Statins in Chronic Kidney Disease—Effects on Atherosclerosis and Cellular Senescence
Abstract
1. Introduction
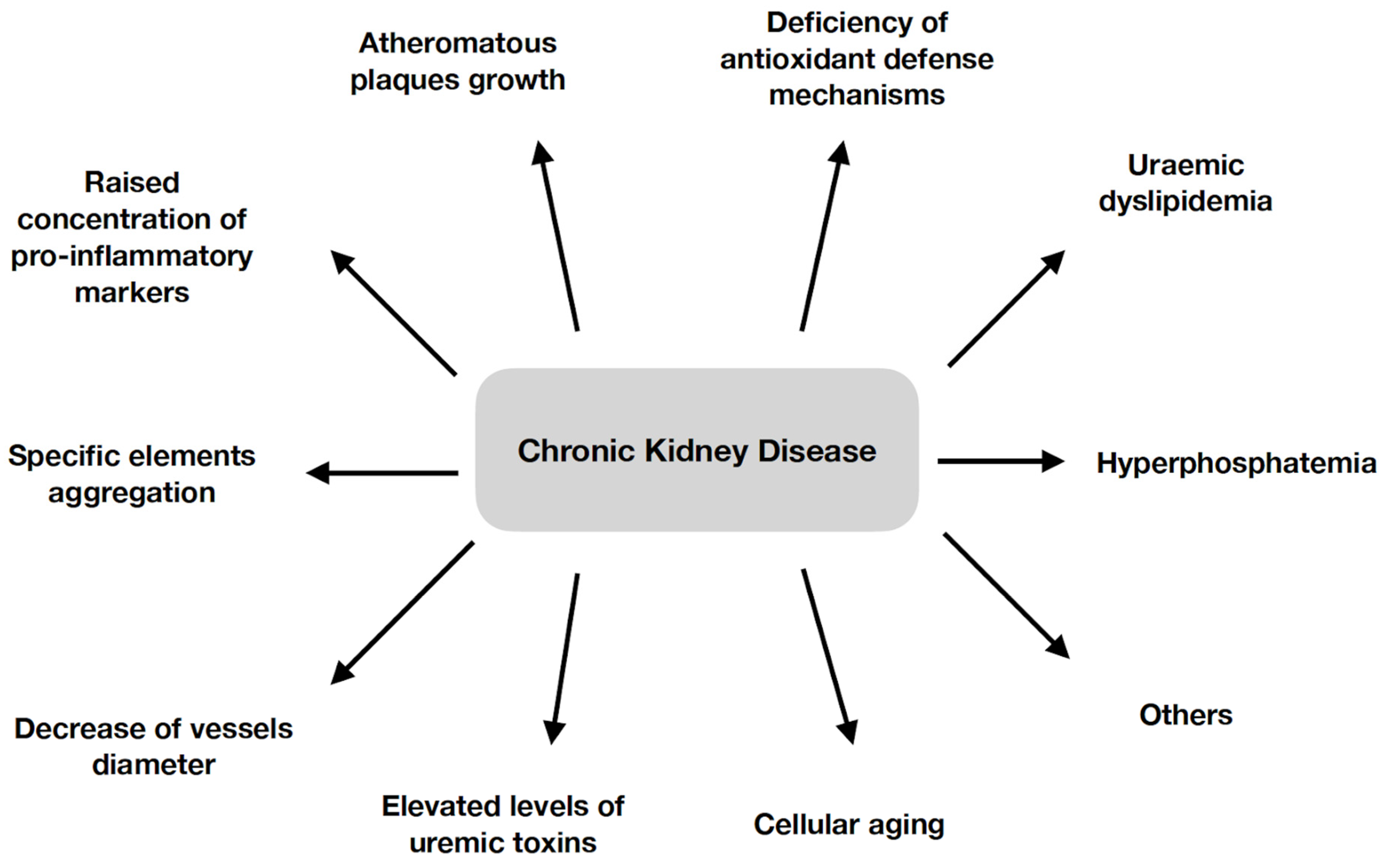
2. Development of Atherosclerosis in Chronic Kidney Disease
3. Statins in Chronic Kidney Disease
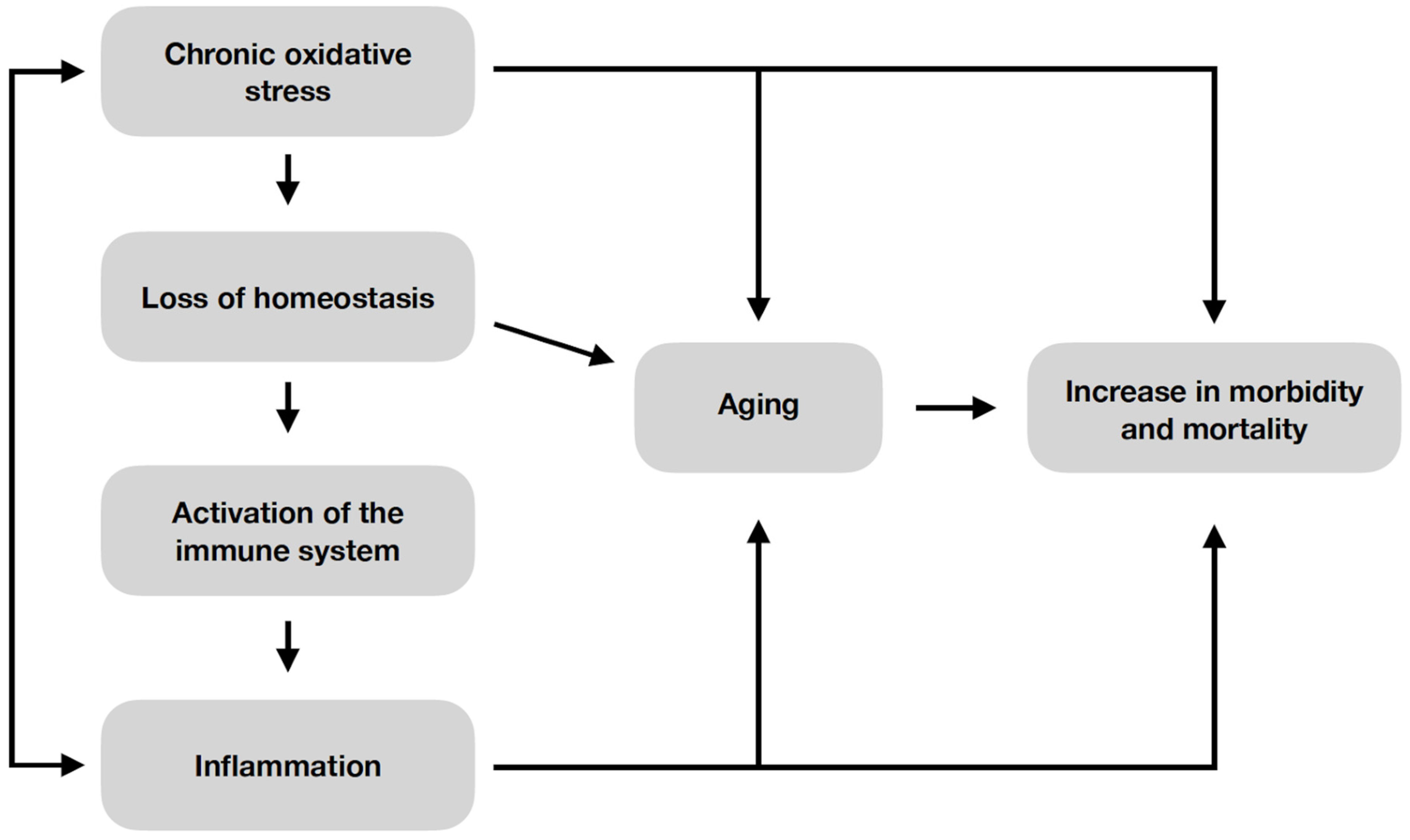
4. Senescence
5. Correlation between Cellular Senescence Process and Atherosclerosis in Chronic Kidney Disease
6. Effects of the Use of Statins on the Process of Cellular Senescence
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Docherty, M.H.; O’Sullivan, E.D.; Bonventre, J.V.; Ferenbach, D.A. Cellular Senescence in the Kidney. J. Am. Soc. Nephrol. 2019, 30, 726–736. [Google Scholar] [CrossRef]
- Shmulevich, R.; Krizhanovsky, V. Cell Senescence, DNA Damage, and Metabolism. Antioxid. Redox Signal. 2021, 34, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Liu, Y.; Men, H.; Zheng, Y. Protective Mechanism of Humanin Against Oxidative Stress in Aging-Related Cardiovascular Diseases. Front. Endocrinol. 2021, 12, 683151. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Simon, F.; Trollet, C.; Santibañez, J.F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies 2016. Oxid. Med. Cell. Longev. 2017, 2017, 4310469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88 Pt B, 314–336. [Google Scholar] [CrossRef]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef]
- Mehdi, M.M.; Solanki, P.; Singh, P. Oxidative stress, antioxidants, hormesis and calorie restriction: The current perspective in the biology of aging. Arch. Gerontol. Geriatr. 2021, 95, 104413. [Google Scholar] [CrossRef]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Sarniak, A.; Lipińska, J.; Tytman, K.; Lipińska, S. Endogenous mechanisms of reactive oxygen species (ROS) generation. Postepy Hig. Med. Dosw. 2016, 70, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Cellular senescence in cardiac diseases. J. Cardiol. 2019, 74, 313–319. [Google Scholar] [CrossRef]
- Shafi, O. Switching of vascular cells towards atherogenesis, and other factors contributing to atherosclerosis: A systematic review. Thromb. J. 2020, 18, 28. [Google Scholar] [CrossRef]
- Jabarpour, M.; Rashtchizadeh, N.; Argani, H.; Ghorbanihaghjo, A.; Ranjbarzadhag, M.; Sanajou, D.; Panah, F.; Alirezaei, A. The impact of dyslipidemia and oxidative stress on vasoactive mediators in patients with renal dysfunction. Int. Urol. Nephrol. 2019, 51, 2235–2242. [Google Scholar] [CrossRef]
- Mok, Y.; Ballew, S.H.; Matsushita, K. Chronic kidney disease measures for cardiovascular risk prediction. Atherosclerosis 2021, 335, 110–118. [Google Scholar] [CrossRef]
