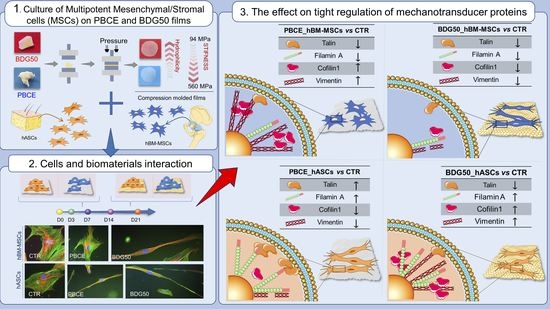
Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness
Abstract
1. Introduction
2. Materials and Methods
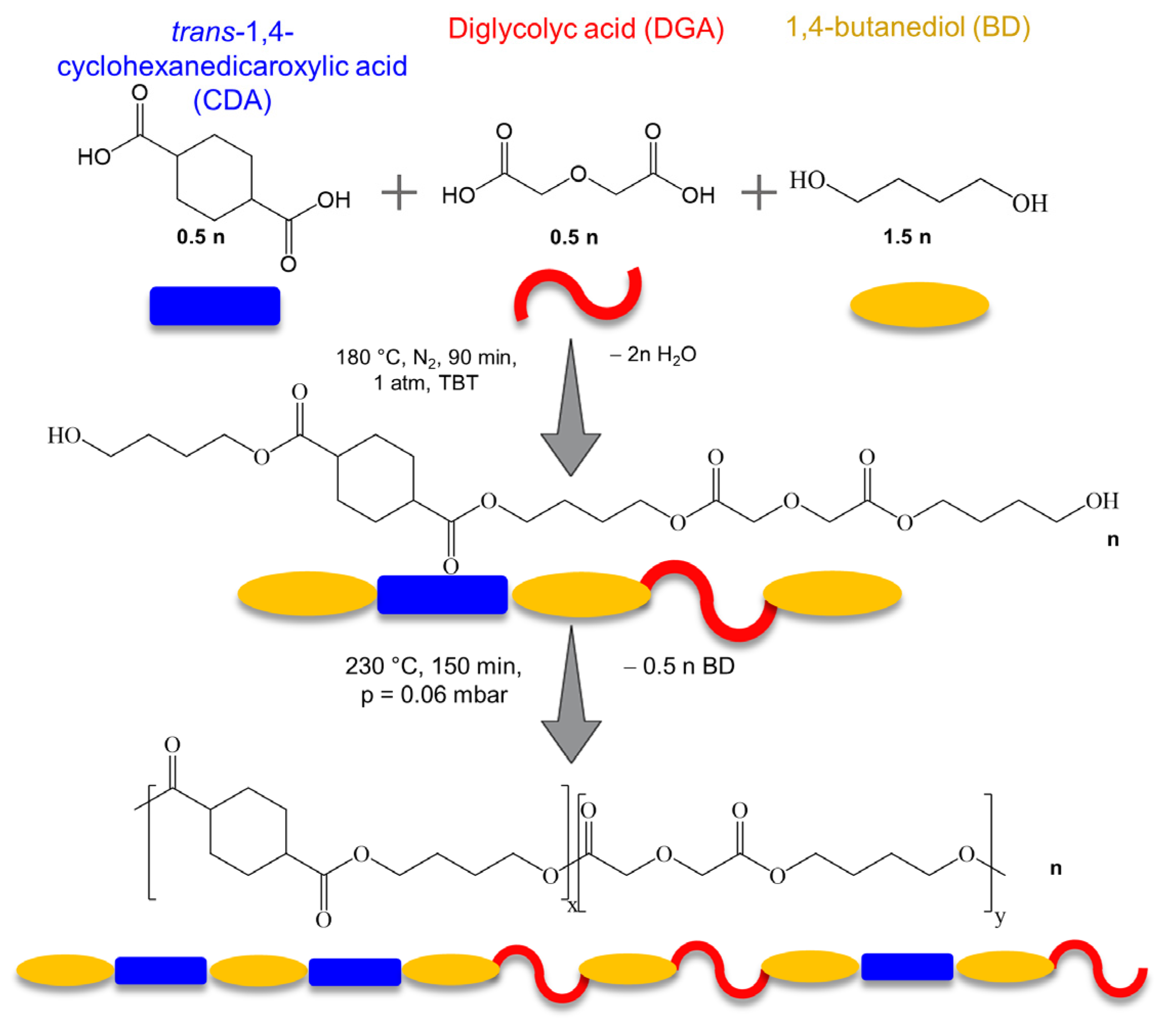
2.1. Materials and PBCE and BDG50 Polymer Synthesis
2.2. Production of PBCE and BDG50 Polymer Films
2.3. PBCE and BDG50 Polymer Film Characterization
2.4. Human Adult Mesenchymal/Stromal Multipotent Cells’ Isolation and In Vitro Culture
2.5. Culture of hBM-MSCs and hASCs on Polymer Films
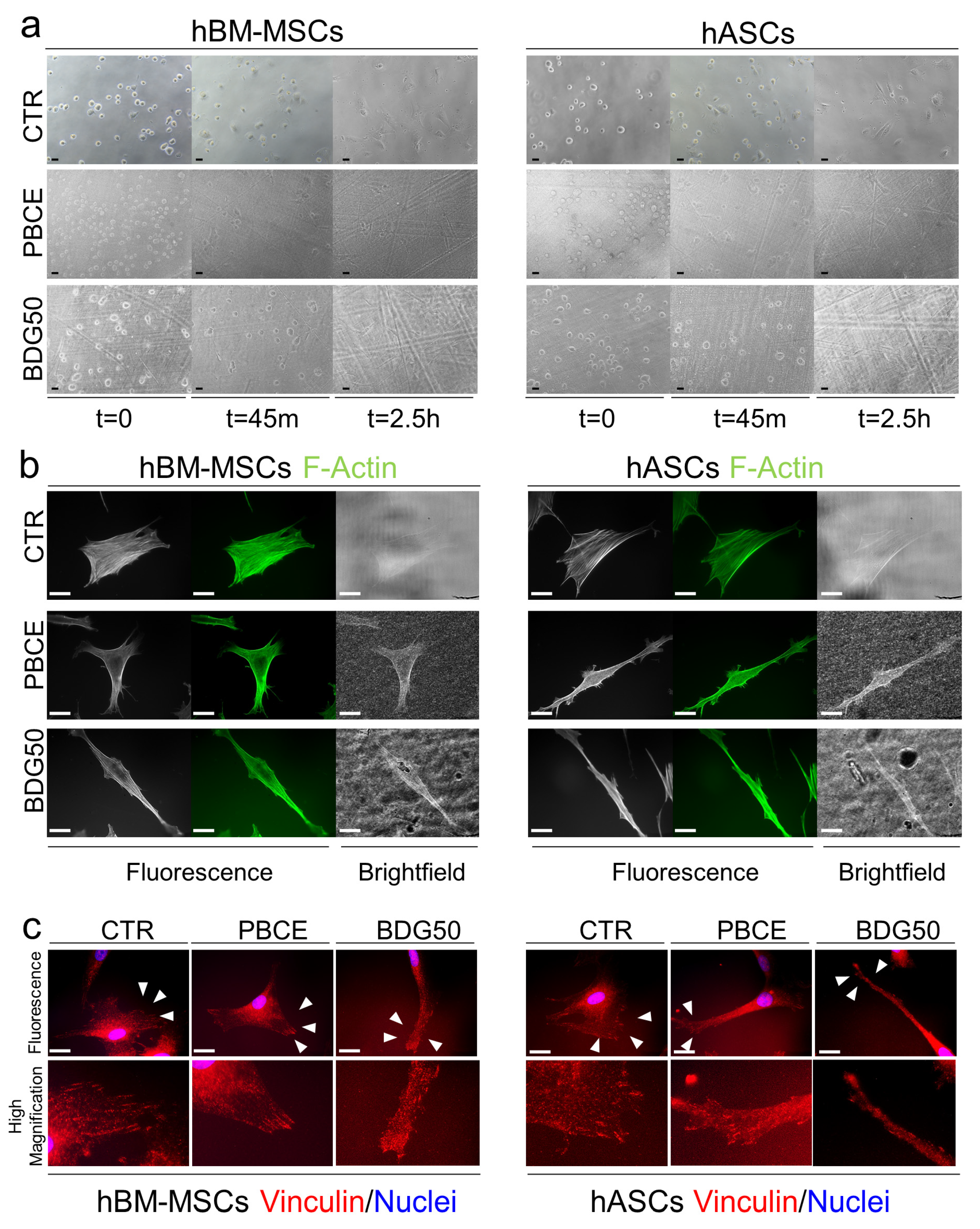
2.6. Adhesion of hBM-MSCs and hASCS on PBCE and BDG50 Films
2.7. Viability Assay
2.8. Cell Proliferation
2.9. Immunofluorescence
2.10. Computational Imaging Analysis
2.11. Cell Extracts
2.12. Western Blotting
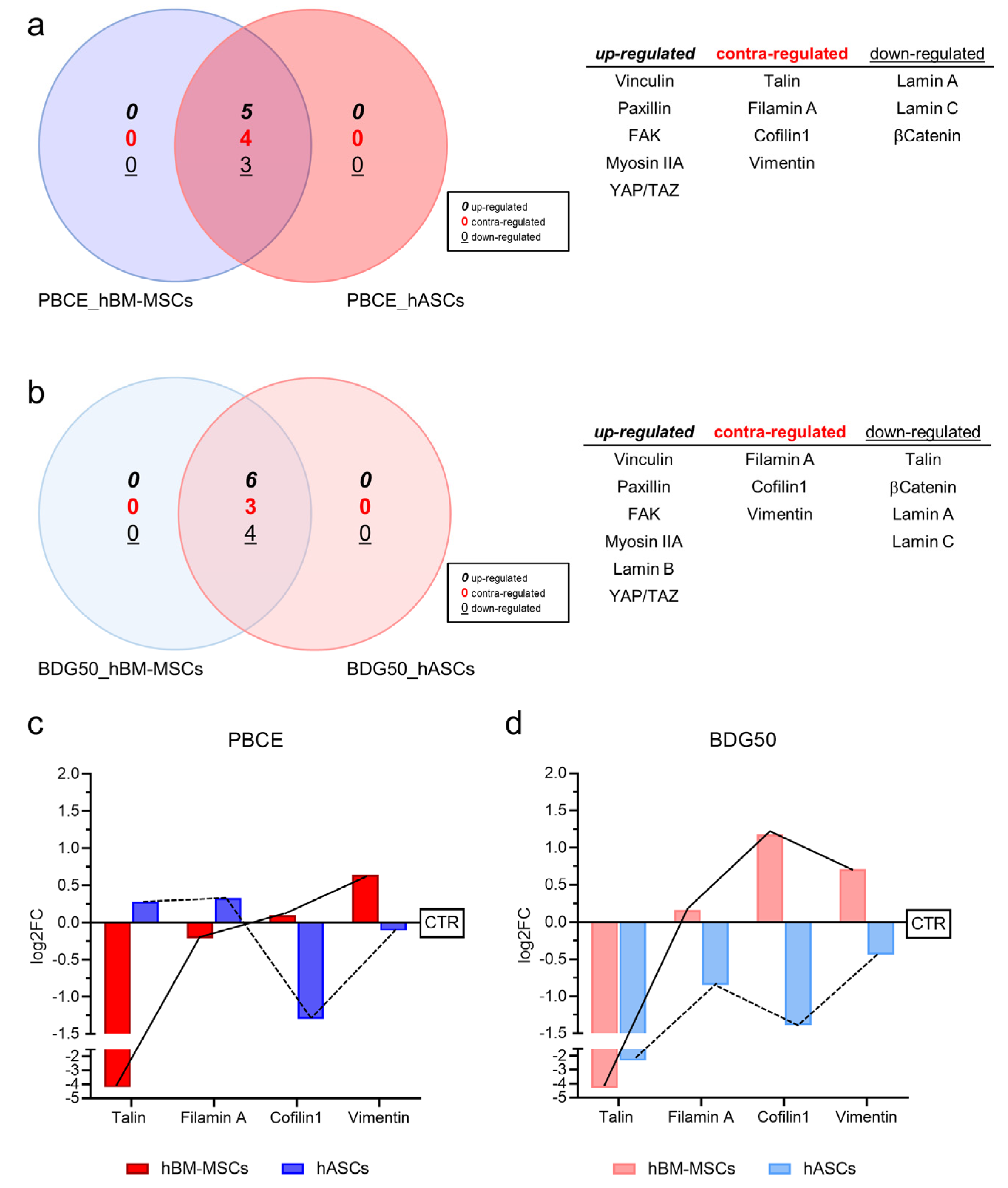
2.13. Venn Diagram Analysis
2.14. Statistical Analysis
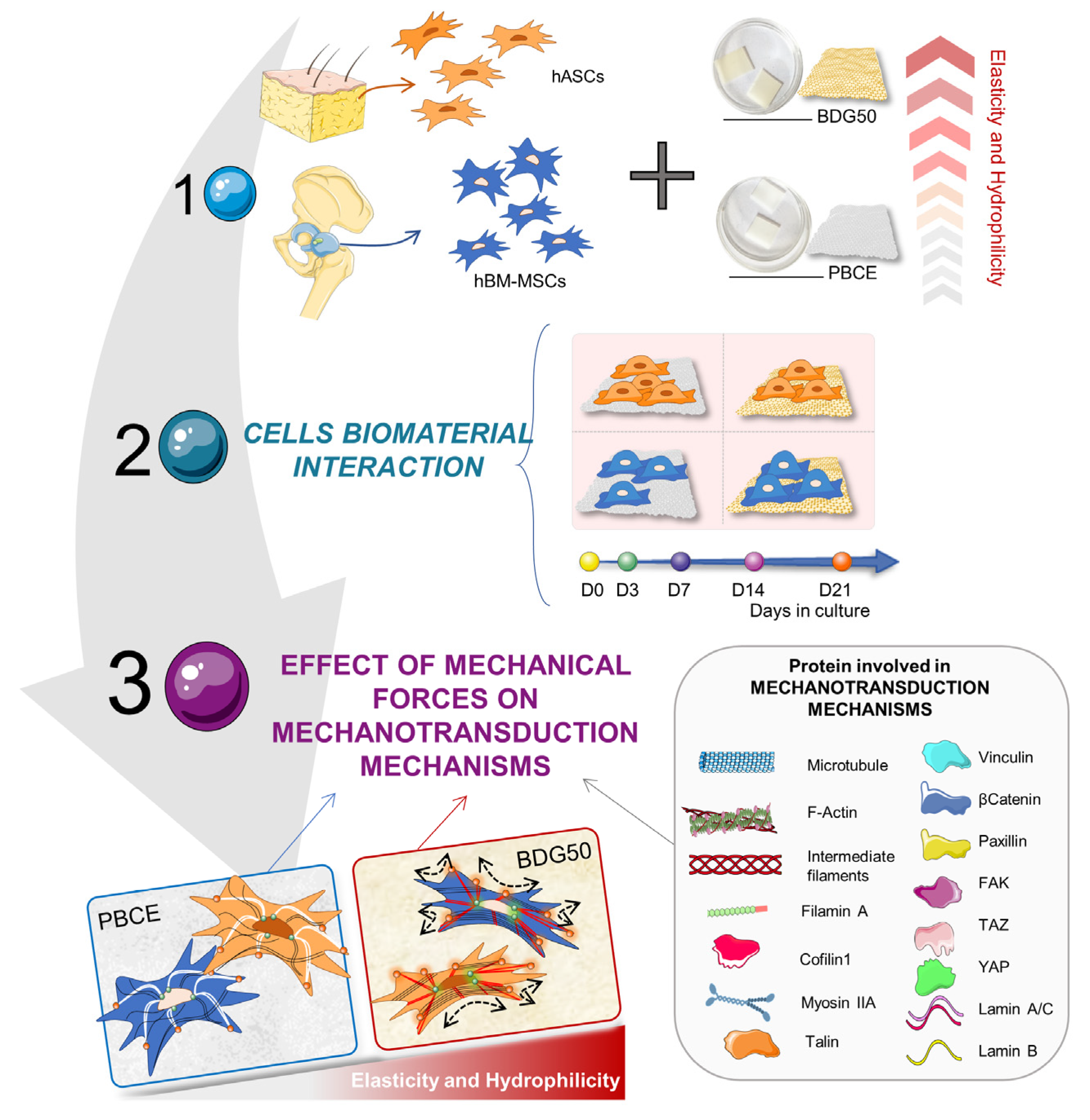
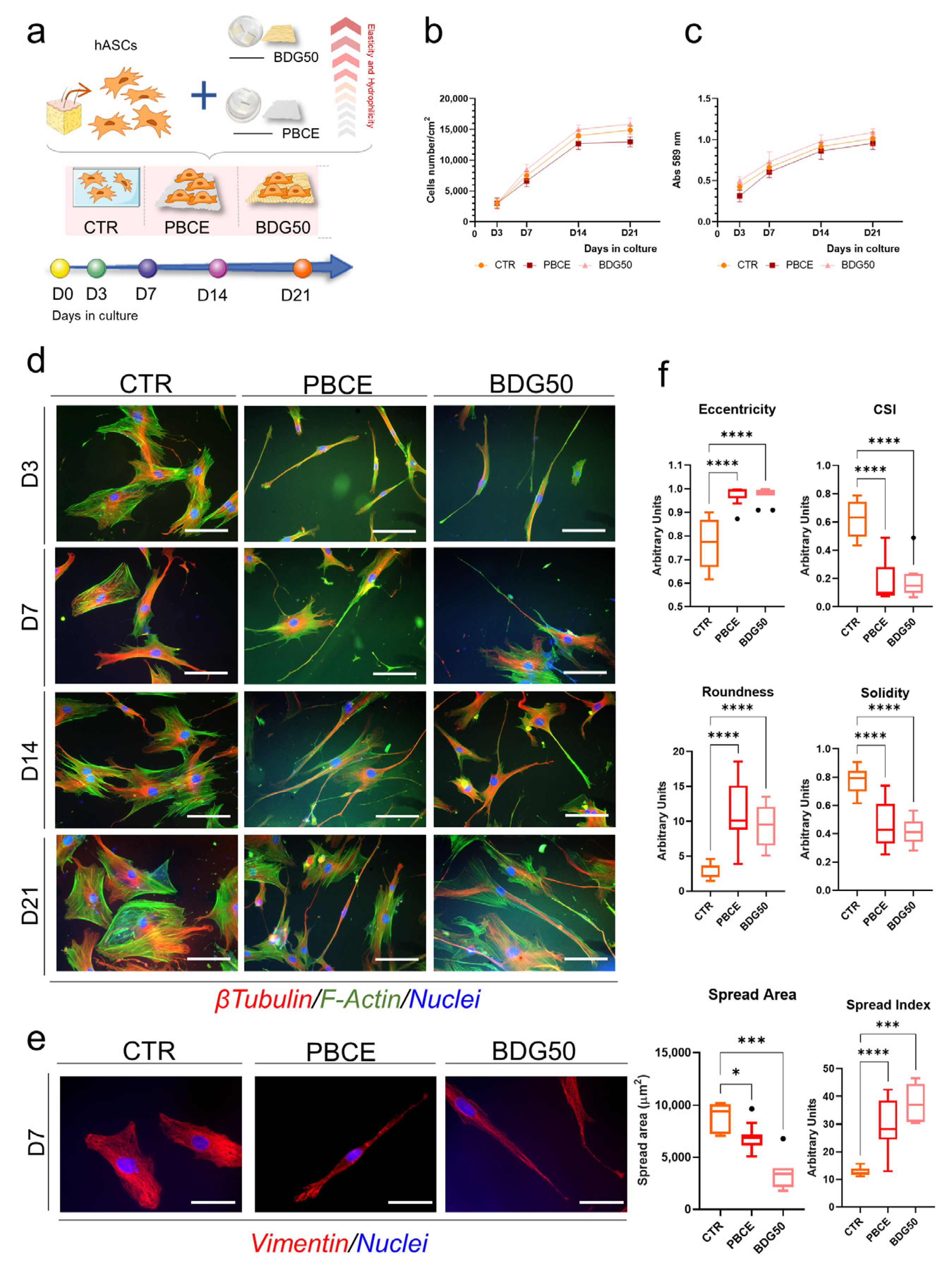
3. Results
3.1. PBCE and BDG50 Film Characterization
3.2. PBCE and BDG50 Are Suitable for the Long-Term Culture of hBM-MSCs and hASCs
3.2.1. hBM-MSCs and hASCs Respond Differently to the Chemical and Physical Properties of PBCE and BDG50
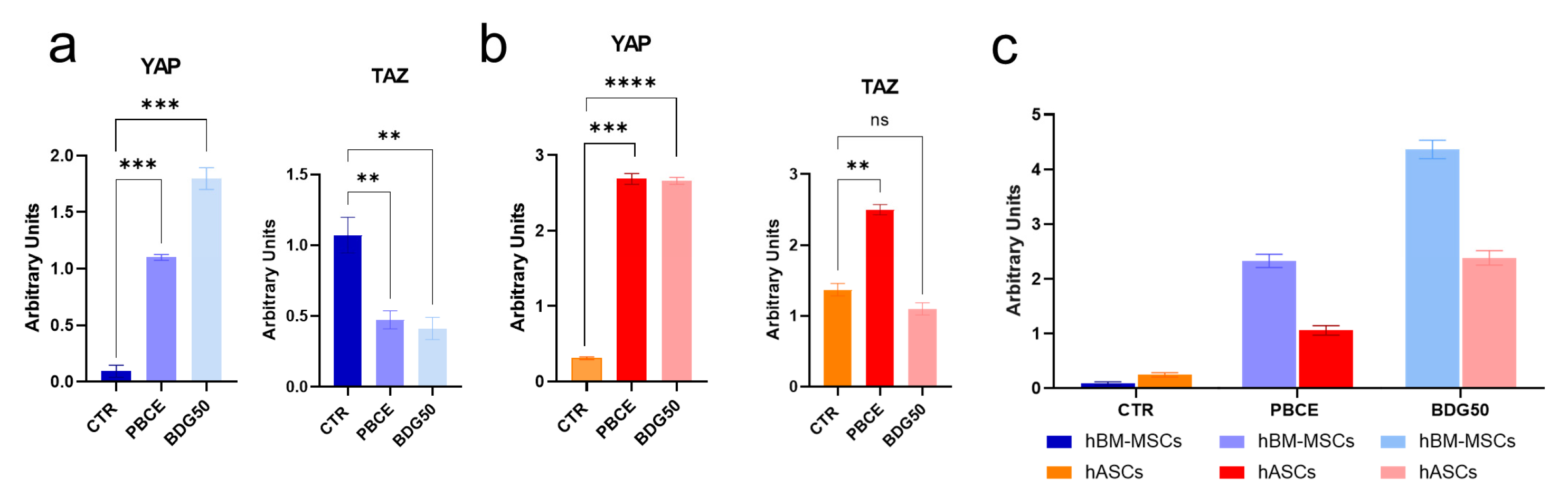
3.2.2. hBM-MSCs and hASCs Respond to PBCE and BDG50 by Activating Distinct Mechanotransduction Pathways
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular mechanotransduction: From tension to function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int. J. Mol. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Imran, S.A.M.; Noordin, K.B.A.A.; Zaman, W.S.W.K.; Nordin, F. Mechanotransduction in Mesenchymal Stem Cells (MSCs) Differentiation: A Review. Int. J. Mol. Sci. 2022, 23, 4580. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Ayad, N.M.E.; Kaushik, S.; Weaver, V.M. Tissue mechanics, an important regulator of development and disease. Philos. Trans. R. Soc. B 2019, 374, 20180215. [Google Scholar] [CrossRef]
- Tortorella, I.; Argentati, C.; Emiliani, C.; Morena, F.; Martino, S. Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis. Cells 2022, 11, 3093. [Google Scholar] [CrossRef]
- Petzold, J.; Gentleman, E. Intrinsic Mechanical Cues and Their Impact on Stem Cells and Embryogenesis. Front. Cell Dev. Biol. 2021, 9, 3112. [Google Scholar] [CrossRef]
- Alonso, J.L.; Goldmann, W.H.; Alonso, J.L.; Goldmann, W.H. Cellular mechanotransduction. AIMS Biophys. 2016, 3, 50–62. [Google Scholar] [CrossRef]
- Hayward, M.K.; Muncie, J.M.; Weaver, V.M. Tissue mechanics in stem cell fate, development, and cancer. Dev. Cell 2021, 56, 1833–1847. [Google Scholar] [CrossRef]
- Hu, D.; Dong, Z.; Li, B.; Lu, F.; Li, Y. Mechanical Force Directs Proliferation and Differentiation of Stem Cells. Tissue Eng. Part B Rev. 2022, 29, 141–150. [Google Scholar] [CrossRef]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10, 2. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Wang, T. How the mechanical microenvironment of stem cell growth affects their differentiation: A review. Stem Cell Res. Ther. 2022, 13, 415. [Google Scholar] [CrossRef]
