X.; Wang, S.; Miao, J.; Wu, L.; Yang, X.; Wang, Y.; Kang, L.; Li, W.; Cui, C.; et al. PKR promotes choroidal neovascularization via upregulating the PI3K/Akt signaling pathway in VEGF expression. Mol. Vis. 2016, 22, 1361–1374. [Google Scholar]
- Vivarini, A.C.; Calegari-Silva, T.C.; Saliba, A.M.; Boaventura, V.S.; Franca-Costa, J.; Khouri, R.; Dierckx, T.; Dias-Teixeira, K.L.; Fasel, N.; Barral, A.M.P.; et al. Systems Approach Reveals Nuclear Factor Erythroid 2-Related Factor 2/Protein Kinase R Crosstalk in Human Cutaneous Leishmaniasis. Front. Immunol. 2017, 8, 1127. [Google Scholar] [CrossRef]
- Liddell, J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef]
- Solis, M.; Romieu-Mourez, R.; Goubau, D.; Grandvaux, N.; Mesplede, T.; Julkunen, I.; Nardin, A.; Salcedo, M.; Hiscott, J. Involvement of TBK1 and IKKepsilon in lipopolysaccharide-induced activation of the interferon response in primary human macrophages. Eur. J. Immunol. 2007, 37, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, E.; Ding, J.L.; Byrne, B. SARM modulates MyD88-mediated TLR activation through BB-loop dependent TIR-TIR interactions. Biochim. Biophys. Acta 2016, 1863, 244–253. [Google Scholar] [CrossRef]
- Yang, L.; Seki, E. Toll-like receptors in liver fibrosis: Cellular crosstalk and mechanisms. Front. Physiol. 2012, 3, 138. [Google Scholar] [CrossRef] [PubMed]
- Schattner, M. Platelet TLR4 at the crossroads of thrombosis and the innate immune response. J. Leukoc. Biol. 2019, 105, 873–880. [Google Scholar] [CrossRef]
- Krikun, G.; Trezza, J.; Shaw, J.; Rahman, M.; Guller, S.; Abrahams, V.M.; Lockwood, C.J. Lipopolysaccharide appears to activate human endometrial endothelial cells through TLR-4-dependent and TLR-4-independent mechanisms. Am. J. Reprod. Immunol. 2012, 68, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Jerez-Dolz, D.; Torramade-Moix, S.; Palomo, M.; Moreno-Castano, A.; Lopez-Vilchez, I.; Hernandez, R.; Badimon, J.J.; Zafar, M.U.; Diaz-Ricart, M.; Escolar, G. Internalization of microparticles by platelets is partially mediated by toll-like receptor 4 and enhances platelet thrombogenicity. Atherosclerosis 2020, 294, 17–24. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Liu, J.; Lv, B.; Chen, F. Extracellular histones induce tissue factor expression in vascular endothelial cells via TLR and activation of NF-kappaB and AP-1. Thromb. Res. 2016, 137, 211–218. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Passam, F.H.; Antoniak, S.; Marshall, S.M.; McDaniel, A.L.; Rudel, L.; Williams, J.C.; Hubbard, B.K.; Dutton, J.A.; Wang, J.; et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J. Clin. Investig. 2012, 122, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Hally, K.E.; Bird, G.K.; La Flamme, A.C.; Harding, S.A.; Larsen, P.D. Platelets modulate multiple markers of neutrophil function in response to in vitro Toll-like receptor stimulation. PLoS ONE 2019, 14, e0223444. [Google Scholar] [CrossRef]
- D’Mello, C.; Almishri, W.; Liu, H.; Swain, M.G. Interactions between Platelets and Inflammatory Monocytes Affect Sickness Behavior in Mice with Liver Inflammation. Gastroenterology 2017, 153, 1416–1428.e2. [Google Scholar] [CrossRef]
