Abstract
Multiple sclerosis (MS) is a chronic, progressive neuroinflammatory disease with a complex pathophysiological background. A variety of diverse factors have been attributed to the propagation of inflammation and neurodegeneration in MS, mainly genetic, immunological, and environmental factors such as vitamin D deficiency, infections, or hormonal disbalance. Recently, the importance of the gut-brain axis for the development of many neurological conditions, including stroke, movement disorders, and neuroinflammatory disorders, has been postulated. The purpose of our paper was to summarize current evidence confirming the role of the gut microbiome in the pathophysiology of MS and related disorders, such as neuromyelitis optica spectrum disorder (NMO-SD). For this aim, we conducted a systematic review of the literature listed in the following databases: Medline, Pubmed, and Scopus, and were able to identify several studies demonstrating the involvement of the gut microbiome in the pathophysiology of MS and NMO-SD. It seems that the most relevant bacteria for the pathophysiology of MS are those belonging to Pseudomonas, Mycoplasma, Haemophilus, Blautia, Dorea, Faecalibacterium, Methanobrevibacter, Akkermansia, and Desulfovibrionaceae genera, while Clostridium perfringens and Streptoccocus have been demonstrated to play a role in the pathophysiology of NMO-SD. Following this line of evidence, there is also some preliminary data supporting the use of probiotics or other agents affecting the microbiome that could potentially have a beneficial effect on MS/NMO-SD symptoms and prognosis. The topic of the gut microbiome in the pathophysiology of MS is therefore relevant since it could be used as a biomarker of disease development and progression as well as a potential disease-modifying therapy.
1. Introduction
Multiple sclerosis (MS) is a progressive neuroinflammatory and neurodegenerative disease that usually has an onset in early adulthood and is predominantly found in females [1]. The main pathophysiological mechanism behind the development of MS is the activation of immunity, which was originally attributed to a T-mediated response, although, more recently, lymphocytes B and microglia have also been found to be involved [2]. As for other factors that have an influence on the initiation and propagation of this process, the importance of such causes as genetic contribution [3], low levels of vitamin D [4], infections, especially a history of Epstein–Barr virus exposure [5], smoking [6], and obesity [7], among others, has been shown. One of the risk factors that has been extensively investigated in the last few years, not only in the context of demyelinating disorders but also a variety of other neurological conditions, is the influence of the gut microbiome and microbiota [8]. While the microbiome includes both the host environment and the variety of microorganisms populating it, the term “microbiota” is used to define only microbiota specific to a particular host or disease [9]. The so-called gut-brain axis [10] is a term used to denote the interaction between the microbiome and the brain. It has been demonstrated that changes in the gut microbiome cause inflammation that affects a variety of systems [11,12], including the central and peripheral nervous systems (CNS) [13,14,15,16]. These interactions have been shown to take place at different levels and involve influences on brain signaling [17], gut-projecting spinal afferent neurons [18], gut-projecting afferent neurons [19], the influence of gut microbiota on the immune system [20], and metabolic systems [21], which, in turn, have an impact on the central and peripheral nervous systems. In the recently published systematic review by Tilocca et al. [22], the authors summarized previous evidence regarding different multi-omics techniques such as metagenomics, metatranscriptomics, metaproteomics, and metabolomics used for the investigation of gut-brain axis interactions. We were able to identify several key components of the gut-brain axis: the CNS, the autonomic nervous system, the enteric nervous system, the hypothalamic-pituitary-adrenal axis, and the immune system.
Especially the impact on the immune system has been postulated as the main mechanism of microbiome influence on the development of MS [23]. However, it is still not clear whether changes in the gut microbiome occur prior to the disease onset in the prodromal stage and could have a causal impact on the disease initiation, whether they are a type of biomarker of the disease progression and occur when the disease propagates, or whether they are a direct consequence of the disease-modifying therapies. It is also possible that multiple mechanisms and bidirectional influences have an impact on the gut-brain axis in MS (Figure 1). One of the suggested mechanisms is activation by the infection [24] either viral [25,26,27], bacterial [27], or fungal [28], that in turn leads to a number of further changes, such as activation of the immune system and changes in the microbiome composition [29]. Another useful approach in this context is offered by metagenomics [30], which comprises both microbiological as well as genetic techniques. As a result, metagenomics enables the identification of DNA specific to particular microorganisms [31].
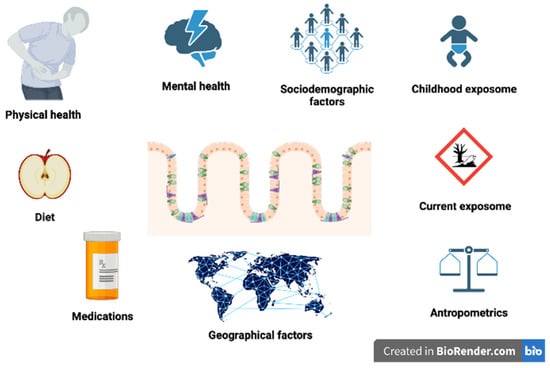
Figure 1.