- Dunn, S.L.; Olmedo, M.L.; Dunn, S.L.; Olmedo, M.L. Mechanotransduction: Relevance to Physical Therapist Practice—Understanding Our Ability to Affect Genetic Expression Through Mechanical Forces. Phys. Ther. 2016, 96, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Frittoli, E.; Palamidessi, A.; Iannelli, F.; Zanardi, F.; Villa, S.; Barzaghi, L.; Abdo, H.; Cancila, V.; Beznoussenko, G.V.; Della Chiara, G.; et al. Tissue fluidification promotes a cGAS–STING cytosolic DNA response in invasive breast cancer. Nat. Mater. 2023, 22, 644. [Google Scholar] [CrossRef]
- Tassinari, R.; Olivi, E.; Cavallini, C.; Taglioli, V.; Zannini, C.; Marcuzzi, M.; Fedchenko, O.; Ventura, C. Mechanobiology: A landscape for reinterpreting stem cell heterogeneity and regenerative potential in diseased tissues. iScience 2023, 26, 105875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Habibovic, P.; Zhang, Y.; Habibovic, P. Delivering Mechanical Stimulation to Cells: State of the Art in Materials and Devices Design. Adv. Mater. 2022, 34, 2110267. [Google Scholar] [CrossRef] [PubMed]
- Argentati, C.; Dominici, F.; Morena, F.; Rallini, M.; Tortorella, I.; Ferrandez-Montero, A.; Pellegrino, R.M.; Ferrari, B.; Emiliani, C.; Lieblich, M.; et al. Thermal treatment of magnesium particles in polylactic acid polymer films elicits the expression of osteogenic differentiation markers and lipidome profile remodeling in human adipose stem cells. Int. J. Biol. Macromol. 2022, 223, 684–701. [Google Scholar] [CrossRef]
- Abbott, R.D.; Kaplan, D.L. Engineering Biomaterials for Enhanced Tissue Regeneration. Curr. Stem Cell Rep. 2016, 2, 140–146. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Wang, J.; Pei, X.; Chen, J.; Wan, Q. Alginate-based biomaterial-mediated regulation of macrophages in bone tissue engineering. Int. J. Biol. Macromol. 2023, 230, 123246. [Google Scholar] [CrossRef]
- Morille, M.; Toupet, K.; Montero-Menei, C.N.; Jorgensen, C.; Noël, D. PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials 2016, 88, 60–69. [Google Scholar] [CrossRef]
- Novikova, L.N.; Kolar, M.K.; Kingham, P.J.; Ullrich, A.; Oberhoffner, S.; Renardy, M.; Doser, M.; Müller, E.; Wiberg, M.; Novikov, L.N. Trimethylene carbonate-caprolactone conduit with poly-p-dioxanone microfilaments to promote regeneration after spinal cord injury. Acta Biomater. 2018, 66, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Allur Subramanian, S.; Oh, S.; Mariadoss, A.V.A.; Chae, S.; Dhandapani, S.; Parasuraman, P.S.; Song, S.Y.; Woo, C.; Dong, X.; Choi, J.Y.; et al. Tunable mechanical properties of Mo3Se3-poly vinyl alcohol-based/silk fibroin-based nanowire ensure the regeneration mechanism in tenocytes derived from human bone marrow stem cells. Int. J. Biol. Macromol. 