- Six, I.; Flissi, N.; Lenglet, G.; Louvet, L.; Kamel, S.; Gallet, M.; Massy, Z.A.; Liabeuf, S. Uremic Toxins and Vascular Dysfunction. Toxins 2020, 12, 404. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Larsson, T.E. Chronic kidney disease: A clinical model of premature aging. Am. J. Kidney Dis. 2013, 62, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of Cardiovascular Disorders in Patients with Chronic Kidney Disease: A Process Related to Accelerated Senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Du, C.; Wang, X.; Wang, X.Y.; Han, W.; Wang, Y.; Qiao, Y.; Zhu, Y.; Ran, L.; Liu, Y.; et al. Mitochondrial Damage-Induced Innate Immune Activation in Vascular Smooth Muscle Cells Promotes Chronic Kidney Disease-Associated Plaque Vulnerability. Adv. Sci. 2021, 8, 2002738. [Google Scholar] [CrossRef]
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef]
- Bourrier, M.; Ferguson, T.W.; Embil, J.M.; Rigatto, C.; Komenda, P.; Tangri, N. Peripheral Artery Disease: Its Adverse Consequences With and Without CKD. Am. J. Kidney Dis. 2020, 75, 705–712. [Google Scholar] [CrossRef]
- Speer, T.; Ridker, P.M.; von Eckardstein, A.; Schunk, S.J.; Fliser, D. Lipoproteins in chronic kidney disease: From bench to bedside. Eur. Heart J. 2021, 42, 2170–2185. [Google Scholar] [CrossRef]
- Simeoni, M.; Borrelli, S.; Garofalo, C.; Fuiano, G.; Esposito, C.; Comi, A.; Provenzano, M. Atherosclerotic-nephropathy: An updated narrative review. J. Nephrol. 2021, 34, 125–136. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Z.; Ouyang, N.; Ruan, X. Hyperphosphatemia and Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 9, 644363. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tandon, S.; Tandon, C. An update on vascular calcification and potential therapeutics. Mol. Biol. Rep. 2021, 48, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Sevinc, C.; Yilmaz, G.; Ustundag, S. The relationship between calcification inhibitor levels in chronic kidney disease and the development of atherosclerosis. Ren. Fail. 2021, 43, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. In Renal Fibrosis: Mechanisms and Therapies. Advances in Experimental Medicine and Biology; Liu, B.C., Lan, H.Y., Lv, L.L., Eds.; Springer: Singapore, 2019; Volume 1165. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Qiu, Y.; Wu, L.; Wang, H.; Wu, L.; Zhao, L.; Xie, D. Statins Have an Anti-Inflammation in CKD Patients: A Meta-Analysis of Randomized Trials. BioMed Res. Int. 2022, 2022, 4842699. [Google Scholar] [CrossRef]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Gunde-Cimerman, N.; Cimerman, A. Pleurotus fruiting bodies contain the inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase-lovastatin. Exp Mycol. 1995, 19, 1–6. [Google Scholar] [CrossRef]