Various environmental factors that have influence on gut microbiome.
The purpose of our paper was to summarize current evidence behind the role of the gut microbiome in the pathophysiology of MS and related disorders. We are also mentioning the potential use of agents that interfere with the composition of the gut microbiome, such as probiotics and fecal transplantation, for the therapy of MS. To identify articles eligible for inclusion, we have searched electronic databases (Pubmed, Medline, and Scopus) with the main focus on original research. We did not use any time restrictions to include the articles.
2. The Role of Gut Microbiome in the Pathogenesis of Multiple Sclerosis
Taking into consideration the fact that the gut microbiota is crucial for the immune system’s growth and maturation [32], it is intuitive to expect its contribution to the pathogenesis of MS [33,34,35].
Animal models provide information that MS-linked microbiota produce factors that precipitate an MS-like autoimmune disease [36]. Molecular mimicry between gut bacterial components and central nervous system (CNS) autoantigens, in concert with gut microbes inducing Th17 cells, acts together to worsen CNS autoimmunity in experimental autoimmune encephalomyelitis (EAE), the most widely used animal model of MS [37]. Therefore, the main mechanism behind the creation of this animal model is based on gut-brain interaction. In the process of developing this model, it has been found that antibiotic administration protected against the disease, which was attributed to Treg- and Th2-cell responses [38].
Following these initial findings, immune cells from mice that received microbiota samples from MS individuals produced less interleukin (IL)-10 than immune cells from mice colonized with healthy samples. This is of particular importance since IL-10 may have a regulatory role in spontaneous CNS autoimmunity [39]. Increased Streptococcus concentration, a higher Firmicutes/Bacteroidetes ratio, and decreased Prevotella concentration are associated with higher disease activity and more abundant intestinal Th17 cells [37]. Proinflammatory responses in human peripheral blood mononuclear cells and in monocolonized mice are related to Akkermansia muciniphila and Acinetobacter calcoaceticus colonization, which are typical for MS patients [40]. On the other hand, Parabacteroides distasonis, which is reduced in MS patients, stimulates anti-inflammatory IL-10 expressing human CD4+CD25+ T cells and IL-10+FoxP3+ Tregs in mice [40]. Microbiota transplantation from MS patients into germ-free mice results in more severe manifestations of EAE compared with mice ‘humanized’ with microbiota from healthy controls (HC) [40]. Moreover, dysbiosis can modulate immunological responses to the microbiota and affect the integrity of the epithelia that comprise cellular barriers vital for the integrity of the intestine and CNS [41]. In the EAE animal model, increased intestinal permeability, overexpression of the tight junction protein zonulin, alterations in intestinal morphology, increased infiltration of proinflammatory Th1/Th17 cells, and a reduced regulatory T cell number in the gut lamina propria, Peyer’s patches, and mesenteric lymph nodes were observed [42]. Germ-free mice are also reported to have greater permeability of the blood-brain barrier (BBB) compared to pathogen-free mice with a normal gut flora [43]. The low-grade microbial translocation to the systemic circulation and ultimately to the brain is a crucial point regarding the contribution of intestinal permeability changes to MS pathophysiology [44]. The change in gut microbiome achieved by exposing germ-free adult mice to a pathogen-free gut microbiota decreased BBB permeability and up-regulated the expression of tight junction proteins [43]. The same study delivered information about the presence of hypermyelinated axons within the prefrontal cortex of germ-free mice, suggesting the role of the microbiota in controlling myelin production in this brain area [43]. In another study by Colpitts et al. [45], they compared gut microbiota composition between acute inflammatory and chronic progressive forms in a murine model of secondary-progressive multiple sclerosis. As a result, the authors found that the mice that developed a severe secondary form of EAE exhibited a dysbiotic gut microbiome when compared with the healthy control mice. The authors also complemented this study with a sub-analysis regarding the influence of antibiotic therapy on the outcomes of the progressive stage of EAE. Interestingly enough, mice receiving antibiotics demonstrated reduced mortality and disease severity.