2022, 210, 196–207. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Armentano, I.; Cacciotti, I.; Tiribuzi, R.; Quattrocelli, M.; Del Gaudio, C.; Fortunati, E.; Saino, E.; Caraffa, A.; Cerulli, G.G.; et al. Tuning multi/pluri-potent stem cell fate by electrospun poly(L-lactic acid)-calcium-deficient hydroxyapatite nanocomposite mats. Biomacromolecules 2012, 13, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Armentano, I.; Montanucci, P.; Argentati, C.; Fortunati, E.; Montesano, S.; Bicchi, I.; Pescara, T.; Pennoni, I.; Mattioli, S.; et al. Design of a nanocomposite substrate inducing adult stem cell assembly and progression toward an Epiblast-like or Primitive Endoderm-like phenotype via mechanotransduction. Biomaterials 2017, 144, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Soccio, M.; Bicchi, I.; Luzi, F.; Torre, L.; Munari, A.; Emiliani, C.; Gigli, M.; Lotti, N.; et al. Unpatterned Bioactive Poly(Butylene 1,4-Cyclohexanedicarboxylate)-Based Film Fast Induced Neuronal-Like Differentiation of Human Bone Marrow-Mesenchymal Stem Cells. Int. J. Mol. Sci. 2020, 21, 9274. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, J.; Cui, W.; Tong, C.; Yin, Z.; Tong, C.; Wang, J.; Cui, W. Advanced Biomaterials for Promoting Endometrial Regeneration. Adv. Healthc. Mater. 2023, 2202490. [Google Scholar] [CrossRef]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Huang, C.M.; Kang, Y.H.; Tsai, C.H.; Shyu, W.C.; Lin, S.Z. Differentiation of stem cells: Strategies for modifying surface biomaterials. Cell Transplant. 2011, 20, 37–47. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Fontana, C.; Tortorella, I.; Emiliani, C.; Latterini, L.; Zampini, G.; Martino, S. Functionalized Silica Star-Shaped Nanoparticles and Human Mesenchymal Stem Cells: An In Vitro Model. Nanomaterials 2021, 11, 779. [Google Scholar] [CrossRef]
- Ben Abdeljawad, M.; Carette, X.; Argentati, C.; Martino, S.; Gonon, M.F.; Odent, J.; Morena, F.; Mincheva, R.; Raquez, J.M. Interfacial Compatibilization into PLA/Mg Composites for Improved In Vitro Bioactivity and Stem Cell Adhesion. Molecules 2021, 26, 5944. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Montanucci, P.; Rallini, M.; Basta, G.; Calabrese, N.; Calafiore, R.; Cordellini, M.; Emiliani, C.; Armentano, I.; et al. Surface Hydrophilicity of Poly(l-Lactide) Acid Polymer Film Changes the Human Adult Adipose Stem Cell Architecture. Polymer 2018, 10, 140. [Google Scholar] [CrossRef]
- Naqvi, S.M.; McNamara, L.M. Stem Cell Mechanobiology and the Role of Biomaterials in Governing Mechanotransduction and Matrix Production for Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 597661. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Challenges with the Development of Biomaterials for Sustainable Tissue Engineering. Front. Bioeng. Biotechnol. 2019, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Merrick, D.; Sakers, A.; Irgebay, Z.; Okada, C.; Calvert, C.; Morley, M.P.; Percec, I.; Seale, P. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 2019, 364, eaav2501. [Google Scholar] [CrossRef]
