“Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios
Abstract
1. Introduction
1.1. Physical and Mental Illnesses: An Etiopathogenetic Bidirectionality on a Large Clinical and Anagraphic Scale
1.2. PNEI: A Symphonic Inter-Systemic Molecular Orchestra?
1.3. Skin as Stress-Triggered Neuroimmunoendocrine Organ
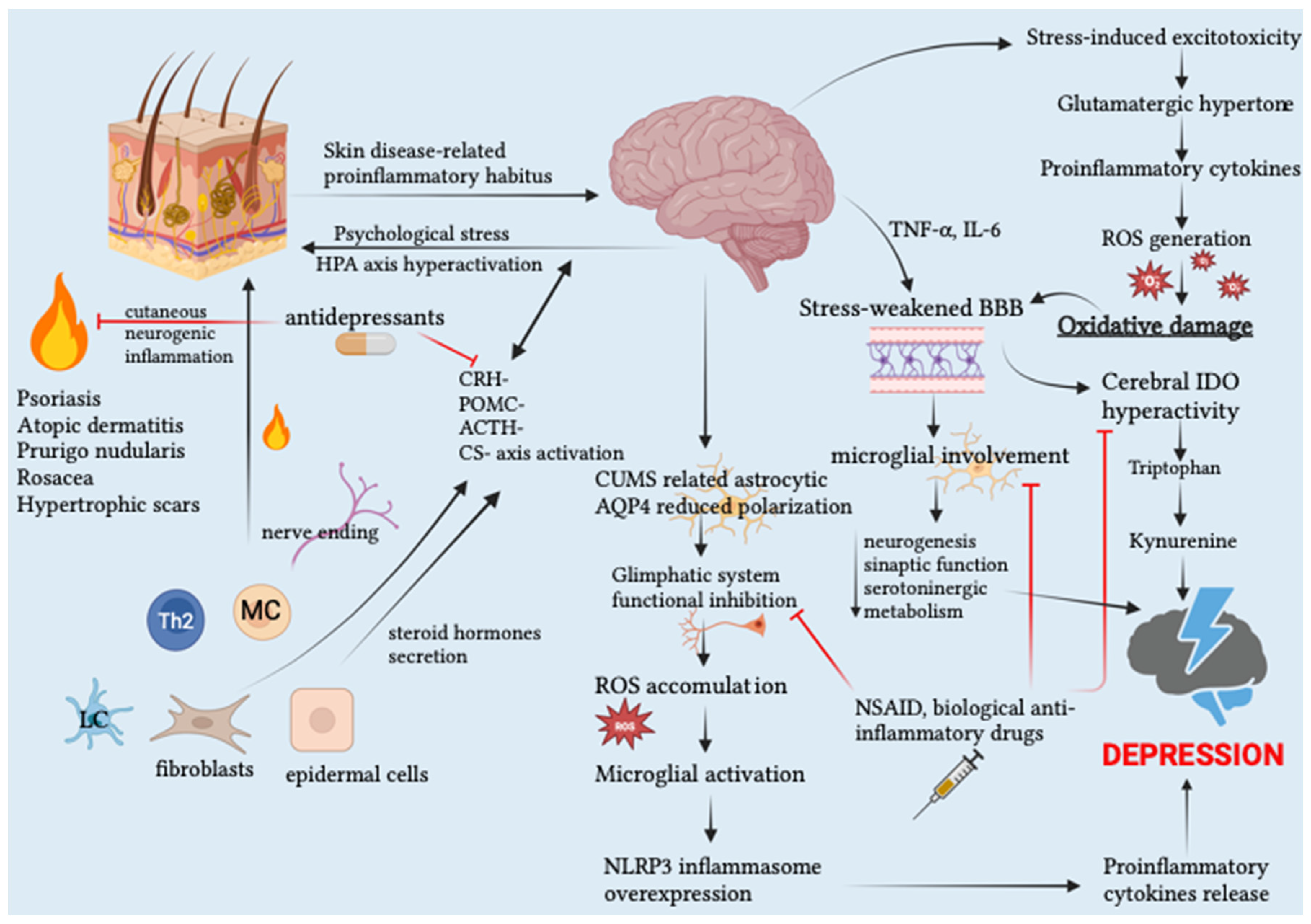
1.4. Skin Disease-Related Pro-Inflammatory Habitus as a Trigger for Mental Disorders
1.5. Tight Junctions Frailty: A Gateway to Skin and Cerebral Inflammation?
1.6. Pathophysiological OS–Inflammation Synergy as a Prerequisite for Etiopathogenetic Synergy between Mental and Organic Disorders
2. Discussion
2.1. Autoimmune Skin Conditions
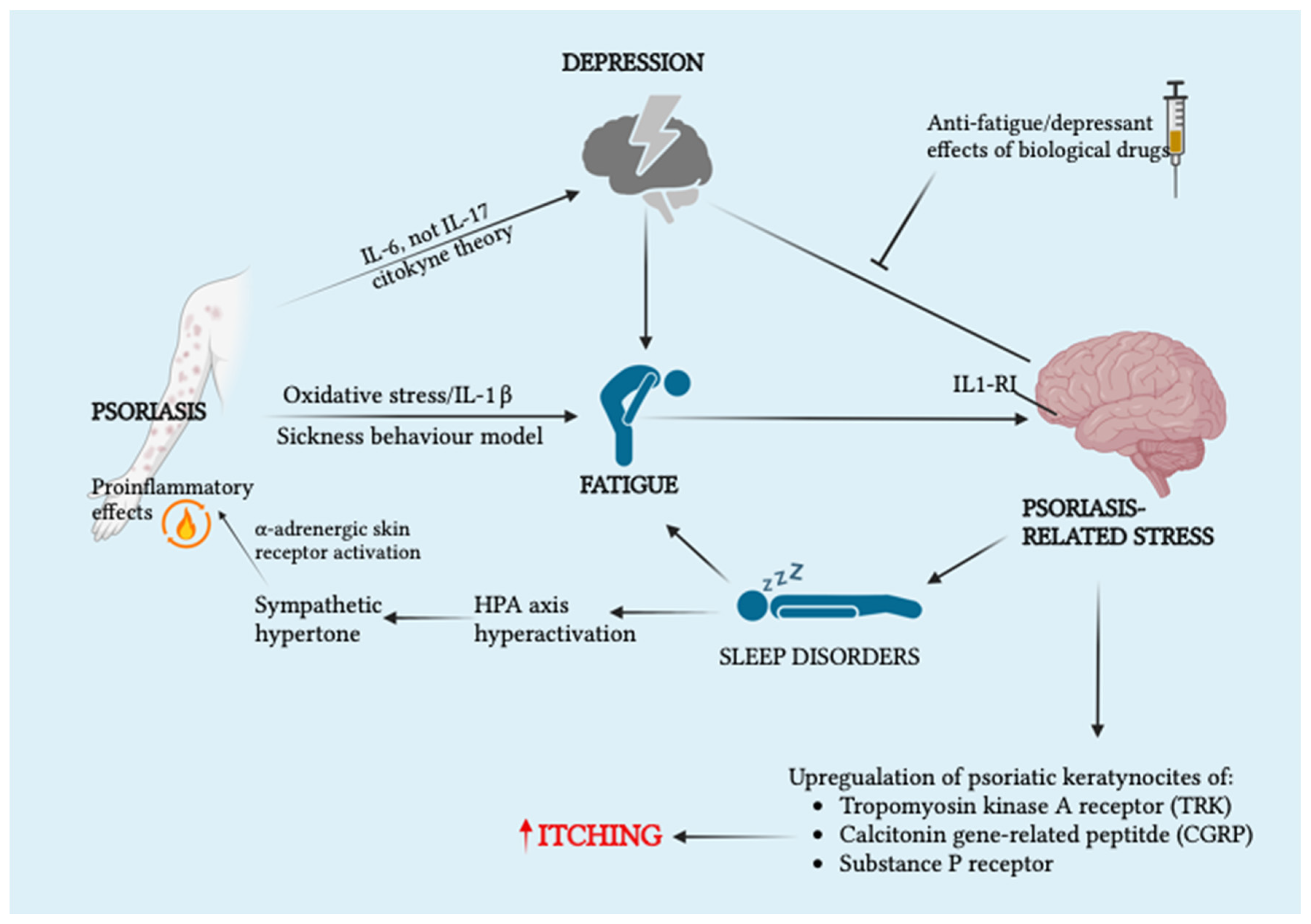
2.1.1. Psoriasis
2.1.2. Alopecia Areata
2.1.3. Immunobullous Skin Diseases
2.1.4. Vitiligo
2.2. Chronic Inflammatory Dermatoses
2.2.1. Lichen Planus
2.2.2. Hidradenitis Suppurativa
2.2.3. Facial Dermatoses
2.2.4. Other Herpesvirus-Related Skin Manifestations
2.2.5. Atopic Dermatitis
2.3. PTSD: A Systemic Inflammatory Disorder
2.4. Inflammatory and Oxidative Markers: Wide-Ranging Clinical Potential
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DeJean, D.; Giacomini, M.; Vanstone, M.; Brundisini, F. Patient Experiences of Depression and Anxiety with Chronic Disease: A Systematic Review and Qualitative Meta-Synthesis. Ont. Health Technol. Assess. Ser. 2013, 13, 1–33. [Google Scholar]
- Cohen, B.E.; Edmondson, D.; Kronish, I.M. State of the Art Review: Depression, Stress, Anxiety, and Cardiovascular Disease. Am. J. Hypertens. 2015, 28, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N. Psychological Stress and Heart Disease: Fact or Folklore? Am. J. Med. 2022, 135, 688–696. [Google Scholar] [CrossRef]
- Hackett, R.A.; Steptoe, A. Type 2 Diabetes Mellitus and Psychological Stress—A Modifiable Risk Factor. Nat. Rev. Endocrinol. 2017, 13, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Sharif, K.; Watad, A.; Coplan, L.; Amital, H.; Shoenfeld, Y.; Afek, A. Psychological Stress and Type 1 Diabetes Mellitus: What Is the Link? Expert Rev. Clin. Immunol. 2018, 14, 1081–1088. [Google Scholar] [CrossRef]
- Bernstein, C.N. Psychological Stress and Depression: Risk Factors for IBD? Dig. Dis. 2016, 34, 58–63. [Google Scholar] [CrossRef]
- Labanski, A.; Langhorst, J.; Engler, H.; Elsenbruch, S. Stress and the Brain-Gut Axis in Functional and Chronic-Inflammatory Gastrointestinal Diseases: A Transdisciplinary Challenge. Psychoneuroendocrinology 2020, 111, 104501. [Google Scholar] [CrossRef]
- Saman, Y.; Arshad, Q.; Dutia, M.; Rea, P. Stress and the Vestibular System. Int. Rev. Neurobiol. 2020, 152, 221–236. [Google Scholar]
- Mravec, B.; Horvathova, L.; Padova, A. Brain Under Stress and Alzheimer’s Disease. Cell. Mol. Neurobiol. 2018, 38, 73–84. [Google Scholar] [CrossRef] [PubMed]
- van Wamelen, D.J.; Wan, Y.-M.; Ray Chaudhuri, K.; Jenner, P. Stress and Cortisol in Parkinson’s Disease. Int. Rev. Neurobiol. 2020, 152, 131–156. [Google Scholar]