According to the literature based on studies in humans, specific bacterial taxa are significantly associated with the pathophysiology of MS. In particular, the following bacteria were found to be more abundant in MS patients than controls: Pseudomonas, Mycoplana, Haemophilus, Blautia, Dorea, Faecalibacterium, Methanobrevibacter, Akkermansia, and Desulfovibrionaceae genera [46,47,48,49]. These taxa have been found to be associated with variations in the expression of genes involved in dendritic cell maturation, interferon signaling, and NF-kB signaling pathways in circulating T cells and monocytes. It has also been postulated that Akkermansia, found more abundantly in MS patients than healthy controls, may be a compensatory response in MS. What is more, progressive MS is uniquely linked to elevated Enterobacteriaceae and Clostridium g24 FCEY and decreased Blautia and Agathobaculum colonization [39]. Moreover, Clostridial species associated with MS might be distinct from those broadly associated with other autoimmune conditions [50]. Differences in the microbiome composition between MS patients and healthy controls concern clostridial species belonging to Clostridia clusters XIVa and IV and Bacteroidetes [50]. As clusters IV and XIVa of the genus Clostridium promote Treg cell accumulation and the colonization of mice by a defined mix of Clostridium strains is described to provide an environment rich in transforming growth factor–b and affect Foxp3+ Treg numbers, it can alter colon function and disrupt immune homeostasis [51]. In addition, several Clostridium species were associated with higher disability scores measured with the expanded disability status scale (EDSS) and fatigue scores [39].
Another important topic is the influence of disease-modifying therapies on the microbiome’s composition. MS patients on disease-modifying therapies have been found to have increased fecal concentrations of Prevotella, Sutterella, and Akkermansia and decreased Sarcina in comparison to untreated individuals [49,52,53]. Glatiramer acetate treatment is described as being linked to differences in microbiome composition in MS patients, expressed in abundance of Bacteroidaceae, Faecalibacterium, Ruminococcus, Lactobacillaceae, Clostridium, and other Clostridiales [49]. On the other hand, in untreated MS patients, an increase in the Akkermansia, Faecalibacterium, and Coprococcus genera after vitamin D supplementation was observed [49].
Studies also show that manipulations of the gut microbiome through the use of probiotics have a positive effect on the health of patients with MS [54,55,56,57]. Administration of probiotics increases the colonization of some taxa known to be depleted in MS, such as Lactobacillus, and reduces the abundance of Akkermansia and Blautia, linked to dysbiosis in MS [56]. Methane metabolism, number of inflammatory monocytes, mean fluorescence intensity (MFI) of CD80 of classical monocytes, and HLA-DR MFI on dendritic cells are also reduced after probiotic intake in MS patients [55]. Moreover, in a healthy population, probiotic administration is linked to decreased expression of the MS risk alleles HLA-DQA1 and HLA-DPB1 [56]. Administration of Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, and Lactobacillus fermentum has favorable effects on disability levels assessed with EDSS, parameters of mental health, inflammatory factors, markers of insulin resistance, and HDL- and total-/HDL-cholesterol levels in MS patients [57]. Probiotic supplementation down-regulates gene expression of IL-8 and tumor necrosis factor–alpha (TNF-a) mRNA in peripheral blood mononuclear cells of patients with MS [57]. It has also been observed that the low-grade inflammation linked to chronic inflammatory diseases is largely combated by nutrients, including nondigestible dietary fibers [58]. Their action is mediated by the gut microbiota, and any microbial change brought on by diet alters host-microbe interactions in a way that either ameliorates or exacerbates the disease, which also applies to MS [57]. What is more, intermittent fasting (IF) ameliorates the clinical course and neurodegenerative changes in EAE [59,60,61]. IF also increased the diversity of gut bacteria, particularly those belonging to the families of Lactobacillaceae, Bacteroidaceae, and Prevotellaceae. IF reduces the IL-17-producing T cells in the gut while increasing the regulatory T cells [59,60,61].
The overview of articles discussed in this section is presented in Table 1.

Table 1.
An overview of studies investigating the role of gut microbiome in the pathogenesis of multiple sclerosis. Studies are presented in chronological order.
3. The Role of Gut Microbiome in the Pathogenesis of Neuromyelitis Optica Spectrum Disorders
Neuromyelitis optica spectrum disorders (NMO-SDs) are a group of chronic autoimmune diseases of the CNS [62]. The symptoms of NMO-SDs are caused by demyelinating lesions occurring mainly in the spinal cord and optic nerves [63,64]. The majority of patients are seropositive for autoantibodies (NMO-SD IgG) against aquaporin-4 (AQP4), a water channel expressed in astrocytes. The AQP4-specific autoantibodies are classified as immunoglobulin (Ig)G1, a T cell-dependent Ig subclass [63]. Although great progress has been made to unravel the pathogenesis of NMO-SDs, the environmental triggers underlying the production of NMO-SD IgG remain unclear [63,65].