- Napier, B.A.; Brubaker, S.W.; Sweeney, T.E.; Monette, P.; Rothmeier, G.H.; Gertsvolf, N.A.; Puschnik, A.; Carette, J.E.; Khatri, P.; Monack, D.M. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J. Exp. Med. 2016, 213, 2365–2382. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sun, X.; Liu, S.; Tang, Y.; Shi, Y.; Bai, Y.; Wang, Y.; Yang, Q.; Yang, Q.; Jiang, W.; et al. Caspase-11-Gasdermin D-Mediated Pyroptosis Is Involved in the Pathogenesis of Atherosclerosis. Front. Pharmacol. 2021, 12, 657486. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Yuan, S.; Wen, S.; Liu, X.; Wang, C.; Qu, Z.; Li, J.; Liu, H.; Sun, L.; et al. TLR4/NF-kappaB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease. Front. Endocrinol. 2019, 10, 603. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Tang, H.; Lin, C.; Shen, Y.; Yan, D.; Tang, X.; Guo, D. Extracellular traps and the role in thrombosis. Front. Cardiovasc. Med. 2022, 9, 951670. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Azmoon, P.; Banki, M.A.; Mantuano, E.; Gonias, S.L. Tissue-type plasminogen activator selectively inhibits multiple toll-like receptors in CSF-1-differentiated macrophages. PLoS ONE 2019, 14, e0224738. [Google Scholar] [CrossRef] [PubMed]
- Kircheis, R.; Planz, O. The Role of Toll-like Receptors (TLRs) and Their Related Signaling Pathways in Viral Infection and Inflammation. Int. J. Mol. Sci. 2023, 24, 6701. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Jager, S.; Sobirey, M.; Escher, F.; Yaulema-Riss, A.; Westermann, D.; Karatas, A.; Heimesaat, M.M.; Bereswill, S.; Dragun, D.; et al. Toll-like receptor-4 modulates survival by induction of left ventricular remodeling after myocardial infarction in mice. J. Immunol. 2008, 180, 6954–6961. [Google Scholar] [CrossRef]
- Satoh, M.; Shimoda, Y.; Maesawa, C.; Akatsu, T.; Ishikawa, Y.; Minami, Y.; Hiramori, K.; Nakamura, M. Activated toll-like receptor 4 in monocytes is associated with heart failure after acute myocardial infarction. Int. J. Cardiol. 2006, 109, 226–234. [Google Scholar] [CrossRef]
- Modhiran, N.; Watterson, D.; Blumenthal, A.; Baxter, A.G.; Young, P.R.; Stacey, K.J. Dengue virus NS1 protein activates immune cells via TLR4 but not TLR2 or TLR6. Immunol. Cell Biol. 2017, 95, 491–495. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cancel, L.M.; Fu, B.M.; Tarbell, J.M. The Glycocalyx and Its Role in Vascular Physiology and Vascular Related Diseases. Cardiovasc. Eng. Technol. 2021, 12, 37–71. [Google Scholar] [CrossRef]
- Tang, T.H.; Alonso, S.; Ng, L.F.; Thein, T.L.; Pang, V.J.; Leo, Y.S.; Lye, D.C.; Yeo, T.W. Increased Serum Hyaluronic Acid and Heparan Sulfate in Dengue Fever: Association with Plasma Leakage and Disease Severity. Sci. Rep. 2017, 7, 46191. [Google Scholar] [CrossRef] [PubMed]
- Suwarto, S.; Sasmono, R.T.; Sinto, R.; Ibrahim, E.; Suryamin, M. Association of Endothelial Glycocalyx and Tight and Adherens Junctions with Severity of Plasma Leakage in Dengue Infection. J. Infect. Dis. 2017, 215, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [PubMed]
- He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006, 13, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, K.; Riteau, N.; Yip, R.; Mayer-Barber, K.D.; Oland, S.; Hieny, S.; Fitzgerald, P.; Oberst, A.; Dillon, C.P.; Green, D.R.; et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 2014, 192, 2029–2033. [Google Scholar] [CrossRef]
- Funchal, G.A.; Jaeger, N.; Czepielewski, R.S.; Machado, M.S.; Muraro, S.P.; Stein, R.T.; Bonorino, C.B.; Porto, B.N. Respiratory syncytial virus fusion protein promotes TLR-4-dependent neutrophil extracellular trap formation by human neutrophils. PLoS ONE 2015, 10, e0124082. [Google Scholar] [CrossRef]
- Cook, C.H.; Trgovcich, J.; Zimmerman, P.D.; Zhang, Y.; Sedmak, D.D. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J. Virol. 2006, 80, 9151–9158. [Google Scholar] [CrossRef]