- Peña-Bautista, C.; Casas-Fernández, E.; Vento, M.; Baquero, M.; Cháfer-Pericás, C. Stress and Neurodegeneration. Clin. Chim. Acta 2020, 503, 163–168. [Google Scholar] [CrossRef]
- Faresjo, M. The Link between Psychological Stress and Autoimmune Response in Children. Crit. Rev. Immunol. 2015, 35, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.M.; Maccauro, G.; Fulcheri, M. Psychological Stress and Cancer. Int. J. Immunopathol. Pharmacol. 2011, 24, 1–5. [Google Scholar] [CrossRef]
- Kruk, J.; Aboul-Enein, B.H.; Bernstein, J.; Gronostaj, M. Psychological Stress and Cellular Aging in Cancer: A Meta-Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 1270397. [Google Scholar] [CrossRef] [PubMed]
- Majnarić, L.T.; Bosnić, Z.; Guljaš, S.; Vučić, D.; Kurevija, T.; Volarić, M.; Martinović, I.; Wittlinger, T. Low Psychological Resilience in Older Individuals: An Association with Increased Inflammation, Oxidative Stress and the Presence of Chronic Medical Conditions. Int. J. Mol. Sci. 2021, 22, 8970. [Google Scholar] [CrossRef] [PubMed]
- Papa, V.; Li Pomi, F.; Borgia, F.; Vaccaro, M.; Pioggia, G.; Gangemi, S. Immunosenescence and Skin: A State of Art of Its Etiopathogenetic Role and Crucial Watershed for Systemic Implications. Int. J. Mol. Sci. 2023, 24, 7956. [Google Scholar] [CrossRef]
- Nusslock, R.; Miller, G.E. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol. Psychiatry 2016, 80, 23–32. [Google Scholar] [CrossRef]
- Scapagnini, U. Psychoneuroendocrinoimmunology: The Basis for a Novel Therapeutic Approach in Aging. Psychoneuroendocrinology 1992, 17, 411–420. [Google Scholar] [CrossRef]
- Benedetti, F. Placebo and the New Physiology of the Doctor-Patient Relationship. Physiol. Rev. 2013, 93, 1207–1246. [Google Scholar] [CrossRef]
- Pregnolato, M.; Damiani, G.; Pereira, A., Jr. Patterns of Calcium Signaling: A Link between Chronic Emotions and Cancer. J. Integr. Neurosci. 2017, 16, S43–S63. [Google Scholar] [CrossRef]
- Messina, G.; Lissoni, P.; Bartolacelli, E.; Magotti, L.; Clerici, M.; Marchiori, P.; Colombo, E. Relationship between Psychoncology and Psychoneuroendocrinoimmunology (PNEI): Enhanced T-Regulatory Lymphocyte Activity in Cancer Patients with Self-Punishement, Evaluated by Rorschach Test. In Vivo 2010, 24, 75–78. [Google Scholar]
- Messina, G.; Lissoni, P.; Rovelli, F. Psychoimmunological Analysis of Cancer Patients: Correlation with the Prognosis. Curr. Aging Sci. 2013, 5, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Bottaccioli, F.; Carosella, A.; Cardone, R.; Mambelli, M.; Cemin, M.; D’Errico, M.M.; Ponzio, E.; Bottaccioli, A.G.; Minelli, A. Brief Training of Psychoneuroendocrinoimmunology-Based Meditation (PNEIMED) Reduces Stress Symptom Ratings and Improves Control on Salivary Cortisol Secretion Under Basal and Stimulated Conditions. Explore 2014, 10, 170–179. [Google Scholar] [CrossRef]