To date, research has shown that T cells recognizing the epitope of AQP4 display cross-reactivity to homologous peptide sequences of commensal bacteria found in human gut flora [66]. In 2012, Varrin-Doyer et al. conducted the first study using peripheral blood T cells obtained from NMO-SD patients and healthy controls (HC) [66]. Both T cells from NMO-SD patients and HC proliferated to intact AQP or discrete AQP4 peptides. The T cells from NMO-SD patients showed markedly higher proliferation, especially when exposed to the peptide p61-80. The peptide p63-76, in turn, exhibited strong homology to a sequence within the Clostridium perfringens adenosine triposphate-binding cassette (ABC) transporter permease. The T cells from NMO-SD patients also proliferated to this homologous bacterial sequence, showing cross-reactivity and supporting the role of molecular mimicry. Moreover, monocytes from NMO-SD patients produced a greater amount of interleukin (IL)-6, which is responsible for T17 polarization in this group of patients [66]. The paper published by Cree et al. also supports the role of C. perfringens in the pathogenesis of NMO-SDs [67]. The stool samples obtained from 16 NMO-SD patients showed a significantly higher concentration of several microbial communities, especially C. perfringens, in comparison to the ones obtained from 16 HC. Most of the NMO-SD patients were treated with immunotherapy, mostly rituximab, which could also influence the gut microbiome. In addition, the authors compared these findings with those of 16 MS patients, five of whom were treated with rituximab. Notably, the gut microbiota in NMO-SD and MS patients differed significantly despite treatment with rituximab in both groups [67]. On the other hand, the study conducted by Gong et al. in China revealed the overrepresentation of Streptococcus sp. in the fecal microbial composition of the NMO-SD patients [68]. Interestingly, the abundance of Streptococcus sp. was positively correlated with disease severity, and the use of immune suppressant medications had a depleting influence on the gut microbiome. Moreover, the patients with NMO-SD showed significant reductions in faecal butyrate, which is believed to have an anti-inflammatory effect [68]. The anti-inflammatory effect of short-chain fatty acids is not limited to the intestinal tract; it also increases the Treg level and inhibits Th17 cell differentiation [68]. The same group from China showed that although in the sigmoid mucosal biopsies collected from 6 NMO-SD patients the diversity of bacterial flora was overall diminished, Streptococcus and Granulicatella sp. were still amply detected in the samples [69]. Furthermore, through the decreased expression of tight junction proteins, the integrity of the intestinal barrier may be impaired. Additionally, the increased number of plasma cells, macrophages, and mast cells found in the lamina propria suggests inflammatory activation of the gut in patients with NMO-SD. Another study from China supports the aforementioned results, showing an overrepresentation of pathogenic species such as Streptococcus and Flavonifractors in the stool samples of NMO-SD patients [70]. Zhang et al. characterized the gut microbiota in both AQP4 seropositive and AQP4 seronegative groups of NMO-SD patients separately and compared these findings with the HC [71]. The microbial composition in NMO-SD showed an increased prevalence of Proteobacteria, Bacteroidetes, and Firmicutes, respectively. Furthermore, butyrate-producing species were abundantly represented in HC compared to NMO-SD patients [71]. Pandit et al. investigated the blood and stool samples of 39 Indian patients with NMO-SD [72]. The prevalence of Clostridium boltae was significantly higher in the stool samples obtained from AQP4 positive patients compared to seronegative specimens. C boltae was not detected in the stool samples collected from HC. Moreover, C boltae peptide p 59–71 showed homology with AQP peptide p92–104. The presence of C boltae correlated with the expression of inflammatory genes associated with B cell chemotaxis as well as Th17 cell activation [72]. Recently, Cheng et al. investigated the role of T follicular helper (Tfh) cells in NMO-SD recurrence and evaluated whether the levels of glycoursodeoxycholic acid (GUDCA), a microbiota metabolite, influenced levels of serum C-X-C motif ligand 13 (CXCL13), which reflect the effects of the Tfh cells on B-cell-mediated humoral immunity [73]. The level of GUDCA was higher in patients with NMOSD with low activity, which was positively correlated with CXCL13. The overview of studies presented in this section is presented in Table 2.

Table 2.
An overview of studies investigating the role of gut microbiome in the pathogenesis of neuromyelitis optica spectrum disorders.
4. Conclusions
The current degree of evidence supports the importance of the gut microbiome in the pathophysiology of MS and related disorders. On the one hand, there are many taxa of bacteria that are found more frequently in patients with MS, but also, disease-modifying therapies used in MS have been shown to change the microbiome composition. One important point that should be considered is whether these changes occur prior to the occurrence of MS or are secondary to the disease itself [74]. It is also possible that genetically determined microbiome changes determine the microbiome composition, which, in turn, leads to disease progression [75,76,77]. Another issue is related to the differences between the microbiome, which constitutes both the host environment as well as all microorganisms encountered in it, and the microbiota, which is limited to microorganisms only [78]. In addition, it is still not clear whether the totality of the patient’s microbiome is equally important as microbiomes in different locations, such as the gut, oral [79], nasal cavity [80], pulmonary tract [81], or vagina [82]. This is of great importance since the environment is the single most important determinant of microbiota composition [83]. Another topic that still needs exploration is the influence of different environmental factors on the microbiome composition and its interaction with MS pathophysiology. Previous studies have demonstrated that dietary changes [84], stressors [85], substance abuse [86], and chronic illnesses [87] co-existing with MS and pharmacotherapy, especially antibiotics [88], influence the microbiome composition.