- Argentati, C.; Tortorella, I.; Bazzucchi, M.; Morena, F.; Martino, S. Harnessing the Potential of Stem Cells for Disease Modeling: Progress and Promises. J. Pers. Med. 2020, 10, 8. [Google Scholar] [CrossRef]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Seo, E.Y.; Chen, J.Y.; Lo, D.; McArdle, A.; Sinha, R.; Tevlin, R.; Seita, J.; Vincent-Tompkins, J.; Wearda, T.; et al. Identification and specification of the mouse skeletal stem cell. Cell 2015, 160, 285–298. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Bazzucchi, M.; Armentano, I.; Emiliani, C.; Martino, S. Adipose Stem Cell Translational Applications: From Bench-to-Bedside. Int. J. Mol. Sci. 2018, 19, 3475. [Google Scholar] [CrossRef]
- Gigli, M.; Lotti, N.; Vercellino, M.; Visai, L.; Munari, A. Novel ether-linkages containing aliphatic copolyesters of poly(butylene 1,4-cyclohexanedicarboxylate) as promising candidates for biomedical applications. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014, 34, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Bloise, N.; Berardi, E.; Gualandi, C.; Zaghi, E.; Gigli, M.; Duelen, R.; Ceccarelli, G.; Cortesi, E.E.; Costamagna, D.; Bruni, G.; et al. Ether-Oxygen Containing Electrospun Microfibrous and Sub-Microfibrous Scaffolds Based on Poly(butylene 1,4-cyclohexanedicarboxylate) for Skeletal Muscle Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 3212. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, L.; Gualandi, C.; Antonioli, D.; Soccio, M.; Liguori, A.; Laus, M.; Lotti, N.; Boccafoschi, F.; Focarete, M.L. Elastomeric Electrospun Scaffolds of a Biodegradable Aliphatic Copolyester Containing PEG-Like Sequences for Dynamic Culture of Human Endothelial Cells. Biomolecules 2020, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hughes, R.; Mullin, N.; Hawkins, R.J.; Holen, I.; Brown, N.J.; Hobbs, J.K. Mechanical Heterogeneity in the Bone Microenvironment as Characterized by Atomic Force Microscopy. Biophys. J. 2020, 119, 502–513. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Morena, F.; Argentati, C.; Calzoni, E.; Cordellini, M.; Emiliani, C.; D’Angelo, F.; Martino, S. Ex-Vivo Tissues Engineering Modeling for Reconstructive Surgery Using Human Adult Adipose Stem Cells and Polymeric Nanostructured Matrix. Nanomaterials 2016, 6, 57. [Google Scholar] [CrossRef]
- Luzi, F.; Tortorella, I.; Di Michele, A.; Dominici, F.; Argentati, C.; Morena, F.; Torre, L.; Puglia, D.; Martino, S. Novel Nanocomposite PLA Films with Lignin/Zinc Oxide Hybrids: Design, Characterization, Interaction with Mesenchymal Stem Cells. Nanomaterials 2020, 10, 2176. [Google Scholar] [CrossRef]