- Bottaccioli, A.G.; Bottaccioli, F.; Carosella, A.; Cofini, V.; Muzi, P.; Bologna, M. Psychoneuroendocrinoimmunology-Based Meditation (PNEIMED) Training Reduces Salivary Cortisol under Basal and Stressful Conditions in Healthy University Students: Results of a Randomized Controlled Study. Explore 2020, 16, 189–198. [Google Scholar] [CrossRef]
- Kim, S.-W.; Su, K.-P. Using Psychoneuroimmunity against COVID-19. Brain Behav. Immun. 2020, 87, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Demori, I.; Piccinno, T.; Saverino, D.; Luzzo, E.; Ottoboni, S.; Serpico, D.; Chiera, M.; Giuria, R. Effects of Winter Sea Bathing on Psychoneuroendocrinoimmunological Parameters. Explore 2021, 17, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Bitzer-Quintero, O.K.; Ortiz, G.G.; Jaramillo-Bueno, S.; Ramos-González, E.J.; Márquez-Rosales, M.G.; Delgado-Lara, D.L.C.; Torres-Sánchez, E.D.; Tejeda-Martínez, A.R.; Ramirez-Jirano, J. Psycho-Neuro-Endocrine-Immunology: A Role for Melatonin in This New Paradigm. Molecules 2022, 27, 4888. [Google Scholar] [CrossRef]
- Rizzi, A.; Saccia, M.; Benagiano, V. Is the Cerebellum Involved in the Nervous Control of the Immune System Function? Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 546–557. [Google Scholar] [CrossRef]
- Bottaccioli, A.G.; Bologna, M.; Bottaccioli, F. Psychic Life-Biological Molecule Bidirectional Relationship: Pathways, Mechanisms, and Consequences for Medical and Psychological Sciences—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 3932. [Google Scholar] [CrossRef]
- Neau, J.-P.; Godeneche, G.; Mathis, S.; Guillet, G. Neurodermatology. Handb Clin Neurol. 2014, 121, 1561–1594. [Google Scholar]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M.; Żmijewski, M.A.; Czajkowski, R.; Cubała, W.J.; Slominski, A.T. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Slominski, A.; Theoharides, T.C.; Peters, E.M.J.; Paus, R. Neuroimmunology of Stress: Skin Takes Center Stage. J. Investig. Dermatol. 2006, 126, 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L. Stress-Induced Interaction of Skin Immune Cells, Hormones, and Neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Eskeland, S.; Halvorsen, J.; Tanum, L. Antidepressants Have Anti-Inflammatory Effects That May Be Relevant to Dermatology: A Systematic Review. Acta Dermato Venereologica 2017, 97, 897–905. [Google Scholar] [CrossRef]
- McPhie, M.L.; Bridgman, A.C.; Kirchhof, M.G. A Review of Skin Disease in Schizophrenia. Dermatology 2021, 237, 248–261. [Google Scholar] [CrossRef]
- Stenger, S.; Grasshoff, H.; Hundt, J.E.; Lange, T. Potential Effects of Shift Work on Skin Autoimmune Diseases. Front. Immunol. 2023, 13, 1000951. [Google Scholar] [CrossRef]
- Filaković, P.; Petek, A.; Koić, O.; Radanović-Grgurić, L.; Degmecić, D. Comorbidity of Depressive and Dermatologic Disorders—Therapeutic Aspects. Psychiatr. Danub. 2009, 21, 401–410. [Google Scholar]