There are several limitations to our review: (i) although we used a detailed search strategy, it is still possible that we did not include some important studies, especially the ones posterior to publication of our review; (ii) another limitation is related to the inclusion of articles that are written only in English; (iii) finally, we mainly focused on the microbiome in our review; however, it is highly dependent on environmental factors [89], especially diet, which also plays an important role in the development of MS [90].
It can therefore be concluded that although there are some preliminary data suggesting that the gut microbiome plays an important role in the pathophysiology of MS, the microbiome composition is determined by too many confounding factors. Future studies should be focused on overcoming this limitation, mainly using methodologies derived from population genetics, such as Mendelian randomization [91].
Author Contributions
Conceptualization, A.D., K.S. and N.S.; methodology, A.D., K.S. and N.S.; investigation, A.D., K.S. and N.S.; resources, A.D., K.S. and N.S.; writing—original draft preparation, A.D., K.S. and N.S.; writing—review and editing, A.D., K.S. and N.S.; visualization, A.D., K.S. and N.S.; supervision, N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
N.S. received support from Biogen.
References
- Ward, M.; Goldman, M.D. Epidemiology and Pathophysiology of Multiple Sclerosis. Contin. Lifelong Learn. Neurol. 2022, 28, 988–1005. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Goris, A.; Vandebergh, M.; McCauley, J.L.; Saarela, J.; Cotsapas, C. Genetics of multiple sclerosis: Lessons from polygenicity. Lancet Neurol. 2022, 21, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Pierrot-Deseilligny, C.; Souberbielle, J.-C. Vitamin D and multiple sclerosis: An update. Mult. Scler. Relat. Disord. 2017, 14, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Jakimovski, D.; Weinstock-Guttman, B.; Ramanathan, M.; Dwyer, M.G.; Zivadinov, R. Infections, Vaccines and Autoimmunity: A Multiple Sclerosis Perspective. Vaccines 2020, 8, 50. [Google Scholar] [CrossRef]
- Arneth, B. Multiple Sclerosis and Smoking. Am. J. Med. 2020, 133, 783–788. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M. Multiple sclerosis and obesity: The role of adipokines. Front. Immunol. 2022, 13, 1038393. [Google Scholar] [CrossRef]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef]
- Shanahan, F.; Ghosh, T.S.; O’Toole, P.W. The Healthy Microbiome-What Is the Definition of a Healthy Gut Microbiome? Gastroenterology 2021, 160, 483–494. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2019, 20, 40–54. [Google Scholar] [CrossRef]
- Alam, R.; Abdolmaleky, H.M.; Zhou, J.R. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 651–660. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, L.J.; Zhang, L.J. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 8243–8250. [Google Scholar] [CrossRef]
- Butler, M.J.; Perrini, A.A.; Eckel, L.A. The Role of the Gut Microbiome, Immunity, and Neuroinflammation in the Pathophysiology of Eating Disorders. Nutrients 2021, 13, 500. [Google Scholar] [CrossRef]
- Bostick, J.W.; Schonhoff, A.M.; Mazmanian, S.K. Gut microbiome-mediated regulation of neuroinflammation. Curr. Opin. Immunol. 2022, 76, 102177. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The gut-brain axis and the microbiome: Mechanisms and clinical implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef]
- Gershon, M.D.; Margolis, K.G. The gut, its microbiome, and the brain: Connections and communications. J. Clin. Investig. 2021, 131, e143768. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Neuhuber, W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000, 85, 1–17. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2020, 12, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Tilocca, B.; Pieroni, L.; Soggiu, A.; Britti, D.; Bonizzi, L.; Roncada, P.; Greco, V. Gut–Brain Axis and Neurodegeneration: State-of-the-Art of Meta-Omics Sciences for Microbiota Characterization. Int. J. Mol. Sci. 2020, 21, 4045. [Google Scholar] [CrossRef] [PubMed]