- Bicchi, I.; Morena, F.; Argentati, C.; Nodari, L.R.; Emiliani, C.; Gelati, M.; Vescovi, A.L.; Martino, S. Storage of mutant human sod1 in non-neural cells from the type-1 amyotrophic lateral sclerosis ratg93a model correlated with the lysosomes’ dysfunction. Biomedicines 2021, 9, 1080. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schwendy, M.; Unger, R.E.; Bonn, M.; Parekh, S.H. Automated cell segmentation in FIJI® using the DRAQ5 nuclear dye. BMC Bioinform. 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; Emiliani, C.; Tancini, B.; Severini, G.M.; Chigorno, V.; Bordignon, C.; Sonnino, S.; Orlacchio, A. Absence of metabolic cross-correction in Tay-Sachs cells: Implications for gene therapy. J. Biol. Chem. 2002, 277, 20177–20184. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Argentati, C.; Trotta, R.; Crispoltoni, L.; Stabile, A.; Pistilli, A.; di Baldassarre, A.; Calafiore, R.; Montanucci, P.; Basta, G.; et al. A Comparison of Lysosomal Enzymes Expression Levels in Peripheral Blood of Mild- and Severe-Alzheimer’s Disease and MCI Patients: Implications for Regenerative Medicine Approaches. Int. J. Mol. Sci. 2017, 18, 1806. [Google Scholar] [CrossRef]
- Cai, H.; Chen, H.; Yi, T.; Daimon, C.M.; Boyle, J.P.; Peers, C.; Maudsley, S.; Martin, B. VennPlex–A Novel Venn Diagram Program for Comparing and Visualizing Datasets with Differentially Regulated Datapoints. PLoS ONE 2013, 8, e53388. [Google Scholar] [CrossRef] [PubMed]
- Atherton, P.; Lausecker, F.; Carisey, A.; Gilmore, A.; Critchley, D.; Barsukov, I.; Ballestrem, C. Force-independent interactions of talin and vinculin govern integrin-mediated mechanotransduction. bioRxiv 2019. preprint. bioRxiv: 629683. [Google Scholar] [CrossRef]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate filaments at the junction of mechanotransduction, migration, and development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The role of vimentin intermediate filaments in the progression of lung cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Legerstee, K.; Geverts, B.; Slotman, J.A.; Houtsmuller, A.B. Dynamics and distribution of paxillin, vinculin, zyxin and VASP depend on focal adhesion location and orientation. Sci. Rep. 2019, 9, 10460. [Google Scholar] [CrossRef]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef]
- Stutchbury, B.; Atherton, P.; Tsang, R.; Wang, D.Y.; Ballestrem, C. Distinct focal adhesion protein modules control different aspects of mechanotransduction. J. Cell Sci. 2017, 130, 1612–1624. [Google Scholar] [CrossRef]
- Stachowiak, M.R.; Smith, M.A.; Blankman, E.; Chapin, L.M.; Balcioglu, H.E.; Wang, S.; Beckerle, M.C.; O’Shaughnessy, B. A mechanical-biochemical feedback loop regulates remodeling in the actin cytoskeleton. Proc. Natl. Acad. Sci. USA 2014, 111, 17528–17533. [Google Scholar] [CrossRef] [PubMed]