- Salim, S. Oxidative Stress: A Potential Link between Emotional Wellbeing and Immune Response. Curr. Opin. Pharmacol. 2016, 29, 70–76. [Google Scholar] [CrossRef]
- Borgia, F.; Li Pomi, F.; Vaccaro, M.; Alessandrello, C.; Papa, V.; Gangemi, S. Oxidative Stress and Phototherapy in Atopic Dermatitis: Mechanisms, Role, and Future Perspectives. Biomolecules 2022, 12, 1904. [Google Scholar] [CrossRef]
- Farzanfar, D.; Dowlati, Y.; French, L.E.; Lowes, M.A.; Alavi, A. Inflammation: A Contributor to Depressive Comorbidity in Inflammatory Skin Disease. Skin Pharmacol. Physiol. 2018, 31, 246–251. [Google Scholar] [CrossRef]
- Gałecki, P.; Talarowska, M. Inflammatory Theory of Depression. Psychiatr. Pol. 2018, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Li, Y.; Jiang, Y.; Huang, J.H.; Wang, F. Glymphatic Dysfunction Induced Oxidative Stress and Neuro-Inflammation in Major Depression Disorders. Antioxidants 2022, 11, 2296. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Scicchitano, M.; Carresi, C.; Scarano, F.; Bosco, F.; Nucera, S.; Ruga, S.; Zito, M.; et al. The “Frail” Brain Blood Barrier in Neurodegenerative Diseases: Role of Early Disruption of Endothelial Cell-to-Cell Connections. Int. J. Mol. Sci. 2018, 19, 2693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zeng, H.; Lei, L.; Tong, X.; Yang, L.; Yang, Y.; Li, S.; Zhou, Y.; Luo, L.; Huang, J.; et al. Tight Junctions and Their Regulation by Non-Coding RNAs. Int. J. Biol. Sci. 2021, 17, 712–727. [Google Scholar] [CrossRef]
- Kramer, N.E.; Cosgrove, V.E.; Dunlap, K.; Subramaniapillai, M.; McIntyre, R.S.; Suppes, T. A Clinical Model for Identifying an Inflammatory Phenotype in Mood Disorders. J. Psychiatr. Res. 2019, 113, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.H.; Koo, J. Psychological Stress and Skin Aging: A Review of Possible Mechanisms and Potential Therapies. Dermatol. Online J. 2013, 19, 18561. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative Stress in the Skin: Impact and Related Protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Atrooz, F.; Liu, H.; Salim, S. Stress, Psychiatric Disorders, Molecular Targets, and More. Prog. Mol. Biol. Transl. Sci. 2019, 167, 77–105. [Google Scholar]
- Thakur, A.; Choudhary, D.; Kumar, B.; Chaudhary, A. A Review on Post-Traumatic Stress Disorder (PTSD): Symptoms, Therapies and Recent Case Studies. Curr. Mol. Pharmacol. 2022, 15, 502–516. [Google Scholar] [CrossRef]
- Pace, T.W.W.; Heim, C.M. A Short Review on the Psychoneuroimmunology of Posttraumatic Stress Disorder: From Risk Factors to Medical Comorbidities. Brain Behav. Immun. 2011, 25, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Skoie, I.M.; Ternowitz, T.; Jonsson, G.; Norheim, K.; Omdal, R. Fatigue in Psoriasis: A Phenomenon to Be Explored. Br. J. Dermatol. 2015, 172, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Bolotna, L.; Sarian, O. Psychopathological disorders as comorbidity in patients with psoriasis (review). Georgian Med. News 2020, 301, 143–147. [Google Scholar]