- Preiningerova, J.L.; Zakostelska, Z.J.; Srinivasan, A.; Ticha, V.; Kovarova, I.; Kleinova, P.; Tlaskalova-Hogenova, H.; Havrdova, E.K. Multiple Sclerosis and Microbiome. Biomolecules 2022, 12, 433. [Google Scholar] [CrossRef] [PubMed]
- Perlejewski, K.; Bukowska-Ośko, I.; Nakamura, S.; Motooka, D.; Stokowy, T.; Płoski, R.; Rydzanicz, M.; Zakrzewska-Pniewska, B.; Podlecka-Piętowska, A.; Nojszewska, M.; et al. Metagenomic Analysis of Cerebrospinal Fluid from Patients with Multiple Sclerosis. Adv. Exp. Med. Biol. 2016, 935, 89–98. [Google Scholar] [CrossRef]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Ep-stein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef]
- Tarlinton, R.E.; Martynova, E.; Rizvanov, A.A.; Khaiboullina, S.; Verma, S. Role of Viruses in the Pathogenesis of Multiple Sclerosis. Viruses 2020, 12, 643. [Google Scholar] [CrossRef]
- Marrodan, M.; Alessandro, L.; Farez, M.F.; Correale, J. The role of infections in multiple sclerosis. Mult. Scler. J. 2019, 25, 891–901. [Google Scholar] [CrossRef]
- Pisa, D.; Alonso, R.; Jiménez-Jiménez, F.J.; Carrasco, L. Fungal infection in cerebrospinal fluid from some patients with multiple sclerosis. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 795–801. [Google Scholar] [CrossRef]
- Yuan, S.; Xiong, Y.; Larsson, S.C. An atlas on risk factors for multiple sclerosis: A Mendelian randomization study. J. Neurol. 2020, 268, 114–124. [Google Scholar] [CrossRef]
- Wang, D. Metagenomics Databases for Bacteria. Methods Mol. Biol. 2023, 2649, 55–67. [Google Scholar] [CrossRef]
- Jovel, J.; O’Keefe, S.; Patterson, J.; Bording-Jorgensen, M.; Wang, W.; Mason, A.L.; Warren, K.G.; Wong, G.K.-S. Cerebrospinal Fluid in a Small Cohort of Patients with Multiple Sclerosis Was Generally Free of Microbial DNA. Front. Cell. Infect. Microbiol. 2017, 6, 198. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Mahad, D.H.; Trapp, B.D.; Lassmann, H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015, 14, 183–193. [Google Scholar] [CrossRef]
- Hemmer, B.; Kerschensteiner, M.; Korn, T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol. 2015, 14, 406–419. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Takewaki, D.; Yamamura, T. Gut microbiome research in multiple sclerosis. Neurosci. Res. 2021, 168, 28–31. [Google Scholar] [CrossRef]
- Miyauchi, E.; Kim, S.W.; Suda, W.; Kawasumi, M.; Onawa, S.; Taguchi-Atarashi, N.; Morita, H.; Taylor, T.D.; Hattori, M.; Ohn, H. Gut microorganisms act together to exac-erbate inflammation in spinal cords. Nature 2020, 585, 102–106. [Google Scholar] [CrossRef]
- Wekerle, H. Brain Autoimmunity and Intestinal Microbiota: 100 Trillion Game Changers. Trends Immunol. 2017, 38, 483–497. [Google Scholar] [CrossRef]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Kirby, T.O.; Kasper, L.H. The Gut Microbiome and Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029017. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Bredberg, A.; Weström, B.; Lavasani, S. Intestinal Barrier Dysfunction Develops at the Onset of Experimental Autoimmune Encephalomyelitis, and Can Be Induced by Adoptive Transfer of Auto-Reactive T Cells. PLoS ONE 2014, 9, e106335. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Buscarinu, M.C.; Fornasiero, A.; Romano, S.; Ferraldeschi, M.; Mechelli, R.; Reniè, R.; Morena, E.; Romano, C.; Pellicciari, G.; Landi, A.C.; et al. The Contribution of Gut Barrier Changes to Multiple Sclerosis Pathophysiology. Front. Immunol. 2019, 10, 1916. [Google Scholar] [CrossRef]
- Colpitts, S.L.; Kasper, E.J.; Keever, A.; Liljenberg, C.; Kirby, T.; Magori, K.; Kasper, L.H.; Ochoa-Repáraz, J. A bidirectional association between the gut microbiota and CNS disease in a biphasic murine model of multiple sclerosis. Gut Microbes 2017, 8, 561–573. [Google Scholar] [CrossRef]