- Holms, R.D. Long COVID (PASC) Is Maintained by a Self-Sustaining Pro-Inflammatory TLR4/RAGE-Loop of S100A8/A9 > TLR4/RAGE Signalling, Inducing Chronic Expression of IL-1b, IL-6 and TNFa: Anti-Inflammatory Ezrin Peptides as Potential Therapy. Immuno 2022, 2, 512–533. [Google Scholar] [CrossRef]
- Fontes-Dantas, F.L.; Fernandes, G.G.; Gutman, E.G.; De Lima, E.V.; Antonio, L.S.; Hammerle, M.B.; Mota-Araujo, H.P.; Colodeti, L.C.; Araujo, S.M.B.; Froz, G.M.; et al. SARS-CoV-2 Spike protein induces TLR4-mediated long-term cognitive dysfunction recapitulating post-COVID-19 syndrome in mice. Cell Rep. 2023, 42, 112189. [Google Scholar] [CrossRef]
- Yoon, S.I.; Kurnasov, O.; Natarajan, V.; Hong, M.; Gudkov, A.V.; Osterman, A.L.; Wilson, I.A. Structural basis of TLR5-flagellin recognition and signaling. Science 2012, 335, 859–864. [Google Scholar] [CrossRef]
- Xiao, W.; Liu, Z.; Lin, J.; Xiong, C.; Li, J.; Wu, K.; Ma, Y.; Gong, Y.; Liu, Z. Association of TLR4 and TLR5 gene polymorphisms with Graves’ disease in Chinese Cantonese population. Hum. Immunol. 2014, 75, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Georgel, A.F.; Cayet, D.; Pizzorno, A.; Rosa-Calatrava, M.; Paget, C.; Sencio, V.; Dubuisson, J.; Trottein, F.; Sirard, J.C.; Carnoy, C. Toll-like receptor 5 agonist flagellin reduces influenza A virus replication independently of type I interferon and interleukin 22 and improves antiviral efficacy of oseltamivir. Antivir. Res. 2019, 168, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Isogawa, M.; Robek, M.D.; Furuichi, Y.; Chisari, F.V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 2005, 79, 7269–7272. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kakimi, K.; Wieland, S.; Guidotti, L.G.; Chisari, F.V. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J. Immunol. 2002, 169, 5188–5195. [Google Scholar] [CrossRef]
- Ruffin, M.; Bigot, J.; Calmel, C.; Mercier, J.; Givelet, M.; Oliva, J.; Pizzorno, A.; Rosa-Calatrava, M.; Corvol, H.; Balloy, V.; et al. Flagellin From Pseudomonas aeruginosa Modulates SARS-CoV-2 Infectivity in Cystic Fibrosis Airway Epithelial Cells by Increasing TMPRSS2 Expression. Front. Immunol. 2021, 12, 714027. [Google Scholar] [CrossRef]
- Owens, A.P., 3rd; Mackman, N. Sources of tissue factor that contribute to thrombosis after rupture of an atherosclerotic plaque. Thromb. Res. 2012, 129 (Suppl. 2), S30–S33. [Google Scholar] [CrossRef][Green Version]
- Tuvim, M.J.; Gilbert, B.E.; Dickey, B.F.; Evans, S.E. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS ONE 2012, 7, e30596. [Google Scholar] [CrossRef]
- Shevlin, E.; Miggin, S.M. The TIR-domain containing adaptor TRAM is required for TLR7 mediated RANTES production. PLoS ONE 2014, 9, e107141. [Google Scholar] [CrossRef]
- Piao, W.; Shirey, K.A.; Ru, L.W.; Lai, W.; Szmacinski, H.; Snyder, G.A.; Sundberg, E.J.; Lakowicz, J.R.; Vogel, S.N.; Toshchakov, V.Y. A Decoy Peptide that Disrupts TIRAP Recruitment to TLRs Is Protective in a Murine Model of Influenza. Cell Rep. 2015, 11, 1941–1952. [Google Scholar] [CrossRef]
- Mukherjee, P.; Winkler, C.W.; Taylor, K.G.; Woods, T.A.; Nair, V.; Khan, B.A.; Peterson, K.E. SARM1, Not MyD88, Mediates TLR7/TLR9-Induced Apoptosis in Neurons. J. Immunol. 2015, 195, 4913–4921. [Google Scholar] [CrossRef]
- Nilsen, K.E.; Skjesol, A.; Frengen Kojen, J.; Espevik, T.; Stenvik, J.; Yurchenko, M. TIRAP/Mal Positively Regulates TLR8-Mediated Signaling via IRF5 in Human Cells. Biomedicines 2022, 10, 1476. [Google Scholar] [CrossRef]