- Torales, J.; Echeverría, C.; Barrios, I.; García, O.; O’Higgins, M.; Castaldelli-Maia, J.M.; Ventriglio, A.; Jafferany, M. Psychodermatological Mechanisms of Psoriasis. Dermatol. Ther. 2020, 33, e13827. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Marangell, L.B.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.J. Depression and Suicidality in Psoriasis: Review of the Literature Including the Cytokine Theory of Depression. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1999–2009. [Google Scholar] [CrossRef]
- Fleming, P.; Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.E.; et al. Effect of Biologics on Depressive Symptoms in Patients with Psoriasis: A Systematic Review. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1063–1070. [Google Scholar] [CrossRef]
- Reszke, R.; Szepietowski, J. Itch and Psyche: Bilateral Associations. Acta Derm. Venereol. 2020, 100, 28–36. [Google Scholar] [CrossRef]
- Amanat, M.; Salehi, M.; Rezaei, N. Neurological and Psychiatric Disorders in Psoriasis. Rev. Neurosci. 2018, 29, 805–813. [Google Scholar] [CrossRef]
- Nowowiejska, J.; Baran, A.; Flisiak, I. Mutual Relationship Between Sleep Disorders, Quality of Life and Psychosocial Aspects in Patients with Psoriasis. Front. Psychiatry 2021, 12, 674460. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A.; Nedoszytko, B.; Żmijewski, M.A.; Cubała, W.J.; Slominski, A.T. The Brain–Skin Axis in Psoriasis—Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int. J. Mol. Sci. 2022, 23, 669. [Google Scholar] [CrossRef]
- Hedemann, T.L.; Liu, X.; Kang, C.N.; Husain, M.I. Associations between Psoriasis and Mental Illness: An Update for Clinicians. Gen. Hosp. Psychiatry 2022, 75, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kuty-Pachecka, M. Psychological and Psychopathological Factors in Alopecia Areata. Psychiatr. Pol. 2015, 49, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Simakou, T.; Butcher, J.P.; Reid, S.; Henriquez, F.L. Alopecia Areata: A Multifactorial Autoimmune Condition. J. Autoimmun. 2019, 98, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Torales, J.; Castaldelli-Maia, J.M.; Ventriglio, A.; Almirón-Santacruz, J.; Barrios, I.; O’Higgins, M.; García, O.; Navarro, R.; Melgarejo, O.; Jafferany, M. Alopecia Areata: A Psychodermatological Perspective. J. Cosmet. Dermatol. 2022, 21, 2318–2323. [Google Scholar] [CrossRef] [PubMed]
- Försti, A.-K.; Huilaja, L.; Schmidt, E.; Tasanen, K. Neurological and Psychiatric Associations in Bullous Pemphigoid-More than Skin Deep? Exp. Dermatol. 2017, 26, 1228–1234. [Google Scholar] [CrossRef]
- Matthews, R.; Ali, Z. Comorbid Mental Health Issues in Patients with Pemphigus Vulgaris and Pemphigus Foliaceus. Clin. Exp. Dermatol. 2022, 47, 24–29. [Google Scholar] [CrossRef]
- Simons, R.E.; Zevy, D.L.; Jafferany, M. Psychodermatology of Vitiligo: Psychological Impact and Consequences. Dermatol. Ther. 2020, 33, e13418. [Google Scholar] [CrossRef]