- Malashicheva, A.; Perepelina, K. Diversity of Nuclear Lamin A/C Action as a Key to Tissue-Specific Regulation of Cellular Identity in Health and Disease. Front. Cell Dev. Biol. 2021, 9, 2834. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Reguant, L.; Blanco, E.; Galan, S.; Le Dily, F.; Cuartero, Y.; Serra-Bardenys, G.; Di Carlo, V.; Iturbide, A.; Cebrià-Costa, J.P.; Nonell, L.; et al. Lamin B1 mapping reveals the existence of dynamic and functional euchromatin lamin B1 domains. Nat. Commun. 2018, 9, 3420. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, T.; Kim, E.; Riehl, B.D.; Esfahani, A.M.; Rosenbohm, J.; Yang, R.; Duan, B.; Lim, J.Y. The LINC complex, mechanotransduction, and mesenchymal stem cell function and fate. J. Biol. Eng. 2019, 13, 68. [Google Scholar] [CrossRef]
- Cai, X.; Wang, K.C.; Meng, Z. Mechanoregulation of YAP and TAZ in Cellular Homeostasis and Disease Progression. Front. Cell Dev. Biol. 2021, 9, 1333. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–184. [Google Scholar] [CrossRef]
- Mitra, S.K.; Hanson, D.A.; Schlaepfer, D.D. Focal adhesion kinase: In command and control of cell motility. Nat. Rev. Mol. Cell Biol. 2005, 6, 56–68. [Google Scholar] [CrossRef]
- Peng, X.; Cuff, L.E.; Lawton, C.D.; DeMali, K.A. Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J. Cell Sci. 2010, 123, 567–577. [Google Scholar] [CrossRef]
- Carisey, A.; Tsang, R.; Greiner, A.M.; Nijenhuis, N.; Heath, N.; Nazgiewicz, A.; Kemkemer, R.; Derby, B.; Spatz, J.; Ballestrem, C. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr. Biol. 2013, 23, 271–281. [Google Scholar] [CrossRef]
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing stem cell niches for differentiation and self-renewal. J. R. Soc. Interface 2018, 15, 20180388. [Google Scholar] [CrossRef]
- Kerch, G. Polymer hydration and stiffness at biointerfaces and related cellular processes. Nanomedicine 2018, 14, 13–25. [Google Scholar] [CrossRef]
- Romani, P.; Valcarcel-Jimenez, L.; Frezza, C.; Dupont, S. Crosstalk between mechanotransduction and metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 22–38. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. StemJournal 2019, 1, 1–25. [Google Scholar] [CrossRef]
- Fernandes, T.G. Design and Fabrication of Artificial Stem Cell Niches. Bioengineering 2022, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Zan, F.; Wei, Q.; Fang, L.; Xian, M.; Ke, Y.; Wu, G. Role of Stiffness versus Wettability in Regulating Cell Behaviors on Polymeric Surfaces. ACS Biomater. Sci. Eng. 2020, 6, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Guo, Z. Bioinspired surfaces with wettability: Biomolecule adhesion behaviors. Biomater. Sci. 2020, 8, 1502–1535. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.Y.M.; Yangben, Y.; Lin, N.J.; Zhong, J.L.; Yang, L. Relationships among cell morphology, intrinsic cell stiffness and cell-substrate interactions. Biomaterials 2013, 34, 9754–9762. [Google Scholar] [CrossRef] [PubMed]