- Gamrekelashvili, J.; Kapanadze, T.; Sablotny, S.; Ratiu, C.; Dastagir, K.; Lochner, M.; Karbach, S.; Wenzel, P.; Sitnow, A.; Fleig, S.; et al. Notch and TLR signaling coordinate monocyte cell fate and inflammation. Elife 2020, 9, e57007. [Google Scholar] [CrossRef]
- Cohen, P. The TLR and IL-1 signalling network at a glance. J. Cell Sci. 2014, 127, 2383–2390. [Google Scholar] [CrossRef]
- Diebold, S.S. Recognition of viral single-stranded RNA by Toll-like receptors. Adv. Drug Deliv. Rev. 2008, 60, 813–823. [Google Scholar] [CrossRef]
- Triantafilou, K.; Orthopoulos, G.; Vakakis, E.; Ahmed, M.A.; Golenbock, D.T.; Lepper, P.M.; Triantafilou, M. Human cardiac inflammatory responses triggered by Coxsackie B viruses are mainly Toll-like receptor (TLR) 8-dependent. Cell. Microbiol. 2005, 7, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Senchenkova, E.Y.; Komoto, S.; Russell, J.; Almeida-Paula, L.D.; Yan, L.S.; Zhang, S.; Granger, D.N. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am. J. Pathol. 2013, 183, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, P.; Roselli, M.; Diodati, A.; Casciani, C.U.; Gazzaniga, P.P. Effects on platelet function by human interferon-beta in carcinoma patients. Anticancer Res. 1994, 14, 2779–2784. [Google Scholar] [PubMed]
- Davizon-Castillo, P.; McMahon, B.; Aguila, S.; Bark, D.; Ashworth, K.; Allawzi, A.; Campbell, R.A.; Montenont, E.; Nemkov, T.; D’Alessandro, A.; et al. TNF-alpha-driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood 2019, 134, 727–740. [Google Scholar] [CrossRef]
- Koupenova, M.; Corkrey, H.A.; Vitseva, O.; Manni, G.; Pang, C.J.; Clancy, L.; Yao, C.; Rade, J.; Levy, D.; Wang, J.P.; et al. The role of platelets in mediating a response to human influenza infection. Nat. Commun. 2019, 10, 1780. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B., Jr.; Meyerholz, D.K.; Perlman, S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef]
- van der Sluis, R.M.; Cham, L.B.; Gris-Oliver, A.; Gammelgaard, K.R.; Pedersen, J.G.; Idorn, M.; Ahmadov, U.; Hernandez, S.S.; Cemalovic, E.; Godsk, S.H.; et al. TLR2 and TLR7 mediate distinct immunopathological and antiviral plasmacytoid dendritic cell responses to SARS-CoV-2 infection. EMBO J. 2022, 41, e109622. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Tripathi, A. Association of toll-like receptor polymorphisms with susceptibility to chikungunya virus infection. Virology 2017, 511, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.E.H.; Kohara, M.; Tsukiyama-Kohara, K. Toll-like Receptor Response to Hepatitis C Virus Infection: A Recent Overview. Int. J. Mol. Sci. 2022, 23, 5475. [Google Scholar] [CrossRef]
- Gorbea, C.; Makar, K.A.; Pauschinger, M.; Pratt, G.; Bersola, J.L.; Varela, J.; David, R.M.; Banks, L.; Huang, C.H.; Li, H.; et al. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J. Biol. Chem. 2010, 285, 23208–23223. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, T.; Komano, J.; Saitoh, Y.; Misawa, T.; Takahama, M.; Kozaki, T.; Uehata, T.; Iwasaki, H.; Omori, H.; Yamaoka, S.; et al. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012, 12, 109–116. [Google Scholar] [CrossRef]
- Kawasaki, A.; Furukawa, H.; Kondo, Y.; Ito, S.; Hayashi, T.; Kusaoi, M.; Matsumoto, I.; Tohma, S.; Takasaki, Y.; Hashimoto, H.; et al. TLR7 single-nucleotide polymorphisms in the 3’ untranslated region and intron 2 independently contribute to systemic lupus erythematosus in Japanese women: A case-control association study. Arthritis Res. Ther. 2011, 13, R41. [Google Scholar] [CrossRef]