- Takemoto, M.; Liao, J.K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1712–1719. [Google Scholar] [CrossRef]
- García-Fernández-Bravo, I.; Torres-Do-Rego, A.; López-Farré, A.; Galeano-Valle, F.; Demelo-Rodriguez, P.; Alvarez-Sala-Walther, L.A. Undertreatment or Overtreatment With Statins: Where Are We? Front. Cardiovasc. Med. 2022, 9, 808712. [Google Scholar] [CrossRef]
- Agarwal, R. Effects of statins on renal function. Mayo Clin. Proc. 2007, 82, 1381–1390, Erratum in Mayo Clin. Proc. 2007, 82, 1579. [Google Scholar] [CrossRef]
- Turner, A.J.; Hick, P.E. Inhibition of aldehyde reductase by acidic metabolites of the biogenic amines. Biochem. Pharmacol. 1975, 24, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Iseki, K.; Yamazato, M.; Tozawa, M.; Takishita, S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002, 61, 1887–1893. [Google Scholar] [CrossRef] [PubMed]
- Janda, S.; Young, A.; Fitzgerald, J.M.; Etminan, M.; Swiston, J. The effect of statins on mortality from severe infections and sepsis: A systematic review and meta-analysis. J. Crit. Care 2010, 25, e7–e22. [Google Scholar] [CrossRef] [PubMed]
- Tleyjeh, I.M.; Kashour, T.; Hakim, F.A.; Zimmerman, V.A.; Erwin, P.J.; Sutton, A.J.; Ibrahim, T. Statins for the prevention and treatment of infections: A systematic review and meta-analysis. Arch. Intern. Med. 2009, 169, 1658–1667, Erratum in Arch Intern Med. 2010, 170, 42. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Sang, Y.; Ballew, S.H.; Astor, B.C.; Hoogeveen, R.C.; Solomon, S.D.; Ballantyne, C.M.; Woodward, M.; Coresh, J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: The Atherosclerosis Risk in Communities study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1770–1777. [Google Scholar] [CrossRef]
- Esmeijer, K.; Dekkers, O.M.; de Fijter, J.W.; Dekker, F.W.; Hoogeveen, E.K. Effect of different types of statins on kidney function decline and proteinuria: A network meta-analysis. Sci Rep. 2019, 9, 16632. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Mosieniak, G. A common signature of cellular senescence; does it exist? Ageing Res. Rev. 2021, 71, 101458. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Targeting cellular senescence with senotherapeutics: Senolytics and senomorphics. FEBS J. 2023, 290, 1362–1383. [Google Scholar] [CrossRef]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and senomorphics: Natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes. Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Motoji, Y.; Fukazawa, R.; Matsui, R.; Abe, Y.; Uehara, I.; Watanabe, M.; Hashimoto, Y.; Miyagi, Y.; Nagi-Miura, N.; Tanaka, N.; et al. Statins Show Anti-Atherosclerotic Effects by Improving Endothelial Cell Function in a Kawasaki Disease-like Vasculitis Mouse Model. Int. J. Mol. Sci. 2022, 23, 16108. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, J.; Badimon, L. Influence of statin use on endothelial function: From bench to clinics. Curr. Pharm. Des. 2007, 13, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Girndt, M.; Seibert, E. Premature cardiovascular disease in chronic renal failure (CRF): A model for an advanced ageing process. Exp. Gerontol. 2010, 45, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, X.; Teng, T.; Ma, Z.G.; Tang, Q.Z. Cellular Senescence in Cardiovascular Diseases: A Systematic Review. Aging Dis. 2022, 13, 103–128. [Google Scholar] [CrossRef]
- Kousios, A.; Kouis, P.; Panayiotou, A.G. Matrix Metalloproteinases and Subclinical Atherosclerosis in Chronic Kidney Disease: A Systematic Review. Int. J. Nephrol. 2016, 2016, 9498013. [Google Scholar] [CrossRef]
- Merino, A.; Buendia, P.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Carracedo, J. Senescent CD14+CD16+ monocytes exhibit proinflammatory and proatherosclerotic activity. J. Immunol. 2011, 186, 1809–1815. [Google Scholar] [CrossRef]
- Ho, C.L.B.; Chih, H.J.; Garimella, P.S.; Matsushita, K.; Jansen, S.; Reid, C.M. Prevalence and risk factors of peripheral artery disease in a population with chronic kidney disease in Australia: A systematic review and meta-analysis. Nephrology 2021, 26, 798–808. [Google Scholar] [CrossRef]
- McCullough, P.A.; Sandberg, K.R.; Dumler, F.; Yanez, J.E. Determinants of coronary vascular calcification in patients with chronic kidney disease and end-stage renal disease: A systematic review. J. Nephrol. 2004, 17, 205–215. [Google Scholar]
- Bahrami, A.; Bo, S.; Jamialahmadi, T.; Sahebkar, A. Effects of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors on ageing: Molecular mechanisms. Ageing Res. Rev. 2020, 58, 101024. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Urbich, C.; Aicher, A.; Hofmann, W.K.; Haendeler, J.; Rössig, L.; Spyridopoulos, I.; Zeiher, A.M.; Dimmeler, S. HMG-CoA reductase inhibitors reduce senescence and increase proliferation of endothelial progenitor cells via regulation of cell cycle regulatory genes. Circ. Res. 2003, 92, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, H.; Onodera, A. Selective Growth Suppressive Effect of Pravastatin on Senescent Human Lung Fibroblasts. Pharmazie 2022, 77, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Uppal, H.; Demaria, M.; Desprez, P.Y.; Campisi, J.; Kapahi, P. Simvastatin suppresses breast cancer cell proliferation induced by senescent cells. Sci. Rep. 2015, 5, 17895. [Google Scholar] [CrossRef] [PubMed]