- Li, K.; He, W.; Hua, H. Characteristics of the Psychopathological Status of Oral Lichen Planus: A Systematic Review and Meta-analysis. Aust. Dent. J. 2022, 67, 113–124. [Google Scholar] [CrossRef]
- Miller, I.M.; Ellervik, C.; Vinding, G.R.; Zarchi, K.; Ibler, K.S.; Knudsen, K.M.; Jemec, G.B.E. Association of Metabolic Syndrome and Hidradenitis Suppurativa. JAMA Dermatol. 2014, 150, 1273. [Google Scholar] [CrossRef]
- Vekic, D.A.; Frew, J.W.; Woods, J.; Cains, G.D. Adopting the Orphan: The Importance of Recognising Hidradenitis Suppurativa as a Systemic Auto-Inflammatory Disease. Australas. J. Dermatol. 2016, 57, 69–70. [Google Scholar] [CrossRef]
- Pescitelli, L.; Ricceri, F.; Prignano, F. Hidradenitis Suppurativa and Associated Diseases. Ital. J. Dermatol. Venereol. 2018, 153, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Li Pomi, F.; Macca, L.; Motolese, A.; Ingrasciotta, Y.; Berretta, M.; Guarneri, C. Neoplastic Implications in Patients Suffering from Hidradenitis Suppurativa under Systemic Treatments. Biomedicines 2021, 9, 1594. [Google Scholar] [CrossRef] [PubMed]
- Misitzis, A.; Goldust, M.; Jafferany, M.; Lotti, T. Psychiatric Comorbidities in Patients with Hidradenitis Suppurativa. Dermatol. Ther. 2020, 33, e13541. [Google Scholar] [CrossRef] [PubMed]
- Caccavale, S.; Tancredi, V.; Boccellino, M.P.; Babino, G.; Fulgione, E.; Argenziano, G. Hidradenitis Suppurativa Burdens on Mental Health: A Literature Review of Associated Psychiatric Disorders and Their Pathogenesis. Life 2023, 13, 189. [Google Scholar] [CrossRef]
- Orion, E.; Wolf, R. Psychologic Factors in the Development of Facial Dermatoses. Clin. Dermatol. 2014, 32, 763–766. [Google Scholar] [CrossRef]
- Haber, R.; El Gemayel, M. Comorbidities in Rosacea: A Systematic Review and Update. J. Am. Acad. Dermatol. 2018, 78, 786–792.e8. [Google Scholar] [CrossRef]
- Woo, Y.R.; Han, Y.J.; Kim, H.S.; Cho, S.H.; Lee, J.D. Updates on the Risk of Neuropsychiatric and Gastrointestinal Comorbidities in Rosacea and Its Possible Relationship with the Gut–Brain–Skin Axis. Int. J. Mol. Sci. 2020, 21, 8427. [Google Scholar] [CrossRef]
- Stamu-O’Brien, C.; Jafferany, M.; Carniciu, S.; Abdelmaksoud, A. Psychodermatology of Acne: Psychological Aspects and Effects of Acne Vulgaris. J. Cosmet. Dermatol. 2021, 20, 1080–1083. [Google Scholar] [CrossRef]
- Sachdeva, M.; Tan, J.; Lim, J.; Kim, M.; Nadeem, I.; Bismil, R. The Prevalence, Risk Factors, and Psychosocial Impacts of Acne Vulgaris in Medical Students: A Literature Review. Int. J. Dermatol. 2021, 60, 792–798. [Google Scholar] [CrossRef]
- Marra, F.; Parhar, K.; Huang, B.; Vadlamudi, N. Risk Factors for Herpes Zoster Infection: A Meta-Analysis. Open Forum Infect. Dis. 2020, 7, ofaa005. [Google Scholar] [CrossRef]
- Sangueza-Acosta, M.; Sandoval-Romero, E. Epstein-Barr Virus and Skin. An. Bras. Dermatol. 2018, 93, 786–799. [Google Scholar] [CrossRef]