- Santos-Sierra, S. Targeting Toll-like Receptor (TLR) Pathways in Inflammatory Arthritis: Two Better Than One? Biomolecules 2021, 11, 1291. [Google Scholar] [CrossRef]
- Javmen, A.; Szmacinski, H.; Lakowicz, J.R.; Toshchakov, V.Y. Blocking TIR Domain Interactions in TLR9 Signaling. J. Immunol. 2018, 201, 995–1006. [Google Scholar] [CrossRef]
- Volpi, C.; Fallarino, F.; Pallotta, M.T.; Bianchi, R.; Vacca, C.; Belladonna, M.L.; Orabona, C.; De Luca, A.; Boon, L.; Romani, L.; et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat. Commun. 2013, 4, 1852. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhan, X.; Tran, K.K.; Shen, H. Selectively targeting the toll-like receptor 9 (TLR9)--IRF 7 signaling pathway by polymer blend particles. Biomaterials 2013, 34, 6464–6472. [Google Scholar] [CrossRef]
- Combes, A.; Camosseto, V.; N’Guessan, P.; Arguello, R.J.; Mussard, J.; Caux, C.; Bendriss-Vermare, N.; Pierre, P.; Gatti, E. BAD-LAMP controls TLR9 trafficking and signalling in human plasmacytoid dendritic cells. Nat. Commun. 2017, 8, 913. [Google Scholar] [CrossRef] [PubMed]
- Shepard, C.R. TLR9 in MAFLD and NASH: At the Intersection of Inflammation and Metabolism. Front. Endocrinol. 2021, 11, 613639. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Abdollahi-Roodsaz, S.; Bohm, C.; Niederreiter, B.; Meyer, B.; Yau, A.C.Y.; Lonnblom, E.; Joosten, L.A.B.; Koenders, M.; Lehmann, C.H.K.; et al. The involvement of Toll-like receptor 9 in the pathogenesis of erosive autoimmune arthritis. J. Cell. Mol. Med. 2018, 22, 4399–4409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, L.; Peng, L.; Qiu, W. TLR9 and its signaling pathway in multiple sclerosis. J. Neurol. Sci. 2017, 373, 95–99. [Google Scholar] [CrossRef]
- Varani, S.; Cederarv, M.; Feld, S.; Tammik, C.; Frascaroli, G.; Landini, M.P.; Soderberg-Naucler, C. Human cytomegalovirus differentially controls B cell and T cell responses through effects on plasmacytoid dendritic cells. J. Immunol. 2007, 179, 7767–7776. [Google Scholar] [CrossRef] [PubMed]
- Zyzak, J.; Mitkiewicz, M.; Leszczynska, E.; Reniewicz, P.; Moynagh, P.N.; Siednienko, J. HSV-1/TLR9-Mediated IFNbeta and TNFalpha Induction Is Mal-Dependent in Macrophages. J. Innate Immun. 2020, 12, 387–398. [Google Scholar] [CrossRef]
- Krug, A.; Luker, G.D.; Barchet, W.; Leib, D.A.; Akira, S.; Colonna, M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 2004, 103, 1433–1437. [Google Scholar] [CrossRef]
- Lund, J.; Sato, A.; Akira, S.; Medzhitov, R.; Iwasaki, A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 2003, 198, 513–520. [Google Scholar] [CrossRef]
- Fiola, S.; Gosselin, D.; Takada, K.; Gosselin, J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 185, 3620–3631. [Google Scholar] [CrossRef]
- Jordi, M.; Marty, J.; Mordasini, V.; Lunemann, A.; McComb, S.; Bernasconi, M.; Nadal, D. IRAK4 is essential for TLR9-induced suppression of Epstein-Barr virus BZLF1 transcription in Akata Burkitt’s lymphoma cells. PLoS ONE 2017, 12, e0186614. [Google Scholar] [CrossRef]
- Qian, J.; Meng, H.; Lv, B.; Wang, J.; Lu, Y.; Li, W.; Zhao, S. TLR9 expression is associated with PD-L1 expression and indicates a poor prognosis in patients with peripheral T-cell lymphomas. Pathol. Res. Pract. 2020, 216, 152703. [Google Scholar] [CrossRef]
- Fernandez-Rodriguez, L.; Cianciaruso, C.; Bill, R.; Trefny, M.P.; Klar, R.; Kirchhammer, N.; Buchi, M.; Festag, J.; Michel, S.; Kohler, R.H.; et al. Dual TLR9 and PD-L1 targeting unleashes dendritic cells to induce durable antitumor immunity. J. Immunother. Cancer 2023, 11, e006714. [Google Scholar] [CrossRef]