- Marcheggiani, F.; Cirilli, I.; Orlando, P.; Silvestri, S.; Vogelsang, A.; Knott, A.; Blatt, T.; Weise, J.M.; Tiano, L. Modulation of Coenzyme Q10 content and oxidative status in human dermal fibroblasts using HMG-CoA reductase inhibitor over a broad range of concentrations. From mitohormesis to mitochondrial dysfunction and accelerated aging. Aging 2019, 11, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Marcheggiani, F.; Kordes, S.; Cirilli, I.; Orlando, P.; Silvestri, S.; Vogelsang, A.; Möller, N.; Blatt, T.; Weise, J.M.; Damiani, E.; et al. Anti-ageing effects of ubiquinone and ubiquinol in a senescence model of human dermal fibroblasts. Free Radic. Biol. Med. 2021, 165, 282–288. [Google Scholar] [CrossRef]

| Disease | SASP Profile |
|---|---|
| Obesity | IL-1α, IL-6, IL-8 |
| Diabetes | IL-1α, IL-6, IL-8 |
| Hypertension | IL-1α, IL-6, IL-8 |
| Atherosclerosis | IL-1α, IL-6, IL-8 |
| Neurodegenerative diseases | p16, MMP i IL-6 |
| Chronic obstructive pulmonary disease | IL-6, IL-8, MMP |
| Biliary cirrhosis | IL-6, IL-8, MMP |
| Cholangitis | IL-6, IL-8, MMP |
| Osteoarthritis | IL-6, IL-8, MMP |
| Anti-Aging Features of the Klotho Protein |
|---|
| Anti-inflammatory effect |
| Protection against oxidative stress |
| Anti-aging of endothelial cells |
| Prevention of vascular calcification |
| Anti-fibrotic properties |
| Suppression of insulin/IGF-1 signaling pathway |
| Regulation of mineral metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fularski, P.; Krzemińska, J.; Lewandowska, N.; Młynarska, E.; Saar, M.; Wronka, M.; Rysz, J.; Franczyk, B. Statins in Chronic Kidney Disease—Effects on Atherosclerosis and Cellular Senescence. Cells 2023, 12, 1679. https://doi.org/10.3390/cells12131679
Fularski P, Krzemińska J, Lewandowska N, Młynarska E, Saar M, Wronka M, Rysz J, Franczyk B. Statins in Chronic Kidney Disease—Effects on Atherosclerosis and Cellular Senescence. Cells. 2023; 12(13):1679. https://doi.org/10.3390/cells12131679
Chicago/Turabian StyleFularski, Piotr, Julia Krzemińska, Natalia Lewandowska, Ewelina Młynarska, Maciej Saar, Magdalena Wronka, Jacek Rysz, and Beata Franczyk. 2023. "Statins in Chronic Kidney Disease—Effects on Atherosclerosis and Cellular Senescence" Cells 12, no. 13: 1679. https://doi.org/10.3390/cells12131679
APA StyleFularski, P., Krzemińska, J., Lewandowska, N., Młynarska, E., Saar, M., Wronka, M., Rysz, J., & Franczyk, B. (2023). Statins in Chronic Kidney Disease—Effects on Atherosclerosis and Cellular Senescence. Cells, 12(13), 1679. https://doi.org/10.3390/cells12131679