- Sausen, D.; Bhutta, M.; Gallo, E.; Dahari, H.; Borenstein, R. Stress-Induced Epstein-Barr Virus Reactivation. Biomolecules 2021, 11, 1380. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Psychological Stress and Immunoprotection versus Immunopathology in the Skin. Clin. Dermatol. 2013, 31, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.; Jaworski, J.C.; Simpson, E.L.; Boguniewicz, M.; Russell, J.J.; Block, J.K.; Tofte, S.; Dunn, J.D.; Feldman, S.R.; Clark, A.R.; et al. Major Comorbidities of Atopic Dermatitis: Beyond Allergic Disorders. Am. J. Clin. Dermatol. 2018, 19, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Yamada, S. Alterations in Brain Neural Network and Stress System in Atopic Dermatitis: Novel Therapeutic Interventions. J. Pharmacol. Exp. Ther. 2023, 385, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C. Effect of Stress on Neuroimmune Processes. Clin. Ther. 2020, 42, 1007–1014. [Google Scholar] [CrossRef]
- Gupta, M.A. Somatization Disorders in Dermatology. Int. Rev. Psychiatry 2006, 18, 41–47. [Google Scholar] [CrossRef]
- Gupta, M.A.; Lanius, R.A.; Van der Kolk, B.A. Psychologic Trauma, Posttraumatic Stress Disorder, and Dermatology. Dermatol. Clin. 2005, 23, 649–656. [Google Scholar] [CrossRef]
- Brown, L.C.; Murphy, A.R.; Lalonde, C.S.; Subhedar, P.D.; Miller, A.H.; Stevens, J.S. Posttraumatic Stress Disorder and Breast Cancer: Risk Factors and the Role of Inflammation and Endocrine Function. Cancer 2020, 126, 3181–3191. [Google Scholar] [CrossRef]
- Oroian, B.A.; Ciobica, A.; Timofte, D.; Stefanescu, C.; Serban, I.L. New Metabolic, Digestive, and Oxidative Stress-Related Manifestations Associated with Posttraumatic Stress Disorder. Oxid. Med. Cell. Longev. 2021, 2021, 5599265. [Google Scholar] [CrossRef]
- Karanikas, E. Psychologically Traumatic Oxidative Stress; A Comprehensive Review of Redox Mechanisms and Related Inflammatory Implications. Psychopharmacol. Bull. 2021, 51, 65–86. [Google Scholar] [PubMed]
- Lushchak, O.; Strilbytska, O.; Koliada, A.; Storey, K.B. An Orchestrating Role of Mitochondria in the Origin and Development of Post-Traumatic Stress Disorder. Front. Physiol. 2023, 13, 1094076. [Google Scholar] [CrossRef] [PubMed]
- Kalinichenko, L.S.; Kornhuber, J.; Müller, C.P. Individual Differences in Inflammatory and Oxidative Mechanisms of Stress-Related Mood Disorders. Front. Neuroendocrinol. 2019, 55, 100783. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, P.; Floridi, L.; Boraschi, D.; Cuadrado, A.; Manda, G.; Levic, S.; D’Acquisto, F.; Hamilton, A.; Athersuch, T.J.; Selley, L. Oxidative Stress and Inflammation Induced by Environmental and Psychological Stressors: A Biomarker Perspective. Antioxid. Redox Signal. 2018, 28, 852–872. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.; Cimino, F. Circulating Advanced Oxidation Protein Products as Oxidative Stress Biomarkers and Progression Mediators in Pathological Conditions Related to Inflammation and Immune Dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef]