- Henke, P.K.; Mitsuya, M.; Luke, C.E.; Elfline, M.A.; Baldwin, J.F.; Deatrick, K.B.; Diaz, J.A.; Sood, V.; Upchurch, G.R.; Wakefield, T.W.; et al. Toll-like receptor 9 signaling is critical for early experimental deep vein thrombosis resolution. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Su, S.B.; Tao, L.; Deng, Z.P.; Chen, W.; Qin, S.Y.; Jiang, H.X. TLR10: Insights, controversies and potential utility as a therapeutic target. Scand. J. Immunol. 2021, 93, e12988. [Google Scholar] [CrossRef] [PubMed]
- Oosting, M.; Cheng, S.C.; Bolscher, J.M.; Vestering-Stenger, R.; Plantinga, T.S.; Verschueren, I.C.; Arts, P.; Garritsen, A.; van Eenennaam, H.; Sturm, P.; et al. Human TLR10 is an anti-inflammatory pattern-recognition receptor. Proc. Natl. Acad. Sci. USA 2014, 111, E4478–E4484. [Google Scholar] [CrossRef] [PubMed]
- Fore, F.; Indriputri, C.; Mamutse, J.; Nugraha, J. TLR10 and Its Unique Anti-Inflammatory Properties and Potential Use as a Target in Therapeutics. Immune Netw. 2020, 20, e21. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.D.; Zahoor, M.A.; Abimiku, A.; Osawe, S.; Rosenthal, K.L. TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection. Front. Immunol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Lee, S.M.; Kok, K.H.; Jaume, M.; Cheung, T.K.; Yip, T.F.; Lai, J.C.; Guan, Y.; Webster, R.G.; Jin, D.Y.; Peiris, J.S. Toll-like receptor 10 is involved in induction of innate immune responses to influenza virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 3793–3798. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Jilma, B.; Kopp, C.W.; Ertl, S.; Gremmel, T.; Koppensteiner, R. Glycocalyx as Possible Limiting Factor in COVID-19. Front. Immunol. 2021, 12, 607306. [Google Scholar] [CrossRef]
- Hong, W.; Yang, J.; Zou, J.; Bi, Z.; He, C.; Lei, H.; He, X.; Li, X.; Alu, A.; Ren, W.; et al. Histones released by NETosis enhance the infectivity of SARS-CoV-2 by bridging the spike protein subunit 2 and sialic acid on host cells. Cell. Mol. Immunol. 2022, 19, 577–587. [Google Scholar] [CrossRef]
- Ligi, D.; Lo Sasso, B.; Giglio, R.V.; Maniscalco, R.; DellaFranca, C.; Agnello, L.; Ciaccio, M.; Mannello, F. Circulating histones contribute to monocyte and MDW alterations as common mediators in classical and COVID-19 sepsis. Crit. Care 2022, 26, 260. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Biswas, I.; Bhagat, S.; Surya Kumari, S.; Khan, G.A. HMGB1 facilitates hypoxia-induced vWF upregulation through TLR2-MYD88-SP1 pathway. Eur. J. Immunol. 2016, 46, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Carestia, A.; Kaufman, T.; Rivadeneyra, L.; Landoni, V.I.; Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Gomez, R.M.; Schattner, M. Mediators and molecular pathways involved in the regulation of neutrophil extracellular trap formation mediated by activated platelets. J. Leukoc. Biol. 2016, 99, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Into, T.; Kanno, Y.; Dohkan, J.; Nakashima, M.; Inomata, M.; Shibata, K.; Lowenstein, C.J.; Matsushita, K. Pathogen recognition by Toll-like receptor 2 activates Weibel-Palade body exocytosis in human aortic endothelial cells. J. Biol. Chem. 2007, 282, 8134–8141. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, N.; Nogami, K.; Yoshizawa, H.; Hayakawa, M.; Isonishi, A.; Matsumoto, M.; Shima, M. Influenza-associated thrombotic microangiopathy with unbalanced von Willebrand factor and a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 levels in a heterozygous protein S-deficient boy. Pediatr. Int. 2016, 58, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Djamiatun, K.; van der Ven, A.J.; de Groot, P.G.; Faradz, S.M.; Hapsari, D.; Dolmans, W.M.; Sebastian, S.; Fijnheer, R.; de Mast, Q. Severe dengue is associated with consumption of von Willebrand factor and its cleaving enzyme ADAMTS-13. PLoS Negl. Trop. Dis. 2012, 6, e1628. [Google Scholar] [CrossRef]