- Mirza, A.; Mao-Draayer, Y. The gut microbiome and microbial translocation in multiple sclerosis. Clin. Immunol. 2017, 183, 213–224. [Google Scholar] [CrossRef]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E.; US Network of Pediatric MS Centers. Gut microbiota in early pediatric multiple sclerosis: A case−control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut Microbiota in Multiple Sclerosis: Possible Influence of Immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.-W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Shima, T.; Imaoka, A.; Kuwahara, T.; Momose, Y.; Cheng, G.; Yamasaki, S.; Saito, T.; Ohba, Y.; et al. Induction of colonic regulatory T cells by indigenous clostridium species. Science 2011, 331, 337–341. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef]
- Pilotto, S.; Zoledziewska, M.; Fenu, G.; Cocco, E.; Lorefice, L. Disease-modifying therapy for multiple sclerosis: Implications for gut microbiota. Mult. Scler. Relat. Disord. 2023, 73, 104671. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Cox, L.M.; Tjon, E.; Kivisakk, P.; Vanande, I.P.; Cook, S.; Gandhi, R.; Glanz, B.; et al. Investigation of probiotics in multiple sclerosis. Mult. Scler. J. 2018, 24, 58–63. [Google Scholar] [CrossRef]
- Atabati, H.; Yazdanpanah, E.; Mortazavi, H.; Bajestani, S.G.; Raoofi, A.; Esmaeili, S.-A.; Khaledi, A.; Saburi, E.; Afshari, J.T.; Sathyapalan, T.; et al. Immunoregulatory Effects of Tolerogenic Probiotics in Multiple Sclerosis. Adv. Exp. Med. Biol. 2021, 1286, 87–105. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Kakhaki, R.D.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2016, 36, 1245–1249. [Google Scholar] [CrossRef]
- Fritsch, J.; Garces, L.; Quintero, M.A.; Pignac-Kobinger, J.; Santander, A.M.; Fernández, I.; Ban, Y.J.; Kwon, D.; Phillips, M.C.; Knight, K.; et al. Low-Fat, High-Fiber Diet Reduces Markers of Inflammation and Dysbiosis and Improves Quality of Life in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 19, 1189–1199.e30. [Google Scholar] [CrossRef]
- Riccio, P.; Rossano, R. Diet, Gut Microbiota, and Vitamins D + A in Multiple Sclerosis. Neurotherapeutics 2018, 15, 75–91. [Google Scholar] [CrossRef]
- Morales-Suarez-Varela, M.; Sánchez, E.C.; Peraita-Costa, I.; Llopis-Morales, A.; Soriano, J.M. Intermittent Fasting and the Possible Benefits in Obesity, Diabetes, and Multiple Sclerosis: A Systematic Review of Randomized Clinical Trials. Nutrients 2021, 13, 3179. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Auto-immunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e6. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Jarius, S.; Ruprecht, K.; Wildemann, B.; Kuempfel, T.; Ringelstein, M.; Geis, C.; Kleiter, I.; Kleinschnitz, C.; Berthele, A.; Brettschneider, J.; et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J. Neuroinflamm. 2012, 9, 14. [Google Scholar] [CrossRef]
- Kleiter, I.; Gahlen, A.; Borisow, N.; Fischer, K.; Wernecke, K.-D.; Wegner, B.; Hellwig, K.; Pache, F.; Ruprecht, K.; Havla, J.; et al. Neuromyelitis optica: Evaluation of 871 attacks and 1153 treatment courses. Ann. Neurol. 2015, 79, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Melamed, E.; Levy, M.; Waters, P.J.; Sato, D.K.; Bennett, J.L.; John, G.R.; Hooper, D.C.; Saiz, A.; Bar-Or, A.; Kim, H.J. Update on biomarkers in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, 134. Available online: https://nn.neurology.org/content/2/4/e134 (accessed on 21 April 2023). [CrossRef]
- Varrin-Doyer, M.; Spencer, C.M.; Schulze-Topphoff, U.; Nelson, P.A.; Stroud, R.M.; Cree, B.A.C.; Zamvil, S.S. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 2012, 72, 53–64. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Bs, C.M.S.; Varrin-Doyer, M.; Baranzini, S.E.; Zamvil, S.S. Gut microbiome analysis in neuromyelitis optica reveals overabundance of Clostridium perfringens. Ann. Neurol. 2016, 80, 443–447. [Google Scholar] [CrossRef]
- Gong, J.; Qiu, W.; Zeng, Q.; Liu, X.; Sun, X.; Li, H.; Yang, Y.; Wu, A.; Bao, J.; Wang, Y.; et al. Lack of short-chain fatty acids and overgrowth of opportunistic pathogens define dysbiosis of neuromyelitis optica spectrum disorders: A Chinese pilot study. Mult. Scler. J. 2018, 25, 1316–1325. [Google Scholar] [CrossRef]
- Cui, C.; Tan, S.; Tao, L.; Gong, J.; Chang, Y.; Wang, Y.; Fan, P.; He, D.; Ruan, Y.; Qiu, W. Intestinal Barrier Breakdown and Mucosal Microbiota Disturbance in Neuromyelitis Optical Spectrum Disorders. Front. Immunol. 2020, 11, 2101. [Google Scholar] [CrossRef]