- Abedi, H.; Zachary, I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997, 272, 15442–15451. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Liu, M.; An, Z.; Li, K.; Gu, X.; Li, P.; Fan, Y. Enhanced hydrophilicity of one-step electrosprayed red blood cell-like PLGA microparticles by block polymer PLGA-PEG-PLGA with excellent magnetic-luminescent bifunction and affinity to HUVECs. Eur. Polym. J. 2023, 191, 112040. [Google Scholar] [CrossRef]
- Fekete, N.; Béland, A.V.; Campbell, K.; Clark, S.L.; Hoesli, C.A. Bags versus flasks: A comparison of cell culture systems for the production of dendritic cell–based immunotherapies. Transfusion 2018, 58, 1800–1813. [Google Scholar] [CrossRef]
- Wang, P.-H.; Mao, B.-H.; Mai Nguyen Thi, K.; Tang, M.-J.; Kamm, R.D.; Tu, T.-Y. The interface stiffness and topographic feature dictate interfacial invasiveness of cancer spheroids. Biofabrication 2023, 15, 015023. [Google Scholar] [CrossRef]
- Du, J.; Wang, Z.; Liu, X.; Hu, C.; Yarema, K.J.; Jia, X. Improving Schwann Cell Differentiation from Human Adipose Stem Cells with Metabolic Glycoengineering. Cells 2023, 12, 1190. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Nakamura, F. Actin-Associated Proteins and Small Molecules Targeting the Actin Cytoskeleton. Int. J. Mol. Sci. 2022, 23, 2118. [Google Scholar] [CrossRef]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Wiggan, O.; Shaw, A.E.; DeLuca, J.G.; Bamburg, J.R. ADF/cofilin regulates actomyosin assembly through competitive inhibition of myosin II binding to F-actin. Dev. Cell 2012, 22, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Bugyi, B.; Carlier, M.F. Control of actin filament treadmilling in cell motility. Annu. Rev. Biophys. 2010, 39, 449–470. [Google Scholar] [CrossRef]
- Ono, S. Cofilin-induced structural changes in actin filaments stay local. Proc. Natl. Acad. Sci. USA 2020, 117, 3349–3351. [Google Scholar] [CrossRef]
- Tanaka, K.; Takeda, S.; Mitsuoka, K.; Oda, T.; Kimura-Sakiyama, C.; Maéda, Y.; Narita, A. Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 2018, 9, 1860. [Google Scholar] [CrossRef]
- Hayakawa, K.; Tatsumi, H.; Sokabe, M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J. Cell Biol. 2011, 195, 721–727. [Google Scholar] [CrossRef]
- Dickmanns, A.; Kehlenbach, R.H.; Fahrenkrog, B. Nuclear Pore Complexes and Nucleocytoplasmic Transport: From Structure to Function to Disease. Int. Rev. Cell Mol. Biol. 2015, 320, 171–233. [Google Scholar] [CrossRef] [PubMed]
- Byfield, F.J.; Wen, Q.; Levental, I.; Nordstrom, K.; Arratia, P.E.; Miller, R.T.; Janmey, P.A. Absence of Filamin A Prevents Cells from Responding to Stiffness Gradients on Gels Coated with Collagen but not Fibronectin. Biophys. J. 2009, 96, 5095. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Kang, X.; An, H.; Lv, Y.; Liu, X. The function and pathogenic mechanism of filamin A. Gene 2021, 784, 145575. [Google Scholar] [CrossRef] [PubMed]