- Bester, J.; Swanepoel, A.C.; Windberger, U. Editorial: Pathological Changes in Erythrocytes During Inflammation and Infection. Front. Physiol. 2022, 13, 943114. [Google Scholar] [CrossRef]
- Maruyama, T.; Hieda, M.; Mawatari, S.; Fujino, T. Rheological Abnormalities in Human Erythrocytes Subjected to Oxidative Inflammation. Front. Physiol. 2022, 13, 837926. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Schorgenhofer, C.; Rieder, T.; Ertl, S.; Pultar, J.; Serles, W.; Sycha, T.; Mayer, F.; Koppensteiner, R.; Gremmel, T.; et al. Microvascular rarefaction in patients with cerebrovascular events. Microvasc. Res. 2022, 140, 104300. [Google Scholar] [CrossRef]
- Wadowski, P.P.; Kautzky-Willer, A.; Gremmel, T.; Koppensteiner, R.; Wolf, P.; Ertl, S.; Weikert, C.; Schorgenhofer, C.; Jilma, B. Sublingual microvasculature in diabetic patients. Microvasc. Res. 2020, 129, 103971. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Steinlechner, B.; Zimpfer, D.; Schloglhofer, T.; Schima, H.; Hulsmann, M.; Lang, I.M.; Gremmel, T.; Koppensteiner, R.; Zehetmayer, S.; et al. Functional capillary impairment in patients with ventricular assist devices. Sci. Rep. 2019, 9, 5909. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Hulsmann, M.; Schorgenhofer, C.; Lang, I.M.; Wurm, R.; Gremmel, T.; Koppensteiner, R.; Steinlechner, B.; Schwameis, M.; Jilma, B. Sublingual functional capillary rarefaction in chronic heart failure. Eur. J. Clin. Investig. 2018, 48, e12869. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, C.; Dayre, A.; Butkowski, E.G.; de Jong, B.; Jelinek, H.F. Inflammation and oxidative stress markers in diabetes and hypertension. J. Inflamm. Res. 2018, 11, 61–68. [Google Scholar] [CrossRef]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pineiro, J.A.; Gonzalez-Rovira, A.; Sanchez-Gomar, I.; Moreno, J.A.; Duran-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.; Schmid, A.I.; Doberer, D.; Schewzow, K.; Weisshaar, S.; Heinze, G.; Bilban, M.; Moser, E.; Wolzt, M. Heme arginate improves reperfusion patterns after ischemia: A randomized, placebo-controlled trial in healthy male subjects. J. Cardiovasc. Magn. Reson. 2012, 14, 55. [Google Scholar] [CrossRef]
- Vartanian, K.B.; Stevens, S.L.; Marsh, B.J.; Williams-Karnesky, R.; Lessov, N.S.; Stenzel-Poore, M.P. LPS preconditioning redirects TLR signaling following stroke: TRIF-IRF3 plays a seminal role in mediating tolerance to ischemic injury. J. Neuroinflammation 2011, 8, 140. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Li, B.; Xia, Y.; Hu, B. Infection and atherosclerosis: TLR-dependent pathways. Cell. Mol. Life Sci. 2020, 77, 2751–2769. [Google Scholar] [CrossRef]
- Tang, A.T.; Choi, J.P.; Kotzin, J.J.; Yang, Y.; Hong, C.C.; Hobson, N.; Girard, R.; Zeineddine, H.A.; Lightle, R.; Moore, T.; et al. Endothelial TLR4 and the microbiome drive cerebral cavernous malformations. Nature 2017, 545, 305–310. [Google Scholar] [CrossRef]
- Means, R.T., Jr. The anaemia of infection. Baillieres Best Pract. Res. Clin. Haematol. 2000, 13, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Akilesh, H.M.; Buechler, M.B.; Duggan, J.M.; Hahn, W.O.; Matta, B.; Sun, X.; Gessay, G.; Whalen, E.; Mason, M.; Presnell, S.R.; et al. Chronic TLR7 and TLR9 signaling drives anemia via differentiation of specialized hemophagocytes. Science 2019, 363, eaao5213. [Google Scholar] [CrossRef]