- Peruzzolo, T.L.; Pinto, J.V.; Roza, T.H.; Shintani, A.O.; Anzolin, A.P.; Gnielka, V.; Kohmann, A.M.; Marin, A.S.; Lorenzon, V.R.; Brunoni, A.R.; et al. Inflammatory and Oxidative Stress Markers in Post-Traumatic Stress Disorder: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2022, 27, 3150–3163. [Google Scholar] [CrossRef]
| Skin Disease | Psychiatric Comorbidities | Pathophysiological Mechanisms |
|---|---|---|
| Alopecia areata | Depressive, personality, and generalized anxiety disorders |
|
| Bullous pemphigoid | Schizophrenia, uni and bipolar disorders, personality disorders, depression, and psychosis | Cross-reactive neurocutaneous autoimmune (BP 180/BP230-mediated) response and subsequent neuroinflammation-neurodegeneration. |
| Pemphigus | Anxiety and depression | Proinflammatory habitus triggered by psychological stress related to previous adverse events. |
| Vitiligo | Depression | Stress-related HPA axis hyperactivation -> inflammation -> melanocytic oxidative damage. |
| Oral lichen planus | Anxiety, depression, and stress |
|
| Hidradenitis suppurativa | Major depression, anxiety, bipolar disorder, psychosis, substance abuse disorder, and suicide | Sharing of inflammatory pathways and involvement of the same cytokines. |
| Acne | Stress, anxiety, depression, obsessive-compulsive disorder, personality disorder, sexual dysfunction, and suicidal ideation/attempt |
|
| Rosacea | Depression and social anxiety |
|
| Herpes simplex virus | // | Emotional stress-induced HPA axis and sympathetic system activation -> stress hormones-induced Th1-Th2 shift -> HSV-specific CD8+ memory T-cells functional inhibition -> viral reactivation. |
| Epstein–Barr virus | // | Chronic stress-induced HPA axis hyperactivation. |
| Atopic dermatitis | Anxiety, depression, attention deficit hyperactivity disorder, emotional problems, conduct disorder, and suicidal ideation |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papa, V.; Li Pomi, F.; Borgia, F.; Genovese, S.; Pioggia, G.; Gangemi, S. “Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios. Cells 2023, 12, 1828. https://doi.org/10.3390/cells12141828
Papa V, Li Pomi F, Borgia F, Genovese S, Pioggia G, Gangemi S. “Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios. Cells. 2023; 12(14):1828. https://doi.org/10.3390/cells12141828
Chicago/Turabian StylePapa, Vincenzo, Federica Li Pomi, Francesco Borgia, Sara Genovese, Giovanni Pioggia, and Sebastiano Gangemi. 2023. "“Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios" Cells 12, no. 14: 1828. https://doi.org/10.3390/cells12141828
APA StylePapa, V., Li Pomi, F., Borgia, F., Genovese, S., Pioggia, G., & Gangemi, S. (2023). “Mens Sana in Cute Sana”—A State of the Art of Mutual Etiopathogenetic Influence and Relevant Pathophysiological Pathways between Skin and Mental Disorders: An Integrated Approach to Contemporary Psychopathological Scenarios. Cells, 12(14), 1828. https://doi.org/10.3390/cells12141828