- Shi, Z.; Qiu, Y.; Wang, J.; Fang, Y.; Zhang, Y.; Chen, H.; Du, Q.; Zhao, Z.; Yan, C.; Yang, M.; et al. Dysbiosis of gut microbiota in patients with neuromyelitis optica spectrum disorders: A cross sectional study. J. Neuroimmunol. 2019, 339, 577126. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.-F.; Wu, L.; Li, H.-F.; Wu, Z.-Y. Characteristic of gut microbiota in southeastern Chinese patients with neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2020, 44, 102217. [Google Scholar] [CrossRef]
- Pandit, L.; Cox, L.M.; Malli, C.; D’Cunha, A.; Rooney, T.; Lokhande, H.; Willocq, V.; Saxena, S.; Chitnis, T. Clostridium bolteae is elevated in neuromyelitis optica spectrum disorder in India and shares sequence similarity with AQP4. Neurol. Neuroimmunol. Neuroinflamm. 2020, 8, e907. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, L.; Li, Z.; Shen, S.; Zhao, Y.; Liu, C.; Zhong, X.; Chang, Y.; Kermode, A.G.; Qiu, W. Gut Microbiome and Bile Acid Metabolism Induced the Activation of CXCR5+ CD4+ T Follicular Helper Cells to Participate in Neuromyelitis Optica Spectrum Disorder Recurrence. Front. Immunol. 2022, 13, 827865. [Google Scholar] [CrossRef]
- Navarro-López, V.; Méndez-Miralles, M.Á.; Vela-Yebra, R.; Fríes-Ramos, A.; Sánchez-Pellicer, P.; Ruzafa-Costas, B.; Núñez-Delegido, E.; Gómez-Gómez, H.; Chumillas-Lidón, S.; Picó-Monllor, J.A.; et al. Article Gut Microbiota as a Potential Predictive Biomarker in Relapsing-Remitting Multiple Sclerosis. Genes 2022, 13, 930. [Google Scholar] [CrossRef]
- Cox, L.M.; Weiner, H.L. The microbiome requires a genetically susceptible host to induce central nervous system autoimmunity. Proc. Natl. Acad. Sci. USA 2020, 117, 27764–27766. [Google Scholar] [CrossRef]
- Maglione, A.; Zuccalà, M.; Tosi, M.; Clerico, M.; Rolla, S. Host Genetics and Gut Microbiome: Perspectives for Multiple Sclerosis. Genes 2021, 12, 1181. [Google Scholar] [CrossRef]
- Elsayed, N.S.; Valenzuela, R.K.; Kitchner, T.; Le, T.; Mayer, J.; Tang, Z.Z.; Bayanagari, V.R.; Lu, Q.; Aston, P.; Anantharaman, K.; et al. Association Between Polygenic Risk Score And Gut Microbiome Of Multiple Sclerosis. bioRxiv 2022. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Zangeneh, Z.; Abdi-Ali, A.; Khamooshian, K.; Alvandi, A.; Abiri, R. Bacterial variation in the oral microbiota in multiple sclerosis patients. PLoS ONE 2021, 16, e0260384. [Google Scholar] [CrossRef]
- Gioacchini, F.M.; Ferlito, S.; Ralli, M.; Scarpa, A.; La Mantia, I.; Re, M.; Romani, L.; Di Stadio, A. Nasal Microbiota and Neuroinflammation: Relationship between Nasal Flora and Multiple Sclerosis Onset/Progression. Life 2022, 12, 2043. [Google Scholar] [CrossRef]
- Montague-Cardoso, K. Lung microbiota and MS. Nat. Neurosci. 2022, 25, 404. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, A.M.; Ireland, D.J.; Payne, M.S. The Vaginal Microbiome in Health and Disease—What Role Do Common Intimate Hygiene Practices Play? Microorganisms 2023, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The Gut Microbiome and Substance Use Disorder. Front. Neurosci. 2021, 15, 1134. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Ramirez, J.; Guarner, F.; Bustos Fernandez, L.; Maruy, A.; Sdepanian, V.L.; Cohen, H. Antibiotics as Major Disruptors of Gut Micro-biota. Front. Cell. Infect. Microbiol. 2020, 10, 731. [Google Scholar] [CrossRef]
- Ahn, J.; Hayes, R.B. Environmental Influences on the Human Microbiome and Implications for Noncommunicable Disease. Annu. Rev. Public Health 2021, 42, 277–292. [Google Scholar] [CrossRef]
- Stoiloudis, P.; Kesidou, E.; Bakirtzis, C.; Sintila, S.A.; Konstantinidou, N.; Boziki, M.; Grigoriadis, N. The Role of Diet and Interventions on Mul-tiple Sclerosis: A Review. Nutrients 2022, 14, 1150. [Google Scholar] [CrossRef]
- Sanderson, E.; Glymour, M.M.; Holmes, M.V.; Kang, H.; Morrison, J.; Munafò, M.R.; Palmer, T.; Schooling, C.M.; Wallace, C.; Zhao, Q.; et al. Mendelian randomization. Nat. Rev. Methods Prim. 2022, 2, 6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).