- Bamburg, J.R.; Bernstein, B.W. Roles of ADF/cofilin in actin polymerization and beyond. F1000 Biol. Rep. 2010, 2, 62. [Google Scholar] [CrossRef]
- Goldman, R.D.; Khuon, S.; Chou, Y.H.; Opal, P.; Steinert, P.M. The function of intermediate filaments in cell shape and cytoskeletal integrity. J. Cell Biol. 1996, 134, 971–983. [Google Scholar] [CrossRef]
- Kumar, A.; Ouyang, M.; Van den Dries, K.; McGhee, E.J.; Tanaka, K.; Anderson, M.D.; Groisman, A.; Goult, B.T.; Anderson, K.I.; Schwartz, M.A. Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J. Cell Biol. 2016, 213, 371–383. [Google Scholar] [CrossRef]
- Austen, K.; Ringer, P.; Mehlich, A.; Chrostek-Grashoff, A.; Kluger, C.; Klingner, C.; Sabass, B.; Zent, R.; Rief, M.; Grashoff, C. Extracellular rigidity sensing by talin isoform-specific mechanical linkages. Nat. Cell Biol. 2015, 17, 1597–1606. [Google Scholar] [CrossRef]



| Antibody | Dilution | Source | Company |
|---|---|---|---|
| Talin | 1:1000 | RABBIT | Cell Signaling Technology, Danvers, MA, USA |
| Paxillin | 1:1000 | RABBIT | Cell Signaling Technology, Danvers, MA, USA |
| Vinculin | 1:1000 | RABBIT | Abcam, Cambridge, UK |
| FAK | 1:200 | RABBIT | Santa Cruz Biotechnology, CA, USA |
| Β-Catenin | 1:200 | RABBIT | Elabscience, Houston, TX, USA |
| Vimentin | 1:1000 | RABBIT | Cell Signaling Technology, Danvers, MA, USA |
| Myosin IIA | 1:1000 | RABBIT | Santa Cruz Biotechnology, CA, USA |
| Filamin A | 1:500 | MOUSE | Santa Cruz Biotechnology, CA, USA |
| Cofilin1 | 1:200 | RABBIT | Santa Cruz Biotechnology, CA, USA |
| Lamin A/C | 1:1000 | MOUSE | Cell Signaling Technology, Danvers, MA, USA |
| Lamin B | 1:200 | GOAT | Santa Cruz Biotechnology, CA, USA |
| YAP | 1:1000 | MOUSE | Cell Signaling Technology, Danvers, MA, USA |
| TAZ | 1:1000 | RABBIT | Cell Signaling Technology, Danvers, MA, USA |
| Actin | 1:200 | RABBIT | Sigma Aldrich, St. Louis, MI, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argentati, C.; Morena, F.; Guidotti, G.; Soccio, M.; Lotti, N.; Martino, S. Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness. Cells 2023, 12, 1746. https://doi.org/10.3390/cells12131746
Argentati C, Morena F, Guidotti G, Soccio M, Lotti N, Martino S. Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness. Cells. 2023; 12(13):1746. https://doi.org/10.3390/cells12131746
Chicago/Turabian StyleArgentati, Chiara, Francesco Morena, Giulia Guidotti, Michelina Soccio, Nadia Lotti, and Sabata Martino. 2023. "Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness" Cells 12, no. 13: 1746. https://doi.org/10.3390/cells12131746
APA StyleArgentati, C., Morena, F., Guidotti, G., Soccio, M., Lotti, N., & Martino, S. (2023). Tight Regulation of Mechanotransducer Proteins Distinguishes the Response of Adult Multipotent Mesenchymal Cells on PBCE-Derivative Polymer Films with Different Hydrophilicity and Stiffness. Cells, 12(13), 1746. https://doi.org/10.3390/cells12131746