- Bouchla, A.; Kriebardis, A.G.; Georgatzakou, H.T.; Fortis, S.P.; Thomopoulos, T.P.; Lekkakou, L.; Markakis, K.; Gkotzias, D.; Panagiotou, A.; Papageorgiou, E.G.; et al. Red Blood Cell Abnormalities as the Mirror of SARS-CoV-2 Disease Severity: A Pilot Study. Front. Physiol. 2021, 12, 825055. [Google Scholar] [CrossRef] [PubMed]
- Bellmann-Weiler, R.; Lanser, L.; Barket, R.; Rangger, L.; Schapfl, A.; Schaber, M.; Fritsche, G.; Woll, E.; Weiss, G. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J. Clin. Med. 2020, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Wadowski, P.P.; Kopp, C.W.; Koppensteiner, R.; Lang, I.M.; Pultar, J.; Lee, S.; Weikert, C.; Panzer, S.; Gremmel, T. Decreased platelet inhibition by P2Y12 receptor blockers in anaemia. Eur. J. Clin. Investig. 2018, 48, e12861. [Google Scholar] [CrossRef]
- Giustino, G.; Kirtane, A.J.; Baber, U.; Genereux, P.; Witzenbichler, B.; Neumann, F.J.; Weisz, G.; Maehara, A.; Rinaldi, M.J.; Metzger, C.; et al. Impact of Anemia on Platelet Reactivity and Ischemic and Bleeding Risk: From the Assessment of Dual Antiplatelet Therapy with Drug-Eluting Stents Study. Am. J. Cardiol. 2016, 117, 1877–1883. [Google Scholar] [CrossRef]
- Averett, D.R.; Fletcher, S.P.; Li, W.; Webber, S.E.; Appleman, J.R. The pharmacology of endosomal TLR agonists in viral disease. Biochem. Soc. Trans. 2007, 35, 1468–1472. [Google Scholar] [CrossRef][Green Version]
- Sun, S.; Rao, N.L.; Venable, J.; Thurmond, R.; Karlsson, L. TLR7/9 antagonists as therapeutics for immune-mediated inflammatory disorders. Inflamm. Allergy Drug Targets 2007, 6, 223–235. [Google Scholar] [CrossRef]
- Proud, P.C.; Tsitoura, D.; Watson, R.J.; Chua, B.Y.; Aram, M.J.; Bewley, K.R.; Cavell, B.E.; Cobb, R.; Dowall, S.; Fotheringham, S.A.; et al. Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model. EBioMedicine 2021, 63, 103153. [Google Scholar] [CrossRef]
- Williams, B.; Neder, J.; Cui, P.; Suen, A.; Tanaka, K.; Zou, L.; Chao, W. Toll-like receptors 2 and 7 mediate coagulation activation and coagulopathy in murine sepsis. J. Thromb. Haemost. 2019, 17, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzer, B.; Kopp, C.W.; Neumayer, C.; Koppensteiner, R.; Jozkowicz, A.; Poledniczek, M.; Gremmel, T.; Jilma, B.; Wadowski, P.P. Toll-like Receptors as Pro-Thrombotic Drivers in Viral Infections: A Narrative Review. Cells 2023, 12, 1865. https://doi.org/10.3390/cells12141865
Panzer B, Kopp CW, Neumayer C, Koppensteiner R, Jozkowicz A, Poledniczek M, Gremmel T, Jilma B, Wadowski PP. Toll-like Receptors as Pro-Thrombotic Drivers in Viral Infections: A Narrative Review. Cells. 2023; 12(14):1865. https://doi.org/10.3390/cells12141865
Chicago/Turabian StylePanzer, Benjamin, Christoph W. Kopp, Christoph Neumayer, Renate Koppensteiner, Alicja Jozkowicz, Michael Poledniczek, Thomas Gremmel, Bernd Jilma, and Patricia P. Wadowski. 2023. "Toll-like Receptors as Pro-Thrombotic Drivers in Viral Infections: A Narrative Review" Cells 12, no. 14: 1865. https://doi.org/10.3390/cells12141865
APA StylePanzer, B., Kopp, C. W., Neumayer, C., Koppensteiner, R., Jozkowicz, A., Poledniczek, M., Gremmel, T., Jilma, B., & Wadowski, P. P. (2023). Toll-like Receptors as Pro-Thrombotic Drivers in Viral Infections: A Narrative Review. Cells, 12(14), 1865. https://doi.org/10.3390/cells12141865







